Abstract
Introduction
The aim of this study is to evaluate whether early (<8 h) surgical decompression is better in improving neurologic outcomes than late (≥8 h) surgical decompression for traumatic spinal cord injury (tSCI).
Methods
The various electronic databases were used to detect relevant articles published up until May 2016 that compared the outcomes of early versus late surgery for tSCI. Data searching, extraction, analysis, and quality assessment were performed according to Cochrane Collaboration guidelines. The results are presented as relative ratio (RR) for binary outcomes and mean difference (MD) for continuous outcomes with 95% confidence intervals (CIs).
Results
Seven studies were finally included in this meta-analysis. There were significant differences between the 2 groups in neurologic improvement (MD = 0.54, 95% CI = −18.52 to −7.02, P < 0.0001) and length of hospital stay (MD = −12.77, 95% CI = 0.34–0.74, P < 0.00001). However, no significant differences were found between the 2 groups in perioperative complications (OR = 0.95, 95% CI = 0.35–2.61, P = 0.92).
Conclusions
Early surgical decompression within 8 h after tSCI was beneficial in terms of neurologic improvement compared with late surgery. Early surgical decompression (within 8 h) is recommended for patients with tSCI.
Level of evidence
Level III, therapeutic study.
Keywords: Spinal cord injuries, Decompression, Timing of surgery, Meta-analysis
Introduction
The prevalence of traumatic spinal cord injury (tSCI) worldwide is approximately 750 per million, with an annual incidence that appears to be rising.1 SCI is thus a critical issue for spinal surgeons, spinal associations, and national health institutions. Prevention of tSCI and treatment strategies that promote neurologic recovery after tSCI are of increasing importance.
The timing of spinal decompression surgery and mega-dose steroid therapy, which is a common pharmacological intervention, are two of the most critical issues faced by spinal surgeons when dealing with patients with tSCI. However, recent large-scale trials have identified controversies concerning the benefits of mega-dose steroid therapy for tSCI; such treatment is controversial regarding both efficacy and the risk of side effects.2, 3 Thus, as mega-dose steroid therapy still remains controversial in relation to neurologic recovery, the timing of surgical decompression is a more important clinical issue for spinal surgeons.
While the role and timing of surgery after tSCI remain controversial, decompression of the spinal cord, stabilization of the vertebrae, and maintenance of blood perfusion are critical in optimizing clinical outcomes after tSCI. Many experimental studies and clinical case series on tSCI support the concepts of primary and secondary injury, and these concepts are regarded as the mechanisms of neurologic injury.4 On the basis of this notion, many surgeons advocate for early surgical decompression in cases of tSCI. A number of studies have reported the results of early spinal decompression surgery versus late spinal decompression surgery for tSCI and concluded that early surgery for tSCI is better at improving neurologic outcomes.5, 6, 7, 8, 9, 10, 11, 12, 13 Although early decompression surgery for tSCI was significantly associated with improved neurologic outcomes in a recent systematic review and meta-analysis, there are as yet no agreed-upon criteria as to what “early timing” entails. A literature review on surgical timing for tSCI revealed that the definition of early timing varied in studies done between the years 2000 and 2015. The recent trend is to adopt decompression within 24 h of injury as the standard for “early surgery.” This definition has been adopted in most studies done since 2010.14
While many studies used 24 h as the cutoff to compare the clinical outcomes of early and late surgery, some studies used shorter time periods as the cutoff. Experimental animal models and clinical investigations (National Acute Spinal Cord Injury Study (NASCIS) −2 and −3) indicate that the time from injury to 8 h is the optimal therapeutic window for spinal cord decompression.2, 3 In addition, spinal cord ischemia caused by swelling and hemorrhage of both the white and the gray matter reaches the peak locally at 8 h after injury.15 Therefore, on the basis of this time window, the purpose of this meta-analysis is to evaluate whether early (within 8 h after injury) surgical decompression is better at improving neurologic outcomes than is late (8 h or more after injury) surgical decompression. We hypothesized that neurologic improvement after traumatic SCI will be superior if surgical decompression for acute SCI is performed in the first 8 h following injury.
Materials and methods
Study selection
To identify relevant studies, we searched the following databases using the controlled vocabulary and free text words described in Appendix 1: MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, and SCOPUS. We attempted to identify all relevant studies regardless of language, publication type (article, poster, conference article, etc.), and publication date. This search was updated in May 2016 and includes reference lists of the studies and any review articles identified. Searched articles had no restrictions for starting dates.
Eligibility criteria
Studies were included in our meta-analysis if: (1) the subjects of the study were patients with acute SCI; (2) the study compared clinical outcomes of early decompression surgery with those of late decompression surgery; (3) early surgery was defined as decompression within 8 h after injury, and late surgery was defined as decompression after more than 8 h; and (4) clinical outcomes included various factors such as neurologic improvement grade, length of hospital stay, length of stay in an intensive care unit (ICU), days of mechanical ventilation care, complications, and mortality.
Data collection and analysis
The two authors (D.L and D.K) independently assessed the title or abstract of studies identified by the search strategy, and then full papers were assessed for final inclusion. Uncertainty about inclusions was resolved through discussion and consensus. Eligible data was abstracted onto predefined forms by the authors independently, and checked for accuracy. We collected information on study characteristics (information about authors, journal, study design, publication year, study period, country, and sample size); patient demographic data (sex, age, injury level, number of subjects, and follow-up time); clinical outcomes length of hospitalization, length of stay in the ICU, days of mechanical ventilation care, complications, mortality, and neurologic improvement as defined by the American Spinal Injury Association (ASIA) scale; definition of timing of decompression surgery in spinal cord injury; mean and standard deviation (SD) of demographic data, and clinical outcomes in the intervention and control groups.
Assessment of methodological quality
The two authors independently determined, via assessment of the methodological qualities of each study using the Newcastle–Ottawa Quality Assessment Scale, the risk of bias in each study cohort.16 Any disagreements between the authors were resolved through discussion or review by the third author. We did not evaluate publication bias because of low statistical power, as the number of included studies was less than 10.
Statistical analysis
The main outcome of our review is neurologic improvement after decompression surgery between the early and late groups. Neurologic status is based on the ASIA impairment scale, and patients in all included studies were classified into grades A-E (categorical variables) depending on motor and sensory function. To evaluate neurologic improvement, we calculated the relative ratio (RR) in the included studies. Furthermore, for a more precise evaluation of improvement in neurologic status, each subject was given a grade between 1 and 5 (continuous variables). The worst grade, 1, was given to class A subjects, and the best grade, 5, was given to class E subjects. Then, we calculated the mean and SD of neurologic status of the early and late decompression surgery groups and analyzed the differences in neurologic improvement between the groups. The Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford) was used to estimate the overall pooled effect size for each outcome. We conducted a meta-analysis of the included studies using a random-effects model. For continuous outcomes, we conducted weighted mean difference (WMD) analysis using the inverse variance method. For binary outcomes, we calculated the RR between groups using the Mantel-Haenszel method. Statistical heterogeneity among the studies was assessed using I-squared (I2), with values of 25%, 50%, and 75% considered low, moderate, and high, respectively, and Cochrane's Q statistic (Chi-square test) for heterogeneity. P value < 0.10 used to define a significant degree of heterogeneity.
Results
Identification of studies
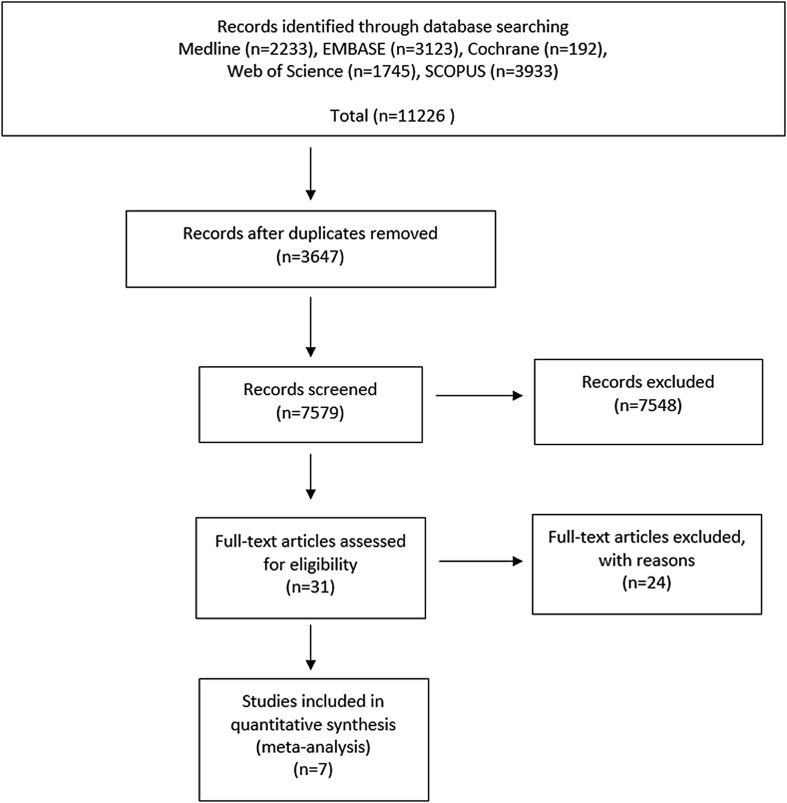
Given the literature search terms, a total of 11,226 relevant articles were initially identified. Of those 11,226 articles, 3647 were duplicated in the databases. After screening the remaining 7579 articles using titles and abstracts, most of the studies were excluded because they were not relevant to the purpose of the present study. So, 31 eligible articles were obtained for full-text review. At last, through full-text review of those 31 articles, 24 articles were excluded after more detailed evaluation because they lacked vital data such as clinical outcomes. The majority of the excluded articles were as follows: (1) did not investigate the effect of the timing of spinal surgical decompression; (2) inappropriate for our study regarding decompression surgery timing; (3) only evaluated patients who underwent decompression surgery within 8 h; (4) only recommended surgical treatment for tSCI within 8 h; or (5) non-surgical treatment was discussed. Finally, 7 articles were included for data extraction and meta-analysis in the present study (Fig. 1).7, 8, 9, 10, 11, 12, 17
Fig. 1.
PRISMA flow diagram.
Quantitative synthesis
Most of the included studies were published in 1999–2015, and they came from 7 different nations. Among the 7 studies, 4 7, 8, 12, 17 (57%) were prospective cohort studies (PCS) and 39, 10, 11 (42%) were retrospective cohort studies (RCS). There were 273 patients in the early (<8 h after injury) surgery group and 377 patients in the late (≥8 h after injury) groups in total. The age and sex of the patients were provided in every study. Subjects who had suffered cervical spinal cord injuries were included in 4 studies8, 9, 11, 12 (57%), and those who had suffered thoracic and lumbar spinal cord injuries were studied in 2 studies7, 10 (28%). A single study17 (14%) did not provide information on the site of injury. The mean follow-up period of the included studies was from 6 months to 67.2 months (Table 1). In addition, use of methylprednisolone before surgery was analyzed in 5 studies7, 8, 11, 12, 17 (71%), the length of hospital stay was reported in 4 studies7, 10, 11, 12 (57%), the length of ICU stay was reported in 3 studies7, 10, 17 (42%), and the use of mechanical ventilator care was reported in 2 studies12, 17 (28%). Detailed information on clinical outcomes was indicated at Table 2.
Table 1.
Study characteristics of the included studies.
| Study | Journal | Study design | Year | Study period | Country | Timing definitions (early/late) | Sample size | Age (years) | Sex (M: F) | Injury level | Follow-up time (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gaebler et al.10 | Spinal cord | RCSa | 1999 | 1985–1992 | Austria | Early: <8 h | 26 | 32.6 | 56: 32 | Thoracic | 67.2 |
| Late: >8 h | 62 | Lumbar | (22.8–111.6) | ||||||||
| Pointillart et al.17 | Spinal cord | PCSb | 2000 | 1990–1995 | France | Early: <8 h | 49 | 30 (20–47) | 108: 12 | – | 12 |
| Late: 8–24 h | 31 | ||||||||||
| Cengiz et al.7 | Arch Orthop Trauma Surg | PCS | 2007 | 2004–2006 | Turkey | Early: <8 h | 12 | 39.67 ± 16.8 | 8: 4 | Thoracic | 14.5 |
| Late: 3–15 days | 15 | 41.44 ± 14.71 | 10: 5 | Lumbar | (12–20) | ||||||
| McCarthy et al.9 | Evidence-Based Spine-Care Journal | RCS | 2011 | Over a 5-year period | Australia | Early: <8 h | 26 | Age matched | Female | Cervical | 6 |
| Late: ≥8 h | 16 | 51.7% | |||||||||
| Chen et al.8 | Neurosurgery-quarterly | PCS | 2012 | – | China | Early: <8 h | 99 | 42.38 ± 13.58 | 1.68: 1 | Cervical | 12 |
| Late: >8 h | 196 | 41.97 ± 13.89 | 1.62: 1 | ||||||||
| Jug et al.12 | Journal of Neurotrauma | PCS | 2015 | 2007–2012 | Slovenia | Early: <8 h | 26 | 44 (30.5–58.5) | 18: 4 | Cervical | 6 |
| Late: 8–24 h | 22 | 52 (25.8–72.8) | 16: 4 | ||||||||
| Grassner et al.11 | Journal of Neurotrauma | RCS | 2015 | 2004–2014 | Germany | Early: <8 h | 35 | 51.9 (±16.4) | 26: 9 | Cervical | 12 |
| Late: ≥8 h | 35 | 50.1 (±18.2) | 33: 2 |
RCS, retrospective cohort study.
PCS, prospective cohort study.
Table 2.
Clinical outcomes between groups in included studies.
| Study | Group | Transferred to hospitals | Used methylprednisolone before surgery | Length of hospital stay (days) | Length of intensive care unit stay (days) | Mechanical ventilation (days) | Complications (n) | Mortality (n) | Neurologic improvement at last follow-up | Improved outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Gaebler et al.10 | Early: <8 h | 55.9 days (11–205) | – | 27.3 (4–101) | 9.6 (1–43) | – | 6 | 7 | Yes | NEUa |
| Late: >8 h | 12 | |||||||||
| Pointillart et al.17 | Early: <8 h | <8 h | Yes | – | 15.5 ± 23.8 | 10.3 ± 16.0 | – | 10 | No | NEU was not significantly different |
| Late: 8–24 h | ||||||||||
| Cengiz et al.7 | Early: <8 h | – | Yes | 12.5 (5–30) | 0 | – | 0 | 0 | Yes | NEU, LOHSb, Complications |
| Late: 3–15 days | 26.0 (14–54) | 0 | 4 | 0 | ||||||
| McCarthy et al.9 | Early: <8 h | – | – | – | – | – | – | 0 | Yes | NEU |
| Late: ≥8 h | 0 | |||||||||
| Chen et al.8 | Early: <8 h | – | Yes | – | – | – | 15 | – | Yes | NEU, Complications |
| Late: >8 h | 40 | |||||||||
| Jug et al.12 | Early: <8 h | 0 (n) | Yes | 38.8 (24.0) | – | 6.5 (1–17) | 3 | 2 | Yes | NEU |
| Late: 8–24 h | 12 (n) | 48.8 (40.3) | 5 (1.25–12) | 5 | 1 | |||||
| Grassner et al.11 | Early: <8 h | – | Yes | 125 (46) | – | – | 4 | 1 | Yes | NEU |
| Late: ≥8 h | 129 (69) | 4 | 1 |
NEU, neurologic improvement.
LOHS, length of hospital stay.
Quality of the included studies
In order to evaluate the methodologic quality of the studies included in our analysis, we used the Newcastle-Ottawa Quality Assessment Scale.16 This scale is a convenient tool for quality assessment of non-randomized studies. The scale assigns a maximum of 9 points for the quality of selection of a study cohort, comparability of cohorts, and assessment of outcomes. As a result, all subjects included in this study presented with a total score of 8 points on the Newcastle–Ottawa Quality Assessment Scale, which indicates that the risk of bias of included studies are low (Table 3).
Table 3.
Newcastle–ottawa quality assessment scales for included studies.
Neurologic improvement
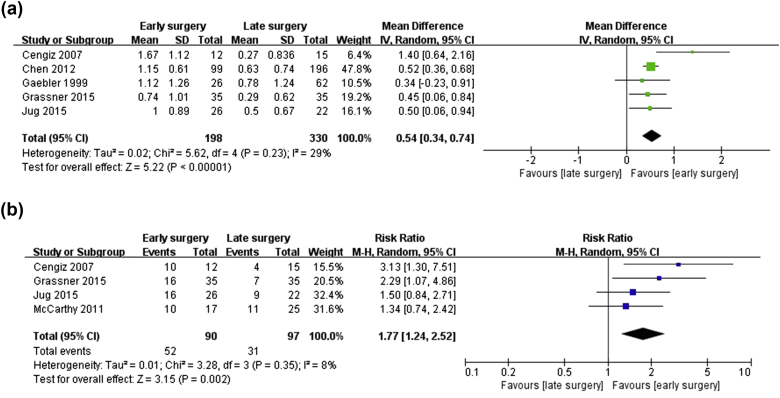
In this meta-analysis, the most critical evaluation basis of clinical outcomes of all 7 included studies was neurologic improvement. When 2 groups were compared in 6 studies7, 8, 9, 10, 11, 12 (86%), the early surgery group presented with significantly increased neurologic improvement, while the remaining study17 (14%) found no significant difference in neurologic improvement between the 2 groups. Even though neurologic improvement was reported in all 7 studies, this meta-analysis only included 5 studies7, 8, 10, 11, 12 (71%) owing to inadequate data. Patients were classified into groups A to E depending on their neurologic examination results. In order to ease calculations, each grade was given a score between 1 and 5. The worst grade, 1, was given for class A, and the best grade, 5, was given for class E. Thus, the degree of neurologic improvement was compared and analyzed by the mean and SD of neurologic grade in 5 studies,7, 8, 10, 11, 12 and the WMD of neurologic improvement between the early and late surgical decompression groups was 0.54 (95% CI: 0.34–0.74, I2 = 29%). In other words, subjects who underwent early decompression surgery gained approximately 0.5 more points of neurologic recovery than did subjects who underwent late decompression surgery (Fig. 2-a). In addition, when an analysis was done based on RR instead of mean and SD, a slightly different selection of studies was made.7, 9, 11, 12 However, in the same manner, the neurologic improvement rate was significantly higher in the early surgery group than in the late surgery group (OR = 1.77, 95% CI = 1.24–2.52, I2 = 8%) (Fig. 2-b). Therefore, two different methods of analysis were used for different types of data, and both led to the conclusion that early surgical decompression for tSCI was associated with increased neurologic improvement during the last-follow up visit.
Fig. 2.
Forest plot of neurologic improvement in the early-surgery group versus the late-surgery group. (a) Neurologic improvement rate given the number of patients (risk ratios, RRs). (b) Degree of neurologic improvement as assessed by improvement score (weighted mean differences, WMD).
Length of hospital stay
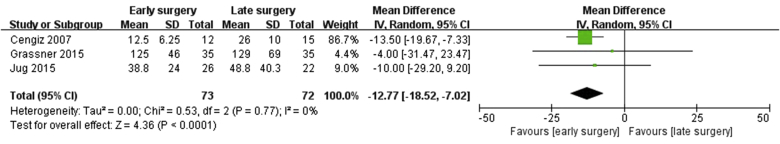
Three studies7, 11, 12 (42%) reported length of hospital stay (73 patients in the early surgery group and 72 patients in the late surgery group). However, 3 studies8, 9, 17 (42%) did not report the length of hospital stay and 1 study10 (14%) only reported the mean length of hospital stay of all groups; thus, the latter 4 studies8, 9, 10, 17 (57%) were excluded from this particular analysis. In addition, although 2 studies10, 17 (28%) reported the length of ICU stay, those data indicated not SD but mean length of hospital stay of all subgroups. So, we could not analyze the effect of surgical timing on the length of ICU stay.
Three studies7, 11, 12 (42%) that included an analysis of the length of hospital stay were not heterogeneous (Chi2 = 0.53; I2 = 0%; p = 0.77). The statistical outcomes of the present study show that a shorter length of hospital stay was detected in the early surgery group compared with the late surgery group. The WMD of the length of hospital stay was −12.77 (95% CI: −18.52, −7.02). This finding means that study subjects who underwent early spinal decompression surgery spent about 13 fewer days in the hospital than did subjects who underwent late surgery (Fig. 3).
Fig. 3.
Forest plot of length of hospital stay (WMD, in days) in the early-surgery group versus the late-surgery group.
Perioperative complications and mortality
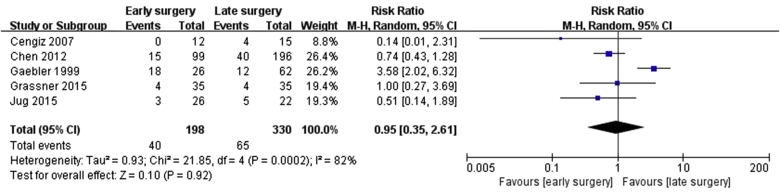
Five studies7, 8, 10, 11, 12 (71%) reported on perioperative complications in early and late surgery groups, consisting of a total of 528 patients (198 patients in the early surgery group and 330 patients in the late surgery group). The other 2 studies9, 17 (28%) did not include information on perioperative complications. Thus, we conducted a meta-analysis of perioperative complications after surgery from the information in those 5 studies. The studies were considered heterogeneous (Chi2 = 21.85; I2 = 82%; p = 0.0002). There were 28 cases of perioperative complications among the 198 patients in the early surgery group and 65 cases among the 330 patients in the late surgery group. However, there were no significant differences in perioperative complications between the early and late surgery groups (OR = 0.95, 95% CI = 0.35–2.61) (Fig. 4).
Fig. 4.
Forest plot of complications after surgery (RRs) in the early-surgery group versus the late-surgery group.
Six studies7, 9, 10, 11, 12, 17 (86%) commented on patient mortality after surgery. However, 2 studies10, 17 (28%) only reported total mortality in all subgroups. Furthermore, another 2 studies7, 9 (28%) reported that none of the patients died after surgery in either group. Thus, a meta-analysis concerning mortality could not be carried out because of insufficient data.
Discussion
Increase in athletic activity, outdoor activity, and the number of traffic accidents has led to an increase in the frequency of tSCI events.18, 19 Traumatic SCI entails social and economic burden, decreases quality of life because of impairments in motor and sensory function, and causes financial problems.20 Thus, research on strategies for restoring neurologic function following tSCI is of great interest to spinal surgeons.
The most important issues for minimizing secondary mechanisms of injury are mega-dose steroid therapy and the timing of surgical decompression. However, the efficacy of mega-dose steroid therapy remains controversial, and side effects from the use of mega-dose steroid therapy prevent it from being authorized in developed countries such as the United State and France, even though it is currently clinically administered.21 Thus, interest in the timing of surgical spinal decompression is on the rise among many spine surgeons, and studies on this issue are making progress. In this study, we performed a meta-analysis of clinical outcomes in accordance with the timing of surgical decompression following tSCI. We concluded that early (within 8 h) surgical decompression led to better neurologic recovery from tSCI than did late surgical decompression, and length of hospital stay appeared to be shorter in the early surgery group. Such results are in agreement with the results of many previous studies on the effect of the timing of spinal surgery after tSCI,6, 13 and also correspond to a recently reported systematic review and meta-analyses on the issue.5, 22, 23 However, in contrast to the results of our study, Liu et al24 reported that early surgical intervention was associated with a higher incidence of mortality and neurological deterioration and did not improve neurological outcomes. Likewise, other authors reported that the timing of surgery does not affect neurological outcomes.17, 25, 26, 27 Therefore, the effect of the timing of surgical decompression on clinical outcomes of patients with tSCI is still under debate, and the issue requires large-scale randomized prospective trials.
One of the limitations of reports on the effect of the timing of surgical decompression for tSCI is that standardized timing is randomly established in each report without there being a consensus on classification into early surgery and late surgery. In an analysis of studies on the timing of surgical decompression in studies published over the course of 2 decades, El Tecle et al14 discovered that the definition of early decompression was highly variable and ranged from 4 h to 96 h after injury. However, an analysis of studies published in the last 5 years (2010–2015) showed that the more recent trend is to adopt decompression within 24 h of injury as a standard for “early decompression”. Likewise, many recent studies13, 28 divided early and late timing using a cut-off point of 24 h, and Liu et al5 reported that early surgical decompression (within 24 h after injury) significantly improved neurologic outcomes compared with late surgery in their meta-analysis. Therefore, many original articles and review articles currently use 24 h as the dividing line between early and late surgery. However, this is merely a recent trend, and there is absolutely no consensus among surgeons, associations, and healthcare institutions, so guidelines for treatment of tSCI patients based on clinical evidence is a matter of essential importance.
Theoretical evidence for early surgical decompression in tSCI patients includes the concept of primary and secondary mechanisms of injury. Primary SCI is the immediate result of dislocation or fracture of structures surrounding vertebral bones. Spinal cord injury may be irreversible, and there is no treatment currently available that reduces the effects of primary injury.4 The primary injury in turn triggers a cascade of secondary mechanisms, including ischemia. Ischemia then causes a cascade of prolonged pathophysiological mechanisms, such as edema, increased excitatory amino acid levels, free radical-mediated injury, electrolyte disturbances, neurotransmitter accumulation, inflammatory mediator production, and lipid peroxidation.4, 29, 30, 31 These events are regarded as secondary mechanisms of SCI. These prolonged secondary mechanisms of injury are the major causes of persistent destruction of the spinal cord. In other words, persistent compression of the spinal cord represents a cause of secondary injury, and if early surgical decompression following primary injury is viable, the injury may potentially be reversible.32, 33 For such reasons, early surgical decompression is essential for minimizing damage to the spinal cord. However, as previously mentioned, how early an “early surgery” must be performed remains unknown, and, though there is a published meta-analysis5 stating that early surgical decompression within 24 h is beneficial, there have been no meta-analysis on surgical decompression performed earlier than 24 h. In experimental animal studies, early surgical decompression in the first 6–8 h after injury enhances the chances of recovery.34, 35, 36 In addition, Donnelly et al15 reported spinal cord ischemia caused by swelling and hemorrhage reaches the peak locally at 8 h after injury. Given these results, an 8-h therapeutic window was established, because it is close to the median time to treatment. In another study, Grossman et al37 suggested that if patients are immediately transported to a trauma center, the majority reach the center within 4 h, so 8 h until surgical decompression is a realistic goal. Therefore, based on such results, we also extracted and analyzed eligible studies in which surgical spinal decompression was performed within 8 h of injury, and we found that early surgical decompression in tSCI patients presented with better clinical outcomes (better neurologic improvement and shorter length of hospital stay), which we consider another line of clinical evidence supporting early management. In addition, not to delay early surgery for tSCI, patients suspected of spinal cord injury should be first transferred to a medical center where spine surgery is possible at the field site for early surgical decompression, and the system should be well-equipped to allow early surgery if a spinal cord injury patient arrives.
In this meta-analysis, the incidence of perioperative complications was not significantly different between the early and late surgery groups. Even though their definition of the timing of early surgery differs from ours, Carreon et al23 and McKinley et al38 reported that the early (72 h or 24 h) surgery group had a lower rate of pulmonary complications, and Liu et al5 also reported that early surgical decompression (within 24 h) was associated with fewer complications. However, unlike those studies, our study did not find any significant difference in the incidence of perioperative complications between the two groups, which encourages early surgery for tSCI, contrary to other studies24, 39 that state that the early surgery group had a heightened risk of mortality.
The 7 included studies compared clinical outcomes such as neurologic improvement, length of hospital stay, and perioperative complications of the early versus late surgery groups for tSCI. Quality assessment of the 7 articles included in this study was done according to the Newcastle–Ottawa Quality Assessment Scale, and all studies scored more than 7 points, which indicates that they have low risk of bias of included studies, thus, eligible for meta-analysis. Furthermore, screening and data extraction were done by two independent, blinded reviewers, which is one strength of our study. However, despite its strengths, there are some limitations to the present study. First, a relatively small number of studies were involved in this meta-analysis. Even though studies, both retrospective studies and prospective studies, included in this meta-analysis are not entirely level-I or II studies, the number of previously published original articles about this topic is inadequate after searching literature which is an absolute limitation. Thus, we concluded that every study result could be valuable clinically, it was all included in the process of analysis. Second, the most important aim of this study was to identify the degree of neurologic improvement following surgical intervention. Six out of the 7 included studies7, 8, 9, 10, 11, 12 found that early surgical decompression led to better neurologic improvement, but 1 study17 stated that there was no significant difference in neurologic improvement between the early and late surgery groups. However, a forest plot of neurologic improvement done for the purpose of identifying neurologic improvement excluded that one study, as it provided insufficient data in terms of the early versus late surgery groups. The effects of early surgical decompression based on 5 studies may have overrated neurologic improvement, so we requested more precise data by contacting the authors through e-mail; an absence of data prevented us from conducting proper analysis. For these reasons, excluding the study that found no difference in neurologic improvement between the 2 groups may be a limitation of our study. Furthermore, the main focus of that study was to evaluate the effect of pharmacological therapy and not the time elapsed from tSCI to surgical intervention. Thus, there is an inherent limitation in directly comparing the results of that study with those of the other studies. Third, as mentioned before, mega-dose steroid therapy or other pharmacologic therapies are used in many institutions in the early stages after an injury; however, use of such medications remains controversial. Although a study which was included in this meta-analysis stated that pharmacologic therapy did not affect neurologic improvement in tSCI, medication may act as a confounding factor in confirming the effect of the timing of surgical decompression, so this factor needs to be controlled in future. Fourth, SCI level was not taken into consideration. Four studies analyzed patients with cervical spinal injuries, two included patients with thoracic and lumbar spinal injuries, and the one study did not provide any information on SCI level. Thoracolumbar junctions T11 through L2 contain a mixture of upper and lower motor neurons, and the healing process may differ from that of other spinal cord levels,40 so SCI level also needs to be controlled in future. Furthermore, there were controversies about the effect of completeness of spinal cord injury lesions on neurologic recovery, and the spinal cord injury characteristics (complete versus incomplete spinal cord injury) of the subjects included in this study were not strictly classified for analysis. Time factor-related RCTs may entail moral issues when surgery is delayed for randomization, so well-controlled RCTs may be implausible. However, if the limitations suggested in this study are correctible, future studies may be able to yield high-quality meta-analyses.
In conclusion, the present study demonstrates that in surgical spinal decompression of tSCI, early surgical decompression, within 8 h of injury, is associated with better neurologic outcomes and shorter length of hospital stay. However, more high-quality clinical trials and more RCTs are needed to strengthen our results.
Conflicts of interest
No funds were received in support of this work. The authors declare that there are no conflicts of interest regarding the publication of this paper.
Funding
No funds were received in support of this work. No relevant financial activities outside the submitted work.
Footnotes
Peer review under responsibility of Turkish Association of Orthopaedics and Traumatology.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.aott.2017.12.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
mmc1
References
- 1.Wyndaele M., Wyndaele J.J. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord. 2006;44(9):523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 2.Bracken M.B., Shepard M.J., Collins W.F. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. results of the second national acute spinal cord injury study. N Engl J Med. 1990;322(20):1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 3.Bracken M.B., Shepard M.J., Holford T.R. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. results of the third national acute spinal cord injury randomized controlled trial. National acute spinal cord injury study. JAMA. 1997;277(20):1597–1604. [PubMed] [Google Scholar]
- 4.Amar A.P., Levy M.L. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44(5):1027–1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 5.Liu J.M., Long X.H., Zhou Y., Peng H.W., Liu Z.L., Huang S.H. Is urgent decompression superior to delayed surgery for traumatic spinal cord Injury? A meta-analysis. World Neurosurg. 2016;87:124–131. doi: 10.1016/j.wneu.2015.11.098. [DOI] [PubMed] [Google Scholar]
- 6.Fehlings M.G., Vaccaro A., Wilson J.R. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) PLoS One. 2012;7(2):e32037. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cengiz S.L., Kalkan E., Bayir A., Ilik K., Basefer A. Timing of thoracolomber spine stabilization in trauma patients; impact on neurological outcome and clinical course. a real prospective (rct) randomized controlled study. Arch Orthop Trauma Surg. 2008;128(9):959–966. doi: 10.1007/s00402-007-0518-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen Qi, Li Feng, Fang Zhong. Timing of surgical decompression for acute traumatic cervical spinal cord injury: a multicenter study. Neurosurg Q. 2012;22:61–68. [Google Scholar]
- 9.McCarthy M.J., Gatehouse S., Steel M., Goss B., Williams R. The influence of the energy of trauma, the timing of decompression, and the impact of grade of SCI on outcome. Evid Base Spine Care J. 2011;2(2):11–17. doi: 10.1055/s-0030-1267100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaebler C., Maier R., Kutscha-Lissberg F., Mrkonjic L., Vecsei V. Results of spinal cord decompression and thoracolumbar pedicle stabilisation in relation to the time of operation. Spinal Cord. 1999;37(1):33–39. doi: 10.1038/sj.sc.3100765. [DOI] [PubMed] [Google Scholar]
- 11.Grassner L., Wutte C., Klein B. Early decompression (<8 h) after traumatic cervical spinal cord injury improves functional outcome as assessed by spinal cord independence measure after one year. J Neurotrauma. 2016;33(18):1658–1666. doi: 10.1089/neu.2015.4325. [DOI] [PubMed] [Google Scholar]
- 12.Jug M., Kejzar N., Vesel M. Neurological recovery after traumatic cervical spinal cord injury is superior if surgical decompression and instrumented fusion are performed within 8 hours versus 8 to 24 hours after injury: a single center experience. J Neurotrauma. 2015;32(18):1385–1392. doi: 10.1089/neu.2014.3767. [DOI] [PubMed] [Google Scholar]
- 13.Umerani M.S., Abbas A., Sharif S. Clinical outcome in patients with early versus delayed decompression in cervical spine trauma. Asian Spine J. 2014;8(4):427–434. doi: 10.4184/asj.2014.8.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Tecle N.E., Dahdaleh N.S., Hitchon P.W. Timing of surgery in spinal cord injury. Spine (Phila Pa 1976) 2016;41(16):E995–E1004. doi: 10.1097/BRS.0000000000001517. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly D.J., Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Pointillart V., Petitjean M.E., Wiart L. Pharmacological therapy of spinal cord injury during the acute phase. Spinal Cord. 2000;38(2):71–76. doi: 10.1038/sj.sc.3100962. [DOI] [PubMed] [Google Scholar]
- 18.Chehensse C., Bahrami S., Denys P., Clement P., Bernabe J., Giuliano F. The spinal control of ejaculation revisited: a systematic review and meta-analysis of anejaculation in spinal cord injured patients. Hum Reprod Update. 2013;19(5):507–526. doi: 10.1093/humupd/dmt029. [DOI] [PubMed] [Google Scholar]
- 19.Qin W., Bauman W.A., Cardozo C. Bone and muscle loss after spinal cord injury: organ interactions. Ann N Y Acad Sci. 2010;1211:66–84. doi: 10.1111/j.1749-6632.2010.05806.x. [DOI] [PubMed] [Google Scholar]
- 20.Ackery A., Tator C., Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21(10):1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 21.Greene K.A., Marciano F.F., Sonntag V.K. Pharmacological management of spinal cord injury: current status of drugs designed to augment functional recovery of the injured human spinal cord. J Spinal Disord. 1996;9(5):355–366. [PubMed] [Google Scholar]
- 22.van Middendorp J.J., Hosman A.J., Doi S.A. The effects of the timing of spinal surgery after traumatic spinal cord injury: a systematic review and meta-analysis. J Neurotrauma. 2013;30(21):1781–1794. doi: 10.1089/neu.2013.2932. [DOI] [PubMed] [Google Scholar]
- 23.Carreon L.Y., Dimar J.R. Early versus late stabilization of spine injuries: a systematic review. Spine (Phila Pa 1976) 2011;36(11):E727–E733. doi: 10.1097/BRS.0b013e3181fab02f. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y., Shi C.G., Wang X.W. Timing of surgical decompression for traumatic cervical spinal cord injury. Int Orthop. 2015;39(12):2457–2463. doi: 10.1007/s00264-014-2652-z. [DOI] [PubMed] [Google Scholar]
- 25.Sapkas G.S., Papadakis S.A. Neurological outcome following early versus delayed lower cervical spine surgery. J Orthop Surg (Hong Kong) 2007;15(2):183–186. doi: 10.1177/230949900701500212. [DOI] [PubMed] [Google Scholar]
- 26.Rahimi-Movaghar V., Yazdi A., Karimi M. Effect of decompression on complete spinal cord injury in rats. Int J Neurosci. 2008;118(10):1359–1373. doi: 10.1080/00207450701392340. [DOI] [PubMed] [Google Scholar]
- 27.Pollard M.E., Apple D.F. Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine (Phila Pa 1976) 2003;28(1):33–39. doi: 10.1097/00007632-200301010-00009. [DOI] [PubMed] [Google Scholar]
- 28.McLain R.F., Benson D.R. Urgent surgical stabilization of spinal fractures in polytrauma patients. Spine (Phila Pa 1976) 1999;24(16):1646–1654. doi: 10.1097/00007632-199908150-00005. [DOI] [PubMed] [Google Scholar]
- 29.Fehlings M.G., Agrawal S. Role of sodium in the pathophysiology of secondary spinal cord injury. Spine (Phila Pa 1976) 1995;20(20):2187–2191. doi: 10.1097/00007632-199510001-00002. [DOI] [PubMed] [Google Scholar]
- 30.Genovese T., Mazzon E., Esposito E. Role of endogenous glutathione in the secondary damage in experimental spinal cord injury in mice. Neurosci Lett. 2007;423(1):41–46. doi: 10.1016/j.neulet.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 31.Carlson S.L., Parrish M.E., Springer J.E., Doty K., Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151(1):77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- 32.Dimar J.R., 2nd, Glassman S.D., Raque G.H., Zhang Y.P., Shields C.B. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976) 1999;24(16):1623–1633. doi: 10.1097/00007632-199908150-00002. [DOI] [PubMed] [Google Scholar]
- 33.Rabinowitz R.S., Eck J.C., Harper C.M. Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine (Phila Pa 1976) 2008;33(21):2260–2268. doi: 10.1097/BRS.0b013e31818786db. [DOI] [PubMed] [Google Scholar]
- 34.Fehlings M.G., Tator C.H. An evidence-based review of decompressive surgery in acute spinal cord injury: rationale, indications, and timing based on experimental and clinical studies. J Neurosurg. 1999;91(Suppl. 1):1–11. doi: 10.3171/spi.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- 35.Delamarter R.B., Sherman J., Carr J.B. Pathophysiology of spinal cord injury. recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77(7):1042–1049. doi: 10.2106/00004623-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Guha A., Tator C.H., Endrenyi L., Piper I. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25(4):324–339. doi: 10.1038/sc.1987.61. [DOI] [PubMed] [Google Scholar]
- 37.Grossman R.G., Frankowski R.F., Burau K.D. Incidence and severity of acute complications after spinal cord injury. J Neurosurg Spine. 2012;17(Suppl.1):119–128. doi: 10.3171/2012.5.AOSPINE12127. [DOI] [PubMed] [Google Scholar]
- 38.McKinley W., Meade M.A., Kirshblum S., Barnard B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85(11):1818–1825. doi: 10.1016/j.apmr.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 39.Kerwin A.J., Frykberg E.R., Schinco M.A., Griffen M.M., Murphy T., Tepas J.J. The effect of early spine fixation on non-neurologic outcome. J Trauma. 2005;58(1):15–21. doi: 10.1097/01.ta.0000154182.35386.7e. [DOI] [PubMed] [Google Scholar]
- 40.Tator C.H. Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery. 2006;59(5):957–982. doi: 10.1227/01.NEU.0000245591.16087.89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
mmc1