Abstract
Eukaryotic genomes consist of several repetitive DNAs, including dispersed DNA sequences that move between chromosome sites, tandem repeats of DNA sequences, and multigene families. In this study, repeated sequences isolated from the genome of Characidium gomesi were analyzed and mapped to chromosomes in Characidium zebra and specimens from two populations of C. gomesi. The sequences were transposable elements (TEs) named retroelement of Xiphophorus (Rex); multigene families of U2 small nuclear RNA (U2 snRNA); and histones H1, H3, and H4. Sequence analyses revealed that U2 snRNA contains a major portion corresponding to the Tx1-type non-LTR retrotransposon Keno, the preferential insertion sites of which are U2 snRNA sequences. All histone sequences were found to be associated with TEs. In situ localization revealed that these DNA sequences are dispersed throughout the autosomes of the species, but they are not involved in differentiation of the specific region of the W sex chromosome in C. gomesi. We discuss mechanisms of TE invasion into multigene families that lead to microstructural variation in Characidium genomes.
Keywords: Mobile DNA, histones, karyotype evolution, snRNA, WZ/ZZ
Introduction
The genomes of all studied eukaryotic species primarily consist of repetitive sequences that are dispersed or found in tandem (Sumner, 2003). Repetitive sequences were identified in fragile sites and evolutionary break point regions, promoting non-B DNA conformations and double-strand breaks, which are involved in chromosomal rearrangements (Eichler and Sankoff, 2003; Szamalek, 2005; Wells, 2007; Barros et al., 2017). Repetitive sequences are also responsible for a significant portion of the karyotype variations observed in many groups of organisms (Kidwell, 2002).
Dispersed DNA sequences can move between chromosome sites, with this movement occurring in the presence or absence of RNA as a transposition intermediate (Tollis and Boissinot, 2012). These mobile segments are called transposable elements (TEs) and are classified as retrotransposons (class I elements, RNA intermediates of the transposition process) or transposons (class II elements, DNA intermediates of the transposition process) (Wicker et al., 2007). These mobile elements can drive genetic and genomic evolution and influence eukaryotic gene regulatory systems (Feschotte, 2008). In addition to consisting of dispersed DNA sequences, eukaryotic genomes are also enriched in tandem repeats of DNA sequences (Hardman, 1986) and groups of repeated and linked genes located at the same chromosomal region, shaping clustered but not tandemly repeated genes such as multigene families (Hentschel and Birnstiel, 1981; Heintz et al., 1991).
A multigene family is described as a group of genes with similar functions and sequences that originate from a common ancestral gene (Nei and Rooney, 2005). The U2 small nuclear RNA (U2 snRNA) sequence represents a multigene family of snRNA that control premessenger RNA intron splicing (Nei and Rooney, 2005). Histone genes do not have introns, and they comprise a multigene family in which the five genes are in the same order but separated by spacer DNA (Hentschel and Birnstiel, 1981). In the rainbow trout (Salmo gairdneri), the histones are present in the order of H4-H2B-H1-H2A-H3, and they are transcribed from the same strand (Connor et al., 1984).
Concerning genome diversification, fish represent an important group for studies of genetic variability. The genus Characidium (Characiformes: Crenuchidae) presents a diversified karyotype microstructure despite its conserved karyotype macrostructure and prevalent diploid number (2n) of 50 (Centofante et al., 2001, 2003; Vicari et al., 2008; Pazian et al., 2013; Scacchetti et al., 2015a; Pucci et al., 2016; Serrano et al., 2017). The Characidium species studied to date exhibited differences mainly in the number of ribosomal DNA sites and sex chromosomes (Pansonato-Alves et al., 2010, 2011, 2014; Pucci et al., 2014; Scacchetti et al., 2015a, Utsunomia et al., 2017), as well as an interesting dynamic of repetitive DNAs (Scacchetti et al., 2015b; Pucci et al., 2016).
The primary goal of this study was to perform sequence analyses and chromosome mapping of some repeated sequences isolated from the genome of C. gomesi. Retroelement of Xiphophorus (Rex) TEs were mapped to chromosomes to elucidate their possible involvement in Characidium karyotype evolution and diversification. The multigene families of U2 snRNA and histones H1, H3, and H4 were also investigated through chromosome mapping and sequence analyses. Our study revealed associations between TEs and the multigene families. The obtained results will improve our understanding of the evolution and diversification of Characidium genomes.
Materials and Methods
Sampling and chromosome preparation
Individuals of the following species were collected at the indicated locations: C. zebra (15 specimens; Paiol Grande Stream, São Bento do Sapucaí, SP) and C. gomesi (nine specimens; Paiol Grande Stream, São Bento do Sapucaí, SP/five specimens; São João River, Carambeí, PR). Chromosomes for analyses were obtained using the ‘air-drying’ procedure (Bertollo et al., 1978). The analyzed specimens were then deposited in the following ichthyology museums: Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupelia), Universidade Estadual de Maringá, and Museu Nacional, Rio de Janeiro, Brazil, voucher numbers (NUP 14577-14580; MNRJ 29183). The processing was performed in accordance with the Ethical Committee on Animal Use (CEUA 29/2016) of the Universidade Estadual de Ponta Grossa and current Brazilian legislation. Chromosome preparations were subjected to conventional Giemsa staining to determine 2n and the chromosome formula.
Sequence isolation
The analyzed sequences were synthesized by polymerase chain reaction (PCR) using genomic DNA from C. gomesi (São João River population), and the reaction mixtures consisted of 100-200 ng of genomic DNA, 0.04-0.2 μM primers, 0.04-0.16 mMdNTPs, 1 U of Taq DNA Polymerase (Invitrogen, Waltham, MA, USA), and 1.5 mM MgCl2 in a 1 reaction buffer (200 mM Tris, pH 8.4, 500 mM KCl). The specific PCR mixtures and primers sequences are summarized in Table S1 (137.1KB, pdf) . The PCR conditions were as follows: (i) Rex1 and Rex3 probes: 95 °C for 5 min, 35 cycles of 95 °C for 1 min, 55 °C for 40 s and 72 °C for 2 min, and a final extension at 72 °C for 5 min; (ii) U2 snRNA probe: 95 °C for 45 s, 30 cycles of 95 °C for 45 s, 52 °C for 45 s and 72 °C for 80 s, and a final extension at 72 °C for 7 min; and (iii) histones H1, H3, and H4: 95 °C for 5 min, 30 cycles of 95 °C for 30 s, 52 °C for 45 s and 72 °C for 80 s, and a final extension at 72 °C for 7 min.
TEs and multigene family sequences: Sequencing and analyses
After the amplification reactions, the PCR products were purified using the GenElute PCR Clean-Up Kit (Sigma Aldrich, St Louis, MO, USA). Rex1 and Rex3 sequences were cloned using pGEM®-T Easy Vector Systems (Promega, Madison, WI, USA). The obtained clones were sequenced using an ABI-PRISM Genetic Analyzer (Applied Biosystems, Carlsbad, CA, USA). The sequences were edited and analyzed using Geneious 7.1.3 software (Kearse et al., 2012), and their identities were confirmed using the CENSOR tool for repeated sequences (Girinst) (Kohany et al., 2006) and BLASTn (NCBI). Finally, the sequences were deposited in GenBank (Table S2 (71.5KB, pdf) ).
Probe preparation
The sequences of Rex3, U2 snRNA, and histones H1 and H4 were labeled with digoxigenin via nick translation using DIG-Nick Translation Mix (Roche Applied Science, Penzberg, Germany), and those of Rex1 and H3 were bio-tinylated using Biotin-Nick Translation Mix (Roche Applied Science). A C. gomesi W-specific chromosome probe was constructed as described by Machado et al. (2011), labeled with digoxigenin 11-dUTP (Roche Applied Science), and used in fluorescence in situ hybridization (FISH) to identify sex chromosomes in the karyotypes.
FISH
Chromosome spreads were subjected to FISH using the constructed probes. FISH was performed under a high stringency of approximately 76% (2.5 ng/μL of each probe, 50% formamide, 2 SSC, 10% dextran sulfate, pH 7.0–7.2, 37 °C overnight) following the general procedure described by Pinkel et al. (1986). Signal detection was performed using an anti-streptavidin antibody conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA) and an anti-digoxigenin antibody conjugated to rhodamine (Roche Applied Science). Chromosomes were counterstained with 4'6-diamidino-2-phenylindole (0.2 μg/mL) in Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA) and observed under an epifluorescence microscope.
Karyotype analysis
Approximately 20 metaphases were analyzed for each species, and karyotypes were determined from the highest-quality images. Chromosomes were classified as metacentric, submetacentric, subtelocentric, or acrocentric according to the arm ratio (Levan et al., 1964) and arranged by decreasing size in the karyotypes.
Results
Analyses of partial sequences of TEs and multigene families
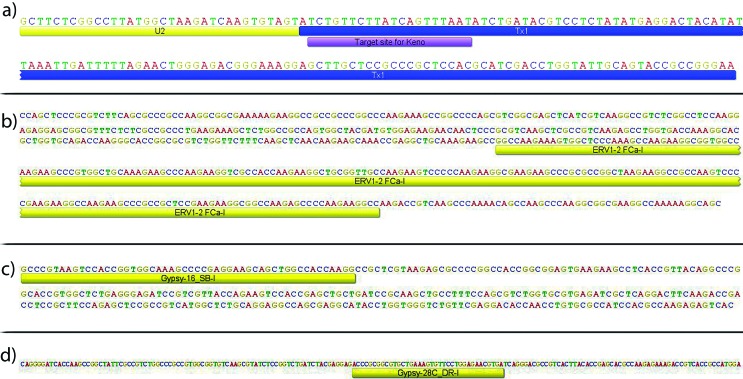
The partial sequences of Rex1, Rex3, U2 snRNA, and the H1, H3, and H4 genes were isolated from the genomes of C. gomesi and C. zebra, and consensus sequence of each gene was constructed (Table S2 (71.5KB, pdf) ). When analyzed using the CENSOR tool, the multigene family sequences displayed high proportions of retrotransposon sequences as follows: U2 snRNA contained the Tx1-type element called Keno-1_SSa (Figure 1a); H1 contained an internal portion (217 bp) of an ERV1-type endogenous retrovirus sequence (Figure 1b); H3 displayed an internal portion (52 bp) of the LTR retrotransposon Gypsy (Figure 1c), although chromosome mapping of this sequence only revealed the main H3 histone clusters with no evidence of dispersed clusters; and H4 contained an internal portion (37 bp) of the LTR retro-transposon Gypsy (Figure 1d).
Figure 1. Partial sequences of multigene families isolated from C. gomesi genome, with TE insertion. (a) Partial sequence of the U2 snRNA gene (yellow), associated with its specific U2-target Keno TE (blue); histone partial sequences, with the internal portion of TEs; (b) H1 with retrotransposon ERV1 (yellow); (c) H3 with retrotransposon Gypsy (yellow); (d) H4 with retrotransposon Gypsy (yellow).
Cytogenetics of Characidium
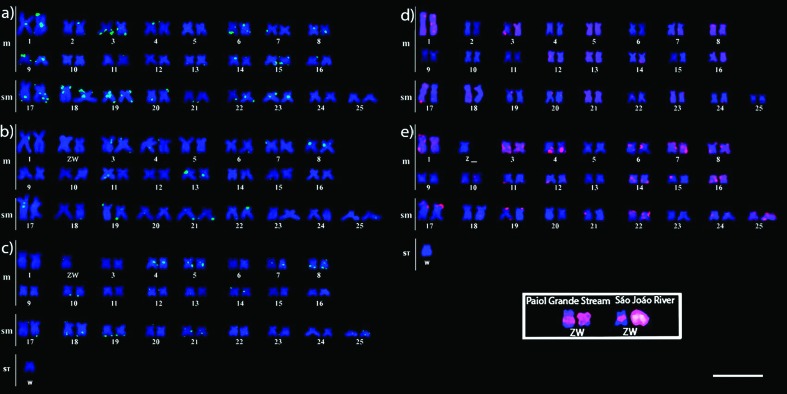
The studied species presented a 2n of 50, and these chromosomes have been cytogenetically described by Machado et al. (2011) and Pucci et al. (2014). Karyotype formulae were organized as 32 metacentric + 18 submeta-centric, excluding females of C. gomesi (São João River population), which were organized as 31 metacentric + 18 submetacentric + 1 subtelocentric. The fundamental number of chromosome arms was 100 in all studied species/populations. No differentiated sex chromosomes were found in the C. zebra population. The C. gomesi W-specific probe revealed sex chromosomes as metacentric pair 2 in C. gomesi from the Paiol Grande Stream population and metacentric Z position 2 and subtelocentric W in C. gomesi from the São João River population (Figure 2, Z and W chromosomes are highlighted in the box).
Figure 2. Karyotypes of Characidium females subjected to fluorescence in situ hybridization (FISH) with TE probes. (a) C. zebra, (b) C. gomesi (PG), (c) C. gomesi (SJ); (d) C. zebra, (e) C. gomesi (SJ). The Rex3 probe did not show any hybridization signals in C. gomesi (PG) chromosomes (not shown). The W and Z sex chromosomes of C. gomesi females are highlighted in the box. PG, Paiol Grande Stream population; SJ, São João River population. Scale bar, 10 μm.
Chromosome mapping of Rex1 and Rex3 on Characidium chromosomes
The non-LTR retrotransposons Rex1 and Rex3 in C. zebra and C. gomesi were observed in a few chromosomes (Figure 2a–e). In C. zebra, Rex1 displayed more prominent hybridization signals in metacentric pair 3 and submeta-centric pairs 18 and 19 (Figure 2a). In C. gomesi from the Paiol Grande Stream population, Rex1 exhibited strong signals in metacentric pairs 8 and 13 (Figure 2b). In C. gomesi from the São João River population, Rex1 exhibited clear marks in metacentric pairs 4, 5, and 8 and submetacentric pair 19 (Figure 2c). However, Rex1 did not display clear marks in the Z and W chromosomes either C. gomesi population (Figure 2b–c). In C. zebra, Rex3 exhibited convincing hybridization signals in metacentric pairs 1, 3, and 8 and submetacentric pair 17 (Figure 2d). In C. gomesi from the São João River population, Rex3 displayed signals in metacentric pairs 1, 3, 4, 6, 7, 8, 14, and 16 and submetacentric pairs 17, 22, and 25 (Figure 2e). Rex3 did not hybridize with the Z and W chromosomes of C. gomesi from the São João River population (Figure 2e), nor did it exhibit hybridization signals in any chromosome of C. gomesi from the Paiol Grande Stream population (data not shown).
Chromosome mapping of multigene families U2 snRNA and the H1, H3, and H4 genes on Characidium chromosomes
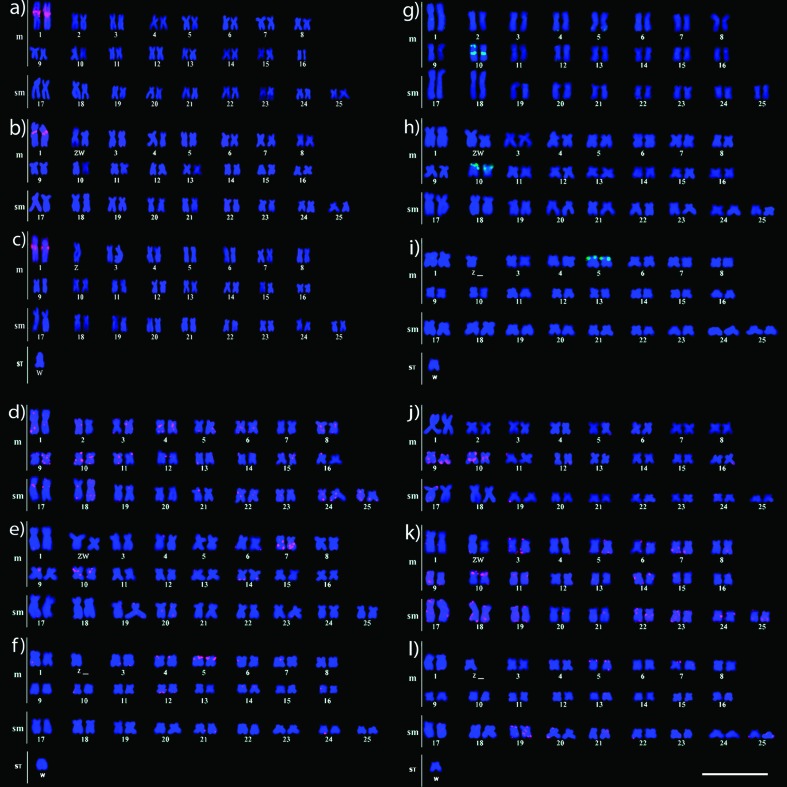
The U2 snRNA probe displayed a single cluster of hybridization signals in the pericentromeric region of meta-centric pair 1 in all analyzed species, with no additional dispersed sites detected (Figure 3a–c).
Figure 3. Karyotypes of Characidium females subjected to fluorescence in situ hybridization (FISH) with multigene family probes. (a) C. zebra, (b) C. gomesi (PG), (c) C. gomesi (SJ); H1 (d) C. zebra, (e) C. gomesi (PG), (f) C. gomesi (SJ); (g) C. zebra, (h) C. gomesi (PG), (i) C. gomesi (SJ); (j) C. zebra, (k) C. gomesi (PG), (l) C. gomesi (SJ). PG, Paiol Grande Stream population; SJ, São João River population. Scale bar, 10 μm.
The H1 histone gene probe displayed primary clusters of hybridization signals in the pericentromeric region and short arm of one chromosome of metacentric pair 10, whereas only one cluster was found in the pericentromeric region of the other chromosome in pair 10 of C. zebra (Figure 3d) and metacentric pair 10 of C. gomesi (Paiol Grande Stream population). An additional cluster was noted in pair 7 of C. gomesi from the Paiol Grande Stream population (Figure 3e) and metacentric pair 5 of C. gomesi from the São João River population (Figure 3f). In addition, each species exhibited weak additional signals in several other autosomes (Figure 3d–f).
The H3 gene probe displayed primary clusters of hybridization signals in the pericentromeric region and short arm of one chromosome of metacentric pair 10 and one cluster in the pericentromeric region of the other chromosome in pair 10 of C. zebra (Figure 3g), the short arm of metacentric pair 10 of C. gomesi from the Paiol Grande Stream population (Figure 3h). One cluster was also found in the short arm of metacentric pair 5 of C. gomesi from the São João River population (Figure 3i).
The H4 gene probe revealed primary clusters of hybridization signals in the pericentromeric region and short arm of one chromosome in metacentric pair 10 and one cluster in the pericentromeric region of the other chromosome in pair 10 of C. zebra, as well as additional marks in metacentric pair 9 (Figure 3j) and the short arm of meta-centric pair 10 of C. gomesi from the Paiol Grande Stream population (Figure 3k) and a weak signal in metacentric pair 5 of C. gomesi from the São João River population (Figure 3l). Marks were also noted in some autosomes of both populations of C. gomesi (Figure 3k–l).
Discussion
Distribution of Rex1 and Rex3 on Characidium chromosomes
Rex elements are non-LTR retrotransposons (Wicker et al., 2007) that are extensively distributed through fish genomes (Ozouf-Costaz et al., 2004; Ferreira et al., 2010; Borba et al., 2013; Schneider et al., 2013; Yano et al., 2014; Sene et al., 2015; Pinheiro et al., 2016), in addition to those of other species. Rex1 and Rex3 are significant sequences in the organization and evolution of the genomes in most of the aforementioned species, as indicated by evident hybridization signals and prominent amounts of these sequences. In this analysis, Rex1 and Rex3 elements were dispersed in small clusters throughout the chromosomes, and they did not display significant chromosome reorganization between Characidium species.
Concerning the distribution of Rex1 and Rex3 in the sex chromosomes, no hybridization sites were identified in the Z and W sex chromosomes of Characidium, whereas these elements are involved in sex chromosome evolution in other species. In particular, Rex3 was detected in the Y chromosome of Chionodraco hamatus (Ozouf-Costaz et al., 2004) and X chromosome of Eigenmannia (Sene et al., 2015); Rex1 and Rex3 were found in the W chromosome of Leporinus (Borba et al., 2013); and Rex1, Rex3, and Rex6 were identified in the Z and W chromosomes of Triportheus (Yano et al., 2014). The Rex1 and Rex3 elements analyzed in the Characidium genome emerged in the ancestral species C. zebra. However, these elements did not exhibit high transposition rates, presenting only small clusters in some autosomes in all analyzed species. Moreover, the Rex3 element was not identified in the genome of C. gomesi from the Paiol Grande Stream population. Natural selection may minimize the transposition rate, promoting vertical inactivation (Lohe et al., 1995), which could be true for Rex elements in Characidium. Another possible explanation for the low transposition rate could be stochastic loss, in which the element is gradually removed from the genome, as observed for mariner-like elements in the Drosophila melanogaster species complex (Lohe et al., 1995) and probably for Rex3 in C. gomesi from the São João River population.
Multigene families and TE insertions
Chromosome mapping of U2 snRNA revealed localized clusters in the first metacentric pair in all studied species. In fact, the distribution pattern of U2 snRNA is highly conserved for Characidium, as described by Scacchetti et al. (2015a), with only some exceptions such as Characidium sp. aff. C. vidali, Characidium sp. 1 (Scacchetti et al., 2015a), and C. alipioi (Serrano et al., 2017). U2 snRNA sequences appear to be conserved in other species, and co-localization and linkage between U2 genes and ribosomal sites has been reported (Cross and Rebordinos, 2005; Manchado et al., 2006; Úbeda-Manzanaro et al., 2010; Scacchetti et al., 2015a). Despite the presence of conserved clusters, sequence analyses of U2 snRNA using the CENSOR tool revealed a major portion corresponding to the Tx1-type non-LTR retrotransposon Keno-1_SSa (Kohany et al., 2006). There are several sequence-specific families in the Tx element group, and Keno is specific for U2 snRNA (Kojima and Fujiwara, 2004). Insertion of the Keno element occurs at a specific site 37 nu-cleotides downstream of U2 snRNA, and its insertion destroys the target (Kojima and Fujiwara, 2004). The Keno-1_SSa (Kohany et al., 2006) element found in the U2 snRNA sequence of Characidium is classified as KenoDr1 because the specific 3’ target sequence (TCTGTTCTTATCAGTTTAAT) localized 37 nucleotides downstream of U2 snRNA (Kojima and Fujiwara, 2004; Kojima and Jurka, 2015). Despite the TE insertion, the U2 snRNA sequence did not exhibit additional clusters.
In situ localization for the H1, H3, and H4 sequences revealed primary clusters in metacentric pair 10 of C. zebra and C. gomesi from the Paiol Grande Stream population as well as metacentric pair 5 of C. gomesi from the São João River population. Additional hybridization signals for H1 and H4 were dispersed through the autosomes of the three populations, although not in the sex chromosomes. Chromosomal rearrangement and the absence of gene flow resulted in the differentiated karyotype of C. gomesi from the São João River population, which exhibited primary clusters of H1, H3, and H4 in metacentric pair 5 (translocation) and subtelocentric sex chromosome W (inversion). The sites of H3 were also localized to metacentric pair 10 in C. alipioi (Serrano et al., 2017), albeit in the long arms, pointing to the occurrence of rearrangements involving these chromosomes. Our analyses of the histone sequences also revealed LTR retrotransposon (Wicker et al., 2007) insertions of ERV1 (H1) and Gypsy (H3 and H4). The LTR retrotransposon Gypsy inserted in the H3 sequence was not involved in the spread of this sequence throughout the genome. Additional clusters of H1 and H4 are probably due to the involvement of TEs. Hence, the major force leading to chromosomal spread of the H1 and H4 sequences in the Characidium karyotypes were probably a consequence of hitchhiking by H1 and H4 with the mobile elements-mediated transposition events. However, these additional H1 and H4 chromosomal marks could represent the Gypsy and ERV1 TE sequences alone without the histone genes adjacent to them.
Insertion of a TE inside or around a gene can alter its expression considerably, increasing or decreasing its expression when the insertion occurs in promoter regions, (Finnegan, 1989), or block gene expression by disrupting normal gene function (Chuong et al., 2016). However, it is difficult at present to determine the consequences of retrotransposon insertions in U2 snRNA and the H3 gene of Characidium, as they are essential for cellular function.
Our results illustrated that the Characidium genome is dynamic concerning TEs. However, these TEs did not promote deep chromosomal reorganization of the Characidium karyotypes, nor were they involved in differentiation of the specific W sex chromosome region in C. gomesi. It is therefore desirable to identify and map other TEs in the Characidium genome to improve our understanding of karyotype and sex chromosome evolution in this fish genus. However, the results presented in this study will enable the detection of innumerous TE insertions/transpositions generating microstructural variation in Characidium genomes, including some TE invasions in gene families.
Acknowledgments
This study was supported by Fundação Araucária (Fundação Araucária de Apoio ao Desenvolvimento Científico e Tecnológico do Estado do Paraná), CNPq (Conselho Nacional de Desenvolvimento Científico e Tec-nológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and SETI-PR (Secretaria de Estado da Ciência, Tecnologia e Ensino Superior do Estado do Paraná).
Supplementary material
The following online material is available for this article:
Footnotes
Associate Editor: Yatio Yonenaga-Yassuda
References
- Barros AV, Wolski MAV, Nogaroto V, Almeida MC, Moreira-Filho O, Vicari MR. Fragile sites, dysfunctional telomere and chromosome fusions: What is 5S rDNA role? Gene. 2017;608:20–27. doi: 10.1016/j.gene.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Bertollo LAC, Takahashi CS, Moreira-Filho O. Cyto-taxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidae) Braz J Genet. 1978;1:103–120. [Google Scholar]
- Borba RS, Silva EL, Parise-Maltempi PP. Chromosome mapping of retrotransposable elements Rex1 and Rex3 in Leporinus Spix, 1829 species (Characiformes: Anostomidae) and its relationships among heterochromatic segments and W sex chromosome. Mob Genet Elements. 2013;3:e27460. doi: 10.4161/mge.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centofante L, Bertollo LAC, Moreira-Filho O. Comparative cytogenetics among sympatric species of Characidium (Pisces, Characiformes). Diversity analysis with the description of a ZW sex chromosome system and natural triploidy. Caryologia. 2001;54:253–260. [Google Scholar]
- Centofante L, Bertollo LAC, Buckup PA, Moreira-Filho O. Chromosomal divergence and maintenance of sympatric Characidium fish species (Crenuchidae, Characidiinae) Hereditas. 2003;138:213–218. doi: 10.1034/j.1601-5223.2003.01714.x. [DOI] [PubMed] [Google Scholar]
- Chuong EB, Elde NC, Feschotte C. Regulatory activities of transposable elements: From conflicts to benefits. Nat Rev Genet. 2016;18:71–86. doi: 10.1038/nrg.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor W, Mezquita J, Winkfein RJ, States JC, Dixon GH. Organization of the histone genes in the rainbow trout (Salmo gairdnerii) J Mol Evol. 1984;20:227–235. doi: 10.1007/BF02104729. [DOI] [PubMed] [Google Scholar]
- Cross I, Rebordinos L. 5S rDNA and U2 snRNA are linked in the genome of Crassostrea angulata and Crassostrea gigas oysters: does the (CT)n(GA)n microsa-tellite stabilize this novel linkage of large tandem arrays? Genome. 2005;48:1116–1119. doi: 10.1139/g05-075. [DOI] [PubMed] [Google Scholar]
- Eichler EE, Sankoff D. Structural dynamics of eukaryotic chromosome evolution. Science. 2003;301:793–797. doi: 10.1126/science.1086132. [DOI] [PubMed] [Google Scholar]
- Ferreira DC, Oliveira C, Foresti F. Chromosome mapping of retrotransposable elements Rex1 and Rex3 in three fish species in the subfamily Hypoptopomatinae (Teleostei, Siluriformes, Loricariidae) Cytogenet Genome Res. 2010;132:64–70. doi: 10.1159/000319620. [DOI] [PubMed] [Google Scholar]
- Feschotte C. Transposable elements and the evolution of regulatory networks. Nat Rev Genet. 2008;9:397–405. doi: 10.1038/nrg2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan DJ. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989;5:103–107. doi: 10.1016/0168-9525(89)90039-5. [DOI] [PubMed] [Google Scholar]
- Hardman N. Structure and function of repetitive DNA in eukaryotes. Biochem J. 1986;234:1–11. doi: 10.1042/bj2340001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N, Zernik M, Roeder RG. The structure of the human histone genes: Clustered but not tandemly repeated. Cell. 1991;24:661–668. doi: 10.1016/0092-8674(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Hentschel CC, Birnstiel ML. The organization and expression of histone gene families. Cell. 1981;25:301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Stur-rock S, Buxton S, Cooper A, Markowitz S, Duran C, et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell MG. Transposable elements and the evolution of genome size in eukaryotes. Genetica. 2002;115:49–63. doi: 10.1023/a:1016072014259. [DOI] [PubMed] [Google Scholar]
- Kohany O, Gentles AJ, Hankus L, Jurka J. Annotation, submission and screening of repetitive elements in Repbase: Repbase Submitter and Censor. BMC Bioinformatics. 2006;25:474. doi: 10.1186/1471-2105-7-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima KK, Fujiwara H. Cross-genome screening of novel sequence-specific non-LTR retrotransposons: Various multicopy RNA genes and microsatellites are selected as targets. Mol Biol Evol. 2004;21:207–217. doi: 10.1093/molbev/msg235. [DOI] [PubMed] [Google Scholar]
- Kojima KK, Jurka J. Ancient origin of the U2 small nuclear RNA gene- targeting non-LTR retrotransposons Utopia . PLoS One. 2015;10:e0140084. doi: 10.1371/journal.pone.0140084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964;52:201–220. [Google Scholar]
- Lohe AR, Moriyama EN, Lidholm DA, Hart DL. Horizontal transmission, vertical inactivation, and stochastic loss of mariner-like transposable elements. Mol Biol Evol. 1995;12:62–72. doi: 10.1093/oxfordjournals.molbev.a040191. [DOI] [PubMed] [Google Scholar]
- Machado TC, Pansonato-Alves JC, Pucci MB, Nogaroto V, Almeida MC, Oliveira C, Foresti F, Bertollo LA, Moreira-Filho O, Artoni RF. Chromosomal painting and ZW sex chromosomes differentiation in Characidium (Characiformes, Crenuchidae) BMC Genetics. 2011;12:65. doi: 10.1186/1471-2156-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchado M, Zuasti E, Cross I, Merlo A, Infante C, Rebor-dinos L. Molecular characterization and chromosomal mapping of the 5S rRNA gene in Solea senegalensis: A new linkage to the U1, U2, and U5 small nuclear RNA genes. Genome. 2006;49:79–86. doi: 10.1139/g05-068. [DOI] [PubMed] [Google Scholar]
- Nei M, Rooney AP. Concerted and birth-and-death evolution of multigene families. Annu Rev Genet. 2005;39:121–152. doi: 10.1146/annurev.genet.39.073003.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozouf-Costaz C, Brandt J, Körting C, Pisano E, Bonillo C, Cou-tanceau J-P, Volff J-N. Genome dynamics and chromosomal localization of the non-LTR retrotransposons Rex1 and Rex3 in Antarctic fish. Antarct Sci. 2004;16:51–57. [Google Scholar]
- Pansonato-Alves JC, Paiva LRS, Oliveira C, Foresti F. Interespecific chromosomal divergences in the genus Characidium (Teleostei: Characiformes: Crenuchidae) Neotrop Ichythyol. 2010;8:77–86. [Google Scholar]
- Pansonato-Alves JC, Vicari MR, Oliveira C, Foresti F. Chromosomal diversification in populations of Characidium cf. gomesi (Teleostei, Crenuchidae) J Fish Biol. 2011;78:183–194. doi: 10.1111/j.1095-8649.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- Pansonato-Alves JC, Serrano EA, Utsunomia R, Camacho JPM, Silva GJC, Vicari MR, Artoni RF, Oliveira C, Foresti F. Single origin of sex chromosomes and multiple origins of B chromosomes in fish genus Characidium . PLoS One. 2014;9:e107169. doi: 10.1371/journal.pone.0107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazian MF, Shimabukuro-Dias CK, Pansonato-Alves JC, Oliveira C, Foresti F. Chromosome painting of Z and W sex chromosomes in Characidium (Characiformes, Crenu-chidae) Genetica. 2013;141:1–9. doi: 10.1007/s10709-013-9701-1. [DOI] [PubMed] [Google Scholar]
- Pinheiro VSS, Carvalho NDM, Carmo EJ, Schneider CH, Feldberg E, Gross MC. Karyoevolution in Potamorhina (Cope, 1878) (Ostariophysi, Curimatidae): Using repetitive DNA for the elucidation of genome organization. Zebrafish. 2016;13:118–131. doi: 10.1089/zeb.2015.1187. [DOI] [PubMed] [Google Scholar]
- Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA. 1986;83:2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci MB, Barbosa P, Nogaroto V, Almeida MC, Artoni RF, Pansonato-Alves JC, Foresti F, Moreira-Filho O, Vicari MR. Population differentiation and speciation in the genus Characidium (Characiformes: Crenuchidae): Effects of reproductive and chromosomal barriers. Biol J Linnean Soc. 2014;111:541–553. [Google Scholar]
- Pucci MB, Barbosa P, Nogaroto V, Almeida MC, Artoni RF, Scacchetti PC, Pansonato-Alves JC, Foresti F, Moreira-Filho O, Vicari MR. Chromosomal spreading of microsatellites and (TTAGGG)n sequences in the Characidium zebra and C. gomesi genomes (Characiformes: Crenuchidae) Cytogenet Genome Res. 2016;149:182–190. doi: 10.1159/000447959. [DOI] [PubMed] [Google Scholar]
- Scacchetti PC, Utsunomia R, Pansonato-Alves JC, Silva GJC, Vicari MR, Artoni RF, Oliveira C, Foresti F. Repetitive DNA sequences and evolution of ZZ/ZW sex chromosomes in Characidium (Teleostei: Characiformes) PLoS One. 2015a;10:e0137231. doi: 10.1371/journal.pone.0137231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scacchetti PC, Utsunomia R, Pansonato-Alves JC, Vicari MR, Artoni RF, Oliveira C, Foresti F. Chromosomal mapping of repetitive DNAs in Characidium (Teleostei, Characiformes): Genomic organization and diversification of ZW sex chromosomes. Cytogenet Genome Res. 2015b;146:136–143. doi: 10.1159/000437165. [DOI] [PubMed] [Google Scholar]
- Schneider CH, Gross MC, Terencio ML, Carmo EJ, Martins C, Feldberg E. Evolutionary dynamics of retro-transposable elements Rex1, Rex3 and Rex6 in Neotropical cichlid genomes. BMC Evol Biol. 2013;13:152. doi: 10.1186/1471-2148-13-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sene VF, Pansonato-Alves JC, Ferreira DC, Utsunomia R, Oli-veira C, Foresti F. Mapping of the retrotrans-posable elements Rex1 and Rex3 in chromosomes of Eigenmannia (Teleostei, Gymnotiformes, Sternopygidae) Cytogenet Genome Res. 2015;146:319–324. doi: 10.1159/000441465. [DOI] [PubMed] [Google Scholar]
- Serrano EA, Utsunomia R, Scudeller PS, Oliveira C, Foresti F. Origin of B chromosomes in Characidium alipioi (Characiformes, Crenuchidae) and its relationship with supernumerary chromosomes in other Characidium species. Comp Cytogenet. 2017;11:81–95. doi: 10.3897/CompCytogen.v11i1.10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner AT. Chromosomes: Organization and Function. Blackwell Publishing Company; London: 2003. p. 287. [Google Scholar]
- Szamalek JM, Goidts V, Chuzhanova N, Hameister H, Cooper DN, Kehrer-Sawatzki H. Molecular characterisation of the pericentric inversion that distinguishes human chromosome 5 from the homologous chimpanzee chromosome. Hum Genet. 2005;117:168–176. doi: 10.1007/s00439-005-1287-y. [DOI] [PubMed] [Google Scholar]
- Tollis M, Boissinot S. The evolutionary dynamics of transposable elements in eukaryote genomes. Genome Dyn. 2012;7:68–91. doi: 10.1159/000337126. [DOI] [PubMed] [Google Scholar]
- Úbeda-Manzanaro M, Merlo MA, Palazón JL, Cross I, Sarasquete C, Rebordinos L. Chromosomal mapping of the major and minor ribosomal genes, (GATA)n and U2 snRNA gene by double-colour FISH in species of the Batrachoididae family. Genetica. 2010;138:787–794. doi: 10.1007/s10709-010-9460-1. [DOI] [PubMed] [Google Scholar]
- Utsunomia R, Scacchetti PC, Hermida M, Fernández-Cebrián R, Taboada X, Fernández C, Bekaert M, Mendes NJ, Robledo D, Mank JE. Evolution and conservation of Characidium sex chromosomes. Heredity. 2017;119:237–244. doi: 10.1038/hdy.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicari MR, Artoni RF, Moreira-Filho O, Bertollo LAC. Diversification of a ZZ/ZW sex chromosome system in Characidium fish (Crenuchidae, Characiformes) Genetica. 2008;134:311–317. doi: 10.1007/s10709-007-9238-2. [DOI] [PubMed] [Google Scholar]
- Wells RD. Non-B DNA conformations, mutagenesis and disease. Trends Biochem Sci. 2007;32:271–278. doi: 10.1016/j.tibs.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, Chalhoub B, Flavell A, Leroy P, Morgante M, Panaud O, et al. A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 2007;8:973–982. doi: 10.1038/nrg2165. [DOI] [PubMed] [Google Scholar]
- Yano CF, Poltronieri J, Bertollo LAC, Artoni RF, Liehr T, de Bello Cioffi M. Chromosomal mapping of repetitive DNAs in Triportheus trifurcatus (Characidae, Characiformes): insights into the differentiation of the Z and W chromosomes. PLoS One. 2014;9:e90946. doi: 10.1371/journal.pone.0090946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Internet Resources
- Girinst, www.girinst.org (October 4, 2016)
- NCBI http://www.ncbi.nlm.nih.gov/blast(October 7, 2016)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.