ABSTRACT
Background: While normal tinnitus is a short-term sensation of limited duration, in 10–15% of the general population it develops into a chronic condition. For 3–6% it seriously interferes with many aspects of life.
Objective: The aim of this trial was to assess effectiveness of a trauma-focused approach, eye movement desensitization and reprocessing (EMDR), in reducing tinnitus distress.
Methods: The sample consisted of 35 adults with high levels of chronic tinnitus distress from five general hospitals in the Netherlands. Participants served as their own controls. After pre-assessment (T1), participants waited for a period of 3 months, after which they were assessed again (T2) before they received six 90 min manualized EMDR treatment sessions in which tinnitus-related traumatic or stressful events were the focus of treatment. Standardized self-report measures, the Tinnitus Functional Index (TFI), Mini-Tinnitus Questionnaire (Mini-TQ), Symptom Checklist-90 (SCL-90) and the Self-Rating Inventory List for Post-traumatic Stress Disorder (SRIP), were completed again halfway through treatment (T3), post-treatment (T4) and at 3 months’ follow-up (T5).
Results: Repeated measures analysis of variance revealed significant improvement after EMDR treatment on the primary outcome, TFI. Compared to the waiting-list condition, scores significantly decreased in EMDR treatment [t(34) = −4.25, p < .001, Cohen’s dz = .72]. Secondary outcomes, Mini-TQ and SCL-90, also decreased significantly. The treatment effects remained stable at 3 months’ follow-up. No adverse events or side effects were noted in this trial.
Conclusions: This is the first study to suggest that EMDR is effective in reducing tinnitus distress. Randomized controlled trials are warranted.
KEYWORDS: Tinnitus, tinnitus distress, EMDR, trauma-focused treatment for tinnitus, Tinnitus Functional Index
HIGHLIGHTS: • Tinnitus is the perception of sound in the absence of auditory stimulation.• For 3–6% of the population it seriously interferes with many aspects of life.• A trauma-focused approach is hypothesized to reduce tinnitus distress.• Treatment with EMDR showed significant results.• Results persisted for up to 3 months (in follow-up).
Antecedentes: mientras que el tinnitus normal es una sensación a corto plazo de duración limitada, en el 10-15% de los pacientes se transforma en una condición crónica. Para el 3-6% de los pacientes interfiere seriamente con muchos aspectos de la vida. El objetivo de este estudio fue evaluar la efectividad de un enfoque centrado en el trauma, la Desensibilización y Reprocesamiento por Movimientos Oculares (EMDR), para reducir el estrés por tinnitus.
Métodos: La muestra consistió en 35 adultos con altos niveles de estrés por tinnitus crónico de cinco hospitales generales en los Países Bajos. Los participantes sirvieron como sus propios controles. Después de la pre-evaluación (T1), los participantes esperaron por un período de 3 meses, después de lo cual fueron evaluados nuevamente (T2) antes de recibir seis sesiones de tratamiento EMDR manualizadas de 90 minutos en las que los eventos traumáticos o estresantes relacionados con el tinnitus fueron el foco del tratamiento. Las medidas de autorreporte estandarizadas, el Índice Funcional de Tinnitus (TFI, por su sigla en inglés), el Mini Cuestionario de Tinnitus (Mini-TQ), la Lista de Chequeo de Síntomas - 90 (SCL-90, por su sigla en inglés) y la Lista de Inventario de Autorreporte para el TEPT (SRIP, por su sigla en inglés) se completaron nuevamente durante el tratamiento (T3), postratamiento (T4) y a los 3 meses de seguimiento (T5).
Resultados: el análisis de varianza de medidas repetidas (ANOVA) reveló una mejora significativa después del tratamiento con EMDR en el resultado primario TFI. Comparado con la condición de lista de espera, los puntajes disminuyeron significativamente en el tratamiento con EMDR, como mostraron las pruebas t de muestras relacionadas (t(34) = -4.25, p <0.001, Cohen’s dz = 0.72). Los resultados secundarios Mini-TQ y SCL-90 también disminuyeron significativamente. Los efectos del tratamiento se mantuvieron estables a los 3 meses de seguimiento. No se observaron eventos adversos o efectos secundarios en este estudio.
Conclusiones: Este es el primer estudio que sugiere que el EMDR es efectivo para reducir el estrés por tinnitus. Se requieren ensayos aleatorios controlados.
PALABRAS CLAVES: Tinnitus, estrés por tinnitus, EMDR, tratamiento focalizado en trauma para el tinnitus.
背景:虽然正常耳鸣是一种持续时间有限的暂时感觉,但一般人群的10-15%里,它会发展成慢性病。对于人群中的3-6%,它对生活的许多方面有严重干扰。
目的:该试验的目的是评估以创伤为焦点的方法 – 眼动脱敏再加工(EMDR)对减少耳鸣痛苦的有效性。
方法:样本由荷兰五家综合医院的35名慢性耳鸣患者组成。被试作为他们自己的控制组。在前测(T1)之后,被试等待3个月进行再次测试(T2),然后接受6次时长90分钟的标准EMDR治疗,其治疗的重点是耳鸣相关的创伤或压力事件。在治疗中途(T3),治疗完成后(T4)和随访3个月(T5)分别对被试症状进行评估。评估中使用标准化的自评量表,包括耳鸣功能指数(TFI),耳鸣问卷迷你版(Mini-TQ),症状检查表(SCL)-90和PTSD自评量表(SRIP)。
结果:重复测量方差分析(ANOVA)显示EMDR治疗后主要结果TFI显著改善。与等待治疗的状况相比,被试在EMDR治疗中的症状得分显著降低(t(34)= -4.25,p < 0.001,Cohen’s dz = 0.72)。次要结果的Mini-TQ和SCL-90也显著下降。随访3个月后治疗效果保持稳定。本试验未发现不良反应或副作用。
结论:这是首个提示EMDR可有效减少耳鸣痛苦的研究,未来有必要进行随机对照试验。
关键词: 耳鸣, 耳鸣痛苦, EMDR, 创伤焦点耳鸣治疗
1. Introduction
Tinnitus, also known as ‘ringing in the ear’, is the perception of sound in the absence of auditory stimulation. It can be differentiated into objective and subjective tinnitus. Subjective tinnitus is idiopathic and may be referred to as a ‘phantom sound’. In the general population, 10–15% have chronic subjective tinnitus (Baguley, McFerran, & Hall, 2013). Some lead a fully functioning life; however, tinnitus becomes a distressing and incapacitating symptom that seriously interferes with many aspects of daily life in 3–6% of the general population (Ahmad & Seidman, 2004; Davis & Rafaie, 2000). The severity of the distress experienced from the tinnitus is determined not by the acoustic characteristics, such as pitch and loudness of tinnitus (Andersson, 2003; Henry & Meikle, 2000; Hiller & Goebel, 2007), but by the cognitive, emotional and behavioural reactions to it (Andersson, 2002; Andersson & Westin, 2008; Cima, Crombez, & Vlaeyen, 2011; McKenna, Handscomb, Hoare, & Hall, 2014). Many tinnitus patients suffer from insomnia (Cronlein et al., 2016), concentration difficulties (Hallam, McKenna, & Shurlock, 2004; Rossiter, Stevens, & Walker, 2006) or headaches (Langguth et al., 2015). Comorbid mental symptoms of anxiety, depression and post-traumatic stress are common (Fagelson, 2007; Hinton, Chhean, Pich, Hofmann, & Barlow, 2006; McCormack et al., 2015; Pattyn et al., 2016; Zoger, Svedlund, & Holgers, 2006). The economic burden of tinnitus to society is substantial. The costs in the Netherlands have been estimated at €6.8 million in 2012 (Maes, Cima, Vlaeyen, Anteunis, & Joore, 2013).
1.1. Models for tinnitus distress
Research on tinnitus distress suggests different factors for causality and prolongation of the disorder. The neurophysiological model of Jastreboff (1990) uses principles of classical (Pavlovian) conditioning to explain how the internal, subjective experience of tinnitus (the conditioned stimulus), which in itself is neutral in origin, can become associated with aversive events (unconditioned stimuli) and so acquire negative meaning (Mckenna, 2004; Wilson, 2006). Hearing the ringing sound (tinnitus) activates the memory representation of the unconditioned stimuli, i.e. aversive tinnitus-related memory representations, and this results in tinnitus distress. Jastreboff and Jastreboff (2006) argue that tinnitus not associated with a negative event (unconditioned stimulus) is not distressing at all.
1.2. Treatments
In the absence of a medical cure for distressing tinnitus, clinical management typically consists of audiological management of hearing loss by sound-masking systems or hearing aids and reducing tinnitus distress by education and cognitive behavioural therapy (CBT) (Tunkel et al., 2014). The latter treatment is directed at the tinnitus-related cognitions and behaviours that are thought to cause and prolong tinnitus-related distress (McKenna et al., 2014). Many studies have shown CBT to be moderately effective in reducing tinnitus distress and increasing quality of life, although reviews are complicated by the heterogeneity of outcome measures and treatment intensity, lack of power and incomplete data reporting (Cima, Andersson, Schmidt, & Henry, 2014; Grewal, Spielmann, Jones, & Hussain, 2014; Hesser, Weise, Westin, & Andersson, 2011; Hoare, Kowalkowski, Kang, & Hall, 2011; Martinez-Devesa, Perera, Theodoulou, & Waddell, 2010). Guidelines suggest that care might best be organized using a stepped-care approach, gradually increasing the intensity of treatment in steps, with education as a first step and specialized multidisciplinary CBT consisting of 60–120 min weekly sessions over 12 weeks as the last step (Cima et al., 2012). In the clinical field, the need is felt for a monodisciplinary treatment that is easily accessible for patients in basic healthcare and reduces tinnitus distress in less time or fewer sessions than multidisciplinary CBT.
1.3. Tinnitus and EMDR
It may be speculated that given the similarities between phantom pain and tinnitus (De Ridder, Elgoyhen, Romo, & Langguth, 2011) and the effectiveness of eye movement desensitization and reprocessing (EMDR) in reducing phantom pain severity (De Roos et al., 2010; Schneider, Hofmann, Rost, & Shapiro, 2007) EMDR might also be an effective intervention for tinnitus distress. EMDR is an evidence-based psychological treatment that is frequently used and effective in post-traumatic stress disorder (PTSD) and its affiliated symptoms (Bisson et al., 2007; Bradley, Greene, Russ, Dutra, & Westen, 2005; Seidler & Wagner, 2006). Desensitization and reprocessing of memories and images that contribute to the symptoms, such as events in which the tinnitus acquired negative meaning, may reduce tinnitus distress. EMDR has not been subject to research in patients with tinnitus to date.
2. Methods
2.1. Design
This multicentre study used a within-group design, where participants were on a waiting list (WL) before treatment and served as their own control. The study design was approved by the Medical Research Ethics Committees United (MEC-U, NL54362.100.15) and registered at the Dutch Trial Registry as ‘EMDR and Tinnitus’ (no. NTR5878).
2.2. Participants
Participants were recruited from five hospitals in the Netherlands (Rivierenland Ziekenhuis Tiel, Noord West Ziekenhuizen Alkmaar, Leids Universitair Medisch Centrum, St. Antonius Ziekenhuizen Utrecht/Nieuwegein and Meander Medisch Centrum Amersfoort). From a priori power calculation, it was estimated that a sample size of n = 32 participants would be sufficient to detect a small main effect (f = .20, β = .80, α = .05; G*power 3). Criteria for study inclusion were: (1) age 18–65 years; (2) tinnitus duration of ≥ 6 months; (3) a score of ≥ 54 (significant problem) on the Tinnitus Functional Index (TFI) questionnaire; (4) referral by the ear, nose and throat (ENT) doctors in general hospitals; (5) no communication problems in Dutch; and (6) no major interfering acute medical or psychiatric condition, such as psychoses or high risk for suicide, as stated in a telephone interview by the research assistant.
2.3. Procedure
Outcomes were measured pre-WL (T1), post-WL/pre-treatment (T2), after three sessions of treatment (T3), post-treatment (T4) and 3 months after treatment (follow-up, T5). All the questionnaires were presented to the patients using Survey Monkey as a digital survey server. As the questionnaires were monitored by a research assistant (not involved in treatment) and the EMDR therapists were blind to the assessment data.
2.4. Measures
Demographics, characteristics of tinnitus, such as duration, start or exacerbation after a specific event, previous treatments for tinnitus and comorbid somatic symptoms were assessed at T1 by a separate questionnaire.
2.5. Primary outcome
The TFI was used to assess tinnitus distress, i.e. the impact of tinnitus symptoms on patients’ lives. The questionnaire consists of 25 questions that cover eight domains: intrusiveness, sense of control, cognitive interference, sleeping problems, hearing problems, relaxation, quality of life and emotional state of mind. It is a reliable and valid survey instrument that is also designed to measure treatment effect (Fackrell, Hall, Barry, & Hoare, 2016; Henry et al., 2016; Meikle et al., 2012). A Dutch version has been validated by Rabau, Wouters, and Van De Heyning (2014). The TFI mean scores (range 0–100) can be stratified into five levels: not a problem, mean (M) = 14 (range 0–17); small problem, M = 21 (range 18–31); moderate problem, M = 42 (range 32–53); significant problem, M = 65 (range 54–72); and very significant problem, M = 78 (range 73–100) (Henry et al., 2016). The cut-off point for entering the study was set at ≥ 54 (significant problem and very significant problem). A smallest detectable change (SDC) of 13 points on the TFI was used as the criterion for clinically significant improvement (CSI) (Henry et al., 2016).
2.6. Secondary outcomes
To facilitate comparison with other research, it was decided to add the Mini-Tinnitus Questionnaire (Mini-TQ), which also measures tinnitus-related distress. The Mini-TQ (12 questions) has good psychometric qualities (Cronbach’s α = .90) (Hiller & Goebel, 2004). The items reflect the most characteristic aspects of tinnitus distress, such as emotional and cognitive distress, intrusiveness, sleep disturbances, somatic complaints and auditory perceptual difficulties.
To assess psychological distress, we used the Symptom Checklist-90 (SCL-90) (Arrindell & Ettema, 1986). The 90 items are rated on a five-point scale of distress (0–4) ranging from ‘not at all’ to ‘extremely’. In this study, we used the total score (range 90–450) and no subscale scores. The SCL-90 is frequently used in both clinical practice and research, including in tinnitus populations (Bauch, Lynn, Williams, Mellon, & Weaver, 2003), and has good psychometric properties (Cronbach’s α = .79–.90).
The Self-Report Inventory for Post-traumatic Stress Disorder (SRIP) consists of 22 questions covering the symptoms of PTSD as described in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (American Psychiatric Association, 2000). Items are scored on a four-point scale ranging from ‘not at all’ to ‘very much’. The sum score of the SRIP ranges from 22 to 88. A cut-off score of ≥ 52 and scores above preset cut-off on the intrusion, avoidance and hyperarousal subscales suggest PTSD. The questionnaire has a high sensitivity and specificity for PTSD (Hovens, Bramsen, & Van Der Ploeg, 2002).
Furthermore, the list of events from the Clinician Administered PTSD Scale (CAPS) interview was used to assess whether patients had experienced traumatic events fulfilling criterion A1 of PTSD according to the DSM-IV.
2.7. EMDR
In the intake session, a standardized case conceptualization was developed by the therapist and participant, consisting of a hierarchy of disturbing tinnitus-related aversive memories and, when present, intrusive images related to other traumatic experiences that directly evoked feelings of powerlessness. EMDR treatment followed the standard eight-phase protocol presented by Shapiro and Forrest (2001; see also De Jongh & Ten Broeke, 2003). Treatment was delivered in six weekly 90 min sessions. Eye movements were used during EMDR sessions and in seven cases additional tactile stimulation (taps or buzzers) were added to enhance taxing of the working memory (cf. EMDR standard protocol). In the first three to five sessions, the focus was on processing disturbing negative (tinnitus- and trauma-related) memories. In the last session or sessions, EMDR was directed at the current tinnitus sensations as experienced during the session. The therapists were licensed (clinical) psychologists who were advanced practitioners in EMDR (four level II trained, one EMDR Europe Practitioner). All EMDR sessions were videotaped. Therapists used a session checklist and were supervised on a monthly basis for 2 h by a registered EMDR consultant (CdR), who used video recordings of sessions to give feedback.
2.8. Data analyses
Analyses were conducted using statistical software (SPSS version 24; IBM Corp., Armonk, NY, USA). An intention-to-treat analysis was performed, including all patients originally enrolled in the study irrespective of whether they completed the therapy or stopped after just one session of EMDR. Missing values were imputed by last observation carried forward (LOCF). After controlling for normal distribution by Shapiro–Wilk and a visual check of the histogram, a series of one-way analyses of variance (ANOVAs) with repeated measures for all outcome measures was computed to determine any general effect. Analyses were controlled for sphericity. When a significant effect for time appeared, post-hoc tests using the Bonferroni correction were conducted to elicit an effect of EMDR treatment (T2–T4). To test the hypotheses, we computed delta (Δ) scores (ΔT1–T2, effect of WL condition; ΔT2–T4, effect of EMDR; ΔT2–T3, effect of first three sessions; and ΔT3–T4, effect of last three sessions) and tested whether these differences were significant using pairwise t-tests. Finally, to evaluate whether the supposed effect was maintained, pairwise t-tests were computed for T4 (post-treatment) and T5 (follow-up).
To determine effect size, we used Cohen’s dz. Lakens (2013) states that Cohen’s dz is an accurate description of effect size in within-group designed research as it takes the correlation between measurements into account, whereas Cohen’s d is used to describe the standardized mean difference of an effect between independent groups. Interpretations of effect sizes of d and dz are equivalent: small (d = .2), medium (d = .5) and large (d = .8).
Number needed to treat (NNT) was calculated as the inverse of absolute risk reduction and was calculated by 1 divided by the percentage of participants with clinically significant improvement (CSI) after treatment minus the percentage of participants with CSI after the WL condition: NNT = 1 /[% CSI (EMDR) - % CSI (WL)].
3. Results
3.1. Participant characteristics
Figure 1 summarizes the participant flow through the study. Of the 226 patients who were seen by the ENT doctor, 43 participants were allocated and 35 started treatment. Thirty participants completed the study follow-up. Five participants (14%) dropped out of treatment at different stages and for different reasons. Three participants (9%) were so-called ‘early completers’ and were without any subjective tinnitus distress after three to five EMDR sessions. T5 was completed by 33 participants (94%).
Figure 1.
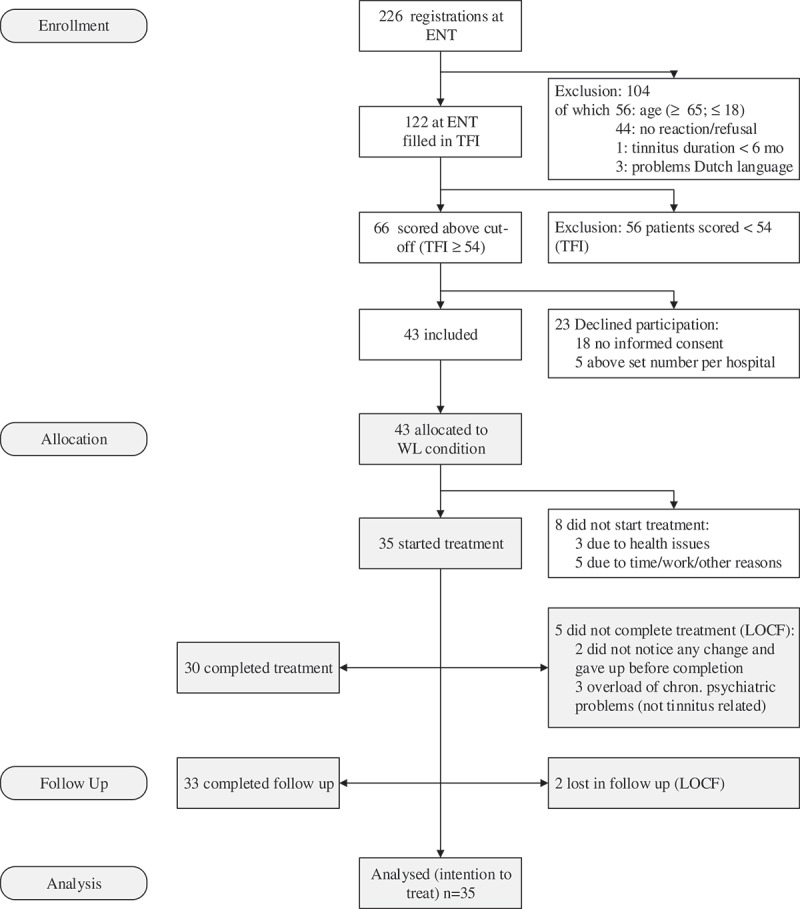
Flow of participants through the trial (CONSORT diagram). ENT, ear, nose and throat; TFI, Tinnitus Functional Index; WL, waiting list; LOCF, last observation carried forward; chron., chronic.
Relevant clinical characteristics of the participants included in the study are presented in Table 1.
Table 1.
Baseline characteristics.
| Characteristics of the sample (n = 35) | n | % |
|---|---|---|
| Gender, male | 19 | 54 |
| Duration of tinnitus (years) | ||
| 0.5–1 | 10 | 29 |
| 1–4 | 12 | 34 |
| > 4 | 13 | 37 |
| Tinnitus started after a specific event | 24 | 68 |
| Acoustic trauma | 9 | 26 |
| Ear complications | 3 | 9 |
| Other somatic problem | 2 | 6 |
| Stressful/traumatic event | 5 | 14 |
| Other or multiple events | 5 | 14 |
| Tinnitus exacerbated after a specific event | 22 | 63 |
| Acoustic trauma | 2 | 6 |
| Ear complications | 4 | 11 |
| Other somatic problem | 2 | 6 |
| Stressful event | 10 | 29 |
| Other or multiple events | 4 | 11 |
| Previous tinnitus treatments (multiple answers possible) | 23 | 66 |
| Audiological treatment (e.g. hearing aid) | 10 | 29 |
| Psycho-education | 3 | 9 |
| Noise mask | 8 | 23 |
| Medication | 1 | 3 |
| Cognitive behavioural therapy (CBT) | 2 | 6 |
| Other treatments (e.g. acupuncture, oxygen) | 8 | 23 |
| Two or more treatments | 7 | 2 |
| Comorbid somatic symptoms (multiple answers possible) | ||
| Sleeping problems | 22 | 63 |
| Headache | 12 | 34 |
| Neck pain | 12 | 34 |
| Other or multiple symptoms | 16 | 46 |
The mean (M) age of the sample was 49.2 years (SD 11.56, range 20–65 years). All cases of tinnitus had an adverse event at the start and/or at exacerbation of the symptom. None of the participants had been previously treated with EMDR.
3.2. Primary outcome measure
Figure 2 and Table 2 show a minimal drop in scores during the WL period, a sharp reduction from the beginning to end of treatment, and a minimal increase at follow-up. A repeated measures ANOVA with Greenhouse–Geisser correction determined that TFI scores showed significant statistical difference between time-points [F(2.48;84.41) = 19.79, p = .001, ηp 2 = .37]. Post-hoc tests using the Bonferroni correction revealed that tinnitus distress in TFI reduced significantly after the EMDR intervention [Wilk’s lambda = .52, F(1;34) = 32.03, p = .001, ηp 2 = .49]. Paired t-tests of scores ΔT1–T2 and ΔT2–T4 showed a significant difference between improvement in the EMDR treatment and WL condition [M = −17.94, SD = 25.0; t(34) = −4.24, p < .001 (two-tailed)]. A medium between-effect size (Cohen’s dz = .72), was found. No significant difference on TFI was revealed between the scores at the end of intervention (T4: M = 45.66, SD = 25.267) and at 3 months’ follow-up [T5: M = 48.77, SD = 25.03; t(34) = 1.52, p = 1.39, not significant (ns)].
Figure 2.
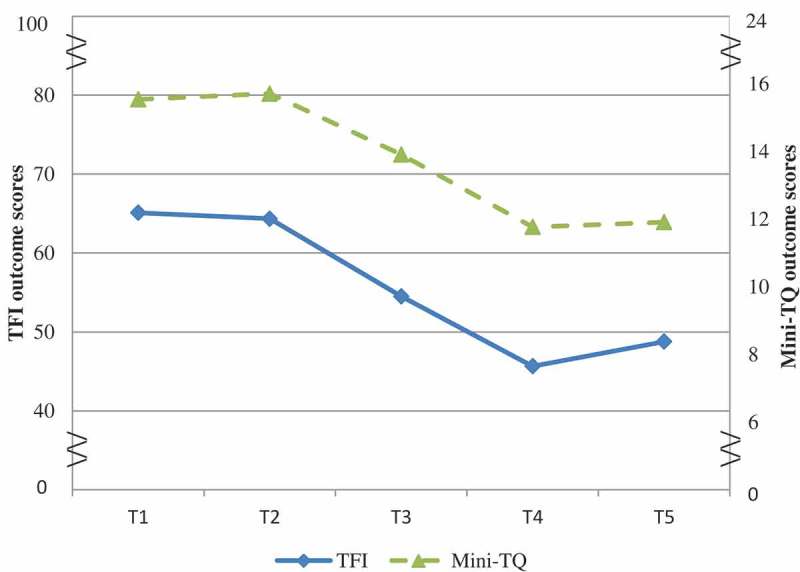
Tinnitus Functional Index (TFI) and Mini-Tinnitus Questionnaire (Mini-TQ) outcomes (TFI: score range 0–100; Mini-TQ: score range 0–24).
Table 2.
Mean (M) and SD for each measurement in the intention-to-treat analysis.
| T1 |
T2 (pre) |
T3 |
T4 (post) |
T5 (FU) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M | SD | M | SD | M | SD | M | SD | M | SD | |
| TFI | 35 | 65.09 | 14.03 | 64.34 | 15.37 | 54.51** | 19.64 | 45.66** | 25.27 | 48.77** | 25.03 |
| Mini-TQ | 35 | 15.54 | 4.64 | 15.71 | 4.72 | 13.91** | 4.93 | 11.77** | 6.4 | 11.91** | 6.32 |
| SCL-90 | 35 | 157.43 | 44.4 | 162.23 | 46.2 | 148.97* | 39.29 | 146.31* | 54.04 | 151.11* | 53.33 |
| SRIP | 35 | 38.51 | 9.32 | 37.91 | 9.44 | 37.06 | 8.65 | 3586 | 9.97 | 35.74 | 10.85 |
TFI, Tinnitus Functional Index; Mini-TQ, Mini-Tinnitus Questionnaire; SCL-90, Symptom Checklist-90; SRIP, Self-Rating Inventory List for Post-traumatic Stress Disorder; FU, follow-up.
*Significant at p = .05 level compared to T2 (start of treatment); **significant at p = .01 level compared to T2 (start of treatment).
3.3. Secondary outcome measures
The Mini-TQ scores (Figure 2 and Table 2) showed a negligible increase after the WL period, dropped sharply from pre- to post-treatment and increased minimally during follow-up. The Mini-TQ scores showed a significant statistical difference between time-points [F(2.07;70.2) = 14.41, p = .001, ηp 2 = .30]. Post-hoc tests revealed that tinnitus distress as measured by the Mini-TQ reduced significantly after the EMDR intervention [Wilk’s lambda = .60, F(1;34) = 22.79, p = .001, ηp 2 = .40]. Paired t-tests (ΔT1–T2 and ΔT2–T4) showed a significant difference between improvement in the EMDR treatment and WL condition [M = −4.12; t(34) = −4.21, p < .001 (two-tailed)]. A medium effect size was found (Cohen’s dz = .71). No significant difference on the Mini-TQ was observed between the scores at the end of the intervention (T4: M = 11.77, SD = 6.40) and at 3 months’ follow-up [T5: M = 11.91, SD = 6.32; t(34) = .92, p = .79, ns].
The scores on the SCL-90 were at all times not normally distributed, as assessed by the Shapiro–Wilk test and a visual histogram. Given the robust nature of the repeated measures ANOVA to violations of normality under the central limit theorem (Donaldson, 1968; Norman, 2010), we performed a repeated measures ANOVA with time (T1–T5) as within-subject variables and SCL-90 as the dependent variable. We also performed a non-parametric test (Friedman) to see whether its results confirmed the outcome of the repeated measures ANOVA. Since both tests gave rise to the same conclusion and the type I error rate was not much affected by violation of the normality assumption, we reported the repeated measures ANOVA in the main test to maximize interpretability of the results. As Table 2 and Figure 3 show, scores increased minimally during the WL period, dropped sharply during treatment and increased slightly during follow-up. The analysis determined that SCL-90 scores showed significant statistical difference between time-points [F(2.66;90.54) = 3.6, p = .02, ηp 2 = .096]. Post-hoc tests revealed that general psychological distress reduced significantly after the EMDR intervention [Wilk’s lambda = .82, F(1;34) = 7.34, p = .01, ηp 2 = .18]. Paired t-tests (ΔT1–T2 and ΔT2–T4) showed a significant difference between improvement in the EMDR treatment and WL condition [M = −20.71, SD = 44.94; t(34) = 2.45, p = .02]. A medium effect size was found (Cohen’s dz = .42). At the 3 month follow-up, no significant difference in SCL-90 was revealed in the scores at the end of the intervention (T4: M = 146.31, SD = 54.04) and at follow-up [T5: M = 151.11, SD = 53.32; t(34) = 1.08, p = .29, ns].
Figure 3.
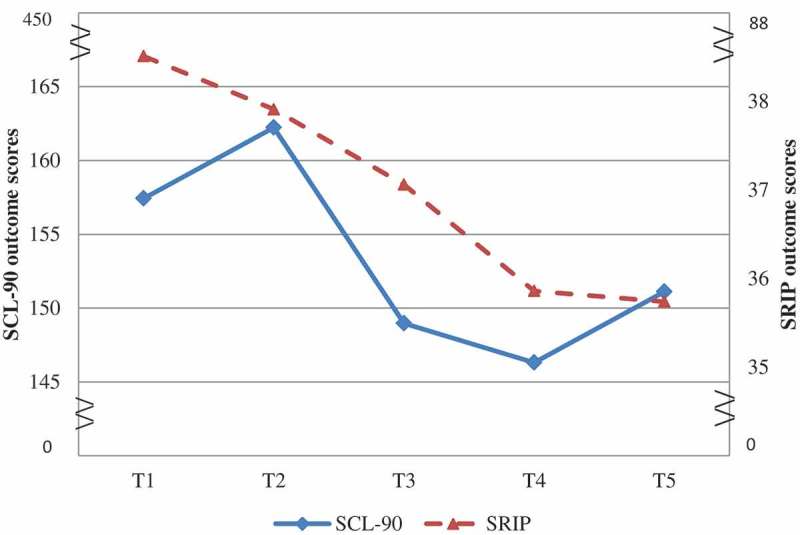
Symptom Checklist-90 (SCL-90) and Self-Rating Inventory List for Post-traumatic Stress Disorder (SRIP) outcome scores (SCL-90: score range 90–450; SRIP: score range 22–88).
SRIP scores were not normally distributed at T3 and T4, as assessed by visual histogram inspection and the Shapiro–Wilk test. Table 3 and Figure 3 show a minimal reduction in scores during the WL period, treatment condition and follow-up. Repeated measures ANOVA revealed no significant effect on SRIP scores [F(2.53;86.03) = 1.94, p = 1.39]. The result of the non-parametric test for the SRIP was not significant. Therefore, SRIP scores were not analysed further.
Table 3.
Delta (Δ) scores and effect sizes for each measurement.
| Delta scoresa |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T1–T2 |
T2–T4 pre–post |
T2–T3 |
T3–T4 |
Cohen’s |
||||||
| N | M | SD | M | SD | M | SD | M | SD | dz | |
| TFI | 35 | 0.74 | 11.62 | 18.6** | 19.53 | 9.83** | 12.85 | 8.86 | 18.70 | 0.72 |
| Mini-TQ | 35 | −0.17 | 3.20 | 3.94** | 4.89 | 1.80** | 0.40 | 2.14 | 0.68 | 0.71 |
| SCL-90 | 35 | −4.80 | 23.31 | 15.91* | 34.77 | 13.26* | 23.02 | 2.66 | 26.21 | 0.41 |
TFI, Tinnitus Functional Index; Mini-TQ, Mini-Tinnitus Questionnaire; SCL-90, Symptom Checklist-90.
aSRIP scores showed no significance in ANOVA, so no Δ scores were computed.
Eye movement desensitization and reprocessing (EMDR) T2–T4 and EMDR T2–T3: *significant at p = .05 level compared to waiting list (WL) T1–T2, **significant at p = .01 level compared to WL T1–T2. EMDR T3–T4: *significant at p = .05 level compared to T2–T3, **significant at p = .01 level compared to T2–T3.
Means, SDs, Δ scores and effect sizes for all measures are presented in Tables 2 and 3.
Outcome scores of the primary and relevant secondary outcome measures at T1–T5 are shown in Figures 2 and 3.
Measurements at T3 were included to provide an impression of the timeline of the effects of EMDR. Visual examination of Figure 2 shows that for TFI and Mini-TQ the gradient of the lines from T2 to T3 seems to be identical to the gradient from T3 to T4. Pairwise t-tests comparing ΔT2–T3 to ΔT3–T4 revealed no significant differences between the first and last parts of the treatment [TFI: t(34) = .23, p = .823; Mini-TQ: t(34) = −454, p = .653]. In Figure 3, the gradient of the line from T2 to T3 of the SCL-90 looks steeper than that from T3 to T4. Pairwise t-tests comparing ΔT2–T3 to ΔT3–T4, however, revealed no significant differences [SCL-90: t(34) = 1.79, p = .082].
3.4. Clinical significance
At post-treatment assessment (T4) and at 3 months’ follow-up (T5), 18 participants (51.4%) showed a response to therapy. The NNT to be classified as a responder according to the SDC at follow-up is 1.95.
3.5. PTSD
Combining the results of the list of events of CAPS and the PTSD criteria according to the SRIP showed that at T1 three participants had PTSD (9%). This was also the case at T4. At T5, four participants had PTSD (11%). All of them suffered from chronic PTSD or acute worsening life circumstances, not tinnitus related. At T5, the results of two participants failed and missing values were imputed.
Post-hoc analyses showed that no demographic factors (age or gender), duration of tinnitus, having been previously treated for tinnitus or somatic comorbidity were correlated with the primary outcome measure at T3 or T4.
4. Discussion
To our knowledge, this is the first study regarding the effectiveness of EMDR on tinnitus distress. The results of our study demonstrated a significant effect of EMDR treatment on a measures of tinnitus distress (the TFI), which was confirmed by the results of the Mini-TQ. Both instruments showed the same pattern of results. When compared to the passive control condition, i.e. the WL period, the effect size of treatment on the outcome measure TFI is medium (Cohen’s dz = .72). Almost one in two patients benefited from EMDR treatment. The effects remained stable after 3 months of follow-up. No adverse events or side effects were found.
The effect sizes on the primary outcome measure of this study, TFI (Cohen’s dz = .72) compare favourably to effect sizes reported in the most recent and largest CBT randomized controlled trial (RCT) (Cima et al., 2012), where between-effect sizes of Cohen’s d = .20 to .32 are reported after 3 months and Cohen’s d = .41–.52 after 8 months of tinnitus distress (tinnitus severity and impairment in the Tinnitus Questionnaire and Tinnitus Handicap Inventory) in a specialized tinnitus centre. Although the lower effect sizes of this study might be explained by the active control condition (usual care), our data show that EMDR may result in a reduction in tinnitus distress in even as few as six sessions of 90 min in a monodisciplinary setting. A significant reduction was already reached in three sessions.
It remains somewhat unclear by what psychological process EMDR yielded positive effects.
The reduction in tinnitus distress after EMDR could not be explained by a decrease in PTSD symptoms. There were only three patients who met the criteria for PTSD at T1 and the mean PTSD scores of our total sample were rather low and well below the clinical cut-off point of 52. Furthermore, there was no significant reduction in SRIP scores post-treatment and at follow-up. Therefore, it is unlikely that a reduction in PTSD symptoms explains the observed improvement in tinnitus distress.
Studies investigating the effect of EMDR on physical symptoms, mostly chronic pain, suggest that treatment aimed at processing unresolved trauma and somatic symptom-related memories can reduce the somatic symptom by reducing the affective dimension of these memories or by integrating the somatic memory components (Tesarz et al., 2014; Van Rood & De Roos, 2009). We suggest that this explanation could also account for the reduction in tinnitus distress after EMDR.
The present study has several strengths. It is the first to use EMDR as treatment for tinnitus distress. The multicentre nature of the trial increases the general applicability of the results. The use of more than one therapist limited therapist bias. The study used a delayed-treatment group to control for spontaneous recovery and fluctuations over time. Therapists and patients were blind to assessment outcomes. Therapists used a manualized treatment protocol, session checklists and video-recorded sessions, which were evaluated and discussed during supervision to enhance treatment integrity. Furthermore, data were analysed under restrictions of intention-to-treat analysis and missing values were imputed by LOCF, resulting in a conservative estimation of effect.
The most important limitation of this study is the within-group design, where patients served as their own control and an independent control group was lacking. Therefore, it cannot be determined whether and to what degree the positive effects can be explained by non-specific effects such as attention or expectancy. Note, however, that if no or minimal change during EMDR had been observed, one could have concluded that EMDR was not a promising treatment and that no further research would have been needed. Given that reductions in tinnitus symptoms were observed immediately after EMDR therapy and that the magnitude of the improvement compared favourably to the effect sizes reported in earlier tinnitus studies, we conclude that EMDR is a promising treatment for tinnitus and that further clinical studies, preferably RCTs, are warranted.
The results found in this study indicate that EMDR is promising in reducing tinnitus distress. The next step would be replication of the results in an RCT to control for non-specific therapy or placebo effects, with a long-term follow-up.
Funding Statement
This work was supported by the Dutch EMDR Association (Vereniging EMDR Nederland, VEN).
Acknowledgements
We thank the participating hospitals for their cooperation in the study and I. M. van der Groep, MSc, university assistant in training at University Utrecht, for statistical advice. This study was partly funded by a small grant from Vereniging EMDR Netherlands (VEN) in 2016–2017.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ahmad N., & Seidman M. (2004). Tinnitus in the older adult. Drugs & Aging, 21(5), 297–10. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, A. P. A (2000). DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association, 75, 78–85. [Google Scholar]
- Andersson G. (2002). Psychological aspects of tinnitus and the application of cognitive-behavioral therapy. Clinical Psychology Review, 22(7), 977–990. [DOI] [PubMed] [Google Scholar]
- Andersson G. (2003). Tinnitus loudness matchings in relation to annoyance and grading of severity. Auris, Nasus, Larynx, 30(2), 129–133. [DOI] [PubMed] [Google Scholar]
- Andersson G., & Westin V. (2008). Understanding tinnitus distress: Introducing the concepts of moderators and mediators. International Journal of Audiology, 47(Suppl 2), S106–111. [DOI] [PubMed] [Google Scholar]
- Arrindell W. A., & Ettema J. (1986). SCL-90: Handleiding bij een multidimensionele psychopathologie-indicator. Lisse: Swets Test Publishers. [Google Scholar]
- Baguley D., McFerran D., & Hall D. (2013). Tinnitus. The Lancet, 382(9904), 1600–1607. [DOI] [PubMed] [Google Scholar]
- Bauch C.D, Lynn S.G, Williams D.E, Mellon M.W, & Weaver A.L. (2003). Tinnitus impact: three different measurement tools. Journal Of The American Academy Of Audiology, 14(4), 181-187. [PubMed] [Google Scholar]
- Bisson J. I., Ehlers A., Matthews R., Pilling S., Richards D., & Turner S. (2007). Psychological treatments for chronic post-traumatic stress disorder. Systematic review and meta-analysis. The British Journal of Psychiatry : the Journal of Mental Science, 190, 97–104. [DOI] [PubMed] [Google Scholar]
- Bradley R., Greene J., Russ E., Dutra L., & Westen D. (2005). A multidimensional meta-analysis of psychotherapy for PTSD. Am J Psychiatry, 162(2), 214–227. [DOI] [PubMed] [Google Scholar]
- Cima R. F., Andersson G., Schmidt C. J., & Henry J. A. (2014). Cognitive-behavioral treatments for tinnitus: A review of the literature. Journal of the American Academy of Audiology, 25(1), 29–61. [DOI] [PubMed] [Google Scholar]
- Cima R. F., Crombez G., & Vlaeyen J. W. (2011). Catastrophizing and fear of tinnitus predict quality of life in patients with chronic tinnitus. Ear and Hearing, 32(5), 634–641. [DOI] [PubMed] [Google Scholar]
- Cima R. F., Maes I. H., Joore M. A., Scheyen D. J., El Refaie A., Baguley D. M., … Vlaeyen J. W. (2012). Specialised treatment based on cognitive behaviour therapy versus usual care for tinnitus: A randomised controlled trial. Lancet, 379(9830), 1951–1959. [DOI] [PubMed] [Google Scholar]
- Cronlein T., Langguth B., Pregler M., Kreuzer P. M., Wetter T. C., & Schecklmann M. (2016). Insomnia in patients with chronic tinnitus: Cognitive and emotional distress as moderator variables. Journal of Psychosomatic Research, 83, 65–68. [DOI] [PubMed] [Google Scholar]
- Davis A., & Rafaie E. A. (2000). Epidemiology of tinnitus Tinnitus handbook (pp. 1–23). Singular San Diego CA. [Google Scholar]
- De Jongh, A, & Ten Broeke, E (2003). Handboek emdr: een geprotocolleerde behandelmethode voor de gevolgen van psychotrauma. Lisse: Swets & Zeilinger. [Google Scholar]
- De Ridder D., Elgoyhen A. B., Romo R., & Langguth B. (2011). Phantom percepts: Tinnitus and pain as persisting aversive memory networks. Proceedings of the National Academy of Sciences of the United States of America, 108(20), 8075–8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roos C., Veenstra A. C., de Jongh A., den Hollander-Gijsman M., van der Wee N. J., Zitman F. G., & van Rood Y. R. (2010). Treatment of chronic phantom limb pain using a trauma-focused psychological approach. Pain Research & Management : the Journal of the Canadian Pain Society = Journal De La Societe Canadienne Pour Le Traitement De La Douleur, 15(2), 65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson T. S. (1968). Robustness of the F-test to errors of both kinds and the correlation between the numerator and denominator of the F-ratio. Journal of the American Statistical Association, 63(322), 660–676. [Google Scholar]
- Fackrell K., Hall D. A., Barry J. G., & Hoare D. J. (2016). Psychometric properties of the Tinnitus Functional Index (TFI): Assessment in a UK research volunteer population. Hearing Research, 335, 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagelson M. A. (2007). The association between tinnitus and posttraumatic stress disorder. American Journal of Audiology, 16(2), 107–117. [DOI] [PubMed] [Google Scholar]
- Grewal R., Spielmann P. M., Jones S. E., & Hussain S. S. (2014). Clinical efficacy of tinnitus retraining therapy and cognitive behavioural therapy in the treatment of subjective tinnitus: A systematic review. The Journal of Laryngology and Otology, 128(12), 1028–1033. [DOI] [PubMed] [Google Scholar]
- Hallam R. S., McKenna L., & Shurlock L. (2004). Tinnitus impairs cognitive efficiency. International Journal of Audiology, 43(4), 218–226. [DOI] [PubMed] [Google Scholar]
- Henry J. A., Griest S., Thielman E., McMillan G., Kaelin C., & Carlson K. F. (2016). Tinnitus functional index: Development, validation, outcomes research, and clinical application. Hearing Research, 334, 58–64. [DOI] [PubMed] [Google Scholar]
- Henry J. A., & Meikle M. B. (2000). Psychoacoustic measures of tinnitus. Journal of the American Academy of Audiology, 11(3), 138–155. [PubMed] [Google Scholar]
- Hesser H., Weise C., Westin V. Z., & Andersson G. (2011). A systematic review and meta-analysis of randomized controlled trials of cognitive-behavioral therapy for tinnitus distress. Clinical Psychology Review, 31(4), 545–553. [DOI] [PubMed] [Google Scholar]
- Hiller W., & Goebel G. (2004). Rapid assessment of tinnitus-related psychological distress using the Mini-TQ. International Journal of Audiology, 43(10), 600–604. [DOI] [PubMed] [Google Scholar]
- Hiller W., & Goebel G. (2007). When tinnitus loudness and annoyance are discrepant: Audiological characteristics and psychological profile. Audiology & Neuro-Otology, 12(6), 391–400. [DOI] [PubMed] [Google Scholar]
- Hinton D. E., Chhean D., Pich V., Hofmann S. G., & Barlow D. H. (2006). Tinnitus among Cambodian refugees: Relationship to PTSD severity. Journal of Traumatic Stress, 19(4), 541–546. [DOI] [PubMed] [Google Scholar]
- Hoare D. J., Kowalkowski V. L., Kang S., & Hall D. A. (2011). Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. The Laryngoscope, 121(7), 1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovens J., Bramsen I., & Van der Ploeg H. (2002). Self-rating inventory for posttraumatic stress disorder: Review of the psychometric properties of a new brief Dutch screening instrument. Perceptual and Motor Skills, 94(3), 996–1008. [DOI] [PubMed] [Google Scholar]
- Jastreboff P. J. (1990). Phantom auditory perception (tinnitus): Mechanisms of generation and perception. Neuroscience Research, 8(4), 221–254. [DOI] [PubMed] [Google Scholar]
- Jastreboff P. J., & Jastreboff M. M. (2006). Tinnitus retraining therapy: A different view on tinnitus, ORL; Journal for Oto-Rhino-Laryngology and Its Related Specialties, 68(1), 23–29. discussion 29–30. [DOI] [PubMed] [Google Scholar]
- Lakens D. (2013). Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Frontiers in Psychology, 4, 863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langguth B., Hund V., Busch V., Jurgens T. P., Lainez J. M., Landgrebe M., & Schecklmann M. (2015). Tinnitus and Headache. BioMed Research International, 797416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes I. H., Cima R. F., Vlaeyen J. W., Anteunis L. J., & Joore M. A. (2013). Tinnitus: A cost study. Ear and Hearing, 34(4), 508–514. [DOI] [PubMed] [Google Scholar]
- Martinez-Devesa P., Perera R., Theodoulou M., & Waddell A. (2010). Cognitive behavioural therapy for tinnitus. Cochrane Database of Systematic Reviews, 9. doi: 10.1002/14651858.CD005233.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A., Edmondson-Jones M., Fortnum H., Dawes P. D., Middleton H., Munro K. J., & Moore D. R. (2015). Investigating the association between tinnitus severity and symptoms of depression and anxiety, while controlling for neuroticism, in a large middle-aged UK population. International Journal of Audiology, 54(9), 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenna L. (2004). Models of tinnitus suffering and treatment compared and contrasted. Audiological Medicine, 2(1), 41–53. [Google Scholar]
- McKenna L., Handscomb L., Hoare D. J., & Hall D. A. (2014). A scientific cognitive-behavioral model of tinnitus: Novel conceptualizations of tinnitus distress. Frontiers in Neurology, 5, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikle M. B., Henry J. A., Griest S. E., Stewart B. J., Abrams H. B., McArdle R., … Turk D. C. (2012). The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear and Hearing, 33(2), 153–176. [DOI] [PubMed] [Google Scholar]
- Norman G. (2010). Likert scales, levels of measurement and the “laws” of statistics. Advances in Health Sciences Education, 15(5), 625–632. [DOI] [PubMed] [Google Scholar]
- Pattyn T., Van den Eede F., Vanneste S., Cassiers L., Veltman D. J., Van de Heyning P., & Sabbe B. C. G. (2016). Tinnitus and anxiety disorders: A review. Hearing Research, 333, 255–265. [DOI] [PubMed] [Google Scholar]
- Rabau S., Wouters K., & Van de Heyning P. (2014). Validation and translation of the Dutch tinnitus functional index. B-ENT, 10(4), 251–258. [PubMed] [Google Scholar]
- Rossiter S., Stevens C., & Walker G. (2006). Tinnitus and its effect on working memory and attention. Journal of Speech, Language, and Hearing Research : JSLHR, 49(1), 150–160. [DOI] [PubMed] [Google Scholar]
- Schneider J., Hofmann A., Rost C., & Shapiro F. (2007). EMDR in the treatment of chronic phantom limb pain. Pain Medicine, 9(1), 76–82. [DOI] [PubMed] [Google Scholar]
- Seidler G. H., & Wagner F. E. (2006). Comparing the efficacy of EMDR and trauma-focused cognitive-behavioral therapy in the treatment of PTSD: A meta-analytic study. Psychological Medicine, 36(11), 1515–1522. [DOI] [PubMed] [Google Scholar]
- Shapiro F., & Forrest M. (2001). EMDR: Eye movement desensitization and reprocessing. New York, NY: Guilford. [Google Scholar]
- Tesarz J., Leisner S., Gerhardt A., Janke S., Seidler G. H., Eich W., & Hartman M. (2014). Effects of eye movement desensitization and reprocessing (EMDR) treatment in chronic pain patients: A systematic review. Pain Medicine (Malden, Mass.), 15, 247–263. [DOI] [PubMed] [Google Scholar]
- Tunkel D. E., Bauer C. A., Sun G. H., Rosenfeld R. M., Chandrasekhar S. S., Cunningham E. R. Jr., … Whamond E. J. (2014). Clinical practice guideline: Tinnitus. Otolaryngology--Head and Neck Surgery : Official Journal of American Academy of Otolaryngology-Head and Neck Surgery, 151(2Suppl), S1–s40. [DOI] [PubMed] [Google Scholar]
- Van Rood Y. R., & de Roos C. (2009). EMDR in the treatment of medically unexplainedsymptoms: A Systematic review. Journal of EMDR Practice and Research, 3(4). [Google Scholar]
- Wilson P. H. (2006). Classical conditioning as the basis for the effective treatment of tinnitus-related distress, ORL; Journal for Oto-Rhino-Laryngology and Its Related Specialties, 68(1), 6–11. discussion 11–13. [DOI] [PubMed] [Google Scholar]
- Zoger S., Svedlund J., & Holgers K. M. (2006). Relationship between tinnitus severity and psychiatric disorders. Psychosomatics, 47(4), 282–288. [DOI] [PubMed] [Google Scholar]


