Abstract
Background:
Hypertensive disorders of pregnancy (HDP) affect 10–15% of women and are associated with a two-fold increased risk of cardiovascular disease (CVD).
Objective:
To determine whether including HDP in an established CVD risk score improves prediction of CVD events in women.
Methods:
The analysis comprised 106,230 ≤10-year observations contributed by 67,406 women, age ≥40, free of prior CVD, with data available on model covariates in the Nurses’ Health Study II. Participants were followed for confirmed myocardial infarction, fatal coronary heart disease, or stroke from 1989–2013. We fit an established CVD risk prediction model (Model A: age, total and HDL cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, diabetes) and compared it to the same model plus HDP and parity (Model B); Cox proportional hazards models were used to obtain predicted probabilities for 10-year CVD risk.
Results:
HDP and parity were associated with 10-year CVD risk independent of established CVD risk factors, overall and at ages 40–49. However, inclusion of HDP and parity in the risk prediction model did not improve discrimination (Model A: C-index=0.691; Model B: Cindex=0.693; p-value for difference=0.31) or risk reclassification (net reclassification improvement=0.4%, 95% CI: -0.2, 1.0%, p=0.26).
Conclusions:
In this first test of the clinical utility of HDP and parity in CVD risk prediction, additional inclusion of HDP and parity in an established risk score did not improve discrimination or reclassification in this low-risk population; this may be due to known associations between HDP and established CVD risk factors in the reference model.
Keywords: Preeclampsia, Pregnancy, Cardiovascular Disease, Cardiovascular Disease Risk Factors
Condensed Abstract:
In this prospective cohort study of 67,406 women age ≥40, hypertensive disorders of pregnancy (HDP) and parity were associated with 10-year cardiovascular disease (CVD) risk independent of established CVD risk factors, overall and at ages 40–49. However, inclusion of HDP and parity in an established risk score did not improve discrimination (Cdifference=0.002, p=0.31) or reclassification (net reclassification improvement=0.4%, 95% CI: 0.2, 1.0%, p=0.26). While HDP and parity did not improve risk prediction in this low-risk population—perhaps due to known associations between HDP and CVD risk factors in the reference model—HDP remains an important CVD risk marker in women.
Introduction
Cardiovascular disease (CVD) is the leading cause of death among women in the United States (1). While established CVD risk factors—including age, total and high-density lipoprotein (HDL) cholesterol, systolic blood pressure (SBP), treatment for high blood pressure, smoking, and diabetes—predict CVD in both men and women, several risk factors specific to women have emerged; these include pregnancy complications, such as hypertensive disorders of pregnancy (HDP; gestational hypertension and preeclampsia) (2–4). During their lifetime, 10–15% of parous women will develop HDP in at least one pregnancy and these women carry a two-fold increased risk of CVD (5–7). The American Heart and Stroke Associations (AHA, ASA) recognize HDP as a major risk factor for CVD and, since 2011, the AHA has recommended clinicians screen women for a history of these complications (8,9).
Clinical risk scores have long been used to direct targeted intervention and prevention. The Pooled Cohort Risk Equations (PCEs) were introduced and endorsed by the American College of Cardiology (ACC) and the AHA in 2013 and, since that time, have been incorporated into clinical practice to predict 10-year risk of first hard atherosclerotic cardiovascular disease (ASCVD) event (10). While these equations advanced prediction of CVD risk by incorporating stroke as an endpoint and creating gender and race/ethnicity-specific equations, they have received criticism and it has been suggested that including novel risk markers may add value to the PCEs in clinical practice (11). Compared to other pregnancy complications, such as gestational diabetes and preterm delivery, HDP is consistently and, in general, more strongly related to future maternal CVD (3,5,6,12). Although previous studies have demonstrated differences in predicted CVD risk between women with and without a history of HDP and called for incorporating HDP as an independent risk marker (7,13), to date, no study has tested the clinical utility of incorporating history of HDP in CVD risk prediction.
To determine if HDP predicts CVD—above and beyond traditionally screened risk factors, or before those risk factors arise—we compared the performance of the PCE, an established CVD risk prediction model, to this model additionally including HDP; given the increased opportunity to develop HDP with each additional pregnancy, we additionally included parity in the model. We hypothesized that 1) incorporating history of HDP into an established CVD risk score might capture high-risk women undetected by current CVD screening and 2) the clinical utility of HDP in risk prediction would be strongest at, or even isolated to, younger ages, before the development of CVD risk factors known to be associated with HDP history and already included in the PCE.
Methods
The Nurses’ Health Study II (NHSII) is a longitudinal cohort study of 116,429 female U.S. registered nurses with repeated assessments of health-related behaviors, reproductive history, and ascertainment of incident disease through biennial questionnaires. Participants were enrolled in 1989, at 25–42 years of age.
Hypertensive Disorders of Pregnancy and Parity Exposures
Pregnancy history—including the number and years of births, pregnancy outcome, gestation length, complications, and infant characteristics—was self-reported for all lifetime pregnancies in 2009. History of HDP was defined as self-report of “pregnancy-related high blood pressure” (i.e. gestational hypertension) or “pre-eclampsia/toxemia.” To assess the validity of self-reported preeclampsia, we reviewed medical records for 598 women who reported preeclampsia on biennial questionnaires from 1991–2001 for provider report of preeclampsia or evidence of gestational hypertension (new onset high blood pressure—SBP ≥140mmHg or DBP ≥90mmHg—after 20 weeks gestation) and proteinuria (≥300mg/24h urine, protein-creatinine ratio ≥0.3, or dipstick reading of ≥1+) (14). There were 411 cases of medical record-confirmed preeclampsia for a positive predictive value (PPV) of 69%. After excluding medical records with insufficient information to confirm or reject a diagnosis (n=136), the PPV increased to 89%.
Established Cardiovascular Disease Risk Factors
Current smoking and anti-hypertensive medication use were self-reported on all biennial questionnaires. Self-reported “current usual blood pressure (if checked within 2 years)” was provided within categories on the 1989 and 1999 biennial questionnaires; the midpoint of each category was assigned as the continuous value for analysis. Previous medical record review of self-reported high blood pressure in NHSII indicated good agreement with 94% sensitivity and 85% specificity (15). Diabetes was self-reported as “diabetes: not during pregnancy” on the 1989 baseline questionnaire and subsequent questionnaires captured incident diagnoses. Diagnoses were confirmed through supplemental questionnaire, which provided information on diagnostic test results, symptoms, and treatment. Cases were then classified into the categories proposed by the National Diabetes Data Group and the American Diabetes Association, as described elsewhere (16–18). In a related cohort, type 2 diabetes diagnosis was confirmed through medical record review in over 98% of women (19).
Measured plasma total and HDL cholesterol values were available for a subset of NHSII women; these values were used to derive predicted total and HDL cholesterol values for all NHSII participants (see Online Appendix). To validate the predicted total and HDL cholesterol values, we compared measured values to predicted values for the 1997 and 1999 biennial questionnaires, which were most proximal to the time of the blood draw. Correlation coefficients between measured and predicted cholesterol values were 0.55 and 0.57 for total cholesterol and 0.52 and 0.50 for HDL cholesterol in 1997 and 1999, respectively.
Cardiovascular Disease Events
At NHSII baseline in 1989, participants reported history of physician-diagnosed “myocardial infarction (MI) or angina” or “stroke (cerebrovascular accident) or transient ischemic attack (TIA).” Subsequent biennial questionnaires captured incident CVD events. Participants or next of kin granted permission for medical record review of incident CVD events during active follow-up. MI was confirmed by applying the World Health Organization criteria of acute symptoms and diagnostic electrocardiographic results or elevated cardiac enzymes (20,21). Fatal coronary heart disease (CHD) was confirmed by hospital or autopsy records or if CHD was listed as the cause of death on the death certificate for an individual with a history of CHD. Stroke was confirmed and classified according to evidence of neurological deficit with sudden or rapid onset, which persisted for more than 24 hours or until death due to vascular cause, according to National Survey of Stroke criteria (22). Silent strokes discovered through radiologic imaging alone and cerebrovascular pathology resulting from infection, trauma, or malignancy were not included. CVD events (non-fatal MI, fatal CHD, non-fatal or fatal stroke) confirmed by medical record review were considered definite cases, while events endorsed by the NHSII participant or a relative, but for which medical records could not be obtained or permission for release was not provided, were considered probable cases. Definite and probable cases of CHD and stroke comprised our CVD outcome of interest.
Exclusions
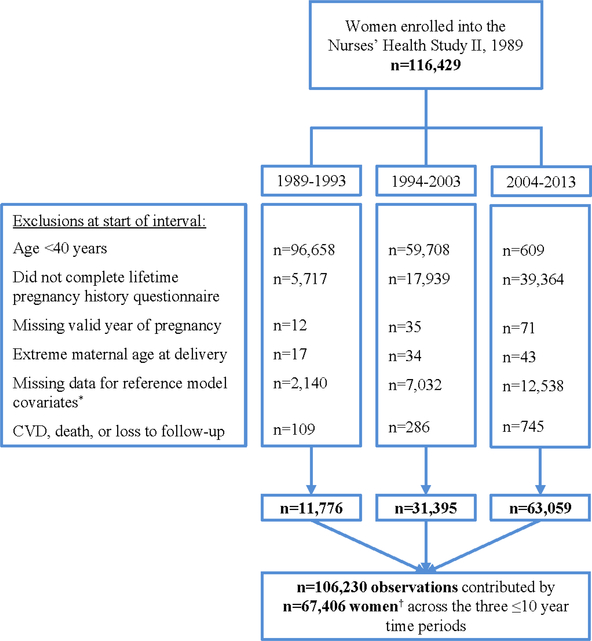
To fully utilize NHSII data in 10-year CVD risk prediction, we divided active follow-up into three independent time periods—1989–1993, 1994–2003, and 2004–2013—and allowed each woman to contribute person-time to the analysis from one or more periods. These three time periods (spanning 25 total years) were constructed backwards from 2013 to enable the two full 10-year time periods to come from the years when women would be at highest risk. Exclusion criteria were applied at the beginning of each time period. As formal estimation of 10-year CVD risk is not recommended before age 40, women were excluded from follow-up during that interval if they were less than 40 years of age (23,24). Women were additionally excluded if they did not complete the 2009 biennial questionnaire (which ascertained lifetime pregnancy history and permitted dating of HDP exposure), had an extreme maternal age at delivery (≤13 or >50 years), were missing a valid year of pregnancy or were missing covariate information required for the reference prediction model, or developed a CVD event, died, or were lost to follow-up before the start of the interval (Figure 1). These exclusions yielded 106,230 observations contributed by 67,406 women across the three time periods. This analysis was approved by the Partners Human Research Committee, Brigham and Women’s Hospital.
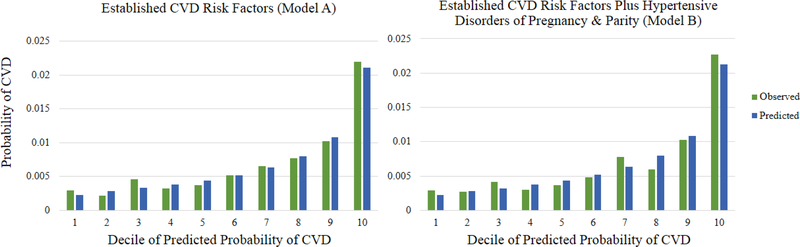
Online Figure 1:
Calibration plot of observed and predicted 10-year probabilities of CVD by decline of predicted probability based on established CVD risk factors (Model A) and on established CVD risk factors plus hypertensive disorders of pregnancy and parity (Model B)
Statistical Analysis
Since the NHSII is comprised predominantly of white women, the PCE for white women served as the reference model (Model A: established CVD risk factors) and included age (years), total cholesterol (mg/dL), HDL cholesterol (mg/dL), SBP (mm Hg), and indicators for antihypertensive medication use, current smoking, and diabetes (see Online Appendix) (10).
For generalizability of our new model, we included both parous and nulliparous women in our primary analysis. Among parous women, each subsequent pregnancy presents an additional opportunity to develop HDP. Furthermore, the severity and outcome of a pregnancy complicated by HDP may affect the likelihood of future pregnancies. Previous research has also shown that the increased risk of cardiovascular death among women with preeclampsia in their first pregnancy is driven primarily by women who do not have another birth (25). Therefore, to flexibly model the complex interrelationship of HDP and parity and to avoid a zero cell (positivity) issue when modeling them separately (as nulliparous women cannot have a history of HDP), we cross-classified these variables into a seven-category exposure variable: nulliparous; normotensive para 1; normotensive para 2 (reference group); normotensive para ≥3; HDP para 1; HDP para 2; and HDP para ≥3. Thus, six indicator variables for HDP and parity were included in our new model (Model B: established CVD risk factors plus HDP and parity).
To assess the potential benefit of incorporating the novel risk markers—history of HDP and parity—into an established CVD risk prediction model, we followed the AHA’s scientific statement on evaluating novel markers of cardiovascular risk (26). First, we fit a Cox proportional hazards model to estimate associations between established CVD risk factors with CVD events (Model A) (27). To assess the independence of HDP and parity in the presence of the established risk factors, we fit the same model additionally including HDP and parity (Model B).
Women contributed person-time to the analysis from the start of each eligible interval (1989–1993, 1994–2003, and/or 2004–2013) until CVD event of interest (non-fatal MI, fatal CHD, non-fatal or fatal stroke), death, last returned questionnaire, or end of the interval. The Cox proportional hazards model is able to accommodate varying lengths of time contributed by participants in estimating 10-year CVD risk (27). We tested for interactions between HDP and parity with both age and calendar time through likelihood ratio tests, comparing a model with and without multiplicative interaction terms between the HDP and parity exposure and 1) age and 2) time. No interactions or violations of the proportional hazards assumption were identified (p=0.17 for age; p=0.22 for time). Model fit, discrimination, and calibration statistics were calculated for each model and the models were compared through the difference in the C-indices and reclassification statistics, using publicly-available macros (http://ncook.bwh.harvard.edu/sasmacros.html; see Online Appendix). Given our pre-specified hypothesis that the model incorporating HDP and parity might perform better at younger ages, we also compared Model A and Model B stratified by age (40–49 and 50–59 years at the start of the interval). Analyses were conducted using SAS software (version 9.4; SAS Institute, Inc., Cary, NC).
Results
Table 1 presents the age-adjusted baseline characteristics of study participants by time period of follow-up. Participants had a mean age of 47.2 years ± 5.1 (standard deviation).
Table 1:
Age-standardized baseline characteristics of Nurses’ Health Study II participants* by time period of follow-up
| Time Period of Follow-up | |||
|---|---|---|---|
| 1989–1993n=11,776(11.1%) | 1994–2003n=31,395(29.6%) | 2004–2013n=63,059(59.3%) | |
| Age, years, mean (SD)† | 41.5 (0.9) | 43.7 (2.3) | 50.0 (4.6) |
| Total cholesterol, mg/dL, mean (SD) | 188.8 (8.1) | 193.0 (14.9) | 202.0 (21.3) |
| HDL-C, mg/dL, mean (SD) | 61.1 (3.9) | 60.1 (6.6) | 62.2 (9.5) |
| Systolic blood pressure, mm Hg, mean (SD) | 115.7 (5.9) | 114.7 (9.0) | 117.2 (11.8) |
| Anti-hypertensive medication use | 8 | 7 | 8 |
| Current smoker | 13 | 11 | 7 |
| Diabetes | 0 | 1 | 2 |
| Hypertensive disorder of pregnancy status | |||
| Normotension | 92 | 91 | 89 |
| Gestational Hypertension | 3 | 3 | 4 |
| Preeclampsia | 5 | 6 | 7 |
| Parity | |||
| Nulliparous | 17 | 18 | 17 |
| 1 birth | 15 | 15 | 13 |
| 2 births | 43 | 40 | 40 |
| ≥3 births | 26 | 27 | 30 |
| Hypertensive disorder of pregnancy and parity | |||
| Nulliparous | 17 | 18 | 17 |
| Normotensive pregnancy and para 1 | 13 | 13 | 11 |
| Normotensive pregnancies and para 2 | 39 | 36 | 35 |
| Normotensive pregnancies and para ≥3 | 23 | 24 | 26 |
| History of HDP and para 1 | 2 | 2 | 2 |
| History of HDP and para 2 | 4 | 5 | 5 |
| History of HDP and para >3 | 3 | 3 | 4 |
Abbreviations: HDL-C: high-density lipoprotein cholesterol. HDP: hypertensive disorder of pregnancy. Values are means (SD) or percentages and are standardized to the age distribution of the study population. Percentages unless otherwise noted. Values of polytomous variables may not sum to 100% due to rounding.
* n=38,324 women contributed person-time to the analysis drawn from one time period, n=19,340 contributed person-time from two time periods, and n=9,742 contributed person-time from all three time periods.
† Value is not age adjusted
Eighty-two percent of participants were parous and approximately 10% had a history of HDP. Women who contributed person-time from a later time period had higher total cholesterol levels, were more likely to be diabetic, and less likely to be current smokers than women who contributed person-time from an earlier time period. Online Table 1 summarizes participant baseline characteristics by age at the start of follow-up (40–49 or 50–59 years).
Online Table 1:
Age-standardized baseline characteristics of Nurses’ Health Study II participantsa by age at start of follow-up
| Age Category at Start of Follow-Up | ||
|---|---|---|
| Interval | ||
| 40–49 years (n=74,052b) |
50–59 years (n=32,178) |
|
| Age, years, mean (SD)c | 44.3 (2.8) | 53.7 (2.3) |
| Total cholesterol, mg/dL, mean (SD) | 139.9 (15.1) | 218.0 (11.1) |
| HDL-C, mg/dL, mean (SD) | 61.1 (7.1) | 63.2 (5.4) |
| Systolic blood pressure, mm Hg, mean (SD) | 115.4 (9.3) | 120.7 (7.0) |
| Anti-hypertensive medication use | 7 | 14 |
| Current smoker | 10 | 8 |
| Diabetes | 1 | 4 |
| Hypertensive disorder in pregnancy status | ||
| Normotension | 90 | 91 |
| Gestational Hypertension | 3 | 3 |
| Preeclampsia | 6 | 6 |
| Parity | ||
| Nulliparous | 18 | 19 |
| 1 birth | 14 | 15 |
| 2 births | 40 | 40 |
| ≥3 births | 28 | 27 |
| Hypertensive disorders in pregnancy and parity | ||
| Nulliparous | 18 | 19 |
| Normotensive pregnancy and para 1 | 12 | 13 |
| Normotensive pregnancies and para 2 | 36 | 35 |
| Normotensive pregnancies and para ≥3 | 25 | 24 |
| History of HDP and para 1 | 2 | 2 |
| History of HDP and para 2 | 5 | 4 |
| History of HDP and para ≥3 | 3 | 3 |
Abbreviations: HDL-C: high-density lipoprotein cholesterol, HDP: hypertensive disorder of pregnancy.
Values are means (SD) or percentages and are standardized to the age distribution of the study population.
Percentages unless otherwise noted. Values of polytomous variables may not sum to 100% due to rounding.
This table contains data on the 106,997 observations contributed by 67,849 women.
n=11,700 observations (15.8%) were drawn from 1989–1993, n=31,546 (42.6%) were drawn from 1994–2003, and n=30,806 (41.6%) were drawn from 2004–2013. n=11,096 women contribute 2 observations to the age 40–49 analysis (e.g., a woman could contribute 2 observations – one from 1989–1993 and one from 1994–2003 – that inform the age 40–49 analysis with baseline ages of 40 in 1989 and 45 in 1994).
Value is not age adjusted
By the end of follow-up, when participants were a median age of 60.6 years (interquartile range (IQR): 57.8, 64.3), there were 685 first CVD events: 359 (52.4%) occurred among women ages 40 to 49 at start of follow-up and 326 (47.6%) occurred among women ages 50 to 59 at start of follow-up. The median age at CVD event was 56.3 years (IQR: 52.3, 60.8).
HDP and parity were associated with 10-year CVD risk in crude analyses (Table 2). Specifically, there were statistically significant increased rates of CVD for women with a history of normotensive pregnancy and one birth (HR: 1.42, 95% CI: 1.12, 1.80) and for women with a history of HDP and two births (HR: 2.02, 95% CI: 1.50, 2.72), compared to women with a history of normotensive pregnancies and two births. When HDP and parity were added to a model with established CVD risk factors, the increased risks associated with 1) a history of normotensive pregnancy and one birth and 2) a history of HDP and two births were attenuated but persisted overall and at ages 40 to 49 (Table 3, Model B). However, HDP and parity were not independently associated with CVD risk in the older age range (50–59 years).
Table 2:
Cumulative incidence and hazard ratios for 10-year risk of CVD by hypertensive disorders of pregnancy and parity, overall and stratified by age at start of interval
| Crude Model | Cases/Person-Years | 10-Year Cumulative Incidence* | HR (95% CI) | P Value |
|---|---|---|---|---|
| Overall | ||||
| Nulliparous | 129/178,380 | 72 | 1.23 (0.98, 1.53) | 0.07 |
| Normotensive pregnancy and para 1 | 104/124,173 | 84 | 1.42 (1.12, 1.80) | <0.01 |
| Normotensive pregnancy and para 2 | 208/352,711 | 60 | 1.00 (ref) | — |
| Normotensive pregnancies and para >3 | 149/241,544 | 62 | 1.05 (0.85, 1.29) | 0.68 |
| History of HDP and para 1 | 18/18,563 | 98 | 1.64 (1.01, 2.66) | 0.04 |
| History of HDP and para 2 | 54/45,346 | 118 | 2.02 (1.50, 2.72) | <0.001 |
| History of HDP and para >3 | 23/31,504 | 74 | 1.24 (0.80, 1.90) | 0.34 |
| Ages 40–49 | ||||
| Nulliparous | 67/120,209 | 55 | 1.30 (0.95, 1.76) | 0.10 |
| Normotensive pregnancy and para 1 | 58/82,163 | 72 | 1.64 (1.19, 2.27) | <0.01 |
| Normotensive pregnancy and para 2 | 103/239,615 | 44 | 1.00 (ref) | — |
| Normotensive pregnancies and para >3 | 76/166,698 | 46 | 1.06 (0.79, 1.43) | 0.70 |
| History of HDP and para 1 | 11/12,741 | 89 | 2.00 (1.08, 3.73) | 0.03 |
| History of HDP and para 2 | 33/31,490 | 104 | 2.43 (1.64, 3.60) | <0.001 |
| History of HDP and para >3 | 11/22,559 | 49 | 1.13 (0.61, 2.11) | 0.70 |
| Ages 50–59 | ||||
| Nulliparous | 62/58,171 | 106 | 1.15 (0.84, 1.57) | 0.39 |
| Normotensive pregnancy and para 1 | 46/42,010 | 108 | 1.18 (0.83, 1.67) | 0.35 |
| Normotensive pregnancy and para 2 | 105/113,096 | 92 | 1.00 (ref) | — |
| Normotensive pregnancies and para >3 | 73/74,846 | 97 | 1.05 (0.78, 1.42) | 0.75 |
| History of HDP and para 1 | 7/5,822 | 120 | 1.29 (0.60, 2.78) | 0.51 |
| History of HDP and para 2 | 21/13,857 | 150 | 1.63 (1.02, 2.61) | 0.04 |
| History of HDP and para >3 | 12/8,945 | 133 | 1.45 (0.80, 2.63) | 0.23 |
Abbreviations: CVD: cardiovascular disease. CI: confidence intervals. HDP: hypertensive disorder of pregnancy. HR: hazard ratio.
*Cumulative incidences per 10,000 women shown above are Kaplan-Meier estimates, which account for censoring.
Table 3:
Hazard ratios, 95% confidence intervals, and P values for 10-year risk of CVD predicted by established CVD risk factors (model A) and established CVD risk factors plus hypertensive disorders of pregnancy and parity (model B), overall and stratified by age at start of interval
| Overall | Ages 40–49 | Ages 50–59 | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Model A: Established CVD Risk Factors | ||||||
| Age, years | 1.03 (1.01, 1.05) | <0.01 | 1.01 (0.93, 1.08) | 0.89 | 0.93 (0.68, 1.27) | 0.64 |
| Age squared | 1.00 (1.00, 1.00) | 0.88 | 0.99 (0.98, 1.01) | 0.41 | 1.01 (0.99, 1.03) | 0.46 |
| Total cholesterol, mg/dL | 1.01 (1.01, 1.02) | <0.001 | 1.01 (1.00, 1.02) | <0.001 | 1.01 (1.01, 1.02) | <0.001 |
| HDL-C, mg/dL | 0.99 (0.98, 0.99) | <0.001 | 0.99 (0.98, 1.00) | 0.03 | 0.98 (0.97, 1.00) | <0.01 |
| Anti-hypertensive medication use (yes/no) | 2.06 (1.60, 2.67) | <0.001 | 2.24 (1.59, 3.14) | <0.001 | 1.76 (1.20, 2.59) | <0.01 |
| Systolic blood pressure, mm Hg | 1.02 (1.01, 1.03) | <0.001 | 1.03 (1.02, 1.04) | <0.001 | 1.01 (1.00, 1.02) | 0.06 |
| Systolic blood pressure * anti-hypertensive medication use | 0.98 (0.97, 0.99) | <0.01 | 0.96 (0.94, 0.98) | <0.001 | 1.00 (0.98, 1.02) | 0.66 |
| Current smoker (yes/no) | 2.40 (1.98, 2.92) | <0.001 | 2.41 (1.86, 3.13) | <0.001 | 2.40 (1.80, 3.20) | <0.001 |
| Diabetes (yes/no) | 1.57 (1.14, 2.17) | <0.01 | 2.06 (1.19, 3.54) | <0.01 | 1.40 (0.94, 2.09) | 0.10 |
| Model B: Established CVD Risk Factors Plus Hypertensive Disorders of Pregnancy and Parity | ||||||
| Age, years | 1.03 (1.01, 1.05) | <0.01 | 1.01 (0.93, 1.09) | 0.85 | 0.93 (0.68, 1.27) | 0.64 |
| Age squared | 1.00 (1.00, 1.00) | 0.88 | 0.99 (0.98, 1.01) | 0.41 | 1.01 (0.99, 1.03) | 0.46 |
| Total cholesterol, mg/dL | 1.01 (1.01, 1.02) | <0.001 | 1.01 (1.00, 1.02) | <0.01 | 1.01 (1.01, 1.02) | <0.001 |
| HDL cholesterol, mg/dL | 0.99 (0.98, 0.99) | <0.001 | 0.99 (0.98, 1.00) | 0.04 | 0.98 (0.97, 1.00) | <0.01 |
| Anti-hypertensive medication use (yes/no) | 2.03 (1.57, 2.63) | <0.001 | 2.19 (1.55, 3.07) | <0.001 | 1.75 (1.19, 2.58) | <0.01 |
| Systolic blood pressure, mm Hg | 1.02 (1.01, 1.03) | <0.001 | 1.03 (1.02, 1.04) | <0.001 | 1.01 (1.00, 1.02) | 0.06 |
| Systolic blood pressure * antihypertensive medication use | 0.98 (0.97, 0.99) | <0.01 | 0.96 (0.94, 0.98) | <0.001 | 1.00 (0.98, 1.02) | 0.66 |
| Current smoker (yes/no) | 2.39 (1.97, 2.90) | <0.001 | 2.39 (1.84, 3.10) | <0.001 | 2.40 (1.80, 3.19) | <0.001 |
| Diabetes (yes/no) | 1.57 (1.14, 2.17) | <0.01 | 2.06 (1.20, 3.55) | <0.01 | 1.40 (0.94, 2.08) | 0.10 |
| Nulliparous | 1.07 (0.86, 1.34) | 0.53 | 1.10 (0.81, 1.51) | 0.53 | 1.04 (0.76, 1.42) | 0.81 |
| Normotensive pregnancy and para 1 | 1.30 (1.03, 1.65) | 0.03 | 1.53 (1.11, 2.11) | 0.01 | 1.09 (0.77, 1.55) | 0.62 |
| Normotensive pregnancies and para >3 | 1.09 (0.88, 1.35) | 0.43 | 1.11 (0.82, 1.49) | 0.50 | 1.07 (0.80, 1.45) | 0.65 |
| History of HDP and para 1 | 1.15 (0.71, 1.87) | 0.58 | 1.37 (0.73, 2.56) | 0.33 | 0.91 (0.42, 1.96) | 0.81 |
| History of HDP and para 2 | 1.51 (1.11, 2.04) | <0.01 | 1.81 (1.22, 2.71) | <0.01 | 1.19 (0.74, 1.92) | 0.47 |
| History of HDP and para >3 | 0.95 (0.62, 1.47) | 0.82 | 0.84 (0.45, 1.58) | 0.60 | 1.07 (0.59, 1.96) | 0.83 |
Abbreviations: CVD: cardiovascular disease. CI: confidence intervals. HDL-C: high-density lipoprotein cholesterol. HDP: hypertensive disorder of pregnancy. HR: hazard ratio. Indicator (yes/no) variables unless otherwise noted. Reference group for hypertensive disorder of pregnancy and parity is normotensive pregnancies and para 2.
Table 4 presents the primary results, comparing Model A, based on established CVD risk factors, to Model B, additionally including HDP and parity, in terms of model fit, discrimination, calibration, and reclassification for 10-year CVD risk prediction. Both models were adequately calibrated with p≥0.05 (Table 4, Online Figure 1). The inclusion of HDP and parity did not obtain a better model fit overall or at ages 50 to 59, but did obtain a significantly better fit to the data at ages 40 to 49 (likelihood ratio test: p=0.03). The change in the C-index was not statistically significant in any model. The overall NRI was 0.4% (95% CI: -0.2, 1.0%, p=0.26) for risk reclassification across three risk groups (<5, 5-<10, and ≥10%). The NRI was not statistically significant overall or in age-stratified models. However, the IDI was statistically significant overall (0.02%, p<0.001) and in age-stratified models (40–49 years: 0.04%, p<0.001; 50–59 years: 0.006%, p=0.04).
Table 4:
Model fit, discrimination, calibration, and reclassification indices in 10-year cardiovascular disease risk prediction based on established CVD risk factors* (Model A) and on established CVD risk factors plus hypertensive disorders of pregnancy and parity (Model B)
| Model A: Without HDP and Parity |
Model B: With HDP and Parity |
P Value† | |
|---|---|---|---|
| Overall | |||
| Model Fit | |||
| BIC | 15,435 | 15,464 | 0.12 |
| Discrimination | |||
| C-index | 0.691 | 0.693 | --- |
| C-difference | --- | 0.002 | 0.31 |
| Calibration | |||
| Hosmer-Lemeshow P value | 0.35 | 0.05 | --- |
| Reclassification | |||
| NRI‡, % | --- | 0.4 | 0.26 |
| IDI, % | --- | 0.02 | <0.001 |
| Ages 40–49 | |||
| Model Fit | |||
| BIC | 7,866 | 7,888 | 0.03 |
| Discrimination | |||
| C-index | 0.669 | 0.673 | --- |
| C-difference | --- | 0.005 | 0.29 |
| Calibration | |||
| Hosmer-Lemeshow P value | 0.63 | 0.31 | --- |
| Reclassification | |||
| NRI‡, % | --- | -0.003 | 0.99 |
| IDI, % | --- | 0.04 | <0.001 |
| Ages 50–59 | |||
| Model Fit | |||
| BIC | 6,658 | 6,691 | 0.99 |
| Discrimination | |||
| C-index | 0.677 | 0.677 | --- |
| C-difference | --- | 0.000 | 0.90 |
| Calibration | |||
| Hosmer-Lemeshow P value | 0.75 | 0.87 | --- |
| Reclassification | |||
| NRI‡, % | --- | 0.6 | 0.13 |
| IDI, % | — | 0.006 | 0.04 |
Abbreviations: BIC: (Schwarz) Bayesian information criterion. NRI: net reclassification improvement. IDI: integrated discrimination improvement. CVD: cardiovascular disease. HDP: hypertensive disorder of pregnancy.
* Established risk factors include age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetes.
†Based on likelihood ratio test for comparison of model fit and 1,000 bootstrap samples for comparison of C-indices and reclassification statistics
‡Low risk: <5%, Intermediate risk: 5%-<10%, and High risk: >10%
Table 5 illustrates the net reclassification of 10-year CVD risk into low (<5%), intermediate (5-<10%), and high risk (≥10%) groups, among women who developed incident CVD and those who did not. The majority of women (99.7%) were classified as low risk by both models. Model B correctly reclassified 0.6% of previously low-risk women who developed CVD (n=4) into a higher risk group, yet it incorrectly reclassified 8.3% (n=1) of previously intermediate risk women as low risk. Online Tables 3 and 4 present the detailed reclassification results stratified by age. Online Tables 5-7 presents the net reclassification of 10-year CVD risk into low and high risk groups based on the dichotomous cut point of 7.5% predicted CVD risk overall and by age.
Table 5:
Net reclassification of 10-year cardiovascular disease risk into low (<5%), intermediate (5% - <10%), and high (≥10%) risk groups, comparing model A (based on established CVD risk factors) to model B (based on established CVD risk factors plus hypertensive disorders of pregnancy and parity) among women with incident CVD and those without incident CVD
| Model B: Established Risk Factors Plus Hypertensive Disorders of Pregnancy and Parity | ||||
| Categories of Predicted 10-Year Risk | ||||
| Model A: Established Risk Factors | Low Risk: | Intermediate Risk: | High Risk: | Total |
| Categories of Predicted 10-Year Risk | <5% | 5%–<10% | >10% | Reclassified |
| Women with incident CVD (n=685) | ||||
| Low Risk: <5% | ||||
| N | 669 | 4 | 0 | 4 |
| % Reclassified | --- | 0.6 | --- | 0.6 |
| Intermediate Risk: 5% - <10% | ||||
| N | 1 | 11 | 0 | 1 |
| % Reclassified | 8.3 | --- | --- | 8.3 |
| High Risk: >10% | ||||
| N | 0 | 0 | 0 | --- |
| % Reclassified | --- | --- | --- | --- |
| Women without incident CVD (n=105,545) | ||||
| Low Risk: <5% | ||||
| N | 105,293 | 60 | 0 | 60 |
| % Reclassified | --- | 0.06 | --- | 0.06 |
| Intermediate Risk: 5% - <10% | ||||
| N | 27 | 153 | 4 | 31 |
| % Reclassified | 14.7 | --- | 2.2 | 16.9 |
| High Risk: >10% | ||||
| N | 0 | 2 | 6 | 2 |
| % Reclassified | --- | 25.0 | --- | 25.0 |
Abbreviations: CVD: cardiovascular disease. Established risk factors include age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetes.
NRI = 0.004; 95% CI: -0.002, 0.010; P = 0.26. NRI for events = 0.004; 95% CI: -0.002, 0.010; P = 0.21. NRI for non-events = -0.0003; 95% CI: -0.0005, -0.0002; P = 0.0003.
Online Table 3:
Net reclassification of 10-year cardiovascular disease risk into low (<5%), intermediate (5% - <10%), and high (≥10%) risk groups, comparing model A (based on established CVD risk factors) to model B (based on established CVD risk factors plus hypertensive disorders of pregnancy and parity) among women with incident CVD and those without incident CVD at ages 40–49
| Model B: Established CVD Risk Factors Plus | ||||
| Hypertensive Disorders of Pregnancy and Parity | ||||
| Categories of Predicted 10-Year Risk | ||||
| Model A: Established CVD Risk Factors | Low Risk: | Intermediate Risk: | High Risk: | Total |
| Categories of Predicted 10-Year Risk | <5% | 5%–<10% | ≥10% | Reclassified |
| Women with incident CVD (n=359) | ||||
| Low Risk: <5% | ||||
| N | 355 | 1 | 0 | --- |
| % Reclassified | --- | 0.3 | --- | --- |
| Intermediate Risk: 5%–<10% | ||||
| N | 1 | 2 | 0 | 1 |
| % Reclassified | 33.3 | --- | --- | 33.3 |
| High Risk: ≥10% | ||||
| N | 0 | 0 | 0 | --- |
| % Reclassified | --- | --- | --- | --- |
| Women without incident CVD (n=73,693) | ||||
| Low Risk: <5% | ||||
| N | 73,637 | 28 | 0 | 28 |
| % Reclassified | --- | 0.04 | --- | 0.04 |
| Intermediate Risk: 5% - <10% | ||||
| N | 9 | 17 | 2 | 11 |
| % Reclassified | 32.1 | --- | 7.1 | 39.2 |
| High Risk: ≥10% | ||||
| N | 0 | 0 | 0 | --- |
| % Reclassified | — | — | — | — |
Abbreviation: CVD: cardiovascular disease. Established risk factors include age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetes. NRI = -0.00003; 95% CI: -0.006, 0.007; P = 0.99. NRI for events = 0.0002; 95% CI: -0.005, 0.008; P = 0.94. NRI for non-events = -0.0003; 95% CI: -0.0004, -0.0001; P = 0.001.
Online Table 4:
Net reclassification of 10-year cardiovascular disease risk into low (<5%), intermediate (5% - <10%), and high (≥10%) risk groups, comparing model A (based on established CVD risk factors) to model B (based on established CVD risk factors plus hypertensive disorders of pregnancy and parity) among women with incident CVD and those without incident CVD at ages 50–59
| Model B: Established CVD Risk Factors | |||||
| Plus Hypertensive Disorders of Pregnancy and Parity | |||||
| Categories of Predicted 10-Year Risk | |||||
| Model A : Established CVD Risk Factors | Low Risk: | Intermediate Risk: | High Risk: | Total | |
| Categories of Predicted 10-Year Risk | <5% | 5%–<10% | ≥10% | Reclassified | |
| Women with incident CVD (n=326) | |||||
| Low Risk: <5% | |||||
| N | 314 | 2 | 0 | 2 | |
| % Reclassified | --- | 0.6 | --- | 0.6 | |
| Intermediate Risk: 5% - <10% | |||||
| N | 0 | 10 | 0 | ||
| % Reclassified | --- | --- | --- | --- | |
| High Risk: ≥10% | |||||
| N | 0 | 0 | 0 | ||
| % Reclassified | --- | --- | --- | --- | |
| Women without incident CVD (n=31,852) | |||||
| Low Risk: <5% | |||||
| N | 31,666 | 15 | 0 | 15 | |
| % Reclassified | --- | 0.05 | --- | 0.05 | |
| Intermediate Risk: 5% - <10% | |||||
| N | 14 | 150 | 0 | 14 | |
| % Reclassified | 8.5 | --- | --- | 8.5 | |
| High Risk: ≥10% | |||||
| N | 0 | 1 | 6 | 1 | |
| % Reclassified | --- | 14.3 | --- | 14.3 | |
Abbreviation: CVD: cardiovascular disease. Established risk factors include age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetes. NRI = 0.006; 95% CI: -0.0002, 0.014; P = 0.13. NRI for events = 0.006; 95% CI: 0.000, 0.014; P = 0.13. NRI for non-events = 0.000; 95% CI: -0.0003, 0.0003; P = 0.99.
Online Table 5:
Net reclassification of 10-year cardiovascular disease risk into low (<7.5%) and high (≥7.5%) risk groups, comparing model A (based on established CVD risk factors) to model B (based on established CVD risk factors plus hypertensive disorders of pregnancy and parity) among women with incident CVD and those without incident CVD
| Model B: Established CVD Risk Factors | |||
| Plus Hypertensive Disorders of Pregnancy and Parity | |||
| Categories of Predicted 10-Year Risk | |||
| Model A: Established CVD Risk Factors | Low Risk: | High Risk: | Total |
| Categories of Predicted 10-Year Risk | <7.5% | ≥7.5% | Reclassified |
| Women with incident CVD (n=685) | |||
| Low Risk: <7.5% | |||
| N | 682 | 2 | 2 |
| % Reclassified | --- | 0.3 | 0.3 |
| High Risk: ≥7.5% | |||
| N | 0 | 1 | --- |
| % Reclassified | --- | --- | --- |
| Women without incident CVD (n=105,545) | |||
| Low Risk: <7.5% | |||
| N | 105,500 | 13 | 13 |
| % Reclassified | --- | 0.01 | 0.01 |
| High Risk: ≥7.5% | |||
| N | 5 | 27 | 5 |
| % Reclassified | 15.6 | --- | 15.6 |
Abbreviation: CVD: cardiovascular disease. Established risk factors include age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetes. NRI = 0.003; 95% CI: -0.0001, 0.006; P = 0.14. NRI for events = 0.003; 95% CI: 0.000, 0.006; P = 0.13. NRI for non-events = -0.0001; 95% CI: -0.0002, -0.000001; P = 0.04.
Online Table 7:
Net reclassification of 10-year cardiovascular disease risk into low (<7.5%) and high (≥7.5%) risk groups, comparing model A (based on established CVD risk factors) to model B (based on established CVD risk factors plus hypertensive disorders of pregnancy and parity) among women with incident CVD and those without incident CVD at ages.50–59
| Model B: Established CVD Risk Factors Plus Hypertensive Disorders of Pregnancy and Parity | |||
| Categories of Predicted 10-Year Risk | |||
| Model A: Established CVD Risk Factors | Low Risk: <7.5% | High Risk: ≥7.5% | Total Reclassified |
| Categories of Predicted 10-Year Risk | |||
| Women with incident CVD (n=326) | |||
| Low Risk: <7.5% | |||
| N | 322 | 1 | 1 |
| % Reclassified | --- | 0.3 | 0.3 |
| High Risk: ≥7.5% | |||
| N | 1 | 2 | 1 |
| % Reclassified | 33.3 | --- | 33.3 |
| Women without incident CVD (n=31,852) | |||
| Low Risk: <7.5% | |||
| N | 31,807 | 9 | 9 |
| % Reclassified | --- | 0.03 | 0.03 |
| High Risk: ≥7.5% | |||
| N | 4 | 32 | 4 |
| % Reclassified | 11.1 | --- | 11.1 |
Abbreviation: CVD: cardiovascular disease. Established risk factors include age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetes. NRI = -0.00002; 95% CI: -0.007, 0.007; P = 0.99. NRI for events = 0.0001; 95% CI: -0.007, 0.008; P = 0.97. NRI for non-events = -0.0002; 95% CI: -0.0004, 0.0001; P = 0.17.
Sensitivity Analyses
To correct for potential overfitting, we adjusted for optimism using 1,000 bootstrap samples; conclusions did not change, with the exception that the IDI statistic was no longer statistically significant (Online Table 2). Given the retrospective report of pregnancy history in 2009, we conducted a sensitivity analysis with follow-up from 2009–2013. Hazard ratios for HDP and parity in univariate models were similar and remained significant for women with a history of HDP and two births (HR=1.75, 95% confidence interval (CI): 1.07, 2.84) but was no longer significant for women with normotensive pregnancy and one birth (HR=1.30, 95% CI: 0.89, 1.91). When HDP and parity were added to the reference model in 2009 (when participants were 45 to 62 years of age), they were no longer statistically significant. To account for the fact that women were able to contribute multiple observations to the analysis across the 25 years of active follow-up, we also ran the Cox proportional hazards regression models utilizing robust sandwich estimates for correlated data and the CIs and p-values were unchanged (data not shown) (28). Restricting the analysis to parous women, utilizing only definite CVD cases, and evaluating preeclampsia, gestational hypertension, and parity separately in Model B did not change conclusions (data not shown). Given the low CVD risk observed in this study population, we also calculated a two-category NRI with the cut-point at the event rate but conclusions were also unchanged (data not shown) (29).
Online Table 2:
Discrimination and reclassification indices in 10-year cardiovascular disease risk prediction based on established CVD risk factorsa (Model A) and on established CVD risk factors plus hypertensive disorders of pregnancy and parity (Model B) and adjusted for optimism
| Model A: Without HDP and Parity |
Model B: With HDP and Parity |
P Valueb | |
|---|---|---|---|
| Overall | |||
| Discrimination | |||
| C-index | 0.689 | 0.688 | --- |
| C-difference | --- | −0.001 | 0.65 |
| Reclassification | |||
| NRI, % (3 categoriesc) | --- | 0.3 | 0.56 |
| NRI, % (2 categoriesd) | --- | 0.2 | 0.53 |
| IDI, % | --- | 0.02 | 0.14 |
| Ages 40–49 | |||
| Discrimination | |||
| C-index | 0.663 | 0.662 | --- |
| C-difference | --- | −0.001 | 0.89 |
| Reclassification | |||
| NRI, % (3 categoriesc) | --- | −0.2 | 0.78 |
| NRI, % (2 categoriesd) | --- | −0.1 | 0.81 |
| IDI, % | --- | 0.04 | 0.15 |
| Ages 50–59 | |||
| Discrimination | |||
| C-index | 0.670 | 0.663 | --- |
| C-difference | --- | −0.007 | 0.07 |
| Reclassification | |||
| NRI, % (3 categoriesc) | --- | 0.3 | 0.79 |
| NRI, % (2 categoriesd) | --- | −0.1 | 0.88 |
| IDI, % | — | 0.006 | 0.80 |
All measures of discrimination and reclassification presented are optimism-adjusted.
Abbreviations: CVD: cardiovascular disease. BIC: (Schwarz) Bayesian information criterion. NRI: net reclassification improvement.
Established risk factors include age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetes.
Based on likelihood ratio test for comparison of model fit and 1,000 bootstrap samples for comparison of Cindices and reclassification statistics
Low risk: <5%, Intermediate risk: 5%-<10%, and High risk: >10%
Low risk: <7.5%, High risk: ≥7.5%
Discussion
To our knowledge, this is the first study to investigate the clinical utility of incorporating history of HDP into CVD risk prediction. HDP and parity remained independently associated with 10-year risk of a first CVD event when added to a model with established CVD risk factors—age, total cholesterol, HDL cholesterol, SBP, treatment for high blood pressure, current smoking, and diabetes. These associations appeared largely driven by the stronger magnitude of relative risks observed among women 40 to 49 years of age. Inclusion of HDP and parity did not improve model discrimination or reclassification between risk groups in this prospective cohort study of 67,406 U.S. women at overall low risk for CVD (Central Illustration).
Figure 1: Flow diagram for Nurses’ Health Study II participants by time interval contributed to 10-year cardiovascular disease (CVD) risk prediction.
To fully utilize NHSII data in 10-year CVD risk prediction, we divided active follow-up into three independent time periods—1989–1993, 1994–2003, and 2004–2013—and allowed each woman to contribute person-time to the analysis from one or more periods. Exclusion criteria were applied sequentially at the beginning of each time period and 67,406 women contributed 106,230 observations across the three time periods. * Current smoking, systolic blood pressure, and predicted total and HDL cholesterol. † n=38,324 women contributed person-time to the analysis drawn from one time period, n=19,340 contributed person-time from two time periods, and n=9,742 contributed person-time from all three time periods.
As no single statistical measure is able to assess all contributions of a novel risk marker, we tested this through several methods: 1) confirming an independent association between HDP and parity with CVD, both alone and in the presence of established CVD risk factors, 2) reporting the discrimination of the new marker and 3) reporting the accuracy of the new marker, with regards to calibration and reclassification (26). Since the C-index is largely insensitive to change, it was not surprising to find that inclusion of HDP and parity did not improve predictive discrimination, despite being statistically significant and independently associated with CVD risk in this population (30,31). Measures of model fit based on the likelihood are more sensitive than the C-index (32). While the BIC was larger for the model including HDP and parity, in part due to the penalty imposed for adding six new indicator variables, the likelihood ratio test indicated that the model with HDP and parity obtained a significantly better fit among women at ages 40 to 49.
Reclassification tables and statistics provide information more relevant to clinical decisions than the C-index which, although popular, should not be relied on solely when evaluating contributions of a novel risk marker (30). HDP and parity did not improve net reclassification across categories of predicted risk, but this should be considered in light of the low underlying risk of CVD in NHSII participants. As only 0.2% of women (n=204) had a predicted 10-year CVD risk greater than or equal to 5% based on Model A, the NHSII population also had little distribution across risk groups.
The increased CVD risk observed among normotensive women with one birth is consistent with previous literature demonstrating a J-shaped relationship between parity and CVD, with the nadir of risk falling around two births (33). In contrast to our finding, a recent study tested candidate reproductive risk factors for inclusion in a CVD risk prediction model and found that parity was not independently associated with coronary heart disease (34). However, information on HDP was not available in this study. Given the interaction between HDP and parity identified in the current study, it may be that the independent association of parity and CVD was not able to be captured without jointly considering a woman’s parity and history of HDP.
The primary limitation of our study is reliance on the nurse participants’ self-reported SBP and the use of predicted, rather than measured, total and HDL cholesterol levels. However, the misclassification induced by assigning the midpoint of self-reported categories as the continuous SBP value and utilizing predicted, rather than measured, cholesterol would be expected to be non-differential with respect to the other risk factors in the models and CVD. Further, self-report of high blood pressure in the NHSII has demonstrated good agreement with medical record values (15) and predicted total and HDL cholesterol appeared well-validated when compared to measured values among women who provided blood samples. Self-reported pregnancy history may result in recall bias and exposure misclassification; however, a validation study of preeclampsia self-report in the cohort showed that the majority of women who selfreported preeclampsia had medical record evidence of the condition. Additionally, the proportions of pregnancies complicated by either gestational hypertension or preeclampsia are consistent with estimates observed elsewhere (7). Although pregnancy history, including HDP and parity, was retrospectively reported in 2009, length of recall has not been consistently associated with accuracy of maternal recall of HDP (35).
Despite the fact that inclusion in our analytic sample was dependent on survival to 2009, which may induce survivor bias, 98.2% of NHSII participants were alive in 2009. While our sensitivity analysis with follow-up from 2009–2013 resulted in some attenuation, HDP and parity remained associated with CVD but were no longer independently associated once controlling for established CVD risk factors. This sensitivity analysis is likely underpowered and also not surprising given the age range of 45 to 62 years in 2009 and the fact that our analysis among 50 to 59 year olds similarly did not demonstrate an independent association of HDP and parity with CVD risk. As the NHSII cohort includes primarily white nurses, these findings may not be generalizable. The use of the PCE for white women was largely appropriate for the NHSII population but the impact of HDP and parity in CVD risk prediction may differ in other race or ethnic groups.
While HDP and parity did not contribute to model discrimination or reclassification of women across pre-established risk groups, they offer practical advantages over some traditional risk factors—ease of ascertainment, low cost, and availability earlier in a woman’s life—which may justify their inclusion in clinical assessment, particularly before women develop clinical CVD risk factors included in the PCE. In a related analysis, we found that the vast majority of the association between HDP and CVD was accounted for by chronic hypertension, type 2 diabetes mellitus, hypercholesterolemia, and weight changes after pregnancy (e.g., proportion mediated for preeclampsia: 71%, 95% CI: 13–98%) (36). This finding, taken together with the fact that HDP and parity are independently associated with CVD at ages 40 to 49 but not at ages 50 to 59, suggests that these women could be identified at early ages to prevent development of established CVD risk factors. The magnitude of associations observed with HDP and parity are also comparable to those observed with other CVD risk factors or markers not incorporated in the PCE, such as family history of CVD. The contribution of HDP and parity in CVD risk prediction model discrimination and reclassification merits further investigation in populations with a greater risk distribution and overall higher CVD risk than the NHSII participants. It may also be the case that other pregnancy complications, such as preterm delivery, that are also associated with CVD but for which we do not see the same extent of mediation of CVD risk by established risk factors may be better positioned to contribute to risk prediction in women (12).
In this first test of the clinical utility of HDP and parity in predicting a woman’s future risk of CVD, the additional inclusion of HDP and parity in risk prediction models did not improve model discrimination or risk reclassification across categories of predicted CVD risk, despite the fact that these novel markers were associated with CVD independent of established risk factors and improved model fit at ages 40 to 49.
EXPANDED METHODS
Total and HDL Cholesterol Prediction
Measured plasma total and HDL cholesterol values were available for women who contributed to previous nested case-control studies of chronic disease and sub-studies of lifestyle exposures. Blood samples were provided between 1996 and 2001 from 29,611 participants at ages 32–54 years. Intra-assay coefficients of variation from blinded, replicate, quality-control samples ranged from 1.3–6.6% for the five laboratory batches that measured HDL cholesterol and from 1.0–6.2% for the eleven laboratory batches that measured total cholesterol. Measured values for total cholesterol were available for 4,299 NHSII participants, after excluding women whose blood samples were not measured for total cholesterol (n=24,923), those who developed cancer before blood draw (n=13), were missing lab information (n=2), or had an extreme measured value (total cholesterol >500 mg/dL, n=1). For HDL cholesterol, measured values were available for n=1,225 women, after excluding women who did not have a measured HDL cholesterol value (n=28,000) or those who developed cancer before blood draw (n=13).
To obtain predicted total and HDL cholesterol for all NHSII participants, we fit multivariable linear regression prediction models separately for total and HDL cholesterol, including the following variables considered predictive of total and HDL cholesterol based on a priori subject matter knowledge: age, race/ethnicity, current smoking, body mass index, self-reported serum total cholesterol (provided within categories on the 1989 questionnaire), physical activity, menopausal status, post-menopausal hormone use, average alcohol intake, dietary variables (total calories, fiber, and percent calories from protein, polyunsaturated, monounsaturated, saturated, and trans fats), and history of chronic hypertension, type 2 diabetes mellitus, or elevated cholesterol. Covariate information was drawn from the blood questionnaire or the biennial questionnaire closest to, but preceding, the blood draw. R-squared values for the final prediction models were 0.32 for total cholesterol and 0.29 for HDL cholesterol.
We calculated predicted total and HDL cholesterol values for each biennial questionnaire cycle by, first, multiplying each regression coefficient by a woman’s covariate value at the time of the questionnaire and, then, summing across all variables included in the prediction model. Extreme and implausible values were handled by recoding the top and bottom 0.5% of the predicted total and HDL cholesterol values for each questionnaire cycle as missing.
Statistical Analysis
Modification of the Pooled Cohort Risk Equation for White Women
For simplicity and interpretation and given a comparable model fit, we did not include the interaction terms between age and total cholesterol, HDL cholesterol, and current smoking nor did we apply the natural log transformation to the continuous variables for our reference model (Model A).
Given the strong relationship between HDP and chronic hypertension after pregnancy(1,2) and to ensure we properly captured the impact of SBP and anti-hypertensive treatment on CVD risk, we assessed the impact of modeling this information: 1) parameterized, as in the PCE, with untreated SBP (equal to 0 for women on anti-hypertensive medication or continuous SBP value for women not on anti-hypertensive medication) and treated SBP (equal to 0 for women not on anti-hypertensive medication or equal to continuous SBP value for women on anti-hypertensive medication) and 2) SBP modeled continuously, anti-hypertensive treatment indicator, and an interaction term between SBP and treatment indicator. These two sets of variables were separately added to a model including all other established CVD risk factors from the PCE and each of those models were then individually compared to a model without information on SBP and anti-hypertensive treatment through a likelihood ratio test. Modeling SBP and treatment information using a separate indicator variable for anti-hypertensive medication use obtained a larger likelihood ratio test statistic and was a significantly better fit to the data, compared to using two variables for treated and untreated SBP. We, therefore, used this parameterization to characterize SBP and treatment of high blood pressure.
Additional Details on Measures of Model Performance
Global model fit was assessed through an LRT, comparing Model B to Model A (which is nested within Model B), and through comparison of the Schwarz Bayesian Information Criterion (BIC). The BIC is a likelihood-based measure of model fit, which imposes a penalty for increasing the number of model variables; a lower BIC value indicates improved model fit.(3)
To evaluate discrimination—the ability of a model to separate individuals who develop the event of interest from those who do not—we calculated Harrell’s C-index for each model, which is analogous to the area under the receiver operating characteristic (ROC) curve, and calculated the change in the C-index. (4,5) Larger values of the C-index indicate a better ability of the model to discriminate women with events from those without events.
To evaluate calibration—how close the predicted probabilities obtained from a model are to the observed risks—we conducted the Greenwood-D’Agostino-Nam test of calibration, which permits censoring, to obtain a Hosmer Lemeshow χ2 statistic and P value comparing predicted and observed risk across deciles for both Model A and Model B;(6) calibration χ2 values greater than 20 and P values less than 0.05 suggest a lack of adequate model calibration.(7)
To evaluate reclassification—the ability of a new model to improve on a previous model—we calculated the categorical net reclassification improvement (NRI; overall and separately for events and non-events) and the integrated discrimination improvement (IDI).(8) Risk reclassification confers information important for the clinical setting; specifically, reclassification statistics inform whether a new model is able to more accurately stratify individuals into higher or lower risk categories.(8,9) The categorical NRI provides a summary measure of change in risk group classification with model B, as compared to model A, while the IDI measures continuous change in the predicted probabilities between the two models.(10) All these reclassification statistics define and account for “good” and “bad” change; “good” change is represented by upward movements for individuals who go on to develop CVD (i.e. predicted probability from the new model, Model B > predicted probability from the old model, Model A) and downward movement for individuals who do not develop CVD (i.e. predicted probability from the new model, Model B < predicted probability from the old model, Model A).(8) For the categorical NRI, we initially examined three categories of 10-year CVD risk: low <5%, intermediate 5% - <10%, and high risk ≥10%. We selected these cut points as women tend to fall into the <5 or <10% 10-year CVD risk categories and generally have lower predicted risk than men.(12,13) We also calculated the NRI using dichotomous risk categorization with a cut point of 7.5%, which was informed by ACC/AHA guidelines for statin therapy initiation.(14)
Central Illustration: Inclusion of hypertensive disorders of pregnancy in an established 10year cardiovascular disease risk prediction model.
* The established CVD risk calculator, based on the Pooled Cohort Risk Equation for white women, was compared across multiple domains of model performance (e.g., discrimination, risk reclassification) with an expanded risk calculator that additionally included history of hypertensive disorders of pregnancy (gestational hypertension or preeclampsia).
Online Table 6:
Net reclassification of 10-year cardiovascular disease risk into low (<7.5%) and high (≥7.5%) risk groups, comparing model A (based on established CVD risk factors) to model B (based on established CVD risk factors plus hypertensive disorders of pregnancy and parity) among women with incident CVD and those without incident CVD at ages 40–49
| Model B: Established CVD Risk Factors Plus Hypertensive Disorders of Pregnancy and Parity | |||
| Categories of Predicted 10-Year Risk | |||
| Model A: Established CVD Risk Factors | Low Risk: <7.5% | High Risk: ≥7.5% | Total Reclassified |
| Categories of Predicted 10-Year Risk | |||
| Women with incident CVD (n=359) | |||
| Low Risk: <7.5% | |||
| N | 358 | 0 | --- |
| % Reclassified | --- | --- | --- |
| High Risk: ≥7.5% | |||
| N | 0 | 1 | --- |
| % Reclassified | --- | --- | --- |
| Women without incident CVD (n=73,693) | |||
| Low Risk: <7.5% | |||
| N | 73,679 | 5 | 5 |
| % Reclassified | --- | 0.01 | 0.01 |
| High Risk: ≥7.5% | |||
| N | 1 | 8 | 1 |
| % Reclassified | 11.1 | --- | 11.1 |
Abbreviation: CVD: cardiovascular disease. Established risk factors include age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, anti-hypertensive medication use, current smoking, and diabetes. NRI = -0.00005; 95% CI: -.0001, 0.000; P = 0.08. NRI for events = inestimable. NRI for non-events = -0.00005; 95% CI: -0.0001, 0.000; P = 0.08.
Perspectives:
Competency in Medical Knowledge:
Hypertensive disorders of pregnancy and parity are independently associated with CVD events, above and beyond established CVD risk factors, particularly at younger ages.
Competency in Patient Care:
Women with a history of preeclampsia or gestational hypertension should be informed that they are increased risk for CVD events.
Translational Outlook:
While inclusion of HDP and parity in an established 10-year risk score did not improve risk prediction in a low-risk population, providers should capture pregnancy history to identify women at risk for CVD events. Further research is needed to test the clinical utility of HDP in CVD risk prediction in more diverse populations as well as in 30-year or lifetime CVD risk scores.
Acknowledgments
Funding: The National Institutes of Health funded this research through these grants: UM1 CA176726, R01 HL088521, R01 HL34594, and R01 CA67262. This work was supported by awards from the American Heart Association (12PRE9110014, 13GRNT17070022). JJS was supported by Training Grant T32HL098048 from the National Heart, Lung, and Blood Institute and by Training Grant T32HD060454 from the National Institute of Child Health and Human Development. LJT was supported by F31HL131222 from the National Heart, Lung, and Blood Institute under the Ruth L. Kirschstein National Research Service Award.
Abbreviations:
- ASCVD
atherosclerotic cardiovascular disease
- CVD
cardiovascular disease
- HDL
high density lipoprotein
- SBP
systolic blood pressure
- HDP
hypertensive disorders of pregnancy
- PCE
Pooled Cohort Risk Equation
- NHSII
Nurses’ Health Study II
- MI
myocardial infarction
- CHD
coronary heart disease
- NRI
net reclassification improvement
Footnotes
Disclosures: There are no author relationships with industry or financial disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This supplemental material has been provided by the authors to give readers additional information about their work.
REFERENCES
- 1.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia: systematic review and meta-analysis. Eur J Epidemiol 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz G Estimating the dimension of a model. Ann Stat 1978;6. [Google Scholar]
- 4.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 5.Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med 2004;23:2109–23. [DOI] [PubMed] [Google Scholar]
- 6.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med 2015;34:1659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Agostino RB, Nam BH. Evaluation of the performance of survival analysis models: discrimination and calibration measures In: Balakrishnan N, Rao CR (editors) Handbook of Statistics. London, United Kingdom: Elsevier, 2004. [Google Scholar]
- 8.Pencina MJ, D'Agostino RB, Sr.,D'Agostino RB, Jr., Vasan RS Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–72; discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 9.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928–35. [DOI] [PubMed] [Google Scholar]
- 10.Pencina MJ, D'Agostino RB, Sr., Steyerberg EW Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pencina MJ, D'Agostino RB, Pencina KM, Janssens AC, Greenland P. Interpreting incremental value of markers added to risk prediction models. Am J Epidemiol 2012;176:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavanaugh-Hussey MW, Berry JD, Lloyd-Jones DM. Who exceeds ATP-III risk thresholds? Systematic examination of the effect of varying age and risk factor levels in the ATP-III risk assessment tool. Prev Med 2008;47:619–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation 2009;120:384–90. [DOI] [PubMed] [Google Scholar]
- 14.Stone NJ, Robinson JG, Lichtenstein AH et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934. [DOI] [PubMed] [Google Scholar]
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2.Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SA. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015;241:211–8. [DOI] [PubMed] [Google Scholar]
- 3.Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 2002;325:157–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre-eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol 2014;63:1815–22. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with preeclampsia: systematic review and meta-analysis. Eur J Epidemiol 2013;28:1–19. [DOI] [PubMed] [Google Scholar]
- 7.Fraser A, Nelson SM, Macdonald-Wallis C, et al. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation 2012;125:1367–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1545–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosca L, Benjamin EJ, Berra K et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation 2011;123:1243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. J Am Coll Cardiol 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook NR, Ridker PM. Calibration of the Pooled Cohort Equations for Atherosclerotic Cardiovascular Disease: An Update. Ann Intern Med 2016;165:786–794. [DOI] [PubMed] [Google Scholar]
- 12.Tanz LJ, Stuart JJ, Williams PL, et al. Preterm Delivery and Maternal Cardiovascular Disease in Young and Middle-Aged Adult Women. Circulation 2017;135:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hermes W, Tamsma JT, Grootendorst DC, et al. Cardiovascular risk estimation in women with a history of hypertensive pregnancy disorders at term: a longitudinal followup study. BMC Pregnancy Childbirth 2013;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ACOG Committee on Practice Bulletins--Obstetrics. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol 2002;99:159–167. [DOI] [PubMed] [Google Scholar]
- 15.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension 2008;52:828–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes 1979;28:1039–57. [DOI] [PubMed] [Google Scholar]
- 17.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes care 1997;20:1183–97. [DOI] [PubMed] [Google Scholar]
- 18.Rich-Edwards JW, Colditz GA, Stampfer MJ, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med 1999;130:278–84. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Colditz GA, Stampfer MJ, et al. A prospective study of maturity-onset diabetes mellitus and risk of coronary heart disease and stroke in women. Arch Intern Med 1991;151:1141–7. [PubMed] [Google Scholar]
- 20.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol 2000;36:959–69. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. IHD Registers: Report of the Fifth Working Group. Copenhagen: World Health Organization. 1971.
- 22.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke 1981;12:I13–44. [PubMed] [Google Scholar]
- 23.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation 2006;113:791–8. [DOI] [PubMed] [Google Scholar]
- 24.Pencina MJ, D'Agostino RB, Sr., et al. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation 2009;119:3078–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skjaerven R, Wilcox AJ, Klungsoyr K, et al. Cardiovascular mortality after preeclampsia in one child mothers: prospective, population based cohort study. BMJ 2012;345:e7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation 2009;119:2408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox D, Oakes D Analysis of Survival Data. New York: Chapman & Hall, 1984. [Google Scholar]
- 28.Lin DY, Wei LJ. The robust inference for the proportional hazards model. J Am Stat Assoc 1989;84:1074–1078. [Google Scholar]
- 29.Pencina MJ, Steyerberg EW, D'Agostino RB, Sr., Net reclassification index at event rate: properties and relationships. Stat Med 2016. [DOI] [PubMed] [Google Scholar]
- 30.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007;115:928–35. [DOI] [PubMed] [Google Scholar]
- 31.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol 2004;159:882–90. [DOI] [PubMed] [Google Scholar]
- 32.Harrell FE Jr. Regression Modeling Strategies. New York, NY: Springer-Verlag, 2001. [Google Scholar]
- 33.Parikh NI, Cnattingius S, Dickman PW, et al. Parity and risk of later-life maternal cardiovascular disease. Am Heart J 2010;159:215–221.e6. [DOI] [PubMed] [Google Scholar]
- 34.Parikh NI, Jeppson RP, Berger JS, et al. Reproductive Risk Factors and Coronary Heart Disease in the Women's Health Initiative Observational Study. Circulation 2016;133:2149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuart JJ, Bairey Merz CN, Berga SL, et al. Maternal recall of hypertensive disorders in pregnancy: a systematic review. Journal of women's health (2002) 2013;22:37–47. [DOI] [PubMed] [Google Scholar]
- 36.Stuart JJ, Tanz LJ, Rimm EB, et al. Hypertensive disorders in first pregnancy and maternal cardiovascular disase: mediation by postpartum cardiovascular risk factors (Abstr). Society for Epidemiologic Research Annual Meeting 2017. [Google Scholar]