Abstract
Background
Excessive drinkers (ED) and patients with alcoholic liver disease (ALD) are several times more susceptible to bacterial and viral infections and have a decrease in antibody responses to vaccinations. Follicular helper T (TFH) cells are essential to select B cells in the germinal center and to produce antibodies. TFH cells express both a membrane-associated and a soluble form of CD40 ligand (sCD40L); in which the latter form is released to circulation upon T cell activation. The effect of alcohol on TFH cells has not been studied.
Objectives
The goals of this study are to determine the levels of TFH and T helper 1 (Th1) cells in ED and those with alcoholic cirrhosis (AC) when compared to healthy controls and to determine the prognostic significance of sCD40L in a cohort of patients with AC.
Methods
Controls, ED, and those with AC were enrolled. Baseline demographic, laboratory tests, and peripheral blood mononuclear cells (PBMCs) were isolated and assessed via flow cytometry for TFH cells. In vitro study was performed to determine the ability of PBMCs to secrete interferon (IFN)-γ upon stimulation. Serum sCD40L were also determined and its prognostic significance was tested in a cohort of AC patients.
Results
The levels of circulating TFH (cTFH) cells were significantly lower in peripheral blood of subjects with ED and AC compared to controls (P<0.05). IFN-γ secretion from PBMCs upon stimulation was also lower in ED and those with cirrhosis. Serum sCD40L was significantly lower in ED and AC when compared to that in controls (P<0.0005). Its level was an independent predictor of mortality.
Conclusions
Patients with AC had significantly lower level of cTFH and sCD40L. The level of sCD40L was an independent predictor of mortality in these patients.
Keywords: Follicular helper T (TFH) cells, Circulating follicular helper T (cTFH) cells, T helper 1 (Th1), Soluble form of CD40 ligand (sCD40L), Alcoholic liver disease (ALD), Alcoholic cirrhosis (AC)
1. Introduction
Excessive alcohol use is one of the most significant risk factors for health problems such as injuries, violence, liver diseases, and cancer.1 Drinking becomes excessive when it causes or elevates the risk for alcohol-related problems or complicates the management of other health problems. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), excessive drinkers (ED) are defined as men who drink more than 4 standard drinks in a day (or more than 14 per week) and women who drink more than 3 drinks in a day (or more than 7 per week).2, 3 Alcoholic liver disease (ALD) develops as a consequence of excessive alcohol use. It is a complex disorder and its pathogenesis is a multi-step and multi-factorial process that progresses through a series of histopathological changes.4 More than 90% of drinkers develop alcoholic steatosis which is reversible upon abstinence.5 However, if alcohol use continues, the disease may progress to alcoholic hepatitis, advanced fibrosis, and alcoholic cirrhosis (AC) in up to 10%–15% of heavy drinkers.4
Excessive alcohol use is strongly associated with immune dysfunction, which can lead to the development of ALD. ED are several times more susceptible to bacterial infections, particularly pneumonia, and viral infections.6 Ethanol is a potent immunosuppressive agent affecting both innate and adaptive immune responses. For instance, antibody responses to hepatitis B vaccination are decreased in those with excessive alcohol use,7 and vaccine titers are decreased even further in patients with ALD.8 Excessive alcohol use decreases the vaccine response in macaques.9 However, little is known about how alcohol affects the adaptive immune system and the mechanisms of how alcohol suppresses the humoral response are not well-studied, especially in human subjects. Alcohol is shown to promote apoptosis of splenic B and T cells in animal models,10 and decreased T helper (Th) cells have been observed in alcoholic patients.8 Patients with ALD develop auto-antibodies against modified liver proteins,11 indicating that with long-term alcohol abuse, there is a functional, antibody response but this response is dysregulated.
Follicular helper T (TFH) cells are a CD4+ T cell lineage uniquely found in the germinal center reaction of secondary lymphoid organs.12 The specific function of TFH cells is to select B cells in the germinal center that produce high-affinity antibodies.12–15 TFH cells develop initially in response to antigen presentation by dendritic cells, and require germinal center B cells for their complete differentiation.12 TFH cells have an activated, effector T cell phenotype and uniquely express high levels of both the chemokine (C-X-C motif) receptor 5 (CXCR5) and the inhibitory receptor programmed death 1 (PD-1).12 TFH cells have been described for both mouse and human, and they control the initiation as well as the outcome of germinal center B cell responses, through CD40 ligand (CD40L).12 On TFH cells, CD40L promotes B cell maturation and function by engaging CD40 on the B cell surface and stimulating B cell secretion of immunoglobulin.16 TFH cells express both a membrane-associated and a soluble form of CD40L (sCD40L); the latter form is released into circulation upon T cell activation.17 TFH cells are required to generate long-lived antibody responses, which confer long term protection to pathogens following infection. Recently, a TFH-like cell population has been identified in the circulation (circulating TFH cells, cTFH cells).18–20 In humans, these cTFH cells can be divided into Th1-, Th2- and Th17-like subsets.21 CXCR3+cTFH cells are related to Th1 cells, CCR6+cTFH cells are related to Th17 cells, and CXCR3−CCR6−cTFH cells are Th2-like.21 Although their origins are not well understood, cTFH cells appear to be precursors to TFH cells, and they form early in an immune response and disseminate to provide key TFH activity to other parts of the body.19 Several studies have shown that the percentage of cTFH cells increases with an ongoing antibody response in autoimmunity, vaccination and infection.18–20
To date, no information is available about cTFH cells and its subsets in patients with excessive alcohol use or those with ALD. The objectives of this study are to (i) determine the levels of CXCR3+ and CXCR3−cTFH cells in subjects with excessive alcohol use and those with end stage ALD, AC, when compared to healthy controls and (ii) determine the prognostic significance of sCD40L in patients with AC.
2. Materials and methods
2.1. Human subject recruitment
2.1.1. Determine the levels of cTFH cells in subjects with excessive alcohol use and those with AC when compared to healthy controls
For these experiments, we recruited 4 healthy controls, 21 ED, and 8 with AC. ED were enrolled from Fairbanks Alcohol and Drug Addiction Treatment Center (Indianapolis, USA). All met the definition of excessive alcohol use as defined by the National Institutes of Health (NIH)/NIAAA criteria.2, 3 All reported drinking excessively until the time of enrollment. They were at least 21 years old without underlying medical illnesses. These subjects had normal hepatic panel and denied past history of jaundice or complications from liver diseases, history of chronic viral hepatitis, and history of any infections within 4 weeks prior to enrollment. Subjects with AC were enrolled from the hepatology clinic at Indiana University (Indianapolis, USA). These patients had history of alcohol consumption averaging at least 80 g per day (for men) or 50 g per day (for women), for at least 10 years.22 This criterion is based on epidemiological evidence of the alcohol consumption and cirrhosis relationship.23 The diagnosis of cirrhosis was made by radiographic imaging compatible with cirrhosis and/or history of ascites, grade 2 or higher hepatic encephalopathy and/or the presence of esophageal varices on upper gastrointestinal endoscopy, or biopsy-proven cirrhosis, with exclusion of other chronic liver diseases. At the time of enrollment, Child-Pugh classification and model for end stage liver disease (MELD) scores were calculated.24 Decompensated cirrhosis patients were those with Child-Pugh class B or C.
2.1.2. Determine the prognostic significance of sCD40L in a cohort of patients with AC
Serum levels of sCD40L were measured in 30 healthy controls, 30 ED, and 83 patients with AC. Patients with AC were recruited between October 2012 and September 2013 and prospectively followed until death, liver transplantation, or study closure date of December 31, 2015. The study was approved by the Institutional Review Board at the Indiana University Purdue University Indianapolis (IUPUI) and Fairbanks Alcohol and Drug Addiction Treatment Center, and Roudebush VAMC Research and Development Program. Written informed consent was obtained from each participant.
2.2. Peripheral blood mononuclear cells (PBMCs) isolation
PBMCs were isolated from a subset of controls, ED, and those with AC as previously described.3 The levels of cTFH cells were determined using flow cytometry. In some experiments, the function of PBMCs was assessed by determining their responses to secrete interferon (IFN)-γ. Briefly, PBMCs from controls, ED and AC were cultured in buffered Roswell Park Memorial Institute (RPMI) 1640 supplemented with 10% (v/v) heat inactivated fetal bovine serum (FBS). Triplicate PBMC cultures (105 cells/mL) were treated with or without 50 ng/mL phorbol 12-myristate 13-acetate (PMA) plus 1 μg/mL ionomycin for 4 hours and the levels of IFN-γ in the supernatant were measured.
2.3. Flow cytometry reagents and cell staining
Anti-human CD3 (clone SP34-2), CD45RA (clone HI500), CD4 (clone RPA-T4), PD-1 (clone MIH4), CXCR5 (clone MU5UBEE) and CXCR3 (clone IC6) antibodies conjugated with appropriate fluorochromes were purchased from eBioscience (San Diego, CA) or BD Pharmingen (San Jose, CA). Cells from different conditions were subjected to surface staining with antibodies at 4 for 30 min. After washing, cells were stained with Fixable Viability Dye eFluor® 780 (Life Technologies) for 5 min in the dark. They were then washed, twice with 2% FBS/phosphate buffered solution (PBS), then subjected to flow cytometry analysis (FACS) using the BD LSR II Flow Cytometer or BD LSR Fortessa (San Diego, CA). Flow cytometry data were analyzed using FlowJo software (Ashland, Oregon). Cell sorting was performed on a BD FACSAria machine.
2.4. Flow cytometric gating strategy
The gating strategy for flow cytometry analysis was as follows: (i) live PBMCs were selected for by gating on viability dye negative cells; (ii) gating for CD4+CD3+ cells; (iii) resulting cells gated on CD45RA−CD4+ cells; (iv) resulting cells gated on CD4+CXCR5+ cells; (iv) resulting cells assessed based on expression of PD-1 and CXCR3.
2.5. Analysis of IFN-γ and sCD40L
Commercially available enzyme-linked immunosorbent assays (ELISAs) were used according to the manufacturers’ protocols for measuring IFN-γ (R&D System, Cat#DIF50, Minneapolis, MN), and sCD40L (R&D System, Cat#SCDL40, Minneapolis, MN) from the supernatant.
2.6. Statistical analysis
The PRISM7 program was used for statistical calculations. Basic descriptive statistics, including mean, standard deviations (SD), and frequencies (percentages) were used. One-way analysis of variance, with Tukey post hoc analysis, was used to compare the mean differences for continuous variables among the study cohorts. For prognostic significance of sCD40L in patients with AC, we examined the time to mortality using log-rank test. Individuals who did not die or underwent liver transplantation were censored. Cox regression models were used to determine the independent predictors of mortality. P< 0.05 was considered statistically significant.
3. Results
3.1. The levels of cTFH cells in ED and subjects with AC
Before analyzing the cTFH cell populations in our patient samples, we first analyzed total CD4+ T cells and the fraction of activated or memory CD4+ T cells in the PBMCs samples. As shown in Fig. 1A, ED showed almost twice as many total CD4+ T cells on average compared to controls; however due to high inter-subject variability, the increase was not statistically significant. Patients with AC, however, showed a significant increase in total CD4+ T cells, over 2-fold on average, compared to controls.
Fig. 1. Levels of total CD4+T cells, CD4+ memory T cells, CXCR3+ and CXCR3−cTFH cells in the study cohorts in relative to frequency of live cells.
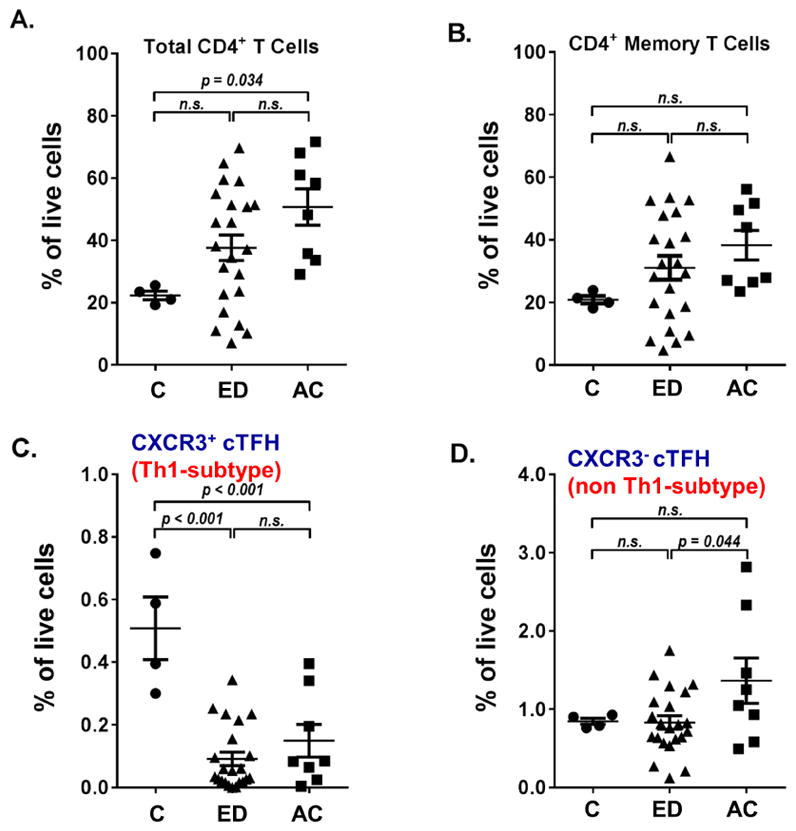
Th cell proportions in Control, ED and AC patients. Whole PBMCs from patients were stained for viability and assessed via flow cytometry. Percentages shown are of live cells. Normal control N =4, ED N = 21, AC N =8. (A) Percentage of CD3+ CD4+ T cells in total live cells. (B) Percentage of CD3+ CD4+ CD45RA− memory T cells in total live cells. (C) Percentage of CD3+ CD4+ CD45RA− CXCR5+ CXCR3+ PD-1+cTFH cells in total live cells. (D) Percentage of CD3+ CD4+ CD45RA− CXCR5+ CXCR3− PD-1+cTFH cells in total live cells. P-values shown via analysis of variance (ANOVA) with Tukey post-hoc analysis. Abbreviation: N.S., not significant.
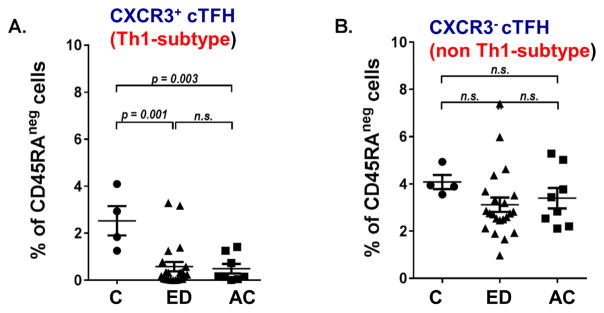
We next determined the level of activated or memory CD4+T cells by staining for CD45RA marker. CD45RA is expressed on naive T cells, as well as the effector CD4+T cells. After antigen experience, activated/memory T cells lose the expression of CD45RA (CD45RA−). Thus CD45RA can be used to generally differentiate the naive from activated/memory T cell populations. Both ED and AC patients showed an average increase in activated/memory CD4+T cells; however, neither increase reached statistical significance (Fig. 1B). We then analyzed CD4+CD45RA−CXCR5+PD-1+cTFH cells by dividing them into CXCR3+ (Th1-like) and CXCR3− (non-Th1-like) fractions. When calculated as a fraction of total live cells, both ED and AC patients showed a highly significant decrease in CXCR3+ cTFH cells compared to controls (Fig. 1C). There were no significant differences in CXCR3−cTFH cells in ED and AC patients, when compared to controls (Fig. 1D). However, AC patients had significantly more of this cell population compared to ED. A similar pattern was seen when CXCR3+ and CXCR3− cTFH cells were calculated as a fraction of the activated/memory CD4+ T cell population (Fig. 2).
Fig. 2. Levels of CXCR3+ and CXCR3−cTFH cells in the study cohorts in relative to frequency of activated/memory CD4 T cell population.
CXCR3 expression on cTFH cells in Control, ED and AC patients. Whole PBMCs from patients were stained for viability, CD3, CD4, and CD45RA and assessed via flow cytometry. Percentages shown are gated on live, CD3+ CD4+ CD45RA− cells. Normal control N =4, ED N = 21, AC N =8. (A) Percentage of CXCR5+ CXCR3+ PD-1+cTFH cells in CD45RA− cells. (B) Percentage of CXCR5+ CXCR3− PD-1+cTFH cells in CD45RA− cells. P-values shown via ANOVA with Tukey post-hoc analysis. Abbreviation: N.S., not significant.
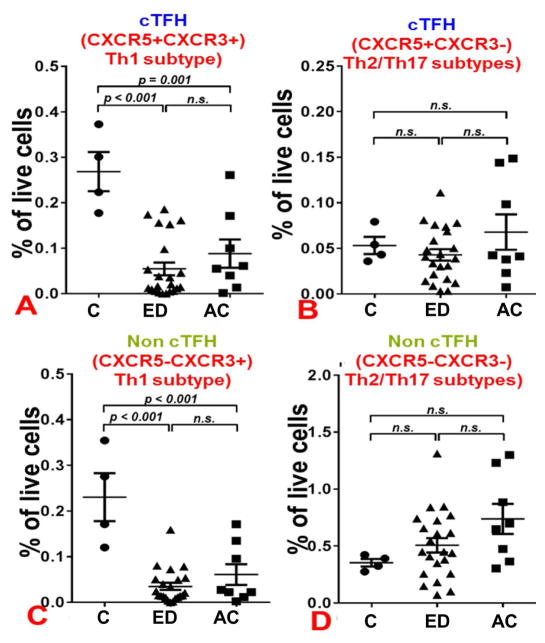
To further analyze the subsets of these T cells population, we next assessed cTFH cells compared to non-cTFH cells, stratified by subtypes of surface markers CXCR3+ (Th1) and CXCR3− (Th2/Th17) populations (Fig. 3). We observed that both Th1-like cTFH cells and Th1 non-cTFH cells were significantly decreased in ED and AC patients, compared to controls (Figs. 3A and C). Conversely, levels of non-Th1-like cTFH cells and non-Th1, non-cTFH cells were not significantly different between patient groups (Figs. 3B and D). A similar pattern was observed when levels of each cell type, as a fraction of the CD45RA− (activated/memory) population, were analyzed (Fig. 4). Taken together, these data show that excessive alcohol consumption significantly decreases Th1 and Th1-like TFH cells circulating in the blood; however, no major differences in these cell types were seen between ED and AC patients.
Fig. 3. Levels of cTFH and non-TFH cells stratified by subtypes in relative to frequency of live cells.
CXCR3 expression on cTFH cells, Th1 cells, and memory cells, as percent of total cells, in Control, ED and AC patients. Whole PBMCs from patients were stained for viability and assessed via flow cytometry. Percentages shown are of live cells. Normal control N =4, ED N = 21, AC N =8. (A) Percentage of CD3+ CD4+ CD45RA− CXCR5+ CXCR3+ PD-1+cTFH cells in total live cells. (B) Percentage of CD3+ CD4+ CD45RA− CXCR5+ CXCR3− PD-1+cTFH cells in total live cells. (C) Percentage of CD3+ CD4+ CD45RA− CXCR5− CXCR3+ PD-1+ Th1 cells in total live cells. (D) Percentage of CD3+ CD4+ CD45RA− CXCR5− CXCR3− PD-1+ cells in total live cells. P-values shown via ANOVA with Tukey post-hoc analysis. Abbreviation: N.S., not significant.
Fig. 4. Levels of cTFH and non-TFH cells stratified by subtypes in relative to frequency of activated/memory CD4 T cell population.
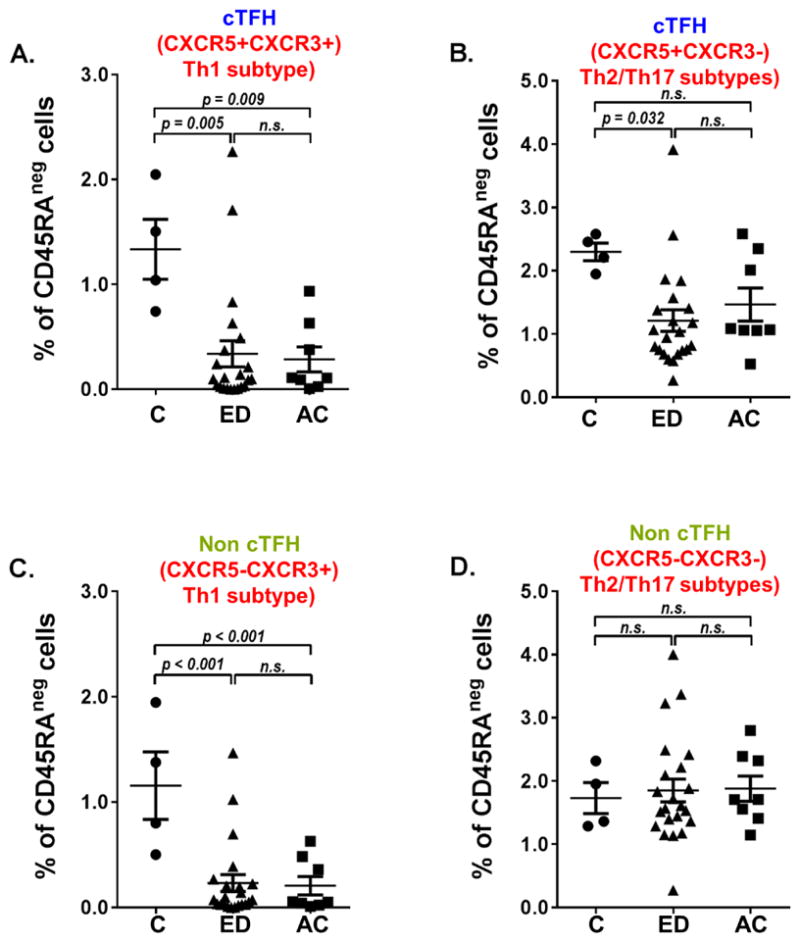
CXCR3 expression on cTFH cells, Th1 cells, and memory cells as percent of activated Th cells in Control, ED and AC patients. Whole PBMCs from patients were stained for viability, CD3, CD4, and CD45RA and assessed via flow cytometry. Percentages shown are gated on live, CD3+ CD4+ CD45RA− cells. Normal control N =4, ED N = 21, ALD N =8. (A) Percentage of CXCR5+ CXCR3+ PD-1+cTFH cells in CD45RA− cells. (B) Percentage of CXCR5+ CXCR3− PD-1+cTFH cells in CD45RA− cells. (C) Percentage of CXCR5− CXCR3+ PD-1+ Th1 cells in CD45RA− cells. (D) Percentage of CXCR5− CXCR3− PD-1+ cells in CD45RA− cells. P-values shown via ANOVA with Tukey post-hoc analysis. Abbreviation: N.S., not significant.
3.2. IFN-γ production by the PBMCs from controls, ED and patients with AC
We next determined the function of PBMCs by determining their responses to secrete IFN-γ upon stimulation with PMA/ionomycin. Triplicate PBMC cultures (105 cells/mL) from 3 study cohorts (N=10 in each group) were treated with or without PMA/ionomycin for 4 hours and the levels of IFN-γ in supernatant were measured (Fig. 5). We found that the level of IFN-γ in the supernatant from ED ((209.3±29.3) pg/mL) and AC ((133.0±37.2) pg/mL) patients were significantly lower than that of controls ((404.3±4.3) pg/mL, P<0.05).
Fig. 5. IFN-γ production by the PBMCs from controls, ED and patients with AC.
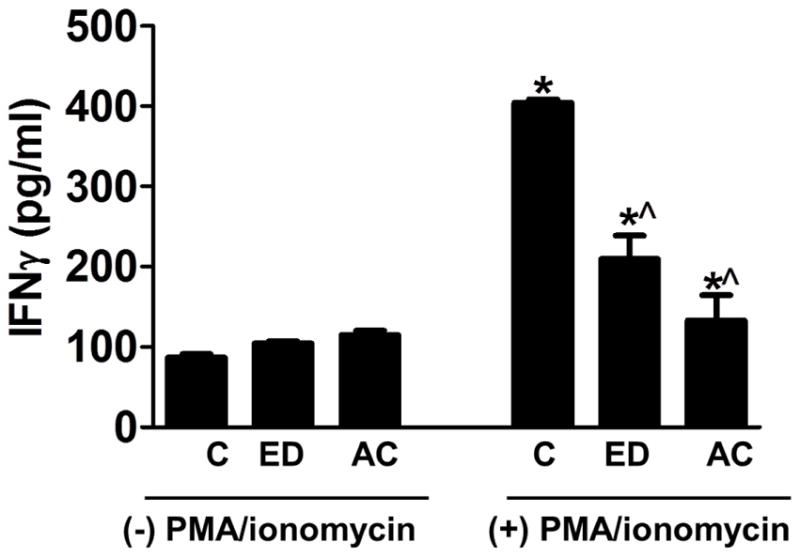
PBMCs were isolated from the whole blood of human subjects and plated at the density of 105 cells/well with or without PMA/ionomycin for 4 hours. The IFN-γ production in the supernatant was measured. *P<0.05 compared to controls and respective samples without PMA/ionomycin, ^P<0.05 compared to Control with PMA/ionomycin.
3.3. Serum levels of sCD40L among controls, ED, and patients with AC
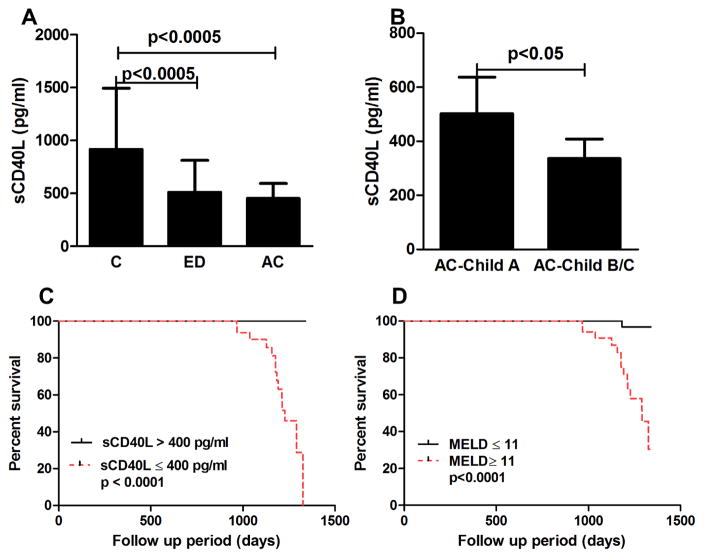
sCD40L is released from the TFH cells upon T cell activation.17 We next determined the level of sCD40L in a large cohort of controls (N=30), ED (N=30), and AC (N=83). Baseline demographic and clinical data were shown in Table 1. The serum level of sCD40L was significantly lower in ED( (511±302 )pg/mL) and AC( (452±142 )pg/mL) when compared to that of controls( (915±579 )pg/mL, P<0.0005) (Fig. 6A). Among patients with AC, sCD40L levels were significantly lower in subjects with decompensated state (Child-Pugh B/C, (336±72) pg/mL) compared to those in Child-Pugh A ((502±135) pg/mL, P<0.05) (Fig. 6B).
Table 1.
Baseline demographic and clinical characteristics of the study cohort.
| Variables | Healthy Controls (N=30) | ED without liver diseases (N=30) | AC (N=83) |
|---|---|---|---|
| Age (years) | 32.9±9.7 | 38.2±12.2 | 50.4±9.2 |
| Men (%) | 63 | 90 | 77 |
| Race, White (%) | 83 | 83 | 93 |
| Total drinks in the last 30 days before enrollment (standard drinks) | N/A | 384.1 ± 185.7 | See footnote below* |
| WBC (cells/mm3) | 4.7±1.4 | 5.5±1.3 | 8.0±11.4 |
| Hemoglobin (g/dL) | 14.3±1.6 | 13.8±1.4 | 11.7±2.9 |
| Platelet counts (cells/mm3) | 238.6±46.6 | 233.9±53.4 | 147.1±74.3 |
| T. Bilirubin (mg/dL) | 0.8±0.3 | 1.0±0.1 | 2.3±3.6 |
| INR | 0.9±0.3 | 0.9±0.1 | 1.3±0.5 |
| AST (U/L) | 19±4 | 21±7 | 37.3±57.9 |
| ALT (U/L) | 21±5 | 20±9 | 45.7±36.8 |
| Albumin (g/dL) | 3.9±0.5 | 3.8±0.3 | 3.3±0.6 |
| Creatinine (mg/dL) | 0.8±0.3 | 1.0±0.2 | 1.1±0.4 |
| Child-Pugh classification(%) | |||
| - A | 69 | ||
| - B | N/A | N/A | 20 |
| - C | 11 | ||
| MELD scores | N/A | N/A | 11.2±6.3 |
History of alcohol consumption averaging at least 80 g per day (for men) or 50 g per day (for women), for at least 10 years. Data are expressed as mean±SD. Abbreviations: WBC, white blood cell; INR, international normalized ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; MELD, model for end stage liver disease; N/A, not applicable.
Fig. 6. Serum levels of sCD40L in the study cohorts and survival curve on the baseline of sCD40L and MELD scores on survival of patients with AC.
(A) Serum levels of sCD40L in the study cohorts. (B) Serum levels of sCD40L in Child A vs. Child B/C. (C–D) Survival curve of sCD40L and MELD scores on the survival in patients with AC. Abbreviation: MELD, model for end stage liver disease.
3.4. Serum levels of sCD40L as an independent predictor of mortality in patients with AC
To determine the prognostic significance of sCD40L, we prospectively followed patients with AC with the follow up of 3.2 years. During the follow up period, 15 (18%) patients died. In the multivariate Cox model adjusting for age, the level of sCD40L (hazard ratio (HR) 0.98, 95% confidence interval (CI) 0.97–0.98, P=0.001) and MELD scores (HR 1.14, 95% CI 1.016–1.221, P=0.02) were the independent predictors of mortality. The baseline sCD40L and MELD scores on the survival of patients with AC is shown in Figs. 6 C and D.
4. Discussion
The major findings of this study provide some mechanistic insights on why ED and those with AC have an increasing risk of bacterial and viral infections.6 We found that (i) excessive alcohol consumption significantly decreased Th1 and Th1-like TFH cells circulating in the blood, (ii) the function of PBMCs to secrete IFN-γ upon stimulation was impaired in ED and patients with AC, (iii) ED and subjects with AC had lower serum level of sCD40L, and (iv) the serum level of sCD40L was an independent predictor of mortality in patients with AC.
Previous studies have shown that excessive alcohol use leads to the increase in the levels of endotoxin (or lipopolysaccharides, LPS), the main driver in the pathogenesis of alcohol-induced liver injury in rodent models of acute or chronic alcohol consumption as well as in humans.25, 26 Once in the circulation, LPS can activate immune cells such as monocytes; resulting in the release of inflammatory cascades.27 Our work provides the additional understanding on the effect of excessive alcohol use on a specific subtype of T cells, called TFH cells. TFH cells are specialized providers of T cell help to B cells, and are essential for germinal center formation, affinity maturation, and the development of most high affinity antibodies and memory B cells.12–15, 28 TFH cells play an important role for the generation of most isotype switched and affinity matured antibodies, and therefore they have an obvious function in protective immunity against pathogens.28 Despite the well described function of TFH cells in antibody responses, little is known about the effects of alcohol on TFH cells especially in humans. We found that excessive alcohol consumption significantly decreased circulating Th1 and Th1-like TFH cells when compared to healthy controls. However, no differences were observed in these cell populations between ED and AC subjects. The effect of alcohol on TFH cells may partly explain the susceptibility to bacterial and viral infection among these patients.6 Further, this may underlie the poor antibody responses after vaccination among excessive alcohol users and those with ALD.7,8 Not only that alcohol interfered with the quantity of circulating Th1 and Th1-like TFH cells, our data also indicated that alcohol also impaired the ability of PBMCs to secrete IFN-γ upon stimulation.
sCD40L, an 18-kDa protein, is normally released from T cells upon activation.17 Its level has been reported to increase in several disease conditions.29, 30 We found that the serum level of sCD40L was lower in ED and those with AC when compared to controls. Whether this observation is an indicator of alcohol-induced T cell suppression should be further investigated. We observed that the level of sCD40L was the lowest in patients with AC. Given that sCD40L can be released from platelets,31 it is possible that the lowest level of sCD40L is secondary to the low platelet counts in these patients compared to controls and ED (Table 1). Another interesting observation in our study was that the level of sCD40L was an independent predictor of mortality in patients with AC. Given the sample size of our patients, we should interpret our results with caution. A future study with a larger cohort of patients and the comprehensive analyses on the outcomes to see whether lower serum sCD40L increasing risk of bacterial/viral infection in patients with AC are needed. Since we only studied patients with AC, one form of ALD, detailed analyses on the levels as well as function of cTFH in another form of ALD, such as alcoholic hepatitis, is warranted.
In summary, we found that excessive alcohol consumption significantly decreased circulating Th1, Th1-like TFH cells, and serum levels of sCD40L. Future studies are needed to further explore the molecular mechanism of these clinical observations and to confirm the prognostic significance of sCD40L in patients with AC.
Acknowledgments
This work was supported by VA Merit Award 1I01BX002634, the NIH R21AA022482, R01DK080440, R01DK104656, R01ES025909, R21CA191507, and P30 DK34989 (to L. Wang), VA Merit Award 1I01CX000361, NIH U01AA021840, NIH R01 DK107682, NIH R01 AA025208, US DOD W81XWH-12-1-0497 (to S. Liangpunsakul), and NIH R21AA024935-01 (to L. Wang and S. Liangpunsakul), and NIH R56 AI112398 (to A. L. Dent).
Footnotes
Authors’ contributions
Study concept and design (K. Hollister, A. L. Dent and S. Liangpunsakul); subject recruitment and sample processing (R. A. Ross, P. Kusumanchi, L. Heathers, K. Chandler, A. Oshodi and S. Teagarden); acquisition of data, database management and experiments (K. Hollister, A. L. Dent and S. Liangpunsakul); data interpretation, drafting and revising the manuscript (K. Hollister, A. L. Dent, L. Wang and S. Liangpunsakul).
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization (WHO) Global status report on alcohol and health 2014. 2015 http://www.who.int/substance_abuse/publications/global_alcohol_report/en/
- 2.US Department of Health and Human Services. National Institute of Health/National Institute on Alcohol Abuse and Alcoholism. Helping Patients who drink too much. A clinician's guide Updated 2005 Edition. 2005 http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf.
- 3.Liangpunsakul S, Toh E, Ross RA, et al. Quantity of alcohol drinking positively correlates with serum levels of endotoxin and markers of monocyte activation. Sci Rep. 2017;7:4462. doi: 10.1038/s41598-017-04669-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liangpunsakul S, Haber P, McCaughan GW. Alcoholic Liver Disease in Asia, Europe, and North America. Gastroenterology. 2016;150:1786–1797. doi: 10.1053/j.gastro.2016.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sozio MS, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis. Semin Liver Dis. 2010;30:378–390. doi: 10.1055/s-0030-1267538. [DOI] [PubMed] [Google Scholar]
- 6.Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–9. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- 7.Degos F, Duhamel G, Brechot C, et al. Hepatitis B vaccination in chronic alcoholics. J Hepatol. 1986;2:402–9. doi: 10.1016/s0168-8278(86)80051-4. [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall C, Roselle GA, Lybecker LA, et al. Hepatitis B vaccination. Response of alcoholic with and without liver injury. Dig Dis Sci. 1988;33:263–9. doi: 10.1007/BF01535747. [DOI] [PubMed] [Google Scholar]
- 9.Messaoudi I, Asquith M, Engelmann F, et al. Moderate alcohol consumption enhances vaccine-induced responses in rhesus macaques. Vaccine. 2013;32:54–61. doi: 10.1016/j.vaccine.2013.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook RT, Schlueter AJ, Coleman RA, et al. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion--absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol Clin Exp Res. 2007;31:1746–58. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albano E. Role of adaptive immunity in alcoholic liver disease. Int J Hepatol. 2012;2012:893026. doi: 10.1155/2012/893026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 13.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–57. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 14.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 15.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nat Immunol. 2010;11:114–20. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rush JS, Hodgkin PD. B cells activated via CD40 and IL-4 undergo a division burst but require continued stimulation to maintain division, survival and differentiation. Eur J Immunol. 2001;31:1150–9. doi: 10.1002/1521-4141(200104)31:4<1150::aid-immu1150>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Graf D, Muller S, Korthauer U, et al. A soluble form of TRAP (CD40 ligand) is rapidly released after T cell activation. Eur J Immunol. 1995;25:1749–54. doi: 10.1002/eji.1830250639. [DOI] [PubMed] [Google Scholar]
- 18.Feng X, Wang D, Chen J, et al. Inhibition of aberrant circulating Tfh cell proportions by corticosteroids in patients with systemic lupus erythematosus. PLoS One. 2012;7:e51982. doi: 10.1371/journal.pone.0051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He J, Tsai LM, Leong YA, et al. Circulating precursor CCR7(lo)PD-1(hi) CXCR5(+) CD4(+) T cells indicate Tfh cell activity and promote antibody responses upon antigen reexposure. Immunity. 2013;39:770–81. doi: 10.1016/j.immuni.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Simpson N, Gatenby PA, Wilson A, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 21.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitfield JB, Rahman K, Haber PS, et al. Brief report: genetics of alcoholic cirrhosis-GenomALC multinational study. Alcohol Clin Exp Res. 2015;39:836–42. doi: 10.1111/acer.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutright P, Fernquist RM. Predictors of per capita alcohol consumption and gender-specific liver cirrhosis mortality rates: thirteen European countries, circa 1970–1984 and 1995–2007. Omega (Westport) 2010;62:269–83. doi: 10.2190/om.62.3.d. [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Ross RA, Zhao S, Tu W, Liangpunsakul S, Wang L. LncRNA AK054921 and AK128652 are potential serum biomarkers and predictors of patient survival with alcoholic cirrhosis. Hep Communications. 2017 doi: 10.1002/hep4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao B, Seki E, Brenner DA, et al. Innate immunity in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2011;300:G516–G525. doi: 10.1152/ajpgi.00537.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaratani H, Tsujimoto T, Douhara A, et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflamm. 2013;2013:495156. doi: 10.1155/2013/495156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–42. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung HW, Lim JB. Clinical significance of elevated serum soluble CD40 ligand levels as a diagnostic and prognostic tumor marker for pancreatic ductal adenocarcinoma. J Transl Med. 2014;12:102. doi: 10.1186/1479-5876-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goules A, Tzioufas AG, Manousakis MN, et al. Elevated levels of soluble CD40 ligand (sCD40L) in serum of patients with systemic autoimmune diseases. J Autoimmun. 2006;26:165–71. doi: 10.1016/j.jaut.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Danese S, Katz JA, Saibeni S, et al. Activated platelets are the source of elevated levels of soluble CD40 ligand in the circulation of inflammatory bowel disease patients. Gut. 2003;52:1435–41. doi: 10.1136/gut.52.10.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]