Abstract
Background
The major decrease in exposure to secondhand smoke (SHS) in public places in recent decades could have contributed to the decline in smoking-related cancer mortality observed in the US population.
Methods
Prospective study among 11,856 non-smoking adults aged ≥40 years who participated in NHANES 1988–1994 or 1999–2004 and were followed for mortality through 2006. We estimated the amount of change in cancer mortality over time attributed to the intermediate pathway of changes in SHS exposure in public places, after adjustment for risk factors and SHS exposure at home.
Results
The adjusted smoking-related cancer mortality rate ratios (95%CI) for a twofold increase in serum cotinine and a 1-hour increase in occupational SHS exposure time were 1.10 (1.03, 1.17) and 1.14 (1.06, 1.24) for all-cancer, and 1.13 (1.03, 1.24) and 1.14 (1.02, 1.26) for smoking-related cancer, respectively. The absolute reduction in mortality comparing 1999–2004 to 1988–1994 was 75.8 (−25.5, 177.0) and 77.0 (2.6, 151.4) deaths/100,000 person-years, for all-cancer and smoking-related cancer, respectively. Among these avoided all-cancer deaths, 45.8 (2.8, 89.5) and 18.1 (−1.2, 39.6)/100,000 person-year were attributable to changes in serum cotinine concentrations and occupational SHS exposure time, respectively. The corresponding numbers of smoking-related cancer avoided deaths were 36.4 (0.7, 72.8) and 9.9 (−3.8, 24.9)/100,000 person-year.
Conclusions
Declines in SHS exposure were associated with reductions in all-cancer and smoking-related cancer mortality, supporting that smoking bans in public places may have reduced cancer mortality among non-smoking adults.
Keywords: Secondhand smoke, cancer mortality, trends, NHANES
INTRODUCTION
Secondhand tobacco smoke (SHS), the combination of the side-stream smoke emitted from the burning end of a tobacco product and the mainstream smoke exhaled by the smoker, contains more than 6,000 chemicals and is carcinogenic to humans.1 The 2006 Surgeon General’s Report on the Health Consequences of Involuntary Exposure to Tobacco Smoke concluded evidence is sufficient to infer that SHS exposure causes lung cancer.2 Other health effects causally linked to SHS exposure include ischemic heart disease and stroke in adults, and lower respiratory tract infections and impaired lung function in children.3 For many other diseases, there is suggestive evidence for causality.2
Exposure to SHS can be assessed by interviews or by measuring the levels of cotinine (a nicotine by-product in the body) in biological samples. According to self-reported information and serum cotinine concentrations, exposure to SHS in the United States has declined intensely during the past three decades.4 This phenomenon has been attributed to downward trends in smoking rates and intensity, and to increases in the number of states with smoke-free policies in public places. As of June 30, 2016, a total of 29 US states and the District of Columbia have enacted statewide bans on smoking in all public places and workplaces, including bars, restaurants and private worksites.5
Cancer death rates in the US, including those that arise at the most common sites (i.e. lung and bronchus, colon and rectum, breast or prostate), have also fallen during the last decades, with an estimated overall decrease of 23% from 1990 to 2012.6 This decline has been attributed to reductions in tobacco consumption, as well as to better surveillance and treatment options. Little is known, however, about the potential contribution of reductions in SHS exposure to the decline of cancer mortality among nonsmokers. Most studies on the health benefits of smoke-free policies have focused on acute cardiovascular and respiratory effects,7;8 and on birth outcomes.9 Moreover, a recently published Cochrane review reported evidence of reduced cardiovascular and respiratory mortality after introduction of national smoke-free bans.10 Recently, though, an ecological study within EU countries reported no statistically significant changes in lung cancer mortality trends after the introduction of smoke-free legislation.11 Although SHS is known to be carcinogenic,1 the long latency period that is required for most cancers to develop makes it difficult to link changes in SHS exposure to changes in cancer rates.
The objective of this study was to evaluate the hypothesis that population changes in the distribution of SHS exposure in public places explain changes in smoking-related cancer mortality over time in two samples of the non-smoking US population, the U.S. National Health and Nutrition Examination Survey (NHANES) 1988–1994 and 1999–2004. For this purpose, we used individual information on serum cotinine and made adjustments for SHS exposure at home, so that our analyses reflect SHS exposure not coming from private settings. Moreover, to differentiate between occupational SHS exposure and SHS exposure from other public places, we also evaluated self-reported occupational SHS exposure time (hours/day). To address the study objectives, we implemented a causal inference mediation approach12 using study period as a major determinant of cancer mortality and changes in SHS exposure as a potential mediator of the relation between period and cancer mortality (see Supplemental Figure S1). This method allows us to estimate the proportion of the decline in smoking-related cancer mortality rates among nonsmokers recruited in 1988–1994 and 1999–2004 that can be independently attributed to changes in SHS exposure.
METHODS
Study population
NHANES is a program of studies designed to assess the health and nutritional status of the general population in the United States. The survey uses a complex multistage sampling design to obtain representative samples of the non-institutionalized US population,13 and it is unique in that it combines interviews and physical examinations. Blood and urine specimens are obtained from NHANES participants who give consent for their specimens to be used in future research studies. Serum specimens are available from NHANES III (1988–1994) up to the present time.13
In this study we used data from NHANES III and NHANES 1999–2004. Because each survey period provides a snapshot of the US population health status, comparing these two periods we can assess how population changes over time in SHS exposure can explain temporal trends in cancer mortality. We initially included 15,973 non-smoking adults (i.e. both never and former smokers) aged ≥40 who completed the NHANES clinical examination. Because self-reported non-smokers or former smokers with serum cotinine levels above 10 ng/mL were considered current smokers, these were not included in the analyses. From these, we excluded pregnant women (n=4 in NHANES 1988–1994 and n=7 in NHANES 1999–2004), as well as participants with missing values in serum cotinine (n=1,183 and 1,190, respectively), occupational SHS exposure (n=63 and n=1, respectively) or SHS exposure at home (n=9 and n=58, respectively). Supplemental Figure 2 shows the flow chart of participant exclusions by study period, which left 11,856 participants for analysis.
Secondhand tobacco smoke measures
Serum cotinine was measured by an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometric method at the Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC). The limits of detection (LOD) for serum cotinine were 0.05ng/mL in NHANES III and 0.015ng/mL in NHANES 1999–2004. For the 11% and 13% of participants <LOD, respectively, cotinine concentrations were replaced by the LOD divided by the √2.14
Exposure at home was defined as the presence of one or more smokers at home. Occupational SHS exposure time was defined as the self-reported daily number of hours of exposure to SHS at the workplace on the basis of the participant’s main paid job within the last week before the interview. The following questions were asked: 1) “At work, how many hours per day are you close enough to people who smoke so that you can smell the smoke?” in NHANES III, and 2) “At this job or business, how many hours per day can you smell the smoke from other people’s cigarettes, cigars and/or pipes?” in NHANES 1999–2004. Individuals who were not working two weeks before the interview (N= 3,533 in NHANES III and N= 3,051 in NHANES 1999–2004) were considered unexposed at work.
Mortality risk factors
NHANES collected information on age, sex, race/ethnicity (Non-Hispanic White (“White”), Non-Hispanic Black (“Black”), Mexican-American, other), education (≥high school, <high school), smoking status (never, former smokers), body mass index (<30, ≥30 kg/m2), alcohol consumption (never, former, current drinkers), physical inactivity (no, yes), and exposure to SHS at home (no, yes). Former smokers were participants who had smoked ≥100 cigarettes in life but were not current smokers (i.e. did not answered yes to the question “Do you smoke cigarettes now?” nor had serum cotinine levels >10 ng/mL).15 According to their alcohol intake, participants were classified as never (<12 drinks in life), former (at least 12 drinks in life but <12 drinks during the previous year), or current drinkers (at least 12 drinks during the previous year). Leisure time physical activity questions included information on the type and frequency of physically active hobbies, sports, or exercises. Four open-ended questions assessed information on physical activities not previously listed. Participants who responded “no” to all leisure time physical activity questions were classified as physically inactive. Obesity was defined as a body mass index (BMI) ≥30 kg/m2.
Mortality follow-up
Cause-specific cancer mortality follow-up data in NHANES 1988–1994 and 1999–2004 was publicly available through December 31, 2006. Vital status and cause of death were determined by probabilistic matching between NHANES records and death certificates from the National Death Index (NDI) based on identifying data elements.13 Cause of death was determined using the underlying cause listed on death certificates, and was coded using the International Classification of Diseases, 10th Revision (ICD-10).16 The primary study endpoints were all-cancer mortality (ICD-10 codes: C00-C97; n=353) and smoking-related cancer mortality (codes C00-C14 [lip, oral cavity and pharynx; n=5]; C32 [larynx; n=2]; C33-C34 [trachea, bronchus, lung; n=81]; C15 [esophagus; n=6]; C16 [stomach; n=16]; C25 [pancreas; n=21]; C22 [liver and intrahepatic bile ducts; n=12]; C18-C21 [colon, rectum and anus; n=47]; C53 [cervix uteri; n=2]; C64-C65 [kidney and renal pelvis; n=9]; and C67 [bladder; n=4]). Smoking-related cancer mortality refers to those cancers for which there is evidence of a causal relationship based on the 2014 Surgeons General’s Report.2 Non-smoking-related cancers were included in the all-cancer mortality group because there is evidence that for some of these cancers (e.g. breast17 or prostate18), smoking adversely affects prognosis, increasing mortality.
We assessed mediation of temporal trends in the context of survival analysis, where the endpoint of interest is “time to death”. Accordingly, follow-up time for each participant was calculated as the difference between the age at date of examination and the age at date of death or age at censoring, whichever occurred first. The length of available follow-up differed by NHANES period (maximum follow-up of 19 years for 1988–1994 and 8 years for 1999–2004). To make the length of follow-up comparable between both survey waves, we censored the follow-up of NHANES 1988–1994 at December 31st, 1996.
Statistical methods
Due to the different selection probabilities of NHANES participants, all analyses were weighted to the underlying US adult population. Statistical methods for the estimation of summary trends and the association between SHS exposure and cancer mortality outcomes can be found in the Supplemental Material, Supplemental Methods. The contribution of changes in SHS exposure between 1988–1994 and 1999–2004 to the absolute change in smoking-related cancer mortality rates (i.e., changes in mortality mediated by changes in SHS exposure) were estimated with two alternative mediation approaches for survival data: 1) the “difference in coefficients” method,19 and 2) the “product of coefficients” method proposed by Lange and Hansen.12 The “difference” and “product” of coefficients methods are expected to give similar results when using Aalen additive hazard models in survival settings, provided that the association of SHS exposure with mortality is similar in both survey periods, and other mediation assumptions hold.20 Thus, the comparison of results from the two methods provides a way to evaluate the robustness of our results to potential violations of the mediation assumption.
In the “difference of coefficients” mediation models shown as main results, we estimated the change in smoking-related cancer mortality rates comparing 1999–2004 to 1988–1994 from the coefficient associated with the survey period indicators in two nested Aalen additive hazard models with individual age-at-death. These models included the same set of confounders, except that one adjusted for SHS exposure measures, and the other did not. The mediated effect was estimated as the difference in the mortality rate changes across survey periods (in absolute and relative terms) estimated comparing the two nested models.
All additive hazard regression models were semi-parametric (i.e. only allowed the baseline hazard to be time-dependent) and were fitted with two increasing levels of adjustment. The first models adjusted for age (as time scale in Aalen models and as restricted cubic splines of baseline age in linear models), sex, race/ethnicity, education and exposure to SHS at home. The adjustment for SHS exposure at home was an attempt to tease out SHS exposure not coming from public places, as our interests focused on the role of changes in SHS exposure in public places. In fully adjusted models we additionally accounted for other potential confounders including smoking and alcohol consumption, obesity, and physical inactivity. In sensitivity analysis, we reanalyzed our data using the “product of coefficient’ method following the same adjustment models, with essentially same results (see Supplemental Material, Supplemental Methods for methodological details and results from the “product of coefficients” method).
For both the difference and the product of coefficient methods, mediated effects were expressed as the absolute decline in mortality rates from 1988–1994 to 1999–2004 attributable to reductions in serum cotinine and in occupational SHS exposure time, as well as the percentage of the adjusted mortality decline across surveys explained by changes in SHS exposure measures. Finally, 95% confidence intervals (CIs) for the mediated effect were derived by simulation from the estimated model coefficients and covariance matrices in the setting of the product of coefficient methods,12 as it is a computationally efficient procedure.
RESULTS
The age, sex and race-adjusted smoking-related cancer mortality rates were 249.1 and 158.4 deaths per 100,000 person-year in 1988–1994 and 1999–2004, respectively (Table 1). The corresponding all-cause cancer mortality rates were 401.3 and 315.3 deaths per 100,000 person-years (data not shown). Smoking-related cancer mortality rates decreased in all subgroups evaluated except in obese participants and in participants exposed to SHS at home.
Table 1.
Sex, age and race-adjusted smoking-related cancer mortality rates (per 100,000 person-years) by NHANES survey period and participants’ characteristics.
| 1988–1994 | 1999–2004 | |||||
|---|---|---|---|---|---|---|
| N (weighted %) | Deaths | Mortality rate | N (weighted %) | Deaths | Mortality rate | |
|
| ||||||
| Overall | 6195 (100) | 140 | 249.1 | 5661 (100) | 60 | 158.4 |
| Sex | ||||||
| Men | 2657 (42) | 81 | 327.5 | 2650 (44) | 36 | 233.9 |
| Women | 3538 (58) | 59 | 190.6 | 3011 (56) | 24 | 103.9 |
| Age | ||||||
| <65 | 3352 (67) | 31 | 121.3 | 3141 (69) | 13 | 54.6 |
| ≥65 | 2843 (33) | 109 | 556.7 | 2520 (31) | 47 | 382.0 |
| Education | ||||||
| ≥ High School | 3450 (73) | 69 | 252.1 | 3866 (84) | 38 | 147.7 |
| < High School | 2745 (27) | 71 | 246.4 | 1795 (16) | 21 | 193.5 |
| Race/ethnicity | ||||||
| White | 3331 (82) | 87 | 248.8 | 3184 (79) | 40 | 153.4 |
| Black | 1147 (7) | 29 | 354.2 | 859 (8) | 11 | 300.7 |
| Mexican -American | 1478 (4) | 22 | 190.6 | 1260 (5) | 7 | 127.3 |
| Other | 239 (7) | 3 | 169.2 | 358 (8) | 2 | 97.0 |
| Physical inactivity | ||||||
| No | 4005 (74) | 94 | 260.1 | 4330 (84) | 43 | 154.7 |
| Yes | 2190 (26) | 46 | 217.8 | 1331 (16) | 17 | 169.5 |
| BMI (kg/m2) | ||||||
| <30 | 4295 (72) | 111 | 263.0 | 3695 (66) | 41 | 126.0 |
| ≥30 | 1900 (28) | 29 | 207.3 | 1966 (34) | 19 | 231.2 |
| Alcohol intake | ||||||
| Never | 1410 (18) | 23 | 185.4 | 1009 (15) | 7 | 81.0 |
| Former | 2687 (40) | 71 | 321.0 | 2511 (41) | 37 | 229.6 |
| Current | 2098 (42) | 46 | 209.7 | 2141 (44) | 16 | 121.6 |
| Smoking status | ||||||
| Never | 3640 (56) | 55 | 165.8 | 3318 (60) | 21 | 76.3 |
| Former | 2555 (44) | 85 | 366.4 | 2343 (40) | 39 | 271.4 |
| Tobacco smoke at home | ||||||
| No | 5339 (87) | 125 | 248.8 | 5311 (94) | 53 | 144.2 |
| Yes | 856 (13) | 15 | 242.6 | 350 (6) | 7 | 406.6 |
| Cotinine concentrations (ng/mL) | ||||||
| <0.05 | 1334 (19) | 31 | 169.6 | 3534 (63) | 31 | 111.6 |
| ≥ 0.05 | 4861 (81) | 109 | 267.6 | 2127 (37) | 29 | 237.0 |
| Occupational SHS* exposure time | ||||||
| 0 | 5410 (83) | 128 | 199.6 | 5353 (94) | 58 | 140.0 |
| ≥ 1 | 785 (17) | 12 | 529.6 | 308 (6) | 2 | 231.2 |
Secondhand tobacco smoke
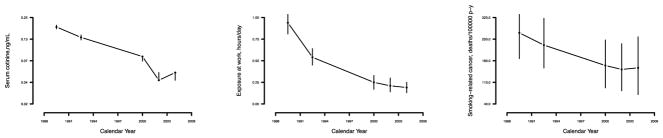
Figure 1 shows the progressive reduction in smoking-related cancer mortality and in SHS exposure over time. As observed, reductions in SHS exposure seem to follow the increase in regulation of smoking in public places in 1986, when reports by the Surgeon General and the NRC concluded that SHS is a cause of lung cancer in nonsmokers. 21 Similarly, since 1988, there has been a pronounced decline in smoking-related cancer deaths.
Figure 1.
Age, sex and race-adjusted geometric means of serum cotinine, self-reported occupational SHS exposure (hours/day), and smoking-related cancer mortality rates across NHANES phases. Vertical bars show 95% confidence intervals based on 15,000 bootstrap resamples.
Table 2 shows a 70% decrease in the unadjusted baseline serum cotinine concentrations [50th (5th, 95th) percentiles: 0.13 (0.04, 1.70) in 1988–1994 and 0.04 (0.01, 1.03) in 1999–2004] and a 59% decrease in baseline prevalence of occupational SHS exposure (define as self-reported occupational SHS exposure time >0 hours/day) [proportion (SE): 16.5 (1.0) in 1988–1994 and 6.7 (0.5) in 1999–2004] between study periods. In both periods, serum cotinine concentrations were higher among non-Hispanic Black, as well as among participants with lower education and participants exposed to SHS at home. Also in both periods, participants with occupational SHS exposure were more often men, younger, physically active, obese, current drinkers, former smokers, and had a higher prevalence of exposure to SHS at home (Table 2).
Table 2.
Distribution of serum cotinine concentrations, percentage of participants with self-reported occupational secondhand smoke exposure (SHS), and occupational SHS exposure time, by NHANES survey period and participants’ characteristics.
| 1988–1994 | 1999–2004 | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Serum cotinine (ng/ml), median (p5, p95) | Occupation al SHS exposure (>0 h/day), % (SE) | Occupational SHS exposure time (h/day), median (p5, p95)* | Serum cotinine (ng/ml), median (p5, p95) * | Occupation al SHS exposure (>0 h/day), % (SE) | Occupational SHS exposure time (h/day), median (p5, p95)* | |
| Overall | 0.13 (0.04, 1.70) | 16.53 (1.0) | 3.0 (1.0, 9.0) | 0.04 (0.01, 1.03) | 6.73 (0.5) | 1.0 (1.0, 8.0) |
| Sex | ||||||
| Men | 0.17 (0.04, 1.75) | 24.09 (1.5) | 3.0 (1.0, 9.0) | 0.04 (0.01, 1.44) | 10.23 (0.9) | 1.0 (1.0, 8.3) |
| Women | 0.12 (0.04, 1.60) | 10.96 (1.1) | 3.9 (1.0, 10.0) | 0.04 (0.01, 0.76) | 3.99 (0.4) | 1.6 (1.0, 8.0) |
| Age | ||||||
| ≤65 | 0.16 (0.04, 1.86) | 23.38 (1.4) | 3.0 (1.0, 9.0) | 0.04 (0.01, 1.12) | 9.12 (0.7) | 1.0 (1.0, 8.0) |
| > 65 | 0.10 (0.04, 1.39) | 2.65 (0.5) | 4.3 (1.0, 10.6) | 0.04 (0.01, 0.70) | 1.36 (0.3) | 2.0 (1.0, 8.2) |
| Education | ||||||
| ≥High School | 0.13 (0.04, 1.42) | 17.96 (1.2) | 3.0 (1.0, 9.0) | 0.04 (0.01, 0.85) | 6.63 (0.5) | 1.0 (1.0, 8.3) |
| < High School | 0.17 (0.04, 2.20) | 12.47 (1.6) | 4.0 (1.0, 9.2) | 0.05 (0.01, 1.50) | 7.23 (1.0) | 2.0 (1.0, 8.0) |
| Race/ethnicity | ||||||
| White | 0.13 (0.04, 1.63) | 16.67 (1.2) | 3.0 (1.0, 9.2) | 0.04 (0.01, 0.92) | 6.14 (0.5) | 1.0 (1.0, 8.0) |
| Black | 0.26 (0.04, 2.41) | 17.31 (1.8) | 4.0 (1.0, 9.3) | 0.07 (0.01, 1.76) | 8.38 (1.2) | 2.0 (1.0, 8.0) |
| Mexican -American | 0.10 (0.04, 1.29) | 14.90 (1.2) | 4.0 (1.0, 10.0) | 0.04 (0.01, 0.46) | 8.42 (0.9) | 1.0 (1.0, 8.0) |
| Other | 0.12 (0.04, 1.42) | 14.70 (4.1) | 6.5 (1.0, 8.0) | 0.04 (0.01, 1.05) | 9.98 (1.8) | 2.0 (1.0, 10.4) |
| Physical inactivity | ||||||
| No | 0.13 (0.04, 1.42) | 17.15 (1.2) | 3.0 (1.0, 9.0) | 0.04 (0.01, 0.86) | 7.05 (0.5) | 1.0 (1.0, 8.0) |
| Yes | 0.16 (0.04, 2.51) | 14.71 (1.5) | 4.0 (1.0, 9.0) | 0.04 (0.01, 1.71) | 5.12 (1.0) | 2.0 (1.0, 8.0) |
| BodyMassIndex | ||||||
| <30 | 0.13 (0.04, 1.59) | 15.60 (1.2) | 4.0 (1.0, 9.2) | 0.04 (0.01, 0.91) | 6.43 (0.5) | 1.0 (1.0, 8.0) |
| ≥30 | 0.15 (0.04, 2.04) | 18.85 (1.7) | 3.0 (1.0, 9.0) | 0.04 (0.01, 1.13) | 7.31 (0.9) | 1.0 (1.0, 9.0) |
| Alcohol intake | ||||||
| Never | 0.11 (0.04, 2.09) | 11.52 (1.2) | 4.0 (1.0, 8.0) | 0.04 (0.01, 0.86) | 4.73 (0.8) | 2.0 (1.0, 10.6) |
| Former | 0.13 (0.04, 1.60) | 14.20 (1.4) | 3.0 (1.0, 9.0) | 0.04 (0.01, 0.90) | 6.94 (0.8) | 1.0 (1.0, 8.8) |
| Current | 0.15 (0.04, 1.67) | 20.80 (1.6) | 3.0 (1.0, 9.9) | 0.04 (0.01, 1.18) | 7.24 (0.7) | 2.0 (1.0, 8.0) |
| Smoking status | ||||||
| Never | 0.12 (0.04, 1.47) | 15.07 (1.0) | 3.0 (1.0, 9.0) | 0.04 (0.01, 0.82) | 6.32 (0.5) | 1.0 (1.0, 8.0) |
| Former | 0.16 (0.04, 1.87) | 18.41 (1.6) | 4.0 (1.0, 10.0) | 0.04 (0.01, 1.33) | 7.34 (0.8) | 1.0 (1.0, 8.0) |
| SHS at home | ||||||
| No | 0.11 (0.04, 0.93) | 15.69 (1.0) | 3.0 (1.0, 9.0) | 0.04 (0.01, 0.54) | 6.56 (0.5) | 1.0 (1.0, 8.0) |
| Yes | 0.82 (0.11, 4.38) | 22.32 (2.2) | 4.0 (1.0, 10.0) | 0.65 (0.04, 4.73) | 9.28 (2.3) | 2.0 (1.0, 8.0) |
Results among participants with self-reported occupational SHS exposure time (N=785 in 1988–1994 and N=308 in 1999–2004).
Comparing 1988–1994 to 1999–2004, the fully adjusted percent reduction in the geometric mean of serum cotinine was 62%. Among participants with self-reported occupational SHS exposure time, the fully adjusted geometric mean percent reduction in SHS exposure time was 32% (Supplemental Material, Supplemental Table 1).
In multivariable analyses, baseline serum cotinine concentrations were positively associated with all-cancer (RRs [95%CI]: 1.10 [1.03, 1.17]) and smoking-related cancer mortality (1.13 [1.03, 1.24]). Occupational SHS exposure time was also positively associated with all-cancer and smoking-related cancer mortality (RR [95%CI] per 1-hour increase in exposure: 1.14 [1.06, 1.24] for all-cancer, and 1.14 [1.02, 1.26] for smoking-related cancer mortality). The corresponding associations were borderline significant (i.e. the lower limit in the 95% confidence interval was relatively close to one) for trachea, bronchus and lung cancer mortality (1.09 [0.95, 1.26] in cotinine models, and 1.13 [0.99, 1.30] in occupational exposure models); and for colon, rectum and anus cancer mortality (1.23 [0.91, 1.66]) in occupational exposure models]) (Table 4); this was expected given the substantially lower number of deaths, which decreases statistical power. The p-values for interactions by survey for the association of serum cotinine and time of exposure at work were 0.75 and 0.13, respectively, for all-cancer; and 0.38 and 0.16, respectively, for smoking-related cancer).
Table 4.
Absolute change in smoking-related cancer mortality rates (per 100,000 person-years) comparing NHANES surveys 1999–2004 to 1988–1994a before and after adjustment for serum cotinine and occupational secondhand smoke exposure (SHS).
| Level of adjustment | All cancer | Smoking related-cancer | ||
|---|---|---|---|---|
| Absolute change in mortality rate (95% CI)** | Difference in change (%)*** | Absolute change in mortality rate (95% CI) ** | Difference in change (%)*** | |
| Adjusted for age, sex, race, low education and exposure at home | −73.6 (−173.8, 26.6) | 0 (reference) | −82.7 (−157.2, −8.3) | 0 (reference) |
| Further adjusted for log cotinine | −26.1 (−131.9, 79.7) | −47.5 (64.5) | −47.1 (−127.3, 33.1) | −35.6 (43.1) |
| Further adjusted for occupational SHS exposure time | −53.4 (−151.5, 44.7) | −20.2 (27.5) | −72.0 (−144.8, 0.8) | −10.7 (13.0) |
| Adjusted for age, sex, race, low education, SHS exposure at home, smoking status, alcohol status, BMI, and physical inactivity | −75.8 (−177.0, 25.5) | 0 (reference) | −77.0 (−151.4, −2.6) | 0 (reference) |
| Further adjusted for log cotinine | −31.5 (−138.8, 75.9) | −44.3 (58.5) | −41.8 (−122.6, 39.1) | −35.2 (45.8) |
| Further adjusted for occupational SHS exposure time | −56.1 (−155.0, 42.9) | −19.7 (26.0) | −66.3 (−139.0, 6.4) | −10.7 (13.9) |
Absolute change in mortality rate by survey period is estimated from Aalen additive hazard models with age at follow-up as the time scale, survey period indicators, and progressive degrees of adjustment.
Mediated effect by serum cotinine and occupational SHS exposure time was calculated using the “difference of coefficient method” as the absolute change in mortality rates between surveys in the reference model, minus that absolute change in the model further adjusted for log-transformed cotinine concentrations or for untransformed occupational SHS exposure time, expressed both in absolute terms (difference in change) and relative to the change in the reference model.
In models adjusted for sociodemographic and lifestyle risk factors, the reduction (rate differences [95%CI]) in all-cancer and smoking-related cancer mortality rates between the two periods were: 75.8 [−25.5, 177.0] and 77.0 [2.6, 151.4]/100.000 person-year, respectively). Further adjustment for the number of smokers at home gave similar findings (data not shown). Following the difference of coefficients method, the adjustment for serum cotinine explained 58.5% (44.3 deaths/100.000 person-year) and 45.8% (35.2 deaths/100.000 person-year) of the respective reductions in mortality. In separate models, adjustment for occupational SHS exposure time explained 26.0% (19.7 deaths/100.000 person-year) and 13.9% (10.7 deaths/100.000 person-year) of the respective reductions in mortality (Table 4).
Consistently, in results from the product of coefficients method, the avoided number of all-cancer deaths comparing 1999–2004 to 1988–1994 that were attributed to the observed decreases in serum cotinine concentrations and in occupational SHS exposure time were 45.8 (95% CI: 2.8, 89.5) and 18.1 (95%CI: −1.2, 39.6) deaths/100,000 person-year, respectively. For smoking-related cancer mortality, the corresponding avoided deaths were 36.4 (95%CI: 0.7, 72.8) and 9.9 (95%CI: −3.8, 24.9) (Supplemental Material, Supplemental Table 2).
DISCUSSION
Smoking-related cancer mortality rates among non-smoking US adults decreased about 36% from 1988–1994 to 1999–2004. After accounting for potential confounders and SHS exposure at home, around 46% of the decrease in cancer mortality could be attributed to declines in SHS exposure in public places based on statistically significant serum cotinine estimates. Our results are unique in that they present the first evidence of the important contribution that smoking bans may have had in the cancer mortality reduction observed in the last decade among US non-smokers. The 59% decrease in the prevalence of self-reported occupational SHS exposure time supports the effective implementation of workplace smoking bans. We estimated that 14% of the overall reduction in smoking-related cancer mortality rates among non-smokers could be attributed to reductions in occupational SHS exposure time, although the evidence for this self-reported measure was only statistically suggestive.
Despite these reductions, the burden of disease from SHS exposure remains large. Results from the last published comprehensive report on the burden of disease associated to SHS exposure showed that in 2004, the last year of our series, SHS exposure caused around 1% of the worldwide mortality (i.e. 379,000 deaths from ischemic heart disease, 165,000 from lower respiratory infections, 36,900 from asthma, and 21,400 from lung cancer), and the loss of 10.9 million Disability-Adjusted Life-Years.22 Specifically, in the US, the CDC estimated that during the period 2005–2009, SHS caused 33,950 deaths from ischemic heart disease and 7,330 from lung cancer among non-smoking adults. Also in the US, in 2016, SHS related deaths resulted in 600,000 years of potential life lost and 6.6 billion dollars of lost productivity.23
The effectiveness of smoke-free policies to reduce SHS exposure and adult smoking is well established,24;25 with strong evidence that implementation of smoking bans prevents cardiovascular disease in the overall population and respiratory outcomes among workers.7;8;26 However, the only previous study on the potential role of smoking bans on cancer mortality showed no changes in lung cancer mortality trends within EU countries from 1994 to 2012 after the implementation of smoke-free legislation.11 Our study, based on individual-level data, extends previous findings of the effectiveness of smoke-free legislation to reduce smoking-related cancer mortality among non-smokers.
Among the main forces driving the observed reductions in SHS exposure during the study period may be the decline on smoking prevalence (from 42% in 1965 to 20% in 2004),27 the recognition of SHS as a cause of disease, and the resulting decline of social acceptance of smoking in public places. Although smoke-free policies did not enter into force until the 2000s, partial restrictions on smoking in public places, government buildings and airplanes started to be implemented in the early 1970s. By 1986, 41 states and the District of Columbia had statutes that restricted smoking to some extent.28 In 1992, following EPA’s conclusion that SHS posed a “serious and substantial public health impact”,29 US states and local governments enacted an increasing number of more restrictive bans which culminated in current comprehensive smoke-free laws (those prohibiting tobacco smoke in all indoor areas of private workplaces, restaurants and bars with no exception). According to the CDC, the number of states in the US that have adopted comprehensive smoke-free laws has increased in the last 15 years from zero in 2000 to 27 in 2015.30 However, despite these great achievements in tobacco control, many US states lack smoke-free laws,30 and even in those with smoke-free laws, disparities in exposure to SHS still exist and legislative and public health efforts are needed to ensure full protection of workers. Worldwide, many countries do not have adequate smoke-free legislation that comprehensively protect workers, and exposure to SHS remains common,31 particularly among hospitality employees.32 Extending public health interventions to reduce SHS exposure is therefore needed in order to reduce its burden of disease, not only in the US, but worldwide.
Our study has several limitations. First, a single measurement of cotinine is an imperfect surrogate of past exposure to SHS because its estimated half-life is approximately 16–19 hours and indicates exposure over the previous 1–2 days. Although there is very recent evidence for epigenetic biomarkers of long-term cumulative exposure,33 these are not available in NHANES. Second, because exposure at work was self-reported, the possibility of some information bias cannot be ruled out. Third, the causal interpretation of mediated effects under all degrees of adjustment is imperfect, since full adjustment may introduce inter-related causal pathways that compromise the ability to identify mediation effects, whereas models adjusting for socio-demographic variables only could leave substantial room for residual confounding. 34 In our data, the difference of coefficients and the product of coefficients methods showed consistent findings under all adjustment models, so results seem to be robust to major violations of the mediation framework assumptions. Fourth, we could not account for the advances in cancer screening, early detection or treatment that may have occurred in the US during the studied period, since NHANES lacks information on these factors. In addition, the enactment of smoke-free legislation during this time period varied across the US and we did not have data on the geographic location of NHANES participants. Moreover, we did not have longitudinal data on SHS exposure in the same subjects, so we cannot discard that some of the period effects can be caused by unmeasured changes in primary and secondary prevention factors in the USA that have likely resulted in a decrease in cancer deaths. Indeed, there was a residual trend in cancer mortality (~40–50% for serum cotinine and ~30–85% for occupational SHS exposure time models, depending on the endpoint) which remained unexplained with the available data. Fifth, even though analyses were adjusted for self-reported SHS exposure at home, we cannot exclude that SHS exposure occurred at other private settings or that accounting for SHS exposure at home is incomplete. Sixth, mortality outcomes were obtained from death certificates, with potential miscoding of the cause of death. Also, changes in death certificates coding over time could affect observed trends in cancer mortality. However, methods for matching NHANES participants with the NDI have been validated and developed with the goal of tracking these changes.13 Seventh, mortality is an imperfect outcome to study cancers with relatively good prognosis. However, many smoking-related cancers (e.g. lung, pancreas) have relatively low survival rates, so their incidence approximates mortality. Finally, excluded participants (n=4417) were more likely to be older, less educated and black, which may underestimate the contribution of changes in secondhand smoke to cancer.
Strengths of this study include the national representativeness of the study sample, relatively large sample size, standardization of the study protocol and extensive laboratory quality control procedures. Additionally, the availability of a specific biomarker of SHS exposure reduces the possibility of exposure misclassification.
Our results support the effectiveness of smoking reductions in indoor public places to reduce smoking-related cancer mortality among non-smoking workers. Given these findings and the fact that SHS remains an important indoor and outdoor air pollutant in many US states and countries, smoke-free laws and interventions must be extended to all public places as well as to private areas in order to further protect the population from unnecessary and involuntary exposure to an established carcinogen.
Supplementary Material
Table 3.
Rate ratios for the association between measures of secondhand smoke exposure (SHS) exposure and cancer mortality endpoints after 8 years of follow-up. Results are expressed for a two-fold increase in baseline serum cotinine concentrations and for a one hour/day increase in time of exposure to SHS at the workplace..
| All-cancer | Smoking-related | Trachea, bronchus and lung | Colon, rectum and annus | Pancreas | ||
|---|---|---|---|---|---|---|
| N Deaths/no deaths | 353/11503 | 200/11656 | 81/11775 | 47/11809 | 21/11835 | |
|
| ||||||
| Serum cotinine | RR (95%CI) | 1.10 (1.03, 1.17) | 1.13 (1.03, 1.24) | 1.09 (0.95, 1.26) | 1.06 (0.85, 1.32) | 1.23 (0.91, 1.66) |
| Occupational SHS exposure time | RR (95%CI) | 1.14 (1.06, 1.24) | 1.14 (1.02, 1.26) | 1.13 (0.99, 1.30) | 1.24 (0.96, 1.60) | 0.96 (0.71, 1.31) |
Models were adjusted for sex, age (continuous), race (White, Black, Mexican-American, Other), education (lower, higher), smoking status (never, former), alcohol status (never, former, current), BMI (<30, ≥30), physical inactivity (yes/no), tobacco exposure at home (yes/no), and survey period.
Highlights.
We investigated the contribution of the decrease in secondhand smoke (SHS) exposure in public places to smoking-related cancer mortality in the US.
We used data from 11,856 non-smoking adults aged ≥40 years who participated in NHANES 1988–1994 or 1999–2004.
Declines in SHS exposure were associated with reductions in all-cancer and smoking-related cancer mortality.
These results support that smoking bans in public places may have reduced cancer mortality among non-smoking adults.
Acknowledgments
Funding: This work was supported by the Strategic Action for Research in Health sciences (CP12/03080; PI15/00071); CIBERESP and CIBEROBN. The Strategic Action for Research in Health Sciences, CIBEROBN and CIBERESP are initiatives from the Carlos the third National Health Institutes in Madrid and the Spanish Ministry of Economy and Competitiveness and are co-funded with European Funds for Regional Development (FEDER).
Footnotes
Authors contributions: MTP, ANA and EGE conceived the study. MTP and AJ performed the statistical analyses. EGE drafted the initial document. All authors reviewed the manuscript for important intellectual content. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. MTP is the guarantor of this study.
Competing interests: None to declare
Consent and Approval: NHANES study protocols were approved by the institutional review board of the National Center for Health Statistics, and written informed consent was obtained from all participants.
Data sharing: Anonymized, non-identifiable participant level cross sectional survey data are publicly available at https://wwwn.cdc.gov/nchs/nhanes/. The statistical code and reduced dataset restricted to the study population and variables used for this analysis is available upon request to the corresponding author.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE LIST
- 1.International Agency for Research on Cancer (IARC) Monographs on the Evaluation of Carcinogenic Risks to Humans. [Last accessed: June, 2017];Personal habits and indoor combustions. 2012 100E Available at: http://monographs.iarc.fr/ENG/Monographs/vol100/mono100E.pdf. [PMC free article] [PubMed] [Google Scholar]
- 2.Moritsugu KP. The 2006 Report of the Surgeon General: the health consequences of involuntary exposure to tobacco smoke. Am J Prev Med. 2007;32:542–543. doi: 10.1016/j.amepre.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 3.Alberg AJ, Shopland DR, Cummings KM. The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. Am J Epidemiol. 2014;179:403–412. doi: 10.1093/aje/kwt335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Disparities in secondhand smoke exposure--United States, 1988–1994 and 1999–2004. MMWR Morb Mortal Wkly Rep. 2008;57:744–747. [PubMed] [Google Scholar]
- 5.CDC (Centers for Disease Control and Prevention) State Tobacco Activities Tracking and Evaluation (STATE) System. Map of Smokefree Indoor Air-Private Worksites, Restaurants and Bars. Available at: http://www.cdc.gov/statesystem/smokefreeindoorair.html.
- 6.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review 1975–2013. National Cancer Institute; Bethesda, MD: Apr, 2016. [Last accessed: May 2017]. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site. [Google Scholar]
- 7.Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012;126:2177–2183. doi: 10.1161/CIRCULATIONAHA.112.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin H, Wang H, Wu W, Lang L, Wang Q, Tian L. The effects of smoke-free legislation on acute myocardial infarction: a systematic review and meta-analysis. BMC Public Health. 2013;13:529. doi: 10.1186/1471-2458-13-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peelen MJ, Sheikh A, Kok M, et al. Tobacco control policies and perinatal health: a national quasi-experimental study. Sci Rep. 2016;6:23907. doi: 10.1038/srep23907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frazer K, Callinan JE, McHugh J, et al. Legislative smoking bans for reducing harms from secondhand smoke exposure, smoking prevalence and tobacco consumption. Cochrane Database Syst Rev. 2016;2:CD005992. doi: 10.1002/14651858.CD005992.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Campos JL, Ruiz-Ramos M, Fernandez E, Soriano JB. Recent lung cancer mortality trends in Europe: effect of national smoke-free legislation strengthening. Eur J Cancer Prev. 2017 doi: 10.1097/CEJ.0000000000000354. [DOI] [PubMed] [Google Scholar]
- 12.Lange T, Hansen JV. Direct and indirect effects in a survival context. Epidemiology. 2011;22:575–581. doi: 10.1097/EDE.0b013e31821c680c. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC), National Centers for Health Statistics (NCHS) [Last accessed: May 2017];National Health and Nutrition Examination Survey (NHANES) Available at: http://www.cdc.gov/nchs/nhanes.htm.
- 14.Hornung R, Reed L. Estimation of average concentration in the presence of nondetectable values. Applied occupational and environmental hygiene. 1990;5:46–51. [Google Scholar]
- 15.Centers for Disease Control and Prevention (CDC) [Last accessed: May 2017];Fourth national report on human exposure to environmental chemicals. Available at: http://www.cdc.gov/exposurereport.
- 16.World Health Organization. [Last accessed: May 2017];WHO International Classification of Diseases (ICD-10) 2014 Available at: http://www.who.int/classifications/icd/en/
- 17.Pierce JP, Patterson RE, Senger CM, et al. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J Natl Cancer Inst. 2014;106:djt359. doi: 10.1093/jnci/djt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones MR, Joshu CE, Kanarek N, Navas-Acien A, Richardson KA, Platz EA. Cigarette Smoking and Prostate Cancer Mortality in Four US States, 1999–2010. Prev Chronic Dis. 2016;13:E51. doi: 10.5888/pcd13.150454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang Z, VanderWeele TJ. When is the difference method conservative for assessing mediation? Am J Epidemiol. 2015;182:105–108. doi: 10.1093/aje/kwv059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology. 2011;22:582–585. doi: 10.1097/EDE.0b013e31821db37e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. The Health Consequences of Involuntary Smoking: A Report of the Surgeon General. Vol. 1986. Rockwille (MD): U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health; 1986. DHHS Publication No. (CDC) 87–8398. [Google Scholar]
- 22.Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet. 2011;377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 23.Max W, Sung HY, Shi Y. Deaths from secondhand smoke exposure in the United States: economic implications. Am J Public Health. 2012;102:2173–2180. doi: 10.2105/AJPH.2012.300805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.International Agency for Research on Cancer (IARC) [Last accessed: May 2017];Evaluating the effectiveness of smoke-free policies. 2009 Available at: http://www.iarc.fr/en/publications/pdfsonline/prev/handbook13/handbook13.pdf.
- 25.Lidon-Moyano C, Fu M, Ballbe M, et al. Impact of the Spanish smoking laws on tobacco consumption and secondhand smoke exposure: A longitudinal population study. Addict Behav. 2017;75:30–35. doi: 10.1016/j.addbeh.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Madureira J, Mendes A, Teixeira JP. Evaluation of a smoke-free law on indoor air quality and on workers’ health in Portuguese restaurants. J Occup Environ Hyg. 2014;11:201–209. doi: 10.1080/15459624.2013.852279. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. [Last accessed: May 2017];Trends in Current Cigarrette Smoking Among High School Students and Adults, United States, 1965–2014. Available at: https://www.cdc.gov/tobacco/data_statistics/tables/trends/cig_smoking/index.htm.
- 28.Institute of Medicine (US) Committee on Secondhand Smoke Exposure and Acute Coronary Events. Making Sense of the Evidence. Washington (DC): National Academies Press (US); 2010. Secondhand Smoke Exposure and Cardiovascular Effects. [PubMed] [Google Scholar]
- 29.Environmental Protection Agency (EPA) Respiratory health effects of passive smoking: lung cancer and other disorders. Tobacco Control. 2018;2:71–79. [Google Scholar]
- 30.Tynan MA, Holmes CB, Promoff G, Hallett C, Hopkins M, Frick B. State and Local Comprehensive Smoke-Free Laws for Worksites, Restaurants, and Bars - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:623–626. doi: 10.15585/mmwr.mm6524a4. [DOI] [PubMed] [Google Scholar]
- 31.Eriksen M, Mackay J, Schluger N, Gomeshtapeh FI, Drope J. The tobacco altas. Atlanta, Georgia USA: American Cancer Socitey; 2015. pp. 9–10. [Google Scholar]
- 32.Jones MR, Wipfli H, Shahrir S, et al. Secondhand tobacco smoke: an occupational hazard for smoking and non-smoking bar and nightclub employees. Tob Control. 2013;22:308–314. doi: 10.1136/tobaccocontrol-2011-050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reynolds LM, Magid HS, Chi GC, et al. Secondhand Tobacco Smoke Exposure Associations With DNA Methylation of the Aryl Hydrocarbon Receptor Repressor. Nicotine Tob Res. 2017;19:442–451. doi: 10.1093/ntr/ntw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lange T, Rasmussen M, Thygesen LC. Assessing natural direct and indirect effects through multiple pathways. Am J Epidemiol. 2014;179:513–518. doi: 10.1093/aje/kwt270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.