Abstract
Macrophages have been found to regulate the effects of biomaterials throughout the entire tissue repair process as an antigen-presenting cell. As a well-defined osteoconductive biomaterial for bone defect regeneration, tricalcium phosphate (TCP) has been found to facilitate a favourable osteoimmunomodulatory response that can shift macrophage polarization towards the M2 phenotype. In the present study, our group discovered that a histone methyltransferase enhancer of zeste1 (EZH1) was drastically downregulated in Thp1 cells stimulated by TCP, indicating that EZH1 may participate in the macrophage phenotype shifting. Furthermore, the NF-κB pathway in macrophages was significantly downregulated through stimulation of TCP, suggesting a potential interaction between EZH1 and the NF-κB pathway. Utilizing gene knock-down therapy in macrophages, it was found that depletion of EZH1 induced M2 macrophage polarization but did not downregulate NF-κB. When the NF-κB pathway was inhibited, the expression of EZH1 was significantly downregulated, suggesting that the inhibition of EZH1 may be regulated by the NF-κB pathway. These novel findings provide valuable insights into a potential gene target system that controls M2 macrophage polarization which ultimately favours a microenvironment suitable for bone repair.
1. Introduction
As a type of antigen-presenting cell, macrophages have been found to regulate implanted biological materials during the tissue repair process [1]. The strong plasticity of macrophages enables their dual function that favours either tissue inflammation or tissue repair, which have made macrophages an ideal research target in the field of tissue engineering [2, 3]. With changes to the surrounding microenvironment, macrophages can polarize into two phenotypes, either M1 (tissue inflammation) or M2 (tissue repair) macrophages. It is generally accepted that the M1 macrophage produces a number of proinflammatory cytokines including IL-6, IL-12, and TNF-alpha, thereby reducing osteogenic differentiation [4]. On the contrary, M2 macrophages are responsible for tissue repair macrophage and hence secrete pro-wound-healing cytokines and growth factors including TGF-β, VEGF, and IFG-1 to promote immunoregulation and tissue repair [5]. Therefore, balancing the microenvironment and macrophage polarization between M1 and M2 phenotypes is critical for proper biomaterial implant integration.
As a well-defined osteoconductive biomaterial for bone regeneration, tricalcium phosphate (TCP) has broad clinical applications [6, 7]. Because of the good absorbability and osteoconduction characteristics of TCP, it enables bone remodelling to occur at the material interface [8–10]. Naturally, however, it is important to understand that prior to bone formation around any biomaterial, immune cells (namely, macrophages) surround the surface of the biomaterial and create a microenvironment that favours either future bone regeneration or fibrous encapsulation [3].
There have been inconsistencies in the literature defining the role of macrophages with respect to their contribution to osteogenesis around TCP bone particles. In a study conducted by Chen et al., it was revealed that macrophage polarization was driven towards the M1 extreme when cobalt was incorporated into β-tricalcium phosphate and this negatively impacted the ability for bone-forming osteoblasts to contribute to new bone formation [11]. Similarly, a study by Tai et al. found that TCP particles promoted macrophage expression of CD86, an M1 surface molecule in macrophages [12]. In contrast, however, a study by Chen et al. found that TCP extracts facilitated a favourable osteoimmunomodulatory response that promoted M2 macrophage polarization including the release of bone morphogenetic protein-2 (BMP2), one of the best-known inducers of osteoblast differentiation of bone marrow stromal cells (BMSCs) [13, 14].
Currently, numerous studies have shown that histone methylation modification plays an important role in the polarization of macrophages [15–17]. Previously, our group revealed that EZH1 can form a nonclassical complex with SUZ12 and UTX, leading to the assembly of RNA polII to the target genes of NF-kappa B [18]. NF-kappa B is a key pathway that mediates macrophage polarizations towards the M1 phenotype, but whether it can regulate EZH1 remains unknown. Furthermore, the direct role of EZH1 during macrophage polarization remains unclear [19, 20].
In the present study, we aimed to investigate the potential role of macrophages during the bone regeneration process in the femurs of rats implanted with TCP. It was revealed that TCP significantly suppresses the activation of the NF-kappa B pathway and causes a decrease in EZH1 expression. The reduction of EZH1 led to lower expression of M1 markers resulting in a lowered release of proinflammatory cytokines. As a result, it was found that this local microenvironment favoured bone regeneration. We then set to characterize the role of EZH1 in the regulation of macrophage polarization and further aimed to clarify the interaction between EZH1 and NF-kappa B during the bone repair process.
2. Materials and Methods
2.1. In Vivo Studies by Using the Femur Defect Model
The tricalcium phosphate utilized in this study was purchased from Olympus Terumo Biomaterials. Six mature Wistar rats whose body weight ranged from 185 to 235 grams were utilized for the in vivo component of this study. All rats utilized in this study were handled in accordance with the policies of the Ethics Committee for Animal Research, Wuhan University, China. Proper sterile conditions and minimally invasive surgical procedures were utilized throughout the entire surgery and approved by the Ethics Committee for Animal Use of the Institute of Biomedical Sciences, under protocol number 134/2012. The animals and surgical protocols were performed according to our previous studies [21]. Briefly, the femoral diaphysis was orientated by a direct incision towards the fascia lata. A bone defect that reached the medullary canal was made by a diamond-tipped drill bit at 4000 rpm irrigated by physiological serum. Then the defect was filled with sterilized TCP. Afterwards, the wound was sutured closed in 2 layers. After one day, 3 rats were sacrificed using sodium pentobarbital (200 mg/kg, ip). After 8 weeks, the remaining three rats were sacrificed.
2.2. Hematoxylin-Eosin Staining
After fixing in 4% formaldehyde for 48 hours, femoral samples were submersed in 10% EDTA, which was changed every three days for 3 weeks to decalcify the samples. Then the samples were embedded in paraffin. Samples were cut in 5 μm thick sections and each slide was prepared with two sections. The sections were stained using hematoxylin-eosin stain (Sigma).
2.3. Cell Culture and Extract Preparation
Thp1 cells were cultured at 37°C with 5% carbon dioxide in HyClone RPMI 1640 medium modified (Thermo Fisher Scientific Inc.), 10% fetal bovine serum (FBS) (Gibco, Life Technologies Corporation), and 100 mg/mL streptomycin (HyClone). TCP was sterilized and placed into 24-well plates (100 mg/well) using a small spatula and then into cultures with 500 μL/well of Thp1 culture medium. After 24 h of incubation, the culture medium was collected as the TCP extract.
Thp1 was first induced to macrophages by culture with 100 ng/mL of phorbol myristate acetate (PMA) (Sigma) at a density of 1 × 106 cells/mL for 48 h. Then the culture medium was changed to culture medium containing TCP extract, 500 ng/mL lipopolysaccharide (LPS) (PeproTech), and 20 ng/mL human interleukin-4 (IL-4) (PeproTech). After incubation for 1 h, the supernatants of Thp1 cells cultured with TCP extract were collected.
Human bone marrow stem cells (HBMSCs) were obtained from patients undergoing iliac bone graft with informed written consent. The procedure was approved by the Ethics Committee at Wuhan University, China. HBMSCs were cultured in a-MEM medium (Thermo Fisher Scientific Inc.) at 37°C with 5% carbon dioxide.
2.4. Gene Silence and Osteogenic Induction
Gene silence in Thp1 was performed as Lipofectamine 3000 reagent protocol (Thermo Fisher Scientific). The target sequence for siRNA was 5′-CCAAAGUGGUCAUGGUGAATT-3′.
Osteogenic-inducing media were comprised of a-MEM with 10% FBS, 100 U/mL penicillin, 100 mg/mL streptomycin, 10 nM dexamethasone, 10 mM beta-glycerophosphate, 50 μg/mL L-ascorbic acid, and TCP extract, Thp1 supernatants.
2.5. Alizarin Red Staining and Alkaline Phosphatase Staining
HBMSCs were first washed with PBS three times and then fixed in 4% formaldehyde for 15 min after being induced with osteogenic differentiation media for 14 days. Then samples were stained with 0.1% alizarin red solution (pH 4.2) at room temperature for 1 h. Alkaline phosphatase (ALP) staining was performed according to the manufacturer's protocol (ALP; Beyotime) after HBMSCs were cultured for 7 days. Quantification of the areas of alizarin red staining and ALP staining was measured by ImageJ software [22]. Stained areas were designated by normalizing the threshold and then analysing the size of the stained areas.
2.6. Protein Extraction and Western Blotting
EZH1 silenced and normal Thp1 cells were treated with TCP extract for 1 h. Then cells were collected with RIPA, mixed with loading buffer, and then heated for 10 min at 95°C to denature total protein for Western blotting. The antibodies against EZH1 (ABclonal, A5818), P65 (Boster, BA0610-2), and P-P65 (Cell Signaling Technology Inc.) were purchased from the indicated companies.
2.7. Reverse Transcription and Quantitative PCR
Thp1 cells were treated with PMA for 48 h, and then the culture medium was replaced with culture medium containing TCP extract, LPS, and IL-4. After incubation for 1 h, the supernatants of Thp1 were collected. Then HBMSC cells were treated with culture medium including the supernatants from the Thp1, LPS, IL-4, and TCP extract which contained osteogenic differentiation medium for 14 days. Thp1 cells were treated with culture medium, LPS, IL-4, and TCP extract for 2 h to evaluate gene expression for macrophage polarization. Total RNA was extracted with TRIzol reagent (TriPure Isolation Reagent, Roche Applied Science, Germany) following the manufacturer's protocol. About 1 μg of total RNA was used in reverse transcription with PrimeScript RT-PCR Kit (TaKaRa, Japan) according to the manufacturer's protocol. The primer sequences used in Q-PCR are shown in Table 1.
Table 1.
The primer sequences used in RT-PCR.
| Gene | Organism | Primer sequence |
|---|---|---|
| IL-10 | Homo sapiens | Forward: 5′-GAGAAGCATGGCCCAGAAATC-3′ Reverse: 5′-GAGAAATCGATGACAGCGCC-3′ |
| IL-1α | Homo sapiens | Forward: 5′-CTCCAGCTGGAGGAAGTTAAC-3′ Reverse: 5′-CTGACTCAAAGCTGGTGGTG-3′ |
| TNF-α | Homo sapiens | Forward: 5′-CTGAACTTCGGGGTGATCGG-3′ Reverse: 5′-GGCTTGTCACTCGAATTTTGAGA-3′ |
| IFN-γ | Homo sapiens | Forward: 5′-GGCCATCAGCAACAACATAAGC-3′ Reverse: 5′-GGGTTGTTGACCTCAAACTTGG-3′ |
| ARG1 | Homo sapiens | Forward: 5′-GCCCTTTGCTGACATCCTA-3′ Reverse: 5′-CACCAGGCTGATTCTTCCGT-3′ |
| CD206 | Homo sapiens | Forward: 5′-GGGTTGCTATCACTCTCTATGC-3′ Reverse: 5′-TTTCTTGTCTGTTGCCGTAGTT-3′ |
| RUNX2 | Homo sapiens | Forward: 5′-CTGTGGCATGCACTTTGACC-3′ Reverse: 5′-GACCCTGACTTTTCGGGGAG-3′ |
| OCN | Homo sapiens | Forward: 5′-GCAAAGGTGCAGCCTTTGTG-3′ Reverse: 5′-GGCTCCCAGCCATTGATACAG-3′ |
| ALP | Homo sapiens | Forward: 5′-TCAGAAGCTAACACCAACG-3′ Reverse: 5′-TTGTACGTCTTGGAGAGGGC-3′ |
| OSX | Homo sapiens | Forward: 5′-CAACCTGCTAGAGATCTGAG-3′ Reverse: 5′-TGCAATAGGAGAGAGCGA-3′ |
| GAPDH | Homo sapiens | Forward: 5′-TCAGCAATGCCTCCTGCAC-3′ Reverse: 5′-TCTGGGTGGCAGTGATGGC-3′ |
2.8. Statistical Analysis
All data analysis was performed using GraphPad Prism software (San Diego, CA). The significance was set at p < 0.05. Data was calculated as a mean, and the statistical significance of differences between different groups was examined by one-way ANOVA and t-test. GAPDH was used as housekeeping gene. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
3. Results
3.1. TCP Scaffolds Promote Bone Regeneration and BMSC Osteogenic Differentiation
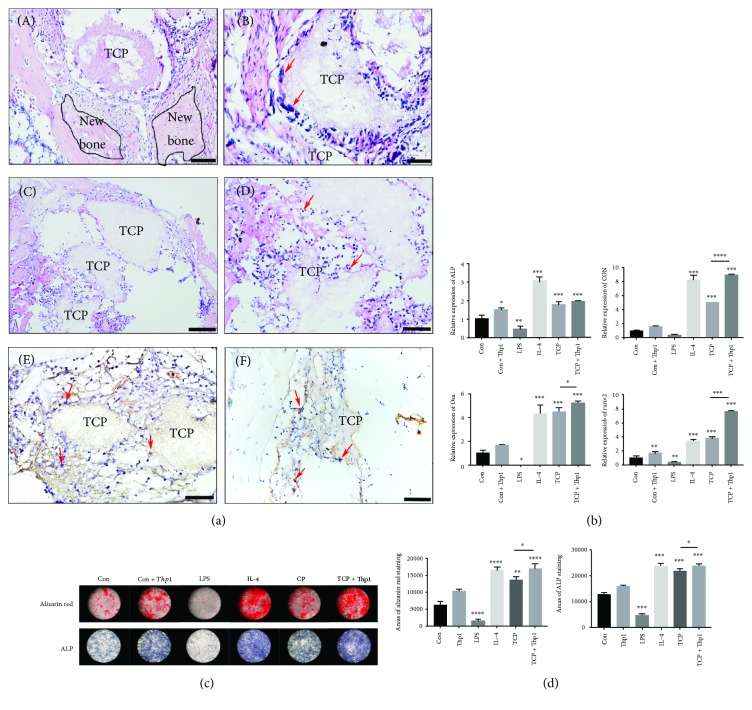
To confirm the ability of TCP scaffolds during bone regeneration and to investigate the potential bone-promoting mechanism, TCP scaffolds were implanted in the femur of rats and sacrificed after 1 day and 8 weeks. After 8 weeks, new bone formation around TCP scaffolds was visible by H&E staining (Figures 1(c) and 1(d)). More in light with the early events taking place during TCP/biomaterial, it was found that after 1 day in vivo, macrophages gathered around the TCP particles prior to bone regeneration (Figures 1(a), 1(b), 1(e), and 1(f)).
Figure 1.
Osteogenic effect and gathering of macrophages at the healing defects filled with TCP. (a) (A–D) Representative sections of hematoxylin-eosin staining. Macrophages are indicated by red arrows. (E) Representative sections of immunohistochemistry staining with F4/80 antibody. Macrophages are indicated by red arrows. (F) Representative sections of immunohistochemistry staining with CD206 antibody. M2 macrophages are indicated by red arrows. (A,C) Scale bar = 50 μm; (B, D, E, and F) scale bar = 20 μm. (b) Relative mRNA expression of ALP, OCN, OSX, and Runx2 of HBMSC stimulated by control medium, supernatant of control Thp1, LPS, IL-4, and TCP extract and supernatant of TCP-induced Thp1. (c) Alizarin red staining, ALP staining, and quantification of HBMSC stimulated by control medium, supernatant of control Thp1, LPS, IL-4, and TCP extract and supernatant of TCP-induced Thp1. (d) Quantification of the areas of alizarin red staining and ALP staining was measured by ImageJ software. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
Experiments from our in vitro data further demonstrated that TCP stimulates BMSC osteogenic differentiation. Firstly, since TCP is mineralized and can be stained with alizarin red (ARS), we utilized TCP extract for all the in vitro experiments. As illustrated in Figures 1(b)–1(d), the extract of TCP scaffolds significantly upregulated osteogenic differentiation markers when compared to the control group (Figures 1(b)–1(d)). Furthermore, the impact of inflammation can be markedly seen through the positive role of anti-inflammatory mediator IL-4, whereas a negative role was observed in the proinflammatory mediators which included LPS during osteoblast differentiation. Compared to the control group, stimulation of Thp1 with the supernatant of the TCP extract induced significantly better osteogenic differentiation (Figure 1). We therefore began to focus on the role of macrophage induced via TCP during BMSC osteogenic differentiation to investigate a potential mechanism for this action.
3.2. TCP Scaffolds Facilitate M2 Macrophage Polarization
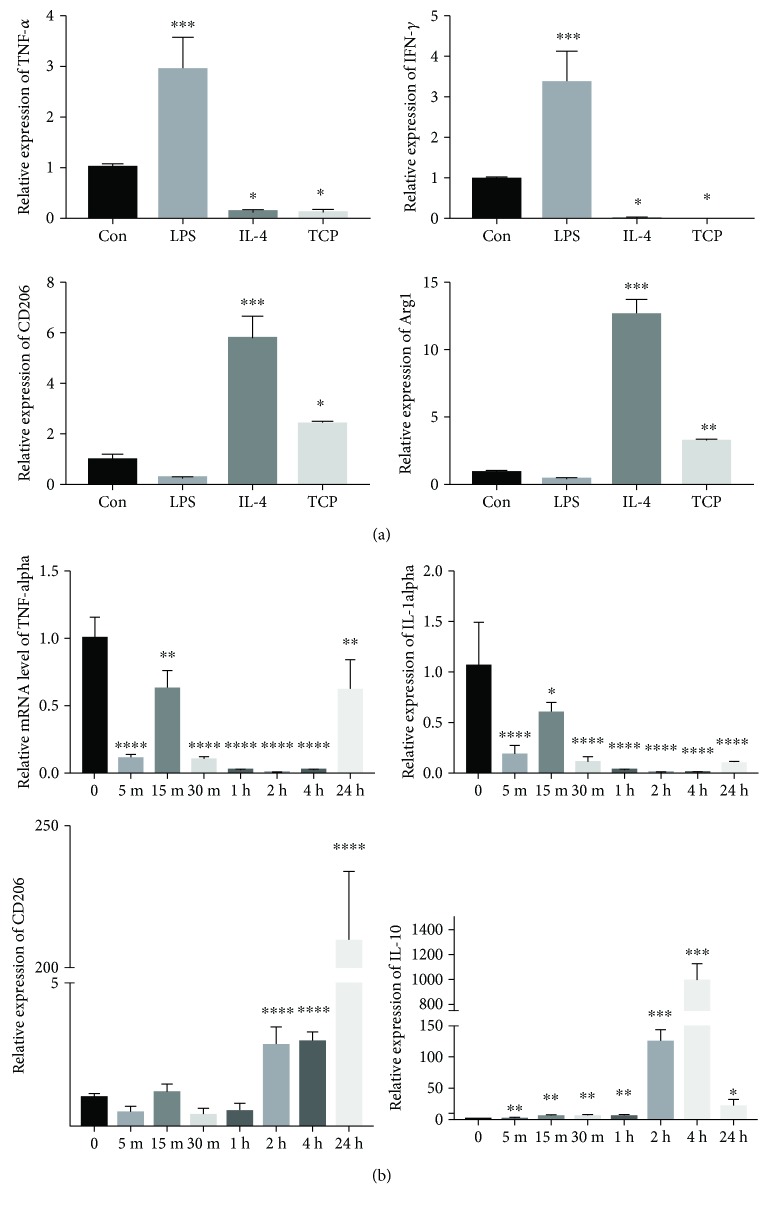
Previous studies have reported that TCP scaffolds can modify the immune microenvironment towards one that favours osteogenesis by switching the macrophage phenotype towards an M2 polarization [23]. Therefore, we decided to evaluate the anti-inflammatory ability of TCP through M1- (TNF-α, IFN-γ) and M2- (CD206, ARG1) associated markers and cytokines. Stimulation of Thp1 cells with TCP extract resulted in a shift towards the M2 phenotype with higher expression of M2 markers and lower expression of M1-associated cytokines (Figure 2(a)). The PCR results (Figure 2(b)) demonstrated that the most significant change in inflammatory gene expression occurred at 2 h, 4 h, and 24 h and therefore confirmed that changes occur rapidly when macrophages are in contact with TCP.
Figure 2.
TCP facilitates M2 macrophage polarization. (a) Relative mRNA expression of TNF-α, IFN-γ, CD206, and ARG1 of Thp1 stimulated by control medium, LPS, IL-4, and TCP extract. (b) Relative mRNA expression of TNF-α, IL-1α, CD206, and IL-10 of Thp1 stimulated by TCP extract at different time points. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
3.3. The Inhibition of EZH1 Caused by TCP Facilitates M2 Macrophage Polarization
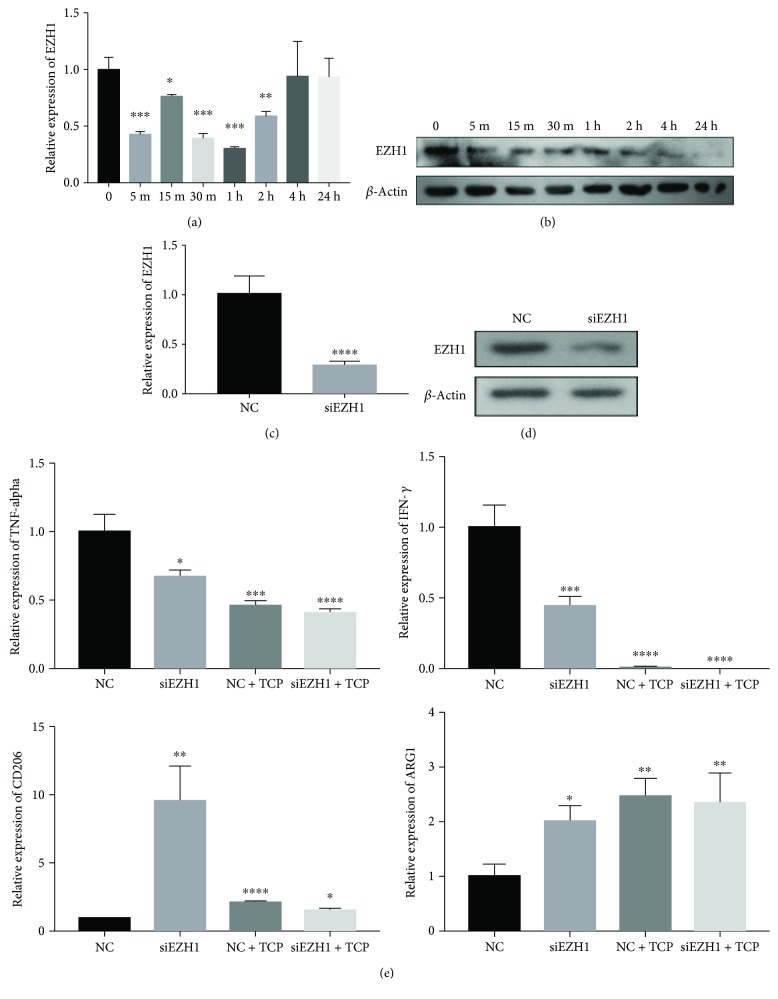
There have been a large volume of published studies describing the role of methylase activity during macrophage polarization [15–17]. EZH1, part of a noncanonical PRC2 complex, specifically catalyzes H3K27 to prevent the derepression of PRC2 target genes. Further study showed that stimulation with TCP extracts significantly inhibited the gene expression and protein production of EZH1 when compared to the control group (Figures 3(a) and 3(b)).
Figure 3.
Inhibition of EZH1 caused by TCP stimulation facilitates M2 macrophage polarization. (a) Relative mRNA expression of EZH1 of Thp1 stimulated by TCP extract at different time points. (b) Western blot of EZH1 expression of Thp1 stimulated by TCP extract at different time points. (c) Relative mRNA expression of EZH1 of Thp1 cells after EZH1 knockdown. (d) Western blot of EZH1 expression of Thp1 cells after EZH1 knockdown. (e) Relative mRNA expression of TNF-α, IFN-γ, CD206, and ARG1 of Thp1 cells after EZH1 knockdown and stimulation by TCP extract. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001.
In order to confirm the role of EZH1 during macrophage polarization, we silenced EZH1 in Thp1 cells with siRNA (Figures 3(c) and 3(d)). By analysing gene expression of macrophage polarization markers, it was found that siEZH1 significantly inhibited M2 macrophage polarization (Figure 3(e)).
3.4. The Inhibition of EZH1 Expression Is Associated with the Inhibition of the NF-κB Pathway
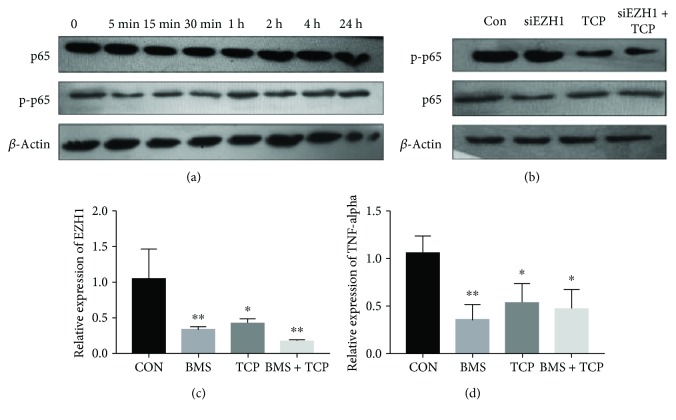
Data from several studies have also identified a role of the NF-κB pathway during macrophage polarization [1]. In the process of M1 polarization, the NF-κB pathway is activated, which activates the transcription of M1-associated factors such as TNF-α and IFN-γ. Since the stimulation of TCP affects macrophages in less than 1 h, significant inhibition of p-p65 (one of the markers of NF-κB pathway activation) was found (Figure 4(a)). However, after the silencing of EZH1, there was no significant difference between the siEZH1 group and the control group (Figure 4(b)). We therefore hypothesized that the NF-κB pathway may be responsible for the inhibition of EZH1.
Figure 4.
The inhibition of EZH1 expression is associated with the inhibition of the NF-κB pathway. (a) Western blot of the NF-κB signalling pathway of Thp1 stimulated by TCP extract at different time points. (b) Western blot of the NF-κB signalling pathway of Thp1 cells after EZH1 knockdown and stimulation by TCP extract. (c) Relative mRNA expression of EZH1 of Thp1 cells after the inhibition of the NF-κB pathway and stimulation by TCP extract. (d) Relative mRNA expression of TNF-α, IFN-γ, CD206, and ARG1 of Thp1 cells after the inhibition of the NF-κB pathway and stimulation by TCP extract. ∗p < 0.05 and ∗∗p < 0.01.
In order to determine the role of the NF-κB pathway during EZH1 inhibition, an inhibitor for the NF-κB pathway (BMS-345541) was utilized. It was found that it significantly affected the potency against IKK-1 and IKK-2 and subsequently inhibited the phosphorylation of p65. When treated with BMS-345541, the expression of EZH1 was significantly downregulated (Figure 4(c)), and since the NF-κB pathway was inhibited, M2 macrophage polarization was upregulated (Figure 4(d)).
4. Discussion
The function of biological materials is associated with their mechanical and physicochemical properties [24]; however, their osteogenic effect is mainly dependent on their histocompatibility once implanted into host tissues. When a biomaterial is implanted, the body's immune system responds quickly, causing a brief, acute inflammatory response that leads to adaptive immunity [25]. The traditional view of the body's immune response is seen as a role of negative regulation to tissue repair function of biomaterials [26]. Recently, however, more studies have found the other sides of the immune regulation to tissue repair.
Macrophages are a group of well-known antigen-presenting cells. During tissue repair, they participate in almost every biological process after biomaterial implantation including the initial immune process, angiogenesis, mesenchymal stem cell recruitment, and differentiation [27–29]. Many in vivo studies have now shown that M1 macrophages dominate in the early process of tissue repair, while the M2 phenotypes are produced later during the process. Macrophages are however able to specifically polarize under various stimuli to either M1 (proinflammatory) or M2 (anti-inflammatory) phenotypes [29].
When it comes to biomaterials as TCP, cells derived from the monocyte/macrophage lineage is well known to be one of the first cell types contacting with biomaterials implanted [3]. As shown in Figure 1(b), here, they are able to specifically polarize under various stimuli to either M1 or M2 phenotypes or differentiate into osteoclasts or mutinucleated osteoclasts around TCP during TCP resorption [30]. There have been various previous papers that characterized these osteoclasts as foreign body giant cells which are associated with biomaterial rejection. Therefore, more recently, phenotypes of these osteoclasts have been associated with tissue regeneration and wound healing by researches demonstrating that even mutinucleated osteoclasts may be characterized as a tissue repair phenotype by demonstrating release of M2-related cytokines and growth factors [31].
In the present study, a positive role of macrophages in regulating bone repair was observed in response to TCP both in vivo and in vitro. Our results showed that macrophages gathered around TCP particles early during the bone repair. Once adhered, they express many surface molecules and cytokines that influences the surrounding microenvironment. To better understand the influence of TCP on macrophage polarization, we stimulated Thp1 cells with extracts from TCP in vitro. The result demonstrated a decreased expression of M1 marker genes (IFN-γ, TNF-α) while an increased expression of M2 markers (TGF-beta, VEGF) was observed.
Histone methylation plays a crucial role in macrophage polarization [32], but the epigenetic mechanism in the process mediated by TCP remains unclear. Previously, our research group found that EZH1 could regulate NF-κB target genes through a nonclassical complex that was associated with SUZ12 and UTX. It was revealed that the polarization process of macrophages was mainly activated by the corresponding transcription factors through the NF-κB and JAK-STAT pathways [1]. After activation, many transcription factors bind to promoter regions of M1 marker genes and begin their transcription [33]. In our study, we also observed that NF-κB was repressed following stimulation with TCP extracts.
Classic NF-κB consists of P50 and RelA (P65); the binding site of RelA 5′-AGAAATTCC-3′ was found in the promoter region of EZH1 gene sequence, but to date, there is no proof regarding the regulation of NF-κB to EZH1 [34]. Therefore, we blocked NF-κB with BMS-345541, an inhibitor of the NF-κB pathway, and found that the expression of EZH1 decreased significantly with/without TCP stimulation. EZH1 belongs to the polycomb family, which can compensate the function of EZH1 in the PRC2 complex to catalyze H3K27me3 through the SET domain, leading to gene transcription inhibition. To investigate the role of EZH1 during macrophage polarization, we silenced EZH1 with siRNA. The expression of M1 markers including TNF-α and IFN-γ was both significantly decreased with or without TCP stimulation, which implied a positive role for EZH1 during the process of macrophages polarizing towards the M1 phenotype. We assume that there may be two potential mechanisms regarding the function of EZH1 as follows: (1) according to our previous study, EZH1 may interact with NF-κB on the promoter regions of NF-κB target genes, which is associated with M1 macrophage polarization and (2) as a marker for gene inhibition, EZH1 may target proximal sites on the promoter region of Tollip, a transcription factor that suppresses the NF-κB pathway [35], which results in an enhancement of the NF-κB pathway.
5. Conclusions
In the present study, it was found that TCP suppressed the activation of the NF-kappa B pathway and caused a decrease in EZH1 expression. The reduction of EZH1 impacted the downregulation of M1 marker genes, which resulted in a lower expression of M1 cytokines. These lower proinflammatory factors promoted a local microenvironment more favourable for bone regeneration. EZH1 seems to play an important role during the process of macrophage polarization and therefore might be an ideal target gene for future therapies aimed at modifying macrophage polarization following biomaterial implantation into host tissues.
Acknowledgments
This work was supported by the funds of the National Natural Science Foundation of China (81771050, 81271108, and 81600906), the Technical Innovation of Hubei Province (2017CFA025 and 2017AHB046), and Fundamental Research Funds for the Central Universities (2042017kf0207).
Data Availability
The data used to support the findings of this study are included within the article.
Disclosure
The authors hereby confirmed that the present work entitled “EZH1 Is Associated with TCP-Induced Bone Regeneration through Macrophages Polarization” has been submitted solely to Journal of Stem Cells International and that it is not concurrently under consideration for publication in another journal. They have been involved in the work leading to the publication of the paper and have read the paper before it is submitted for publication. The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this article. This original research is free of conflict of interest.
Authors' Contributions
Study design was performed by Yufeng Zhang. Study conduct was performed by Xiaoshi Jia and Hudi Xu. Data collection was performed by Xiaoshi Jia, Chengcheng Yin, and Richard J. Miron. Data analysis was performed by Xiaoshi Jia, Hudi Xu, and Richard J. Miron. Data interpretation was performed by Xiaoshi Jia, Hudi Xu, Xiaoxin Zhang, Yufeng Zhang, and Richard J. Miron. Drafting of the manuscript was performed by Xiaoshi Jia, Hudi Xu, and Richard J. Miron. Revision of the manuscript content was performed by all authors. Approval of the final version of the manuscript was made by all authors. Xiaoshi Jia and Hudi Xu take responsibility for the integrity of the data analysis.
References
- 1.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. Journal of Clinical Investigation. 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vi L., Baht G. S., Whetstone H., et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. Journal of Bone and Mineral Research. 2015;30(6):1090–1102. doi: 10.1002/jbmr.2422. [DOI] [PubMed] [Google Scholar]
- 3.Miron R. J., Bosshardt D. D. OsteoMacs: key players around bone biomaterials. Biomaterials. 2016;82:1–19. doi: 10.1016/j.biomaterials.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Spiller K. L., Nassiri S., Witherel C. E., et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207. doi: 10.1016/j.biomaterials.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence T., Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature Reviews Immunology. 2011;11(11):750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 6.Chappard D., Guillaume B., Mallet R., Pascaretti-Grizon F., Baslé M. F., Libouban H. Sinus lift augmentation and β-TCP: A microCT and histologic analysis on human bone biopsies. Micron. 2010;41(4):321–326. doi: 10.1016/j.micron.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Gaasbeek R. D. A., Toonen H. G., van Heerwaarden R. J., Buma P. Mechanism of bone incorporation of beta-TCP bone substitute in open wedge tibial osteotomy in patients. Biomaterials. 2005;26(33):6713–6719. doi: 10.1016/j.biomaterials.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 8.Nicholas R. W., Lange T. A. Granular tricalcium phosphate grafting of cavitary lesions in human bone. Clinical Orthopaedics and Related Research. 1994;306:197–203. [PubMed] [Google Scholar]
- 9.Zerbo I. R., Bronckers A. L. J. J., de Lange G. L., Burger E. H., van Beek G. J. Histology of human alveolar bone regeneration with a porous tricalcium phosphate - a report of two cases. Clinical Oral Implants Research. 2001;12(4):379–384. doi: 10.1034/j.1600-0501.2001.012004379.x. [DOI] [PubMed] [Google Scholar]
- 10.Suba Z., Takács D., Gyulai-Gaál S., Kovács K. Facilitation of β-tricalcium phosphate-induced alveolar bone regeneration by platelet-rich plasma in beagle dogs: a histologic and histomorphometric study. International Journal of Oral & Maxillofacial Implants. 2004;19(6):832–838. [PubMed] [Google Scholar]
- 11.Chen Z., Yuen J., Crawford R., Chang J., Wu C., Xiao Y. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated β-tricalcium phosphate. Biomaterials. 2015;61:126–138. doi: 10.1016/j.biomaterials.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Tai S., Cheng J. Y., Ishii H., et al. Effects of beta-tricalcium phosphate particles on primary cultured murine dendritic cells and macrophages. International Immunopharmacology. 2016;40:419–427. doi: 10.1016/j.intimp.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z., Wu C., Gu W., Klein T., Crawford R., Xiao Y. Osteogenic differentiation of bone marrow MSCs by β-tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials. 2014;35(5):1507–1518. doi: 10.1016/j.biomaterials.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q., Zhang L. L., Yang Y., Lin Y. Z., Miron R. J., Zhang Y. F. Improvement of implant placement after bone augmentation of severely resorbed maxillary sinuses with ‘Tent-Pole’ grafting technique in combination with rhBMP-2. The Chinese Journal of Dental Research. 2017;20(1):9–17. doi: 10.3290/j.cjdr.a37737. [DOI] [PubMed] [Google Scholar]
- 15.Ishii M., Wen H., Corsa C. A. S., et al. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood. 2009;114(15):3244–3254. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao Y., Kang K., Giannopoulou E., Fang C., Ivashkiv L. B. IFN-γ induces histone 3 lysine 27 trimethylation in a small subset of promoters to stably silence gene expression in human macrophages. Cell Reports. 2016;16(12):3121–3129. doi: 10.1016/j.celrep.2016.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu G., Liu G., Xiong S., Liu H., Chen X., Zheng B. The histone methyltransferase Smyd2 is a negative regulator of macrophage activation by suppressing interleukin 6 (IL-6) and tumor necrosis factor α (TNF-α) production. Journal of Biological Chemistry. 2015;290(9):5414–5423. doi: 10.1074/jbc.M114.610345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lui J. C., Garrison P., Nguyen Q., et al. EZH1 and EZH2 promote skeletal growth by repressing inhibitors of chondrocyte proliferation and hypertrophy. Nature Communications. 2016;7 doi: 10.1038/ncomms13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J., Liu D., Guo B., et al. Role of biphasic calcium phosphate ceramic-mediated secretion of signaling molecules by macrophages in migration and osteoblastic differentiation of MSCs. Acta Biomaterialia. 2017;51:447–460. doi: 10.1016/j.actbio.2017.01.059. [DOI] [PubMed] [Google Scholar]
- 20.Krausgruber T., Blazek K., Smallie T., et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nature Immunology. 2011;12(3):231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 21.Liao L., Yang S., Miron R. J., Wei J., Zhang Y., Zhang M. Osteogenic properties of PBLG-g-HA/PLLA nanocomposites. PLoS One. 2014;9(9, article e105876) doi: 10.1371/journal.pone.0105876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao L. QSIM: quantitative structured illumination microscopy image processing in ImageJ. Biomedical Engineering Online. 2015;14(1):p. 4. doi: 10.1186/1475-925X-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z., Mao X., Tan L., et al. Osteoimmunomodulatory properties of magnesium scaffolds coated with β-tricalcium phosphate. Biomaterials. 2014;35(30):8553–8565. doi: 10.1016/j.biomaterials.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 24.Stevens M. M. Exploring and engineering the cell-surface interface. Biophysical Journal. 2011;100(3):p. 189a. doi: 10.1016/j.bpj.2010.12.1248. [DOI] [Google Scholar]
- 25.Arnold L., Henry A., Poron F., et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. Journal of Experimental Medicine. 2007;204(5):1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trindade R., Albrektsson T., Tengvall P., Wennerberg A. Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clinical Implant Dentistry and Related Research. 2016;18(1):192–203. doi: 10.1111/cid.12274. [DOI] [PubMed] [Google Scholar]
- 27.Brown B. N., Valentin J. E., Stewart-Akers A. M., McCabe G. P., Badylak S. F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30(8):1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown B. N., Londono R., Tottey S., et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials [Acta Biomaterialia 8 (2012) 978–987] Acta Biomaterialia. 2012;8(7):2871–2871. doi: 10.1016/j.actbio.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez F. O., Sica A., Mantovani A., Locati M. Macrophage activation and polarization. Frontiers in Bioscience. 2008;13(13):p. 453. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 30.Miron R. J., Zohdi H., Fujioka-Kobayashi M., Bosshardt D. D. Giant cells around bone biomaterials: osteoclasts or multi-nucleated giant cells? Acta Biomaterialia. 2016;46:15–28. doi: 10.1016/j.actbio.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Vasconcelos D. P., Costa M., Amaral I. F., Barbosa M. A., Águas A. P., Barbosa J. N. Modulation of the inflammatory response to chitosan through M2 macrophage polarization using pro-resolution mediators. Biomaterials. 2015;37:116–123. doi: 10.1016/j.biomaterials.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 32.Yang X., Wang X., Liu D., Yu L., Xue B., Shi H. Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Molecular Endocrinology. 2014;28(4):565–574. doi: 10.1210/me.2013-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satoh T., Takeuchi O., Vandenbon A., et al. The Jmjd 3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nature Immunology. 2010;11(10):936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]
- 34.Su S. K., Li C. Y., Lei P. J., et al. The EZH1–SUZ12 complex positively regulates the transcription of NF-κB target genes through interaction with UXT. Journal of Cell Science. 2016;129(12):2343–2353. doi: 10.1242/jcs.185546. [DOI] [PubMed] [Google Scholar]
- 35.Humbert-Claude M., Duc D., Dwir D., et al. Tollip, an early regulator of the acute inflammatory response in the substantia nigra. Journal of Neuroinflammation. 2016;13(1):p. 303. doi: 10.1186/s12974-016-0766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.