Abstract
Older age, obesity, hypertension, snoring, and excessive daytime sleepiness have been associated with sleep apnea. The objective of this study was to determine the prevalence (crude and adjusted), as well as the risk factors, of sleep apnea in the adult Canadian population. Data from the 2009 Sleep Apnea Rapid Response (SARR) questionnaire were used to identify the risk factors, and all sleep-related questions in the SARR questionnaire were used. The outcome variable of interest was health professional-diagnosed sleep apnea. Covariates of interest were demographic variables, population characteristics, respiratory and cardiovascular diseases, and enabling resources. The multiple logistic regression model adjusted for the clustering effect was used to analyze the data. Sleep apnea was diagnosed in 858,913 adults (3.4% of the population), and more men (65.4%) than women (34.6%) were diagnosed with sleep apnea. Multivariable logistic regression analysis indicated that age (45 and older), loud snoring, sudden awakening with gasping/choking (rare/sometimes and once or more a week), and nodding off/falling asleep in driving in the past 12 months were significantly associated with diagnosed sleep apnea. Predictive probability demonstrated that in overweight and obese persons, ≥15 minutes of daily exercise significantly decreased the risk of diagnosed sleep apnea. The conclusion of this study is that in the Canadian population, sleep apnea is associated with older age, loud snoring, and sleeping problems. The protective effect of exercise warrants further investigation.
1. Introduction
Sleep apnea is defined as the complete cessation of airflow in the nose or mouth for 10 or more seconds during sleep [1]. Three types of sleep apnea, namely central [2], obstructive [3, 4], and mixed [5, 6] have been identified. Central sleep apnea occurs when the brain temporarily fails to send a signal to the muscles responsible for breathing control [2], and mixed sleep apnea occurs when both central and obstructive sleep apnea are present [5, 6]. Obstructive sleep apnea (OSA) is the most common form of sleep apnea and is defined as a disorder in which a person frequently stops breathing during sleeping because of an obstruction of the upper airway due to poor motor tone of the tongue and/or airway dilator muscles [3, 4].
Sleep apnea is a worldwide phenomenon [7–15]. Risk factors for sleep apnea include older age [4, 7, 16–20]; cardiovascular risk factors, namely, obesity [4, 7, 9, 10, 16, 17, 20–26], sedentary lifestyle [16, 24], hypertension [9, 15, 16, 18, 25, 27–29], and diabetes [9, 16–18, 25, 30–32]; alcohol use [9, 10, 18, 24, 30]; smoking [4, 10, 33, 34]; chronic pulmonary disease [35–38]; snoring [39–43]; excessive daytime sleepiness (EDS) [3, 23, 44]; anxiety [45–48]; depression [45, 48]; and low socioeconomic status [18, 25, 49]. Sleep apnea has been associated with cardiovascular diseases, namely, congestive heart failure [7, 9, 50, 51] and myocardial infarction [7, 52], sex (being male) [4, 9, 16, 18, 19, 23, 24], inferior driving and motor vehicle accidents [10, 53, 54], lower work efficiency [54–56], and high mortality [10, 26, 57].
Demographic [4, 7, 9, 16–20, 23, 24], environmental, and population characteristics [18, 25, 49, 54–56], predisposing as well as enabling resources, are associated with sleep apnea. The objective of this study was to determine the prevalence (crude and adjusted), as well as the risk factors, of sleep apnea in the adult Canadian population.
2. Materials and Methods
2.1. Design
The data of this study were from the 2009 Sleep Apnea Rapid Response (SARR) questionnaire [58], a component of the 2009 Canadian Community Health Survey (CCHS) [59]. The SARR was the first cross-sectional survey to estimate sleep breathing disorder among Canadians [8, 60]. Based on the sampling frame of the SARR module, 9523 Canadians of age 12 years and older were interviewed over two months (January and February 2009) for this survey. Residents of Indian Reserve, Crown lands, and the Territories, as well as full-time members of the Canadian Forces were excluded. The weighted sample amounted to about 98% of the Canadian population. This study concentrated on adult participants who were 18 years and older.
2.2. Statistical Analysis
From the literature review, we identified important variables that might be associated with the outcome prior to the analyses of the data. Sampling weights, which referred to the unequal probability of being selected in the survey, were applied in all estimates. The bootstrap method with 500 replications was used to compute the standard errors of regression coefficients in order to account for clustering inherited in the study design of the survey. Logistic regression was used to predict the univariate association of prevalence of sleep apnea and relevant variables. Following that, we identified the covariates that show an association or borderline association with the dependent variable and then performed multivariate analyses. According to the bivariable analysis results, variables with p < 0.20 were reserved in a multivariable model. Multivariable analysis was completed by utilizing logistic regression models, based on a maximum likelihood approach, to analyze the data. All significant independent variables (p < 0.05) and critical variables were retained in the final multivariable model. Predictive probability was used to draw the interaction graphs.
The outcome variable of interest was health professional-diagnosed sleep apnea. All sleep-related questions in SARR questionnaire were used. The covariates of interest were demographic variables (age, sex, ethnicity, birthplace, residence within Canada, and home ownership), socioeconomic characteristics (education, employment, and household income etc.), and health status (chronic bronchitis, chronic obstructive pulmonary disease, cardiovascular diseases, and other diseases). Interactions were examined using predictive probability graphs. The interactions between factor variables were measured by margins effects in Stata [61].
3. Results
More than 25 million (25,378,352) Canadians, 51.5% female and 48.5% male, completed the 2009 CCHS, including the SARR. Eighty-one percent of them were “white,” and 19% were grouped as “others”. The following results were calculated on the participants that completed the different sections: (1) education—almost sixty-nine percent (68.6%) had postsecondary or higher, 15.3% secondary, and 16.1% less than secondary education, (2) employment—55.8% was employed full-time, 10.4% part-time, and 33.8% was unemployed, (3) body mass index (BMI, kg/m2)—33.7% was overweight (BMI = 25–30), and 16.8% was obese (BMI > 30), (4) smoking—current smoking was present in 21.2%, ex-smoking in 41.4%, and 37.4% never smoked, (5) physical activity—only 31.7% did more than 15 minutes of physical activity daily, (6) household income—14.7% earned less than $30,000, 49.5% between $30,000 and $99,999, and 25.5% more than $99,999, and (7) geographic location—Atlantic Canada 7.0%, Quebec 24.1%, Ontario 38.5%, Prairies 16.8%, and British Columbia (BC) 13.6%. 3.4% of the adult population was diagnosed with sleep apnea, and more men (65.4%) than women (34.6%) were diagnosed with sleep apnea.
In Table 1, ethnicity is recoded into two groups—white and others (Aboriginals, South Asian, Southeast Asian, Black, and others). Unadjusted univariate analysis (Table 1) showed that sex (male (p < 0.001)), age (45–64 (p < 0.001), ≥65 (p < 0.001)), ethnicity (others (0.018)), marital status (married/common-in-law (p=0.030)), BMI (overweight (p < 0.001), obese (p < 0.001)), ex-smoker (p=0.008), hypertension (p < 0.001), diabetes (p < 0.001), heart disease (p < 0.001), anxiety (p=0.006), pain and discomfort (p < 0.001), loud snoring (p < 0.001), “trouble going to/staying asleep most of the time” (p=0.020), “how often awakened suddenly with gasping or choking” (“rarely or sometimes” (p < 0.001), “once a week or more” (p < 0.001)), and “feeling tired or sleepy during daytime” (p=0.008) were significantly associated with diagnosed sleep apnea.
Table 1.
Univariate associations between sleep apnea and independent variables of interest.
| Predictors | Diagnosed sleep apnea | p value | |
|---|---|---|---|
| No (%) | Yes (%) | ||
| 96.6 | 3.4% | ||
| Sex | |||
| Female | 51.5 | 34.6 | — |
| Male | 48.5 | 65.4 | <0.001 |
| Age | |||
| 18–44 | 48.9 | 24.3 | — |
| 45–64 | 35.2 | 55.1 | <0.001 |
| ≥65 | 15.4 | 20.6 | <0.001 |
| Ethnicity | |||
| White | 81.0 | 89.3 | — |
| Others | 19.0 | 10.7 | 0.018 |
| Born in Canada | |||
| In Canada | 74.7 | 78.3 | — |
| Outside Canada | 25.3 | 21.7 | 0.404 |
| Marital status | |||
| Single/separate/widow/divorced | 36.1 | 26.4 | — |
| Married/common-in-law | 63.9 | 73.6 | 0.030 |
| Education | |||
| Less than secondary | 16.1 | 17.4 | — |
| Secondary graduation | 15.3 | 13.9 | 0.658 |
| Postsecondary and above | 68.6 | 68.7 | 0.825 |
| Employment | |||
| Unemployed | 33.8 | 38.0 | — |
| Full-time employed | 55.8 | 52.2 | 0.330 |
| Part-time employed | 10.4 | 9.8 | 0.573 |
| BMI | |||
| Normal (<25) | 49.6 | 10.6 | — |
| Overweight (25–30) | 33.7 | 48.4 | <0.001 |
| Obesity (≥30) | 16.8 | 41.0 | <0.001 |
| Smoking status | |||
| Nonsmoker | 37.4 | 25.9 | — |
| Ex-smoker | 41.4 | 51.4 | 0.008 |
| Current smoker | 21.2 | 22.6 | 0.080 |
| Five or more alcohol drinks in past 12 months | |||
| None | 59.6 | 62.9 | — |
| Once or less in a month | 28.8 | 29.3 | 0.859 |
| Twice or more in a month | 11.6 | 7.7 | 0.131 |
| Daily 15 min physical activity | |||
| No | 68.3 | 73.8 | — |
| Yes | 31.7 | 26.2 | 0.186 |
| Own the dwelling place | |||
| No | 25.5 | 26.3 | — |
| Yes | 74.5 | 73.7 | 0.849 |
| Household income | |||
| <$30,000 | 14.7 | 14.2 | — |
| $30,000 to 99,999 | 49.5 | 46.4 | 0.902 |
| ≥100,000 | 25.5 | 28.1 | 0.608 |
| Not stated | 10.3 | 11.3 | 0.806 |
| Geographic location | |||
| Atlantic area | 7.0 | 6.8 | — |
| Quebec | 24.1 | 12.9 | 0.087 |
| Ontario | 38.5 | 52.8 | 0.259 |
| Prairie area | 16.8 | 12.3 | 0.424 |
| BC | 13.6 | 15.1 | 0.710 |
| Hypertension | |||
| No | 81.4 | 66.0 | — |
| Yes | 18.6 | 34.0 | <0.001 |
| Migraine headache | |||
| No | 89.6 | 88.8 | — |
| Yes | 10.4 | 11.2 | 0.738 |
| COPD ∗ | |||
| No | 96.3 | 90.5 | — |
| Yes | 3.7 | 9.5 | 0.001 |
| Diabetes | |||
| No | 94.1 | 84.0 | — |
| Yes | 5.9 | 16.0 | <0.001 |
| Heart disease | |||
| No | 95.3 | 88.6 | — |
| Yes | 4.7 | 11.4 | <0.001 |
| Anxiety disorder | |||
| No | 94.8 | 88.6 | — |
| Yes | 5.2 | 11.4 | 0.006 |
| Pain or discomfort | |||
| No | 82.9 | 69.0 | — |
| Yes | 17.1 | 31.0 | <0.001 |
| Loud snoring | |||
| Snoring not louder than talking | 85.0 | 51.0 | — |
| Snoring louder than talk | 15.0 | 49.0 | <0.001 |
| Trouble of going/staying asleep | |||
| None of the time | 34.1 | 35.2 | — |
| Some of the time | 50.1 | 35.4 | 0.090 |
| Most of the time | 15.8 | 29.4 | 0.020 |
| How often awakened suddenly with gasping or choking | |||
| Never | 94.3 | 74.4 | — |
| Rarely or sometimes | 4.3 | 14.4 | <0.001 |
| Once a week or more | 1.5 | 11.2 | <0.001 |
| Feeling tired or sleepy during daytime | |||
| No | 54.7 | 40.4 | — |
| Yes | 45.3 | 59.6 | 0.006 |
| Nodded or fallen asleep in driving in the past 12 months | |||
| No | 86.6 | 87.4 | — |
| Yes | 4.4 | 8.0 | 0.084 |
| Does not drive | 9.1 | 4.6 | 0.055 |
∗COPD only diagnosed in persons who were 35 years and older.
One of the prerequisites for the diagnosis of chronic obstructive pulmonary disease (COPD) in the SARR was an age of 35 and above, and therefore, COPD was excluded from the final model even though it was associated with a high risk of diagnosed sleep apnea in the univariate analysis. The multivariable logistic regression analysis (Table 2) indicated that age (45–64 (p < 0.013), ≥65 (p < 0.027)), loud snoring (p < 0.001), sudden awakening with gasping/choking (rare or sometimes (p < 0.001), once a week or more (p < 0.001)), and nodding off/falling asleep in driving in the past 12 months (p=0.034) were significantly associated with diagnosed sleep apnea.
Table 2.
Multivariate logistic regression of the association between sleep apnea and independent variables of interest.
| Predictors | Diagnosed sleep apnea OR (95% conf. interval) | p value |
|---|---|---|
| Age | ||
| 18–44 | 1.00 | — |
| 45–64 | 1.94 (1.15–3.26) | 0.013 |
| ≥65 | 1.94 (1.08–3.51) | 0.027 |
| Ethnicity | ||
| White | 1.00 | — |
| Others (includes Aboriginals) | 0.76 (0.37–1.55) | 0.453 |
| Smoking status | ||
| Nonsmoker | 1.00 | — |
| Ex-smoker | 1.10 (0.67–1.79) | 0.713 |
| Current smoker | 1.22 (0.69–2.15) | 0.497 |
| Hypertension | ||
| No | 1.00 | — |
| Yes | 1.08 (0.68–1.71) | 0.734 |
| Loud snoring | ||
| Snoring not louder than talking | 1.00 | — |
| Snoring louder than talk | 3.11 (1.95–4.96) | <0.001 |
| How often awakened suddenly with gasping or choking | ||
| Never | 1.00 | — |
| Rarely or sometimes | 3.52 (1.92–6.46) | <0.001 |
| Once a week or more | 7.92 (3.74–16.74) | <0.001 |
| Nodded or fallen asleep in driving in the past 12 months | ||
| No | 1.00 | — |
| Yes | 2.41 (1.07–5.43) | 0.034 |
| Does not drive | 0.53 (0.18–1.53) | 0.238 |
| Interaction (Geographic location ∗ Sex) | ||
| Quebec | ||
| Female | 1.00 | — |
| Male | 0.83 (0.19–3.56) | 0.803 |
| Ontario | ||
| Female | 1.00 | — |
| Male | 1.12 (0.34–3.66) | 0.856 |
| Prairie area | ||
| Female | 1.00 | — |
| Male | 0.80 (0.16–4.01) | 0.791 |
| BC | ||
| Female | 1.00 | — |
| Male | 4.83 (1.20–19.43) | 0.03 |
| Interaction (BMI ∗ daily 15 min physical activity) | ||
| Overweight (25–30) | ||
| No | 1.00 | — |
| Yes | 0.17 (0.05–0.60) | 0.006 |
| Obesity (≥30) | ||
| No | 1.00 | — |
| Yes | 0.14 (0.04–0.49) | 0.002 |
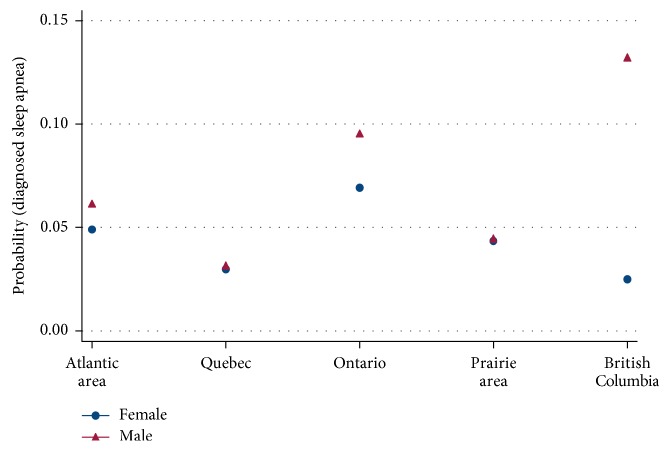
The predictive margins of geographic locations and sex are displayed in Figure 1 with the probability of diagnosed sleep apnea on the Y-axis and the geographic locations (from east to west Canada) on the X-axis. The Atlantic area includes Nova Scotia, Prince Edward Island, New Brunswick, and Newfoundland and Labrador, and the Prairie area includes Manitoba, Saskatchewan, and Alberta. In this figure, it can be seen that there were no statistical significant differences between diagnosed sleep apnea and male and female sex in all the provinces except for BC. Men in BC had a significant (p=0.03) higher risk of being diagnosed with sleep apnea.
Figure 1.
The predictive margins of geographic locations and sex. Y-axis—probability of diagnosed sleep apnea. X-axis—geographic locations (from east to west Canada). Atlantic area includes Nova Scotia, Prince Edward Island, New Brunswick, and Newfoundland and Labrador, and Prairie area includes Manitoba, Saskatchewan, and Alberta.
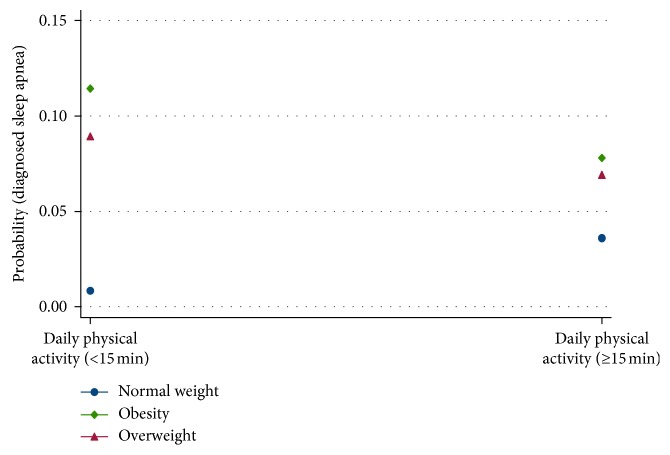
Predictive margins of BMI and physical activity are displayed in Figure 2 with the probability of diagnosed sleep apnea on the Y-axis and the daily physical activity on the X-axis. The survey data demonstrated that for the candidates who were overweight, ≥15 minutes of daily exercise significantly (p=0.006) decreased the risk of diagnosed sleep apnea. In the obese class, the significance was even higher (p=0.002). Among persons of normal weight, the time spent in physical activity did not significantly impact the diagnosis of sleep apnea.
Figure 2.
Predictive margins of BMI and physical activity. Y-axis—probability of diagnosed sleep apnea. X-axis—daily physical activity.
4. Discussion
Strong associations between older age, loud snoring, sudden awakenings due to gasping or choking, and nodding off or falling asleep while driving and diagnosed sleep apnea in the Canadian population were established in this study. The strong interactions between location and sleep apnea in men were demonstrated in the higher incidence of sleep apnea in men in BC. Strong interactions were also shown between BMI, physical activity, and sleep apnea in overweight and obese persons, ≥15 minutes of daily exercise significantly decreased the risk of diagnosed sleep apnea.
Increasing age is associated with an increased risk for sleep apnea [4, 7, 16, 62]. In two American studies, Pan et al. [18] found that the prevalence of sleep apnea in men and women increased with age, 0.86% in the 18–25 age group, 3.5% in the 26–64 age group, and 4.47% in the ≥65 age group, and Bixler et al. [14] in their study of sleep-disordered breathing in women found a higher prevalence of sleep apnea in those aged ≥65 compared with those aged 45 to 64. Interestingly, in this study, although the prevalence was significantly higher in both the 45–64-year-old group and the >65-year-old group, it was the highest in the younger of these two groups. This is similar to the findings of Bixler et al. [13] in their study of men with a higher prevalence of sleep apnea in the 45–64-year-olds as compared to the 65–100-year-olds.
Snoring is caused by turbulent airflow through a narrowed airway [63]. There is a definitive change in airflow during the hypopnea episode, and this affects the characteristics of snoring sounds. The resumption of breathing after apnea is usually accompanied by a sudden change in airflow [64]. Snoring has been recognized as a key indicator of OSA [12]. Loud snoring was identified in this study as significantly associated with sleep apnea. The association of the intensity of snoring and the severity of OSA has been recognized in several studies [40–43]. Acar et al. [42] identified a significantly higher snoring intensity in persons with severe OSA (apnea-hypopnea index (AHI) ≥ 30) as compared to persons with mild to moderate OSA (<30 AHI ≥ 5). Specifically how the severity of OSA causes an increase in the intensity of snoring is still unknown. Kim et al. [43] suggested that as the severity of OSA increases, the pressure generated in the airway during apnea might be higher and might cause higher snoring intensity.
The significant association of “sudden awakenings due to gasping or choking” and sleep apnea in this study agrees with the finding of Zhang et al [65]. Awakenings due to gasping or choking are common in OSA [66], and are a reliable indicator of OSA [67]. Gasping/choking causes poor quality and/or quantity of sleep, which often results in EDS [66]. Although not as strong as “loud snoring” and “sudden awakenings due to gasping or choking,” “nodding off or falling asleep while driving” was also strongly associated with sleep apnea. The group with severe OSA in Arita et al. [68] study reported being involved in accidents due to falling asleep. In the European study of Goncalves et al. [69], falling asleep at the wheel was contributed to poor sleep the previous night and general poor sleeping habits.
The interaction between location and sex indicated a significantly higher (p=0.03) association of men and diagnosed sleep apnea in BC. We hypothesized that there might be an association between sleep apnea and altitude. Almost all of the cities with the largest populations in BC (where most probably most of the participants in the survey lived) are in the Lower Mainland, with just Kelowna in the interior. The altitude in the Lower Mainland cities ranges from 12 meters (Richmond) to 150 meters (Burnaby). In a cross-sectional population study conducted by Ruiz et al. [70] on the prevalence of sleep complaints in three Colombian cities, Santa Marta, Bucaramanga, and Bogota, they found that the risk for severe sleep apnea, OSA, and EDS was the highest in Santa Maria at 15 meters above sea level. To investigate the effect of exposure to moderate altitude on nocturnal hypoxemia, sleep and breathing disorders, and daytime functioning, Nussbaumer-Ochsner et al. conducted an RCT on patients with OSA (32 men and 2 women) living at low altitudes and discontinuing their CPAP treatment for a few days at high altitudes. They found that the exposure to altitude exacerbated the hypoxemias and led to more sleep-related breathing disorders because of numerous central apnea/hypopnea episodes [71]. As the two referenced studies showed contrasting results, further investigation is needed. The fact that this association was only identified in men required investigation. According to Bloch et al., the apneas/hypopneas linked with sporadic episodes of hypoxemia in OSA patients sleeping near sea level are mainly due to upper airway collapse [72]. Male sex [4, 16, 19, 23] and obesity [4, 7, 10, 17, 23] have been identified as major risk factors for OSA. Men usually gain weight more centrally than women, and this results in more fat stored in the upper airway structures in men [73]. The likelihood of airway collapse is affected by fat deposited to the anterior neck and submandibular areas [74]. The longer airway in men (independent of body weight) provides another explanation for the increased tendency for airway collapse [75]. The critical closure pressure, the pressure at which the upper airway collapses, is higher in men than in women for any given body mass index [76], and therefore, it is reasonable to assume that anatomical factors predispose men to pharyngeal collapse.
An interaction between BMI, the time spent doing physical activity, and sleep apnea was also identified. In the overweight class, 15 minutes of physical activity every day led to significantly (p=0.006) less diagnosed sleep apnea. In the obese class, the significance was even higher (p=0.002). The systematic review and meta-analysis of six studies by Iftikhar et al. [77] demonstrated a statistically significant effect (pooled estimate of mean pre- to postexercise reduction in AHI = −6.27 events/h, p < 0.001) of exercise in reducing the severity of sleep apnea in patients with OSA with minimal changes in body weight. Exercise also had significant effects on cardiorespiratory fitness, daytime sleepiness, sleep efficiency, and the management of OSA. In a review by De Andrade et al. [78] on the effects of exercise in OSA patients, they found that the physiological adjustments caused by physical exercise led to increased upper airway dilator muscle tone and deep sleep time; and decreased build-up of fluid in the neck, systemic inflammatory response, and body weight. The exercise programs included in this review contained primarily aerobic exercises for durations of 30–45 minutes to 60–90 minutes for three to five days a week [79–84]. Barnes et al. [85] used resistance exercises, and in certain programs, resistance exercises were added to the aerobic exercises [80, 82, 84]. The major benefits of exercise programs for persons with OSA were a decrease in the severity of the condition and daytime sleepiness and increased sleep efficiency and oxygen consumption, regardless of weight loss. Dobrosielski et al. [86] invited persons older (>60 years), overweight, with untreated OSA, and not in a training program, to participate in a 12-week training program. At the end of the program, they found decreases in body weight and percentage of total body and trunk fat, as well as significant improvements in aerobic capacity nocturnal SaO2 and AHI (decreased by 10 events per hour). In a case-control study of over 2,000 persons, Simpson et al. [24] investigated the effect of low levels of physical activity on the prevalence of OSA, OSA-related symptoms, and cardiometabolic risk. When compared to the moderate-exercise group, the odds ratio for moderate-severe OSA was 0.6 in the high-exercise group, 1.6 in the low-exercise group, and 2.7 in the no-exercise group. They also found that persons with OSA that exercise had significantly lower levels of doctor-diagnosed depression, symptoms of fatigue, systolic and diastolic blood pressure, and C-reactive protein.
The protective effect of exercise is potentially a modifiable risk factor, and instead of implementing single interventions in isolation, which if often ineffective, the development of public policy is important. The impact of public policies on epidemics such as overweight (especially in children) has had qualified success depending on the specific interventions [87, 88].
A few more factors were identified in the univariate analysis to be significantly associated with sleep apnea, namely, marital status (married/common-law), ethnicity (others), smoking status (ex-smoker), hypertension, COPD, diabetes, heart disease, anxiety disorder, pain and discomfort, “trouble falling asleep most of the time,” and “feeling tired or sleepy during the daytime.” Further discussion will be limited to the factors that were highly (≤0.001) associated with sleep apnea.
Hypertension has been identified as a risk factor for sleep apnea in numerous studies [16–18, 20, 21]. Luyster et al. identified a significantly higher cardiovascular risk (BMI ≥ 30 kg/m2, sedentary lifestyle, hypertension, and diabetes) in the group with sleep apnea alone [16]. Both Pan et al. [18], in their study on alcohol consumption, chronic diseases and sleep apnea, and hypertension, and Wang et al. [20], in their study on the prevalence of hypertension and circadian blood pressure variations in Chinese patients, found hypertension to be significantly associated with sleep apnea. Asha'ari et al. also identified this significant association in a young (mean age of 27) population [21]. Sleep apnea has been associated with congestive heart failure [7, 9, 50, 51] and myocardial infarction [7, 52], but as “heart disease” in the SARR data was not defined, it is difficult to discuss this finding.
The restriction of the diagnosis of COPD to persons over the age of 35 led to the exclusion of this variable from the multivariate analysis. In numerous studies [35–38], however, a strong association between sleep apnea and COPD has been demonstrated. In their study of older men with moderate to severe COPD, Soler et al. [37] found that light sleep (stage 1 sleep) was significantly higher in subjects with COPD-OSA than in subjects with only COPD. Subjective sleep quality was poor among patients in both groups; however, they found no differences in measures of dyspnea, exercise tolerance, health-related quality of life, quality of sleep, and sleepiness. The presence of OSA correlated with BMI, but not with the “Epworth Sleepiness Scale,” insomnia index, sleep quality, dyspnea scale, anxiety/depression scales, exercise tolerance, or FEV1. Surprisingly, in the group of community-dwelling older men in the “Outcomes of sleep disorders in older men study,” Zhao et al. [36] found that obstructive airway disease was associated with a lower prevalence of sleep apnea.
Associations between sleep apnea and diabetes, pain and discomfort, and anxiety were identified in this study. Type 2 diabetes is frequently associated with OSA, with obesity as a common risk factor [32]. In persons with OSA and chronic musculoskeletal pain, Nadeem et al. [89] found that the pain significantly shortened sleep time and lessened the quality of sleep. Asghari et al. [48] found no association between OSA and the severity of depression and anxiety symptoms; however, Rezaeitalab et al. [45] found that 53.9% of the study population experienced anxiety and 46.1% depression, and that OSA severity was associated with the frequency of anxiety.
4.1. Limitations of the Study
The large data we were working with had a few limitations: (1) no objective sleep data; (2) no data on the treatment for OSA or any other treatment; (3) the exercise duration was only categorized according to a 15/min cutoff of daily activity, without any further specification of duration or intensity; and (4) all the data were self-reported including those regarding sleepiness at the wheel. Especially the limited information on types and duration of exercise limited our ability for recommendations on exercise prescription.
5. Conclusions
This study investigated the prevalence and possible risk factors of sleep apnea in the Canadian population. Strong relationships between older age, loud snoring, sudden awakenings due to gasping or choking, and nodding off or falling asleep while driving and sleep apnea, as well as strong interactions between location and sleep apnea in men, and BMI, physical activity and sleep apnea, were demonstrated. The strong association between BMI, physical activity, and sleep apnea merits investigation into the introduction of physical activity programs in the treatment of not only overweight but also sleep apnea. The protective effect of exercise found in this large dataset is potentially a modifiable risk factor and important for public policy.
Acknowledgments
Research assistant was reimbursed from one of the main author's research funds.
Data Availability
The surveys used, the 2009 Canadian Community Health Survey (CCHS) and Sleep Apnea Rapid Response (SARR), are available from Statistics Canada.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this manuscript.
References
- 1.Berry R. B., Budhiraja R., Gottlieb D. J., et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Journal of Clinical Sleep Medicine. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javaheri S. Central sleep apnea. Clinics in Chest Medicine. 2010;31(2):235–248. doi: 10.1016/j.ccm.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Park J. G., Ramar K., Olson E. J. Updates on definition, consequences, and management of obstructive sleep apnea. Mayo Clinic Proceedings. 2011;86(6):549–554. doi: 10.4065/mcp.2010.0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Punjabi N. M. The epidemiology of adult obstructive sleep apnea. Proceedings of the American Thoracic Society. 2008;5(2):136–143. doi: 10.1513/pats.200709-155mg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J., Wang Y., Feng J., Chen B. Y., Cao J. Complex sleep apnea syndrome. Patient Preference and Adherence. 2013;7:633–641. doi: 10.2147/ppa.s46626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaheri S., Smith J., Chung E. The prevalence and natural history of complex sleep apnea. Journal of Clinical Sleep Medicine. 2009;5(3):205–211. [PMC free article] [PubMed] [Google Scholar]
- 7.Vozoris N. T. Sleep apnea-plus: prevalence, risk factors, and association with cardiovascular diseases using United States population-level data. Sleep Medicine. 2012;13(6):637–644. doi: 10.1016/j.sleep.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Evans J., Skomro R., Driver H., et al. Sleep laboratory test referrals in Canada: sleep apnea rapid response survey. Canadian Respiratory Journal. 2014;21(1):e4–e10. doi: 10.1155/2014/592947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kent B. D., Grote L., Ryan S., et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: the European Sleep Apnea Cohort (ESADA) study. Chest. 2014;146(4):982–990. doi: 10.1378/chest.13-2403. [DOI] [PubMed] [Google Scholar]
- 10.Young T., Peppard P. E., Gottlieb D. J. Epidemiology of obstructive sleep apnea: a population health perspective. American Journal of Respiratory and Critical Care Medicine. 2002;165(9):1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 11.Woods C. E., Usher K., Maguire G. P. Obstructive sleep apnoea in adult indigenous populations in high-income countries: an integrative review. Sleep and Breathing. 2015;19(1):45–53. doi: 10.1007/s11325-014-1032-7. [DOI] [PubMed] [Google Scholar]
- 12.Young T., Palta M., Dempsey J., Skatrud J., Weber S., Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New England Journal of Medicine. 1993;328(17):1230–1235. doi: 10.1056/nejm199304293281704. [DOI] [PubMed] [Google Scholar]
- 13.Bixler E. O., Vgontzas A. N., Ten Have T., Tyson K., Kales A. Effects of age on sleep apnea in men: I. prevalence and severity. American Journal of Respiratory and Critical Care Medicine. 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 14.Bixler E. O., Vgontzas A. N., Lin H. M., et al. Prevalence of sleep-disordered breathing in women: effects of gender. American Journal of Respiratory and Critical Care Medicine. 2001;163(3):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 15.Duran J., Esnaola S., Rubio R., Iztueta A. Obstructive sleep apnea-hypopnea and related clinical features in a population-based sample of subjects aged 30 to 70 yr. American Journal of Respiratory and Critical Care Medicine. 2001;163(3):685–689. doi: 10.1164/ajrccm.163.3.2005065. [DOI] [PubMed] [Google Scholar]
- 16.Luyster F. S., Kip K. E., Buysse D. J., Aiyer A. N., Reis S. E., Strollo P. J., Jr. Traditional and nontraditional cardiovascular risk factors in comorbid insomnia and sleep apnea. Sleep. 2014;37(3):593–600. doi: 10.5665/sleep.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrscher T. E., Overland B., Sandvik L., Westheim A. S., Akre H. High cardiovascular risk profile in patients with sleep apnea. Laryngoscope. 2014;124(1):306–310. doi: 10.1002/lary.24304. [DOI] [PubMed] [Google Scholar]
- 18.Pan Y., Wang W., Wang K. S. Associations of alcohol consumption and chronic diseases with sleep apnea among US adults. International Journal of High Risk Behaviors and Addiction. 2014;3(2) doi: 10.5812/ijhrba.19088.e19088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng X., Gu W., Li Y., Liu M., Li Y., Gao X. Age-group-specific associations between the severity of obstructive sleep apnea and relevant risk factors in male and female patients. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0107380.e107380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Li C., Feng L., Feng J., Cao J., Chen B. Prevalence of hypertension and circadian blood pressure variations in patients with obstructive sleep apnoea-hypopnoea syndrome. Journal of International Medical Research. 2014;42(3):773–780. doi: 10.1177/0300060513516756. [DOI] [PubMed] [Google Scholar]
- 21.Asha’ari Z. A., Hasmoni M. H., Ab Rahman J., Yusof R. A., Ahmad R. A. The association between sleep apnea and young adults with hypertension. Laryngoscope. 2012;122(10):2337–2342. doi: 10.1002/lary.23379. [DOI] [PubMed] [Google Scholar]
- 22.Goodfriend T. L. Obesity, sleep apnea, aldosterone, and hypertension. Current Hypertension Reports. 2008;10(3):222–226. doi: 10.1007/s11906-008-0042-x. [DOI] [PubMed] [Google Scholar]
- 23.Soylu A. C., Levent E., Sariman N., Yurtlu S., Alparslan S., Saygi A. Obstructive sleep apnea syndrome and anthropometric obesity indexes. Sleep and Breathing. 2012;16(4):1151–1158. doi: 10.1007/s11325-011-0623-9. [DOI] [PubMed] [Google Scholar]
- 24.Simpson L., McArdle N., Eastwood P. R., et al. Physical inactivity is associated with moderate-severe obstructive sleep apnea. Journal of Clinical Sleep Medicine. 2015;11(10):1091–1099. doi: 10.5664/jcsm.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y. C., Hung S. Y., Wang H. K., et al. Sleep apnea and the risk of chronic kidney disease: a nationwide population-based cohort study. Sleep. 2015;38(2):213–221. doi: 10.5665/sleep.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall N. S., Wong K. K., Liu P. Y., Cullen S. R., Knuiman M. W., Grunstein R. R. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 27.Peppard P. E., Young T., Palta M., Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. New England Journal of Medicine. 2000;342(19):1378–1384. doi: 10.1056/nejm200005113421901. [DOI] [PubMed] [Google Scholar]
- 28.Nieto F. J., Young T. B., Lind B. K., et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 29.Bixler E. O., Vgontzas A. N., Lin H. M., et al. Association of hypertension and sleep-disordered breathing. Archives of Internal Medicine. 2000;160(15):2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 30.Liu C. L., Wu C. S. Assessing whether the association between sleep apnea and diabetes is bidirectional. Canadian Journal of Diabetes. 2017;41(2):197–203. doi: 10.1016/j.jcjd.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 31.Manin G., Pons A., Baltzinger P., et al. Obstructive sleep apnoea in people with type 1 diabetes: prevalence and association with micro- and macrovascular complications. Diabetic Medicine. 2015;32(1):90–96. doi: 10.1111/dme.12582. [DOI] [PubMed] [Google Scholar]
- 32.Reichmuth K. J., Austin D., Skatrud J. B., Young T. Association of sleep apnea and type II diabetes: a population-based study. American Journal of Respiratory and Critical Care Medicine. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim K. S., Kim J. H., Park S. Y., et al. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. Journal of Clinical Sleep Medicine. 2012;8(4):367–374. doi: 10.5664/jcsm.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashyap R., Hock L. M., Bowman T. J. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep and Breathing. 2001;5(4):167–172. doi: 10.1055/s-2001-18805. [DOI] [PubMed] [Google Scholar]
- 35.Jen R., Li Y., Owens R. L., Malhotra A. Sleep in chronic obstructive pulmonary disease: evidence gaps and challenges. Canadian Respiratory Journal. 2016;2016:5. doi: 10.1155/2016/7947198.7947198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y. Y., Blackwell T., Ensrud K. E., et al. Sleep apnea and obstructive airway disease in older men: outcomes of sleep disorders in older men study. Sleep. 2016;39(7):1343–1351. doi: 10.5665/sleep.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soler X., Gaio E., Powell F. L., et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Annals of the American Thoracic Society. 2015;12(8):1219–1225. doi: 10.1513/annalsats.201407-336oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mieczkowski B., Ezzie M. E. Update on obstructive sleep apnea and its relation to COPD. International Journal of Chronic Obstructive Pulmonary Disease. 2014;9:349–362. doi: 10.2147/copd.s42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiz J. A., Jane R., Sola-Soler J., Abad J., Garcia M. A., Morera J. Continuous analysis and monitoring of snores and their relationship to the apnea-hypopnea index. Laryngoscope. 2010;120(4):854–862. doi: 10.1002/lary.20815. [DOI] [PubMed] [Google Scholar]
- 40.Azarbarzin A., Moussavi Z. Snoring sounds variability as a signature of obstructive sleep apnea. Medical Engineering and Physics. 2013;35(4):479–485. doi: 10.1016/j.medengphy.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Lee L. A., Lo Y. L., Yu J. F., et al. Snoring sounds predict obstruction sites and surgical response in patients with obstructive sleep apnea hypopnea syndrome. Scientific Reports. 2016;6(1) doi: 10.1038/srep30629.30629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Acar M., Yazici D., Bayar Muluk N., Hanci D., Seren E., Cingi C. Is there a relationship between snoring sound intensity and frequency and OSAS severity? Annals of Otology, Rhinology & Laryngology. 2016;125(1):31–36. doi: 10.1177/0003489415595640. [DOI] [PubMed] [Google Scholar]
- 43.Kim J. W., Lee C. H., Rhee C. S., Mo J. H. Relationship between snoring intensity and severity of obstructive sleep apnea. Clinical and Experimental Otorhinolaryngology. 2015;8(4):376–380. doi: 10.3342/ceo.2015.8.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottlieb D. J., Whitney C. W., Bonekat W. H., et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. American Journal of Respiratory and Critical Care Medicine. 1999;159(2):502–507. doi: 10.1164/ajrccm.159.2.9804051. [DOI] [PubMed] [Google Scholar]
- 45.Rezaeitalab F., Moharrari F., Saberi S., Asadpour H., Rezaeetalab F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. Journal of Research in Medical Sciences. 2014;19(3):205–210. [PMC free article] [PubMed] [Google Scholar]
- 46.Shapiro A. L. Anxiety in middle-aged men with obstructive sleep apnea: state of the science. Journal of the American Association of Nurse Practitioners. 2014;26(12):689–695. doi: 10.1002/2327-6924.12118. [DOI] [PubMed] [Google Scholar]
- 47.Lehto S. M., Sahlman J., Soini E. J., et al. The association between anxiety and the degree of illness in mild obstructive sleep apnoea. Clinical Respiratory Journal. 2013;7(2):197–203. doi: 10.1111/j.1752-699x.2012.00304.x. [DOI] [PubMed] [Google Scholar]
- 48.Asghari A., Mohammadi F., Kamrava S. K., Tavakoli S., Farhadi M. Severity of depression and anxiety in obstructive sleep apnea syndrome. European Archives of Oto-Rhino-Laryngology. 2012;269(12):2549–2553. doi: 10.1007/s00405-012-1942-6. [DOI] [PubMed] [Google Scholar]
- 49.Ansarin K., Sahebi L., Sabur S. Obstructive sleep apnea syndrome: complaints and housing characteristics in a population in the United States. Sao Paulo Medical Journal. 2013;131(4):220–227. doi: 10.1590/1516-3180.2013.1314451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gottlieb D. J., Yenokyan G., Newman A. B., et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the Sleep Heart Health Study. Circulation. 2010;122(4):352–360. doi: 10.1161/circulationaha.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Javaheri S., Javaheri S., Javaheri A. Sleep apnea, heart failure, and pulmonary hypertension. Current Heart Failure Reports. 2013;10(4):315–320. doi: 10.1007/s11897-013-0167-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mooe T., Franklin K. A., Holmstrom K., Rabben T., Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. American Journal of Respiratory and Critical Care Medicine. 2001;164(10):1910–1913. doi: 10.1164/ajrccm.164.10.2101072. [DOI] [PubMed] [Google Scholar]
- 53.May J. F., Porter B. E., Ware J. C. The deterioration of driving performance over time in drivers with untreated sleep apnea. Accident Analysis and Prevention. 2016;89:95–102. doi: 10.1016/j.aap.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Leger D., Bayon V., Laaban J. P., Philip P. Impact of sleep apnea on economics. Sleep Medicine Reviews. 2012;16(5):455–462. doi: 10.1016/j.smrv.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Mulgrew A. T., Ryan C. F., Fleetham J. A., et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Medicine. 2007;9(1):42–53. doi: 10.1016/j.sleep.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Sivertsen B., Overland S., Glozier N., Bjorvatn B., Maeland J. G., Mykletun A. The effect of OSAS on sick leave and work disability. European Respiratory Journal. 2008;32(6):1497–1503. doi: 10.1183/09031936.00044908. [DOI] [PubMed] [Google Scholar]
- 57.Fonseca M. I., Pereira T., Caseiro P. Death and disability in patients with sleep apnea: a meta-analysis. Arquivos Brasileiros de Cardiologia. 2015;104(1):58–66. doi: 10.5935/abc.20140172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Public Health Agency of Canada. Fast Facts from the Canadian Community Health Survey—Sleep Apnea Rapid Response. Ottawa, Canada: Public Health Agency of Canada; 2009. http://www.phac-aspc.gc.ca/cd-mc/sleepapnea-apneesommeil/pdf/sleep-apnea.pdf. [Google Scholar]
- 59.Statistics Canada. Canadian Community Health Survey (CCHS): Detailed Information for 2005 (Cycle 3.1) 2007. http://www23.statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SurvId=3226&SurvVer=0&InstaId=15282&InstaVer=3&SDDS=3226&lang=en&db=imdb&adm=8&dis=2. [Google Scholar]
- 60.What is the Impact of Sleep Apnea on Canadians? 2010. http://www.phac-aspc.gc.ca/cd-mc/sleepapnea-apneesommeil/ff-rr-2009-eng.php. [Google Scholar]
- 61.Williams R. Using the margins command to estimate and interpret adjusted predictions and marginal effects. Stata Journal. 2012;12(2):308–331. [Google Scholar]
- 62.Young T., Shahar E., Nieto F. J., et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Archives of Internal Medicine. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 63.Remmers J. E., de Groot W. J., Sauerland E. K., Anch A. M. Pathogenesis of upper airway occlusion during sleep. Journal of Applied Physiology. 1978;44(6):931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- 64.Younes M., Ostrowski M., Atkar R., Laprairie J., Siemens A., Hanly P. Mechanisms of breathing instability in patients with obstructive sleep apnea. Journal of Applied Physiology. 2007;103(6):1929–1941. doi: 10.1152/japplphysiol.00561.2007. [DOI] [PubMed] [Google Scholar]
- 65.Zhang P., Zhang R., Zhao F., et al. The prevalence and characteristics of obstructive sleep apnea in hospitalized patients with type 2 diabetes in China. Journal of Sleep Research. 2015;25(1):39–46. doi: 10.1111/jsr.12334. [DOI] [PubMed] [Google Scholar]
- 66.Ioachimescu O. C., Collop N. A. Sleep-disordered breathing. Neurologic Clinics. 2012;30(4):1095–1136. doi: 10.1016/j.ncl.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 67.Myers K. A., Mrkobrada M., Simel D. L. Does this patient have obstructive sleep apnea?: The Rational Clinical Examination systematic review. JAMA. 2013;310(7):731–741. doi: 10.1001/jama.2013.276185. [DOI] [PubMed] [Google Scholar]
- 68.Arita A., Sasanabe R., Hasegawa R., et al. Risk factors for automobile accidents caused by falling asleep while driving in obstructive sleep apnea syndrome. Sleep and Breathing. 2015;19(4):1229–1234. doi: 10.1007/s11325-015-1145-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goncalves M., Amici R., Lucas R., et al. Sleepiness at the wheel across Europe: a survey of 19 countries. Journal of Sleep Research. 2015;24(3):242–253. doi: 10.1111/jsr.12267. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz A. J., Sepulveda M. A., Martinez P. H., et al. Prevalence of sleep complaints in Colombia at different altitudes. Sleep Science. 2016;9(2):100–105. doi: 10.1016/j.slsci.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nussbaumer-Ochsner Y., Schuepfer N., Ulrich S., Bloch K. E. Exacerbation of sleep apnoea by frequent central events in patients with the obstructive sleep apnoea syndrome at altitude: a randomised trial. Thorax. 2010;65(5):429–435. doi: 10.1136/thx.2009.125849. [DOI] [PubMed] [Google Scholar]
- 72.Bloch K. E., Latshang T. D., Ulrich S. Patients with obstructive sleep apnea at altitude. High Altitude Medicine and Biology. 2015;16(2):110–116. doi: 10.1089/ham.2015.0016. [DOI] [PubMed] [Google Scholar]
- 73.Whittle A. T., Marshall I., Mortimore I. L., Wraith P. K., Sellar R. J., Douglas N. J. Neck soft tissue and fat distribution: Comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54(4):323–328. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartz A. R., Patil S. P., Squier S., Schneider H., Kirkness J. P., Smith P. L. Obesity and upper airway control during sleep. Journal of Applied Physiology. 2010;108(2):430–435. doi: 10.1152/japplphysiol.00919.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malhotra A., Huang Y., Fogel R. B., et al. The male predisposition to pharyngeal collapse: importance of airway length. American Journal of Respiratory and Critical Care Medicine. 2002;166(10):1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 76.Kirkness J. P., Schwartz A. R., Schneider H., et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. Journal of Applied Physiology. 2008;104(6):1618–1624. doi: 10.1152/japplphysiol.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iftikhar I. H., Kline C. E., Youngstedt S. D. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175–184. doi: 10.1007/s00408-013-9511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Andrade F. M., Pedrosa R. P. The role of physical exercise in obstructive sleep apnea. Jornal Brasileiro de Pneumologia. 2016;42(6):457–464. doi: 10.1590/s1806-37562016000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Norman J. F., Von Essen S. G., Fuchs R. H., McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Research Online. 2000;3(3):121–129. [PubMed] [Google Scholar]
- 80.Kline C. E., Crowley E. P., Ewing G. B., et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sengul Y. S., Ozalevli S., Oztura I., Itil O., Baklan B. The effect of exercise on obstructive sleep apnea: a randomized and controlled trial. Sleep and Breathing. 2011;15(1):49–56. doi: 10.1007/s11325-009-0311-1. [DOI] [PubMed] [Google Scholar]
- 82.Servantes D. M., Pelcerman A., Salvetti X. M., et al. Effects of home-based exercise training for patients with chronic heart failure and sleep apnoea: a randomized comparison of two different programmes. Clinical Rehabilitation. 2012;26(1):45–57. doi: 10.1177/0269215511403941. [DOI] [PubMed] [Google Scholar]
- 83.Ackel-D’Elia C., da Silva A. C., Silva R. S., et al. Effects of exercise training associated with continuous positive airway pressure treatment in patients with obstructive sleep apnea syndrome. Sleep and Breathing. 2012;16(3):723–735. doi: 10.1007/s11325-011-0567-0. [DOI] [PubMed] [Google Scholar]
- 84.Schutz T. C., Cunha T. C., Moura-Guimaraes T., et al. Comparison of the effects of continuous positive airway pressure, oral appliance and exercise training in obstructive sleep apnea syndrome. Clinics. 2013;68(8):1168–1174. doi: 10.6061/clinics/2013(08)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barnes M., Goldsworthy U. R., Cary B. A., Hill C. J. A diet and exercise program to improve clinical outcomes in patients with obstructive sleep apnea–a feasibility study. Journal of Clinical Sleep Medicine. 2009;5(5):409–415. [PMC free article] [PubMed] [Google Scholar]
- 86.Dobrosielski D. A., Patil S., Schwartz A. R., Bandeen-Roche K., Stewart K. J. Effects of exercise and weight loss in older adults with obstructive sleep apnea. Medicine and Science in Sports and Exercise. 2015;47(1):20–26. doi: 10.1249/mss.0000000000000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Collins C. E., Warren J. M., Neve M., McCoy P., Stokes B. Systematic review of interventions in the management of overweight and obese children which include a dietary component. International Journal of Evidence-Based Healthcare. 2007;5(1):2–53. doi: 10.1111/j.1479-6988.2007.00061.x. [DOI] [PubMed] [Google Scholar]
- 88.Kersh R., Stroup D. F., Taylor W. C. Childhood obesity: a framework for policy approaches and ethical considerations. Preventing Chronic Disease. 2011;8(5):p. A93. [PMC free article] [PubMed] [Google Scholar]
- 89.Nadeem R., Bawaadam H., Asif A., et al. Effect of musculoskeletal pain on sleep architecture in patients with obstructive sleep apnea. Sleep and Breathing. 2014;18(3):571–577. doi: 10.1007/s11325-013-0920-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The surveys used, the 2009 Canadian Community Health Survey (CCHS) and Sleep Apnea Rapid Response (SARR), are available from Statistics Canada.