Abstract
The proportion of obese or diabetic population has been anticipated to increase in the upcoming decades, which rises the prevalence of nonalcoholic fatty liver disease (NAFLD) and its progression to nonalcoholic steatohepatitis (NASH). Recent evidence indicates that NASH is the main cause of chronic liver diseases and it is an important risk factor for development of hepatocellular carcinoma (HCC). Although the literature addressing NASH-HCC is growing rapidly, limited data is available about the etiology of NASH-related HCC. Experimental studies on the molecular mechanism of HCC development in NASH reveal that the carcinogenesis is relevant to complex changes in signaling pathways that mediate cell proliferation and energy metabolism. Genetic or epigenetic modifications and alterations in metabolic, immunologic, and endocrine pathways have been shown to be closely related to inflammation, liver injury, and fibrosis in NASH along with its subsequent progression to HCC. In this review, we provide an overview on the current knowledge of NASH-related HCC development and emphasize molecular signaling pathways regarding their mechanism of action in NASH-derived HCC.
1. Introduction
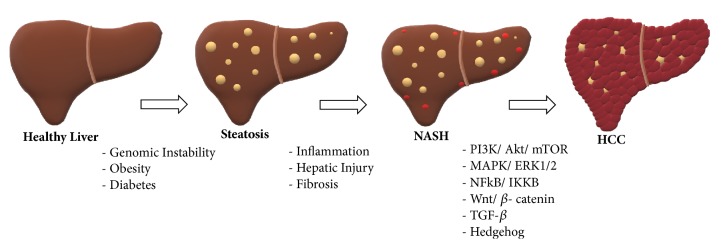
Hepatocellular carcinoma (HCC) is an aggressive cancer with poor prognosis and its incidence increases exponentially in developing countries. The most common underlying causes of HCC are chronic liver diseases and cirrhosis, largely occurring due to hepatitis B, hepatitis C virus (HCV), or alcoholic liver disease [1]. In recent years, nonalcoholic fatty liver disease (NAFLD) also becomes one of leading etiologies for HCC. NAFLD is a spectrum of liver diseases ranging from simple steatosis to liver injury. The initial stage of an inflammatory phase in NAFLD is defined as nonalcoholic steatohepatitis (NASH) [2]. NASH is characterized by inflammation, hepatocellular damage, and fibrosis, which increase the risk of HCC with high rates of mortality (Figure 1). The emergence of HCC in NASH patients with or without cirrhosis is still controversial, such that HCC can also be seen in NASH patients without cirrhosis [3].
Figure 1.
Development of NASH and HCC from healthy liver.
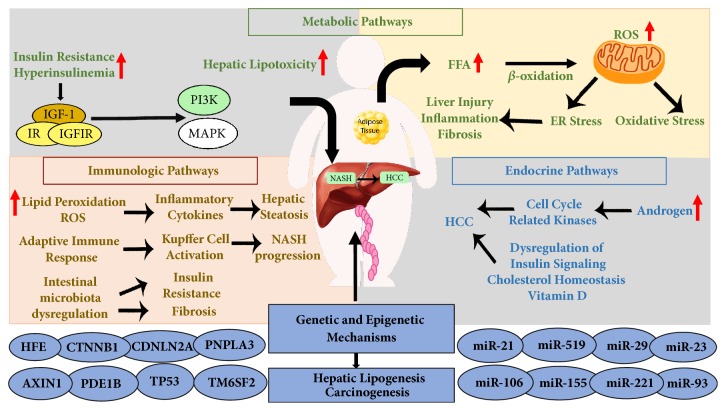
The progression of NASH-related HCC is a complex and multifactorial process, including several risk factors such as genomic instability, obesity, or diabetes [4, 5]. Involvement of the mechanisms related to these factors appears to cause changes in some common signaling pathways that lead to transition of dysplastic hepatocytes into hepatocellular carcinoma. Currently, the proposed mechanisms include genetic, metabolic, immunologic, and endocrine pathways, which subsequently activate oncogenic mechanisms [6] (Figure 2). In this review, we attempt to summarize recent knowledge in NASH progression and particularly focus on molecular signaling pathways involved in the conversion of NASH into hepatocarcinogenesis.
Figure 2.
The proposed mechanisms in NASH-related HCC progression.
2. Cellular Mechanisms in NASH Progression
Clinical and epidemiological studies support a concept that multiple mechanisms derive NAFLD, NASH, and HCC development. So far, the detailed mechanism of the progression from NAFLD to NASH has not been completely explained, yet a “two-hit hypothesis” was initially proposed [7, 8]. According to this hypothesis, the first hit was insulin resistance, and steatosis was the initiative cause of NASH progression [9]. Insulin resistance enhances lipolysis and increases the level of serum free fatty acid (FFA). Elevation of FFA leads to delivering triglycerides from the liver to peripheral organs, which induce hyper-synthesis of lipid thus causing excessive lipid storage in the liver, called steatosis. Meanwhile, accumulation of triglycerides promotes the appearance of the second hit, oxidative stress, that shows steatohepatitis because of increased level of fatty acid oxidation [10]. Oxidative stress triggers lipid peroxidation, release of proinflammatory molecules, and mitochondrial damage [11], which are the cellular mechanisms involved in the formation of hepatocellular damage, inflammation, and fibrosis in NASH pathology [12, 13].
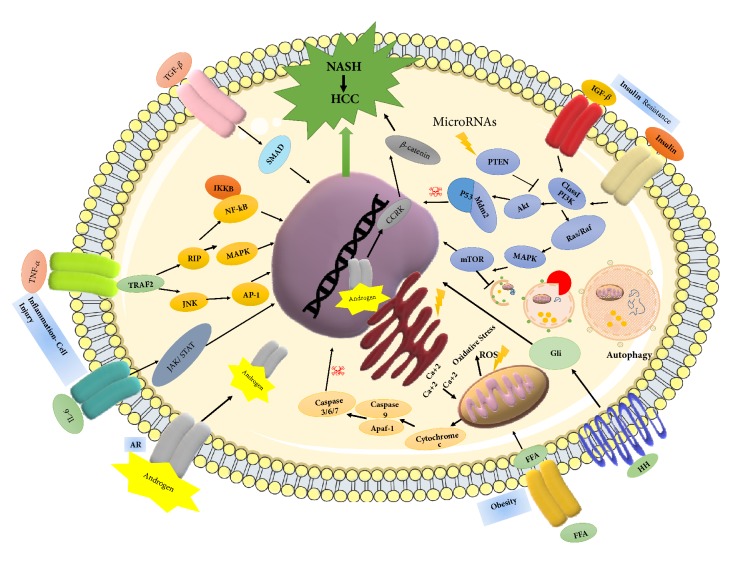
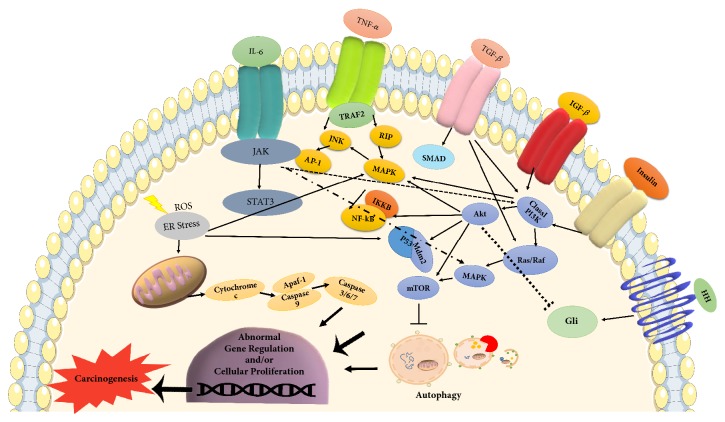
Even though a large number of researches have supported the two-hit hypothesis, it is still unclear whether NASH develops sequentially on the background of a fatty liver, or it is rather a de novo response to the accumulated lipotoxicity. Instead of the two-hit hypothesis, there is a new consensus on the multiparallel hit theory, which better explains NASH development and its progression to HCC [14]. This theory suggests that NASH is the consequence of numerous conditions acting in parallel, including genetic variations, abnormal lipid metabolism, oxidative and/or endoplasmic reticulum stress, mitochondrial dysfunction, altered immune responses, and imbalance in gut microbiota [15]. According to this theory, hepatic inflammation is the first cause of fibrosis progression in NASH rather than steatosis [16]. The following section will focus on detailed mechanisms at molecular level and their related signaling pathways in NASH-dependent HCC progression (Figures 3 and 4).
Figure 3.
Molecular signaling pathways involved in NASH-related HCC.
Figure 4.
Interaction of oncogenic pathways in NASH-HCC progression.
3. Molecular Mechanisms Involved in NASH-Related HCC
3.1. Genetic and Epigenetic Mechanisms
Recent advances in genetic technology allow obtaining comprehensive data on the genetic alterations associated with HCC. Differential gene expression results from gene mutations in regulatory elements or epigenetic changes, which plays an important role in susceptibility to the development of HCC.
Genetic mutation in the gene encoding patatin-like phospholipase domain-containing protein 3 (PNPLA3) on chromosome 22 is a well-known factor in NASH-related HCC progression [17]. The variant (rs738409 c.444 C>G, p.I148M) causes a cytosine to guanine mutation resulting in isoleucine to methionine conversion. This mutation correlates with increased lipid accumulation in liver and predisposes individuals to fatty liver-associated diseases, from simple steatosis to steatohepatitis, NASH, and HCC [18]. Although the physiological and biological functions of PNPLA3 within the liver are not fully elucidated, the association of PNPLA3 mutations with HCC is evident [19]. Overexpression of I148M variant in mouse liver promotes accumulation of triacylglycerol, increases synthesis of fatty acids, and impairs triacylglycerol hydrolysis [20]. Moreover, the PNPLA3 genotype has been reported to influence liver storage of retinol and retinol serum levels in obese subjects [21] suggesting a potential role of PNPLA3 in regulating retinol metabolism and hepatic stellate cells (HSCs) biology. Similarly, PNPLA3 has been shown to be expressed in HSCs [22], but its role in HCC progression in these cells still needs to be investigated [23]. There is an increased prevalence of another mutation in the transmembrane 6 superfamily member 2 gene (TM6SF2) in NASH patients. Carriage of a genetic variant in TM6SF2 (rs58542926 c.449 C>T, p.E167K) on chromosome 19 (19p13.11) has been reported to correlate with steatosis and advanced fibrosis in NASH patients [24, 25], independently of diabetes, obesity, or PNPLA3 genotype. Although, conflicting data exists regarding its role in HCC progression, the TM6SF2 variant is thought to be associated with liver injury in NASH-related HCC pathogenesis [26]. Hemochromatosis gene (HFE) mutations (C282Y and H63D) in NASH increased the susceptibility to more severe form of disease with fibrosis or cirrhosis [27, 28] and implicated HCC development in these patients. Particularly, H63D mutation was found in noncirrhotic HCC and led to hepatic inflammation, fibrosis, and carcinogenesis due to increased iron load in these patients [29]. Recently, the rs641738 genotype, encoding the membrane bound O-acyltransferase domain-containing 7 (MBOAT7), was associated with more severe liver damage and increased risk of fibrosis in NASH patients; however, these findings need further investigation regarding HCC progression [30, 31]. In addition to various single mutations, the genetic instability in NASH patients was reported much higher than in NAFLD patients, and this was considered as one of the inducements for NASH-related HCC. Quantitative analysis revealed abundant amplifications of DNA, where the genes involved in oncogenic mechanisms are located. These genes encode telomerase reverse transcriptase (TERT), vascular endothelial growth factor A (VGFA), MET, and MYC proteins that are known to have a role in tumor growth. Moreover, exome-sequencing analysis of HCC showed the highest prevalence of mutation in oncogenic genes, like CTNNB1, AXIN1 (involved in β-catenin/WNT signaling pathway), albumin (ALB), TP53, and CDKN2A [32]. Furthermore, differential expressions of the exportin 4 (XPO4) and phosphodiesterase 1B (PDE1B) genes were identified in HCC as well as in NASH; however, the physiological role of these genes in NASH-related HCC is still unknown [33, 34].
Epigenetic changes, causing aberrant DNA methylation, have been considered another important mechanism in NASH progression [35]. It occurs through the enzyme methyltransferases (DNMTs), leading to silence of genes related to DNA damage and repair, lipid and glucose metabolism, and fibrosis progression [36]. The methylation of the CpG island near the PDE1B gene was shown to be linked with survival in HCC patients; nevertheless, the only epigenetic change that has clearly been linked to NASH-related HCC is the gene encoding chromodomain helicase DNA-binding protein 1 (CHD1) [37].
MicroRNAs (miRNAs) are endogenous, small noncoding RNAs, having a role in the regulation of gene expression. Convincing evidence showed that expression of miRNAs is dysregulated in many cancers through various mechanisms and they may function as either oncogenes or tumor suppressors under certain conditions [38]. So far, no studies have yet significantly focused on miRNA expression in human NASH-associated HCC; however, genome-wide analysis revealed 23 miRNAs, differentially expressed in NASH patients. Among them, liver specific miR-122 expression is reduced in NASH patients and, thus, negatively regulates hepatic lipogenesis [39]. Downregulation of miR-122 was also demonstrated in a mouse model of NASH-HCC, indicating direct role of this miRNA in NASH-associated HCC [40]. To date, most of the studies indicate a critical role of several miRNAs (miR-21, miR-29, miR-23, miR-155, miR-221, miR-222, miR-106, miR-93, miR-519) in NASH-associated carcinogenesis [32]. Strikingly, altered expression of these miRNAs have been found to be involved in major hepatocarcinogenic pathways, including the TGF-β, Wnt/β-catenin, mitogen-activate protein kinase (MAPK), and phosphatidylinositol 3-kinases (PI3K)/AKT/mTOR that regulate proliferation and energy metabolism in the cell [41]. Importantly, several of these miRNAs target the main inhibitor of the PI3K/AKT pathway, PTEN protein, and its mutations were found in HCC patients [42]. In accordance, PTEN deficient mice have been shown to develop steatosis, hepatomegaly, and HCCs [43, 44].
3.2. Metabolic Pathways
The common association of high-fat diet, obesity, and diabetes with NASH and HCC pathogenesis indicates that the molecular link between energy balance and cell cycle control in hepatocytes is the key mechanism for the progression of NASH-related HCC. Indeed, these metabolic factors are closely related to insulin resistance and hyperinsulinemia, which activates insulin receptor signaling via PI3K and MAPK pathway.
Experimental evidence indicated that insulin resistance and hyperinsulinemia increased the expression of insulin and insulin-like growth factor-1 (IGF-1) [45]. Binding of insulin or IGF-1 to their respective receptors, namely, insulin receptor (IR) and insulin-like growth factor-1 receptor (IGF1R), triggers signaling cascade via insulin receptor substrate-1 (IRS-1) that results in activation of its downstream PI3K and MAPK pathways. In fact, these pathways play a significant role in the carcinogenesis of HCC by induction of cell proliferation and inhibition of apoptosis [46, 47]. The role of PI3K pathway in the progression of HCC is mainly mediated by its effect on cyclin D1-dependent cell cycle, Mdm2/p53-dependent apoptosis, and mTOR-dependent cell growth [48]. On the other hand, MAPK pathway affects cell growth by inducing the transcription of protooncogenes, c-fos, and c-jun. In addition, MAPK pathway eventually activates the Wnt/β-catenin signaling cascade, which leads to fibrosis and carcinogenesis in liver [49].
Another important consequence of insulin resistance is excessive lipid accumulation in liver. In other words, imbalance in energy metabolism increases hepatic lipotoxicity, resulting in excessive production of FFAs [50]. Indeed, β-oxidation of these FFAs in mitochondria induces the formation of reactive oxygen species (ROS). Overproduction of ROS causes respiratory chain disruption and further functional defect in mitochondria, which is the main event for cytochrome c release and triggering apoptotic death signal. Recently, RIP1- and RIP3-activated JNK (Jun-(N)-terminal kinase) has been proposed as an apoptotic pathway responsible for the emergence of liver injury, inflammation, and fibrosis in NASH patients as well as in mouse model of steatohepatitis [51, 52].
Insulin signaling and lipotoxicity in mitochondria are connected to several other mechanisms, such as oxidative and endoplasmic reticulum (ER) stress, that contribute to hepatic cell injury and ultimately carcinogenesis in NASH [53]. Certainly, there is significant cross-talk between ROS production, oxidative and/or ER stress, and cell death mechanisms, correlating to the development of progressive disease conditions in NASH and HCC. ROS and oxidative stress disrupt ER functions via increased release of calcium from ER stores. Excess amount of calcium level induces mitochondrial and lysosomal permeabilization, which in turn increased further mitochondrial ROS release and potentiate sequential activation of proapoptotic pathway initiated by executive caspases 9 and 3 [54, 55]. Under normal catabolic condition in cells, the superoxides (incompletely reduced forms of oxygen) are converted into nontoxic water by glutathione peroxidase and catalase. The biochemical function of these enzymes is to protect the organism from oxidative damage by reducing the amount of free hydrogen peroxide. The level of iron is an important factor for glutathione peroxidase and catalase activities, which is upregulated during intake of excess iron; otherwise, it induces oxidative stress by enhancing FA oxidation. Accordingly, elevated level of iron is observed in NASH patients and considered as a risk factor for HCC development [56].
Autophagy is one of the important stress response pathways in cells, supporting cell survival by recycling metabolic components. This mechanism reduces cytosolic organelles or macromolecules by sequestering them in double-membrane vesicles and delivering them to the lysosomes for degradation. Recent discoveries showed a molecular connection between lipolysis and autophagy mechanisms. In the liver, autophagy suppresses protein aggregate, lipid accumulation, oxidative stress, chronic cell death, and inflammation. On the contrary, autophagy regulates adipogenesis and adipose tissue differentiation [57]. Now, the emerging role of autophagy in NASH and NASH-derived HCC is a double-edged sword. On the one hand, autophagy enables the hepatocytes to tolerate stress and promote tumorigenesis. On the other hand, autophagy plays an important role in damage mitigation in response to stress that can limit tumorigenesis [58, 59]. Although there is controversy whether autophagy promotes or inhibits NASH progression, its role in energy metabolism via PI3K/mTOR pathway strongly supports the idea that autophagy may be an ideal candidate for therapeutic purposes. Therefore, further investigations are needed to determine the exact role of autophagy in NASH-associated HCC.
3.3. Immunologic Pathways
Mitochondrial dysfunction and stimulation of stress mediators not only facilitate the production of ROS, but also contribute to the progression of HCC by immune reactions. Insulin resistance and oxidative stress stimulate IKKβ- (inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase beta) dependent NF-κB (nuclear factor kappa-light-chain-enhancer of activated B-cells) signaling pathway and promote hepatocyte survival in addition to their crucial role in liver inflammatory responses [60]. It has been shown that ROS along with products of lipid peroxidation increases the release of several inflammatory and inhibitory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), leptin, and adiponectin [61]. TNF-α activates prooncogenic pathways via JNK and IKKβ that promote the synthesis of AP-1 and NF-κB. Phosphorylation and subsequent degradation of IKKβ lead to the nuclear entry of NF-κB, triggering inflammatory cascades, which in turn aggravate NF-κB activation. Extracellular lipid can also activate IKKβ by engaging TLRs (Toll-like receptors). The TLR-deficient mice studies revealed the attenuation of severe steatosis, indicating TLR as an important proinflammatory mediator in NASH progression [62]. On the other hand, IL-6 activates STAT-3 (signal transducer and activator of transcription 3), an oncogenic transcription factor that induces cell proliferation and antiapoptotic pathways, and was found to be important for NASH-related HCC development [63]. Leptin has been described as profibrotic and proangiogenic factor in liver carcinogenesis by initiating an intracellular signaling cascade of proinflammatory cytokines (TNF-α and IL-6). Moreover, binding of leptin to its respective receptor in HCC cells activates JAK2/STAT, MAPK, and PI3K signaling pathways [64]. Interestingly, leptin has also been shown to upregulate the TERT and thereby lead to immortalization of tumor cells in HCC [65]. Adiponectin is an anti-inflammatory cytokine, specifically produced in adipose tissue. Under normal physiological conditions, it inhibits angiogenesis via modulation of apoptosis [66]. However, insulin resistance reduced level of adiponectin and the release of TNF-α and IL-6 that further inhibit adiponectin production and thus potentiate HCC development [67]. Adiponectin and leptin act antagonistically on liver fibrogenesis and inflammation [68]. However, reports of serum levels of adiponectin and the expression of its receptor are inconsistent [69]. Therefore, further investigations are necessary to clarify the function of adipokines in NASH and HCC development.
Immune activation is a prerequisite for the development of NASH, which is also linked to adaptive immune responses. In several animal models, the potential role of CD8+ T-lymphocytes, and CD4+ T-lymphocytes in liver damage and carcinogenesis was demonstrated [70, 71]. Moreover, liver damage stimulates the recruitment of different types of immune cells to the site of injury. Kupffer cell (KC) activation is critical in NASH and precedes the recruitment of other cells, therefore contributing to NASH progression [72]. In NASH, a number of ligands and cytokines can also activate Natural Killer (NK) cells; however, data obtained from animal models are contradictory, indicating that two different phenotypes of NK cells have been associated with liver disease and act oppositely during inflammation [73, 74]. The involvement of adaptive immune system was demonstrated in response to liver injury and inflammation, but its exact role in NASH-related HCC is still unknown.
Acute cell injury triggers another signaling pathway, Hedgehog, a complex cellular pathway for liver repair and regeneration. This pathway induces mobilization of hepatic progenitor cells at the site of injury and replaces damaged hepatocytes [75]. Current data suggest that abnormal Hedgehog signaling results in dysregulated cellular repair and malignant transformation in HCC progression. Moreover, the development of HCC has been described as a contrary function of Hedgehog pathway, in which hyperactivation of progenitor cells could survive independently from regulation of NF-κB, thereby being less susceptible to NF-κB-driven apoptosis [76].
The gut microflora plays an important role in the development and function of the host immune system. Through the portal circulation, liver is directly exposed to gut-derived products, being the first line of defense against bacterial toxins [77]. The studies in both animal models and human showed that alteration in intestinal microflora triggers an immune response, inflammation, and immune cell infiltration of liver and adipose tissue. Modulation of gut microbiota induces insulin resistance by inhibiting expression of gut-secreted anorectic hormones, such as GLP-1 and PYY. In addition, the reduced expression of a LPL- (lipoprotein lipase-) suppressor FIAF (fasting-induced adipose factor) prevents FA release leading to FA and triglyceride accumulation [78]. The shift on the bacterial community prevalence in gut microbiota results in release of pathogen-associated molecular patterns (PAMPs). PAMPs are recognized by TLRs and other pattern recognition receptors (PRRs) and potentiate innate immune responses. Lipopolysaccharides (LPSs), a major component of outer membrane of gram negative bacteria, are considered the prototypical class of PAMPs. While LPSs are specifically recognized by TLR4, the other PAMPs such as flagellin, lipoteichoic acid, peptidoglycan, nucleic acid variants (dsRNA), or unmethylated CpG motifs are recognized by other receptors, such as TLR2, TLR3, TLR5, and TLR9 [79]. Similarly, human TLR2, TLR4, and TLR9 are involved in the pathogenesis of NASH [80]. Interaction of LPSs and TLR4 with the monocyte differentiation antigen CD14 system on Kupffer cells triggers inflammatory cascade, which activates NF-κB pathway and induces the production of TNF-α, IL-1, and IL-6 cytokines [81]. The stimulation of this pathway was demonstrated in animal model of NASH, and elevated TNF-α expression as well as serum LPS-binding proteins was detected [82]. In HSCs, the activation of TRL4-dependent pathway was shown to be involved in fibrosis progression [83]. Although further investigations are necessary to show the generation of secondary bile acids by gut microbiota in NASH-HCC, the studies have shown the induction of DNA damage by one of the secondary bile acids, sDCA [84, 85].
3.4. Endocrine Pathways
The incidence of NASH and HCC is higher in males irrespective of the etiology. This suggests that the differential endocrine signaling might increase the tendency of HCC development in NASH patients. Both estrogen and androgen are steroid hormones that mediate their action by binding to nuclear receptors and acting as transcription factors to regulate the expression of multiple genes. It was suggested that androgen and androgen receptors (ARs) might promote HCC progression and/or that estrogen and estrogen receptors might suppress HCC development [86]. The AR gene encodes AR molecule, which is a transcriptional factor able to bind DNA with its DNA-binding domain. AR is activated directly by androgen hormone and induces the transcription of cell cycle-related kinase (CCRK) that upregulates β-catenin/T-cell factor signaling, leading to promotion of HCC [87]. ARs can also be activated by other signaling pathways such as MAPK and PI3K, which are well-known in the development of HCC in NASH [88]. Although ARs are extensively studied in HCC, their role in NASH is still under investigation. Several animal studies demonstrated the development of liver steatosis, insulin resistance, altered lipid metabolism, and progression of NASH to HCC via either SREB1 (sterol regulatory element-binding protein), PEPCK (phosphoenolpyruvate carboxykinase), and PTB-1B (protein tyrosine phosphatase 1B) or SREB2 and CYP27A1 [89]. These molecules play significant role in insulin signaling, cholesterol homeostasis, and vitamin D3 metabolism through activation of the JNK pathway [90].
4. Conclusion and Future Perspectives
NASH is the aggressive form of NAFLD and its prevalence is progressively increasing due to the growing epidemic of obesity and diabetes. Accumulated evidence is likely to make NASH one of the most common causes of HCC in upcoming years. Recent advances in whole genome association study (WGAS) and next generation sequencing (NGS) allow clarifying remarkable genetic changes in signaling pathways related to energy metabolism and cell proliferation that are directly linked to carcinogenesis. Currently, the data obtained from various clinical and in vivo molecular studies achieve the consensus that genomic instability, abnormal lipid metabolism, uncontrolled stress mediators, and altered immune responses are coordinately acting mechanisms, prompting inflammation, liver injury, and fibrosis along with HCC. Our understanding of the underlying molecular basis in the NASH-related HCC development is that the signaling pathways involved in NASH pathogenesis seem to act simultaneously in HCC development. In this complex scenario, key molecules involved in reciprocal interaction between several pathways lead to overactivation of prooncogenic mechanism and, meanwhile, inactivate tumor-suppressive or antioncogenic mechanisms. Ongoing clinical trials of a wide range of molecules, targeting different pathways, have been shown to reduce the NASH-HCC progression in several pathogenic aspects, yet the translation of these findings into personalized therapy is still a major challenge. Thus, a better understanding of the molecular signaling pathways involved in NASH-related HCC will allow the discovery of novel targeting molecules for therapeutic and preventive approaches.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Baffy G., Brunt E. M., Caldwell S. H. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. Journal of Hepatology. 2012;56(6):1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver International. 2017;37:81–84. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- 3.Michelotti G. A., Machado M. V., Diehl A. M. NAFLD, NASH and liver cancer. Nature Reviews Gastroenterology & Hepatology. 2013;10(11):656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 4.Ascha M. S., Hanouneh I. A., Lopez R., Tamimi T. A.-R., Feldstein A. F., Zein N. N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51(6):1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 5.Said A., Ghufran A. Epidemic of non-alcoholic fatty liver disease and hepatocellular carcinoma. World Journal of Clinical Oncology. 2017;8(6):429–436. doi: 10.5306/wjco.v8.i6.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margini C., Dufour J. F. The story of HCC in NAFLD: From epidemiology, across pathogenesis, to prevention and treatment. Liver International. 2016;36(3):317–324. doi: 10.1111/liv.13031. [DOI] [PubMed] [Google Scholar]
- 7.Day C. P., James O. F. W. Steatohepatitis: A Tale of Two ‘Hits’? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 8.Gentile C. L., Pagliassotti M. J. The role of fatty acids in the development and progression of nonalcoholic fatty liver disease. The Journal of Nutritional Biochemistry. 2008;19(9):567–576. doi: 10.1016/j.jnutbio.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sumida Y., Niki E., Naito Y., Yoshikawa T. Involvement of free radicals and oxidative stress in NAFLD/NASH. Free Radical Research. 2013;47(11):869–880. doi: 10.3109/10715762.2013.837577. [DOI] [PubMed] [Google Scholar]
- 10.Kawano Y., Cohen D. E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. Journal of Gastroenterology. 2013;48(4):434–441. doi: 10.1007/s00535-013-0758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Busafi S. A., Bhat M., Wong P., Ghali P., Deschenes M. Antioxidant therapy in nonalcoholic steatohepatitis. Hepatitis Research and Treatment. 2012;2012:8. doi: 10.1155/2012/947575.947575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browning J. D., Horton J. D. Molecular mediators of hepatic steatosis and liver injury. The Journal of Clinical Investigation. 2004;114(2):147–152. doi: 10.1172/JCI200422422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunt E. M., Kleiner D. E., Wilson L. A., Belt P., Neuschwander-Tetri B. A. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53(3):810–820. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tilg H., Moschen A. R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- 15.Takaki A., Kawai D., Yamamoto K. Multiple hits, including oxidative stress, as pathogenesis and treatment target in non-alcoholic steatohepatitis (NASH) International Journal of Molecular Sciences. 2013;14(10):20704–20728. doi: 10.3390/ijms141020704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peverill W., Powell L. W., Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. International Journal of Molecular Sciences. 2014;15(5):8591–8638. doi: 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sookoian S., Pirola C. J. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53(6):1883–1894. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- 18.Dongiovanni P., Donati B., Fares R., et al. PNPLA3 I148M polymorphism and progressive liver disease. World Journal of Gastroenterology. 2013;19(41):6969–6978. doi: 10.3748/wjg.v19.i41.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yopp A. C., Choti M. A. Non-alcoholic steatohepatitis-related hepatocellular carcinoma: A growing epidemic? Digestive Diseases. 2015;33(5):642–647. doi: 10.1159/000438473. [DOI] [PubMed] [Google Scholar]
- 20.Smagris E., BasuRay S., Li J., et al. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology. 2015;61(1):108–118. doi: 10.1002/hep.27242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondul A., Mancina R. M., Merlo A., et al. PNPLA3 I148M variant influences circulating retinol in adults with nonalcoholic fatty liver disease or obesity. Journal of Nutrition. 2015;145(8):1687–1691. doi: 10.3945/jn.115.210633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pirazzi C., Valenti L., Motta B. M., et al. PNPLA3 has retinyl-palmitate lipase activity in human hepatic stellate cells. Human Molecular Genetics. 2014;23(15):4077–4085. doi: 10.1093/hmg/ddu121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruschi F. V., Tardelli M., Claudel T., Trauner M. PNPLA3 expression and its impact on the liver: current perspectives. Hepatic Medicine: Evidence and Research. 2017;Volume 9:55–66. doi: 10.2147/HMER.S125718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozlitina J., Smagris E., Stender S., et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nature Genetics. 2014;46(4):352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y.-L., Reeves H. L., Burt A. D., et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nature Communications. 2014;5, article 4309 doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L. Z., Xia H. H., Xin Y. N., Lin Z. H., Xuan S. Y. TM6SF2 E167K Variant, a novel genetic susceptibility variant, contributing to nonalcoholic fatty liver disease. Journal of Clinical and Translational Hepatology. 2015;3(4):265–270. doi: 10.14218/JCTH.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bugianesi E., Manzini P., D'Antico S., et al. Relative contribution of iron burden, HFE mutation and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39(1):179–187. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- 28.Nelson J. E., Bhattacharya R., Lindor K. D., et al. HFE C282Y mutations are associated with advanced hepatic fibrosis in caucasians with nonalcoholic steatohepatitis. Hepatology. 2007;46(3):723–729. doi: 10.1002/hep.21742. [DOI] [PubMed] [Google Scholar]
- 29.Ye Q., Qian B.-X., Yin W.-L., Wang F.-M., Han T. Association between the HFE C282Y, H63D polymorphisms and the risks of non-alcoholic fatty liver disease, liver cirrhosis and hepatocellular carcinoma: An updated systematic review and meta-analysis of 5,758 cases and 14,741 controls. PLoS ONE. 2016;11(9) doi: 10.1371/journal.pone.0163423.e0163423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mancina R. M., Dongiovanni P., Petta S., et al. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230. doi: 10.1053/j.gastro.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thabet K., Chan H. L. Y., Petta S., et al. The membrane-bound O-acyltransferase domain-containing 7 variant rs641738 increases inflammation and fibrosis in chronic hepatitis B. Hepatology. 2017;65(6):1840–1850. doi: 10.1002/hep.29064. [DOI] [PubMed] [Google Scholar]
- 32.Schulze K., Imbeaud S., Letouzé E., et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nature Genetics. 2015;47(5):505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang X.-T., Pan K., Chen M.-S., et al. Decreased expression of XPO4 is associated with poor prognosis in hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 2011;26(3):544–549. doi: 10.1111/j.1440-1746.2010.06434.x. [DOI] [PubMed] [Google Scholar]
- 34.Zain S. M., Mohamed R., Cooper D. N., et al. Genome-wide analysis of copy number variation identifies candidate gene loci associated with the progression of non-alcoholic fatty liver disease. PLoS ONE. 2014;9(4) doi: 10.1371/journal.pone.0095604.e95604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iacobazzi V., Castegna A., Infantino V., Andria G. Mitochondrial DNA methylation as a next-generation biomarker and diagnostic tool. Molecular Genetics and Metabolism. 2013;110(1-2):25–34. doi: 10.1016/j.ymgme.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Tryndyak V. P., Han T., Muskhelishvili L., et al. Coupling global methylation and gene expression profiles reveal key pathophysiological events in liver injury induced by a methyl-deficient diet. Molecular Nutrition & Food Research. 2011;55(3):411–418. doi: 10.1002/mnfr.201000300. [DOI] [PubMed] [Google Scholar]
- 37.Liu F., Li H., Chang H., Wang J., Lu J. Identification of hepatocellular carcinoma-associated hub genes and pathways by integrated microarray analysis. TUMORI. 2015;101(2):206–214. doi: 10.5301/tj.5000241. [DOI] [PubMed] [Google Scholar]
- 38.Erstad D. J., Fuchs B. C., Tanabe K. K. Molecular signatures in hepatocellular carcinoma: A step toward rationally designed cancer therapy. Cancer. 2018;124(15):3084–3104. doi: 10.1002/cncr.31257. [DOI] [PubMed] [Google Scholar]
- 39.Cheung O., Puri P., Eicken C., et al. Nonalcoholic steatohepatitis is associated with altered hepatic microRNA expression. Hepatology. 2008;48(6):1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takaki Y., Saito Y., Takasugi A., et al. Silencing of microRNA-122 is an early event during hepatocarcinogenesis from non-alcoholic steatohepatitis. Cancer Science. 2014;105(10):1254–1260. doi: 10.1111/cas.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Conti A., Ortega J. F., Tryndyak V., et al. MicroRNA deregulation in nonalcoholic steatohepatitisassociated liver carcinogenesis. Oncotarget . 2017;8(51):88517–88528. doi: 10.18632/oncotarget.19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalid A., Hussain T., Manzoor S., Saalim M., Khaliq S. PTEN: A potential prognostic marker in virus-induced hepatocellular carcinoma. Tumor Biology. 2017;39(6) doi: 10.1177/1010428317705754.101042831770575 [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Qi X., Zeng Z., et al. CRISPR/Cas9-mediated p53 and Pten dual mutation accelerates hepatocarcinogenesis in adult hepatitis B virus transgenic mice. Scientific Reports. 2017;7(1, article 2796) doi: 10.1038/s41598-017-03070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu Z., Hu J., Cao H., et al. Loss of Pten synergizes with c-Met to promote hepatocellular carcinoma development via mTORC2 pathway. Experimental & Molecular Medicine. 2018;50(1, article e417) doi: 10.1038/emm.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Minicis S., Agostinelli L., Rychlicki C., et al. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS ONE. 2014;9(5) doi: 10.1371/journal.pone.0097136.e97136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janku F., Kaseb A. O., Tsimberidou A. M., Wolff R. A., Kurzrock R. Identification of novel therapeutic targets in the PI3K/AKT/mTOR pathway in hepatocellular carcinoma using targeted next generation sequencing. Oncotarget . 2014;5(10):3012–3022. doi: 10.18632/oncotarget.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang S., Liu G. Targeting the RAS/RAF/MEK/ERK pathway in hepatocellular carcinoma. Oncology Letters. 2017;13(3):1041–1047. doi: 10.3892/ol.2017.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kudo Y., Tanaka Y., Tateishi K., et al. Altered composition of fatty acids exacerbates hepatotumorigenesis during activation of the phosphatidylinositol 3-kinase pathway. Journal of Hepatology. 2011;55(6):1400–1408. doi: 10.1016/j.jhep.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 49.Chettouh H., Lequoy M., Fartoux L., Vigouroux C., Desbois-Mouthon C. Hyperinsulinaemia and insulin signalling in the pathogenesis and the clinical course of hepatocellular carcinoma. Liver International. 2015;35(10):2203–2217. doi: 10.1111/liv.12903. [DOI] [PubMed] [Google Scholar]
- 50.Hirsova P., Ibrabim S. H., Gores G. J., Malhi H. Thematic review series: Lipotoxicity: Many roads to cell dysfunction and cell death lipotoxic lethal and sublethal stress signaling in hepatocytes: Relevance to NASH pathogenesis. Journal of Lipid Research. 2016;57(10):1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Afonso M. B., Rodrigues P. M., Carvalho T., et al. Necroptosis is a key pathogenic event in human and experimental murine models of non-alcoholic steatohepatitis. Clinical Science. 2015;129(8):721–739. doi: 10.1042/CS20140732. [DOI] [PubMed] [Google Scholar]
- 52.Gautheron J., Vucur M., Reisinger F., et al. A positive feedback loop between RIP3 and JNK controls non-alcoholic steatohepatitis. EMBO Molecular Medicine. 2014;6(8):1062–1074. doi: 10.15252/emmm.201403856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu S., Yang L., Li P., et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473(7348):528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bozaykut P., Sahin A., Karademir B., Ozer N. K. Endoplasmic reticulum stress related molecular mechanisms in nonalcoholic steatohepatitis. Mechanisms of Ageing and Development. 2016;157:17–29. doi: 10.1016/j.mad.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 55.Novo E., Parola M. Redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis & Tissue Repair. 2008;1, article 5 doi: 10.1186/1755-1536-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nelson J. E., Wilson L., Brunt E. M., et al. Relationship between the pattern of hepatic iron deposition and histological severity in nonalcoholic fatty liver disease. Hepatology. 2011;53(2):448–457. doi: 10.1002/hep.24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Onal G., Kutlu O., Gozuacik D., Dokmeci Emre S. Lipid droplets in health and disease. Lipids in Health and Disease. 2017;16, article 128 doi: 10.1186/s12944-017-0521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L., Liao J.-Z., He X.-X., Li P.-Y. The role of autophagy in hepatocellular carcinoma: Friend or foe. Oncotarget . 2017;8(34):57707–57722. doi: 10.18632/oncotarget.17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mao Y., Yu F., Wang J., Guo C., Fan X. Autophagy: a new target for nonalcoholic fatty liver disease therapy. Hepatic Medicine: Evidence and Research. 2016;8:27–37. doi: 10.2147/HMER.S98120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He G., Karin M. NF-κB and STAT3- key players in liver inflammation and cancer. Cell Research. 2011;21(1):159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Park E. J., Lee J. H., Yu G.-Y., et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jia L., Vianna C. R., Fukuda M., et al. Hepatocyte toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nature Communications. 2014;5, article 3878 doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Min H., Mirshahi F., Verdianelli A., et al. Activation of the GP130-STAT3 axis and its potential implications in nonalcoholic fatty liver disease. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2015;308(9):G794–G803. doi: 10.1152/ajpgi.00390.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun Q., Jiang N., Sun R. Leptin signaling molecular actions and drug target in hepatocellular carcinoma. Drug Design, Development and Therapy. 2014;8:2295–2302. doi: 10.2147/DDDT.S69004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stefanou N., Papanikolaou V., Furukawa Y., Nakamura Y., Tsezou A. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC Cancer. 2010;10, article 442 doi: 10.1186/1471-2407-10-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xing S.-Q., Zhang C.-G., Yuan J.-F., Yang H.-M., Zhao S.-D., Zhang H. Adiponectin induces apoptosis in hepatocellular carcinoma through differential modulation of thioredoxin proteins. Biochemical Pharmacology. 2015;93(2):221–231. doi: 10.1016/j.bcp.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 67.Shen J., Yeh C.-C., Wang Q., Gurvich I., Siegel A. B., Santella R. M. Plasma adiponectin and hepatocellular carcinoma survival among patients without liver transplantation. Anticancer Reseach. 2016;36(10):5307–5314. doi: 10.21873/anticanres.11103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carbone F., la Rocca C., Matarese G. Immunological functions of leptin and adiponectin. Biochimie. 2012;94(10):2082–2088. doi: 10.1016/j.biochi.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 69.Nannipieri M., Cecchetti F., Anselmino M., et al. Pattern of expression of adiponectin receptors in human liver and its relation to nonalcoholic steatohepatitis. Obesity Surgery. 2009;19(4):467–474. doi: 10.1007/s11695-008-9701-x. [DOI] [PubMed] [Google Scholar]
- 70.Ma C., Kesarwala A. H., Eggert T., et al. NAFLD causes selective CD4+ T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531(7593):253–257. doi: 10.1038/nature16969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wolf M., Adili A., Piotrowitz K., et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26(4):549–564. doi: 10.1016/j.ccell.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Lanthier N. Targeting Kupffer cells in non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: why and how? World Journal of Hepatology. 2015;7(19):2184–2188. doi: 10.4254/wjh.v7.i19.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin-Murphy B. V., You Q., Wang H., et al. Mice lacking natural killer T cells are more susceptible to metabolic alterations following high fat diet feeding. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0080949.e80949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian Z., Chen Y., Gao B. Natural killer cells in liver disease. Hepatology. 2013;57(4):1654–1662. doi: 10.1002/hep.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng X., Zeng W., Gai X., et al. Role of the Hedgehog pathway in hepatocellular carcinoma (Review) Oncology Reports. 2013;30(5):2020–2026. doi: 10.3892/or.2013.2690. [DOI] [PubMed] [Google Scholar]
- 76.Della Corte C. M., Viscardi G., Papaccio F., et al. Implication of the Hedgehog pathway in hepatocellular Carcinoma. World Journal of Gastroenterology. 2017;23(24):4330–4340. doi: 10.3748/wjg.v23.i24.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Noverr M. C., Huffnagle G. B. Does the microbiota regulate immune responses outside the gut? Trends in Microbiology. 2004;12(12):562–568. doi: 10.1016/j.tim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 78.Żak-Gołąb A., Olszanecka-Glinianowicz M., Kocełak P., Chudek J. The role of gut microbiota in the pathogenesis of obesity. Postepy Higieny i Medycyny Doswiadczalnej. 2014;68:84–90. doi: 10.5604/17322693.1086419. [DOI] [PubMed] [Google Scholar]
- 79.Valentini M., Piermattei A., Di Sante G., Migliara G., Delogu G., Ria F. Immunomodulation by gut microbiota: Role of toll-like receptor expressed by T cells. Journal of Immunology Research. 2014;2014:8. doi: 10.1155/2014/586939.586939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brandl K., Schnabl B. Intestinal microbiota and nonalcoholic steatohepatitis. Current Opinion in Gastroenterology. 2017;33(3):128–133. doi: 10.1097/MOG.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bashiardes S., Shapiro H., Rozin S., Shibolet O., Elinav E. Non-alcoholic fatty liver and the gut microbiota. Molecular Metabolism. 2016;5(9):782–794. doi: 10.1016/j.molmet.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruiz A. G., Casafont F., Crespo J., et al. Lipopolysaccharide-binding protein plasma levels and liver TNF-alpha gene expression in obese patients: evidence for the potential role of endotoxin in the pathogenesis of non-alcoholic steatohepatitis. Obesity Surgery. 2007;17(10):1374–1380. doi: 10.1007/s11695-007-9243-7. [DOI] [PubMed] [Google Scholar]
- 83.Brun P., Castagliuolo I., Pinzani M., Palù G., Martines D. Exposure to bacterial cell wall products triggers an inflammatory phenotype in hepatic stellate cells. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2005;289(3):G571–G578. doi: 10.1152/ajpgi.00537.2004. [DOI] [PubMed] [Google Scholar]
- 84.Borrelli A., Bonelli P., Tuccillo F. M., et al. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biology. 2018;15:467–479. doi: 10.1016/j.redox.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshimoto S., Loo T. M., Atarashi K., et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499(7456):97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 86.Nagasue N., Yu L., Yukaya H., Kohno H., Nakamura T. Androgen and oestrogen receptors in hepatocellular carcinoma and surrounding liver parenchyma: Impact on intrahepatic recurrence after hepatic resection. British Journal of Surgery. 1995;82(4):542–547. doi: 10.1002/bjs.1800820435. [DOI] [PubMed] [Google Scholar]
- 87.Awuah P. K., Monga S. P. Cell cycle-related kinase links androgen receptor and β-catenin signaling in hepatocellular carcinoma: Why are men at a loss? Hepatology. 2012;55(3):970–974. doi: 10.1002/hep.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kanda T., Jiang X., Yokosuka O. Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers. World Journal of Gastroenterology. 2014;20(28):9229–9236. doi: 10.3748/wjg.v20.i28.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin H.-Y., Yu I.-C., Wang R.-S., et al. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology. 2008;47(6):1924–1935. doi: 10.1002/hep.22252. [DOI] [PubMed] [Google Scholar]
- 90.Norlin M., Pettersson H., Tang W., Wikvall K. Androgen receptor-mediated regulation of the anti-atherogenic enzyme CYP27A1 involves the JNK/c-jun pathway. Archives of Biochemistry and Biophysics. 2011;506(2):236–241. doi: 10.1016/j.abb.2010.11.023. [DOI] [PubMed] [Google Scholar]