Abstract
Objectives of the Study
Summary of observational studies concerning the pharmacological management of diabetic macular edema (DME).
Methods
A literature review was conducted using the PubMed database on 1 February 2018 to identify studies evaluating the efficacy of anti-VEGF and dexamethasone (DEX) implants for DME. Studies with more than 10 patients and follow-up of more than 6 months were selected. Analyses were carried out on the overall population and on subgroups defined according to baseline visual acuity (BVA) and the patients' naïve or non-naïve status.
Results
Thirty-two studies evaluating the efficacy of anti-VEGF and 31 studies evaluating the efficacy of DEX-implants were retained, concerning 6,842 and 1,703 eyes, respectively. A mean gain of +4.7 letters for a mean of 5.8 injections (mean follow-up: 15.6 months) and +9.6 letters for a mean of 1.6 injections (10.3 months) was found in the anti-VEGF and DEX-implant studies, respectively. Final VA appears to be similar for both treatment (62 letters for anti-VEGF, 61.2 letters for DEX-implant), and BVA appears lower for DEX-implant, which may partially explain the greater visual gain. The DEX-implant studies show greater gains in VA compared to the anti-VEGF studies, especially for higher BVA. Indeed, mean gains for the subgroups of patients with BVA<50 letters, 50<BVA<60 letters, and BVA>60 letters are +4.3, +5.8, and +3.1 letters, respectively, in the anti-VEGF studies and +10.5, +9.3, and +8.8 letters, respectively, in the DEX-implant studies. Regarding the patient's initial status, only naïve status appears to confer the best functional response in DEX-implant studies.
Conclusion
Observational studies investigating DEX-implant report clinically similar final VA when compared to anti-VEGF, but superior visual gains in real-life practice. This latter difference could be due to the better BVA, but also to the fact that less injections were administered in the anti-VEGF observational studies than in the interventional studies.
1. Introduction
Diabetic macular edema (DME) is one of the clinical manifestations of diabetic retinopathy (DR). It is the leading cause of reduced visual acuity and visual impairment in diabetic patients [1, 2]. It develops in approximately 30% of patients who have had diabetes for at least 20 years [3]. Diabetes decreases life expectancy by a mean of 8 years, and 50% of patients with diabetes will die from a cardiovascular event [4]. DME in these patients increases cardiovascular morbidity and mortality by a factor of 2 [5].
The management of DME has changed significantly in recent years. For several decades, laser photocoagulation [6] was the standard treatment for patients with clinically significant DME, leaving macular scars that increase in size over time and can cause secondary vision loss [7]. Since the advent of new pharmacological treatments such as anti-VEGF (vascular endothelial growth factor) and corticosteroids, the management of DME patients has evolved considerably. Intravitreal pharmacological treatments have now become one of the first-line treatments for DME. They come with the benefit of less local side effects, but doubts remain about their possible systemic side effects [8]. Three anti-VEGFs, bevacizumab (Avastin®, Genentech Inc., San Francisco, CA, USA), ranibizumab (Lucentis®, Novartis, Basel, Switzerland), and aflibercept (Eylea®, Bayer, Leverkusen, Germany), and a slow release corticosteroid implant (Ozurdex®, Allergan Inc., Irvine, California) delivering 700 micrograms of dexamethasone (DEX) into the vitreous are used to treat DME. Ranibizumab, aflibercept, and the DEX-implant have been granted European Marketing Authorization (EMA) worldwide for this indication, but this is not the case for bevacizumab. This authorization was obtained based on the data from the pivotal interventional studies. Most of the relevant interventional clinical trials have been performed on carefully selected patient populations. The argument for this strict selection of patients is to ensure that confounding factors do not mask the effect of the treatment. Nevertheless, findings based on a strictly selected patient population cannot be extrapolated to a broader panel of unselected patients. This is a major problem when treatment guidelines are intended for use in routine practice. Indeed, these randomized, controlled studies, with their necessarily stringent inclusion and exclusion criteria, do not obtain data on patients with very high or very low baseline visual acuity (BVA) or with certain comorbidities or on non-naïve patients. In addition, these patients constitute a motivated and observant population; for example, in the pivotal studies patients received a monthly injection over a prolonged time period of 36 months [9, 10]. One can legitimately wonder if the patients treated in routine clinical practice resemble the populations included in the clinical studies. In addition, real-life treatment regimens, i.e., in observational studies, cannot be as stringent as in interventional studies, which often leads to poorer adherence [11, 12]. It is therefore useful to inform our daily practice, not only with the results of interventional studies, but also with the findings of the so-called “real-life” observational studies. The drawbacks of the latter are the potential biases (patients lost to follow-up, missing data) and the lower level of evidence compared to interventional studies. It is therefore vital to consider a significant number of real-life studies, in order to draw valid conclusions.
The objective of this work is therefore to synthetize the available observational studies concerning the pharmacological management of DME.
2. Methods
A review of the literature was conducted on the PubMed database on February 1, 2018, to identify all articles investigating the efficacy of anti-VEGF and DEX-implants for treating DME. The key words used were as follows: diabetic macular edema (DME) AND ranibizumab, DME AND Lucentis, DME AND bevacizumab, DME AND Avastin, DME AND aflibercept, DME AND Eylea, DME AND dexamethasone implant, DME AND Ozurdex, DME AND ranibizumab AND aflibercept AND bevacizumab AND dexamethasone implant, and finally DME AND Lucentis AND Avastin AND Eylea AND Ozurdex.
Only articles published in English were selected. Only the ranibizumab, aflibercept, bevacizumab, and dexamethasone implant molecules were retained. Two study designs were found: randomized pivotal studies and observational “real-life” studies. Only the observational studies were selected for this work. Of the studies investigating the efficacy of anti-VEGF and DEX-implant, only series with an initial enrollment of more than 10 patients and follow-up of more than six months were included in the final analysis. For any given study, if different anti-VEGF drugs were used or different types of patients included (for instance naïve versus non-naïve patients), we presented the results separated into different groups of treatment.
The visual acuity (VA) or gain values used for this work were the primary objectives from each study. For the anti-VEGF studies, the VA or gain values used were the end-of-study data, and for the DEX-implant studies, the primary effectiveness endpoints were the maximum mean change in best corrected visual acuity (BCVA) (best improvement) from baseline after each DEX injection. This criterion for DEX-implant was validated by the Food and Drug Administration (FDA) and used in the Reinforce study [13]. VA data expressed in logMAR were converted to the ETDRS score in order to evaluate VA gain or loss relative to the baseline data.
In order to report on functional efficacy, a comparison of VA gains, final VA, and the number of anti-VEGF injections or DEX-implants was initially conducted on the overall study population. Secondary analyses of subgroups, formed according to BVA (less than 50 letters, between 50 and 60 letters, and greater than 60 letters) and the naïve or non-naïve status of the patient at baseline, were also performed. In the case of switching therapy, a minimum wash out time of 1 month was observed in all the studies. The results are presented with the mean gain value and range (minimum and maximum gain observed in studies).
3. Results
Our PubMed search initially screened 189 studies, 129 studies of anti-VEGF, and 60 studies of DEX-implants. After eliminating the interventional studies and applying our search criteria (follow-up ≥ 6 months and a minimum of 10 patients included), a total of 32 studies (38 groups of treatment) evaluating the efficacy of anti-VEGF [14–45] and 31 studies (35 groups of treatment) evaluating the efficacy of DEX-implants [13, 26, 46–74] were retained, concerning a total of 6,842 eyes and 1,703 eyes, respectively, for the period between 2005 and 2016.
3.1. Overall Population
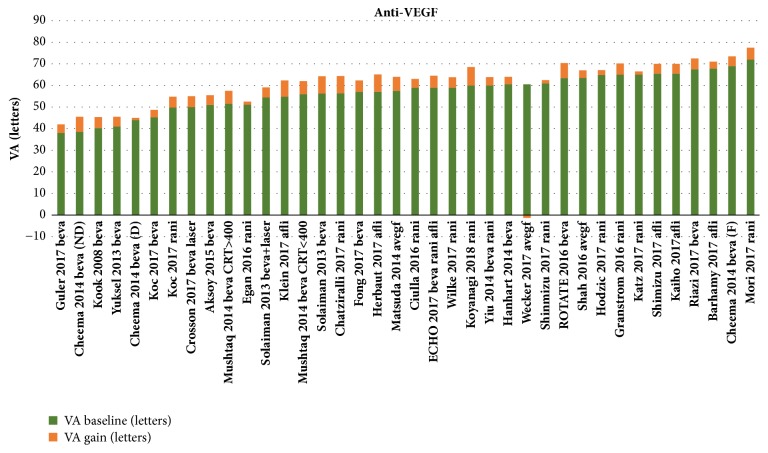
For the anti-VEGF studies, patients had a mean BVA of 57.3 letters (range 38-72 letters). Mean follow-up was 15.6 months (6-48 months). During follow-up, a mean gain of + 4.7 letters (-5 - +8.5 letters) (median 4.7 letters) was observed for a mean of 5.8 intravitreal injections (IVI) (1.3-17) (Figures 1 and 2). The mean final VA was 62 letters (42-77.5 letters). Figure 1 shows the BVA and gain values obtained during follow-up, and the sum of the two which corresponds to final VA, for all of these studies.
Figure 1.
Summary of observational studies investigating the efficacy of anti-VEGF in the treatment of diabetic macular edema.
Figure 2.
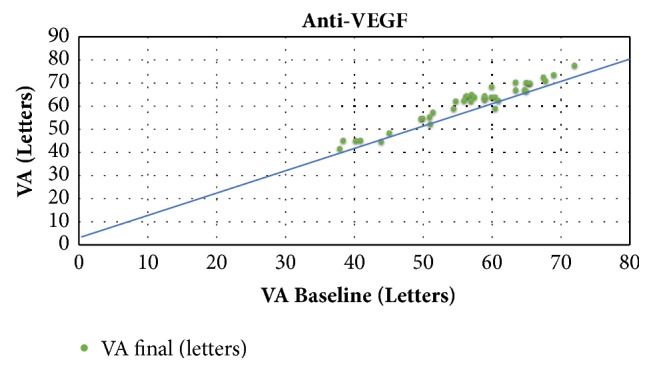
Final visual acuity as a function of baseline visual acuity in studies evaluating the efficacy of anti-VEGF.
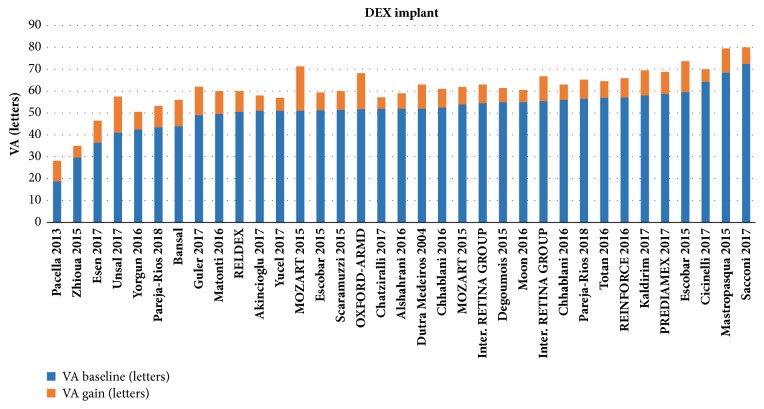
For the DEX-implant studies, patients had a mean BVA of 51.5 letters (range 18.8-72.5 letters). Mean follow-up was 10.3 months (6-36 months). During follow-up, a mean maximum gain of + 9.6 letters (+5.2 - +20.2 letters) was observed for a mean number of 1.6 IVI (1-3.9) (Figures 3 and 4). The maximum mean VA was 61.2 letters (28.2-80 letters).
Figure 3.
Summary of observational studies investigating the efficacy of the dexamethasone implant in the treatment of diabetic macular edema.
Figure 4.
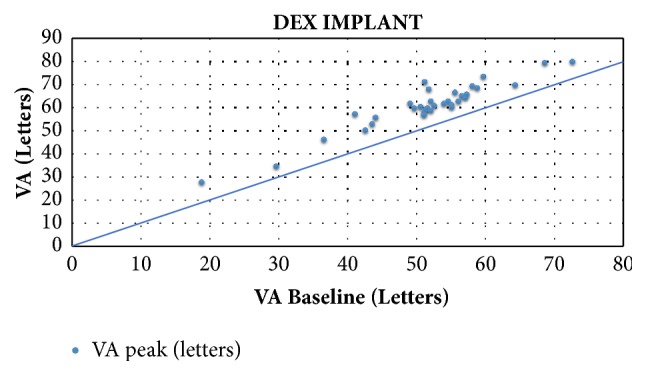
Final visual acuity as a function of baseline visual acuity in studies evaluating the efficacy of the dexamethasone implant.
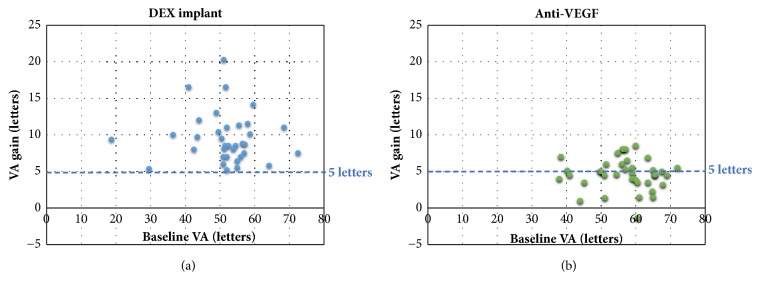
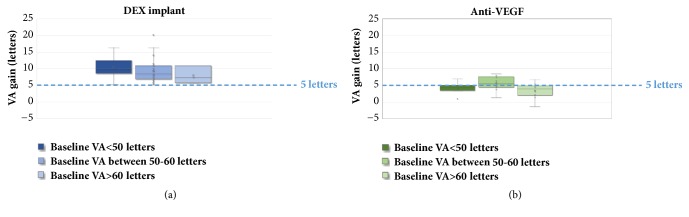
Figure 5 illustrates the mean gain on the same scale of visual acuity gains, according to the BVA in the different observational studies assessing the efficacy of anti-VEGF and dexamethasone implants for treating diabetic macular edema.
Figure 5.
Comparison of mean gain in the different observational studies investigating the efficacy of dexamethasone implant (Figure 5(a)) and anti-VEGF (Figure 5(b)).
3.2. Segmentation according to the Patient's Baseline Status
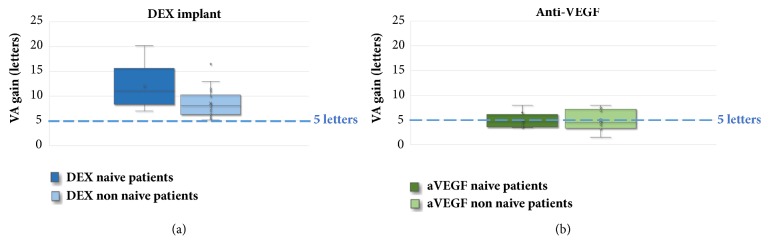
By analyzing the results according to the patient's baseline status, we found, in the anti-VEGF studies, a mean gain of + 5 letters for 5.2 IVI in naïve patients (BVA 56 letters) [15, 19, 24, 27, 28, 30, 37] and + 4.8 letters for 6.2 IVI in patients non-naïve to one or more previous treatments (BVA 56.9 letters) [16, 20, 21, 23, 29, 31, 32, 38, 42].
In the DEX-implant studies, there was a mean gain of + 12 letters for 1.9 IVI in naïve patients (BVA 57.9 letters) [50, 54, 56, 59, 62, 65] and + 8.6 letters for 1.4 IVI in non-naïve patients (BVA 48.7 letters) [26, 46, 47, 49–57, 64, 69–73] (Figure 6).
Figure 6.
Visual acuity gain in ETDRS letters according to the patients' naïve or non-naïve status in the different observational studies with dexamethasone implant (a) and anti-VEGF (b).
In the anti-VEGF studies, the non-naïve patients had received a mean of at least 10.7 treatments (other anti-VEGF, Triamcinolone IVT, DEX IVT, focal laser) prior to inclusion, compared to 5.4 in the DEX-implant studies.
3.3. Segmentation according to BVA
For subgroups with low BVA (<50 letters), there is a mean gain of +4.3 letters in the anti-VEGF studies [26, 28, 29, 32, 34] (mean BVA of 42.4 letters) and +10.5 letters in the DEX-implant studies [48, 55, 56, 61, 65, 66, 71, 73] (mean BVA of 39.4 letters). Mean follow-up was 13 months for a mean of 3 IVI and 9 months for a mean of 1.2 IVI, respectively.
For subgroups with BVA of between 50 and 60 letters, there is a mean gain of + 5.8 letters in the anti-VEGF studies [14, 15, 21, 23–25, 33, 35, 37–39, 45] (mean BVA of 55.7 letters) and + 9.3 letters in the DEX-implant studies [13, 46, 47, 49, 50, 52–54, 56, 57, 61–63, 65–67, 69, 71, 74] (mean BVA of 54.1 letters) with mean follow-up of 16.3 months for a mean of 5.8 IVI and 11.6 months for a mean of 1.75 IVI, respectively.
Finally, for subgroups with high BVA (>60 letters), there is a mean gain of + 3.1 letters in the anti-VEGF studies [16–18, 20, 22, 30, 31, 34, 40–42, 44] (mean BVA of 65.3 letters) and + 8.8 letters in the DEX-implant studies [51, 59, 60, 74] (BVA mean of 68.4 letters). Mean follow-up was, respectively, 13.5 months for a mean of 6.5 IVI and 9 months for a mean of 1.8 IVI (Figure 7) (Table 1).
Figure 7.
Visual acuity gain (in letters ETDRS) according to the 3 baseline visual acuity subgroups in the observational studies with dexamethasone implant (a) and anti-VEGF (b).
Table 1.
Summary of BVA, gain, final VA and mean number of injections in the overall population and subgroups for anti-VEGF and DEX-implant observational studies (BVA: baseline visual acuity, IVI: intravitreal injection.
| Anti-VEGF | DEX-implant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of eyes | BVA (letters) | Mean gain (letters) | Final VA (letters) | Mean follow-up (months) | Mean IVI | Number of eyes | BVA (letters) | Mean gain (letters) | Max VA (letters) | Mean follow-up (months) | Mean IVI | |
| Overall population | 6842 | 57.3 | +4.7 | 62 | 15.6 | 5.8 | 1703 | 51.5 | +9.6 | 61.2 | 10.3 | 1.6 |
| BVA | ||||||||||||
| ≤ 50 letters | 449 | 42.4 | +4.3 | 46.7 | 13 | 3 | 363 | 39.4 | +10.5 | 49.9 | 9 | 1.2 |
| 50-60 letters | 4773 | 55.7 | +5.8 | 61.7 | 16.3 | 5.8 | 1218 | 54.1 | +9.3 | 63.7 | 11.6 | 1.75 |
| ≥ 60 letters | 1620 | 65.3 | +3.1 | 68.3 | 13.5 | 6.5 | 122 | 68.4 | +8.8 | 76.5 | 9 | 1.8 |
| Initial Status | ||||||||||||
| Naive patients | 781 | 56 | +5 | 61 | 12.3 | 5.2 | 176 | 57.9 | + 12 | 69.9 | 10.8 | 1.9 |
| Non-naïve patients | 413 | 56.9 | + 4.8 | 61.8 | 12.1 | 6.2 | 801 | 48.7 | +8.6 | 57.3 | 9.2 | 1.4 |
Table 1 summarizes all the results of this study.
4. Discussion
Since anti-VEGF and DEX-implants came onto the market, the therapeutic practices for DME have evolved, and laser photocoagulation treatments have gradually been abandoned in favor of IVI. Indeed, the impressive results obtained in clinical trials have encouraged practitioners to use these pharmacological treatments which offer much higher VA gains [9, 10, 75–85] than laser treatment. Indeed, the latter, although it does reduce macular thickness by 50% at 3 years, only rarely improves visual acuity [86]. However, the patients included in the therapeutic trials investigating these pharmacological treatments are selected according to very specific criteria, which do not necessarily correspond to all the patients treated in routine practice. The aim of the present study was to synthetize all the observational studies investigating ranibizumab, aflibercept, bevacizumab, and DEX-implants for DME, in order to evaluate their effectiveness in “real-life” situations.
Indeed, these types of studies have numerous advantages over interventional studies: they provide a more accurate reflection of routine practice; confirm the effectiveness of a treatment under real conditions; include unselected patients under a regimen based on day-to-day practice (actual injection intervals and follow-up); provide complementary data to the interventional studies (notably on the state of practice and possible comparisons between countries); and also provide long-term data. On the other hand, these studies have a number of potential drawbacks, including the possibility of introducing bias (missing data, patients lost to follow-up) and a lower level of evidence. It is therefore essential to analyze a significant number of observational studies and to synthetize their findings, in order to draw scientifically valid conclusions from them.
Real-life observational studies tend to confirm the level of efficacy obtained in interventional studies of pharmaceutical treatments for DME. Indeed, observational studies of anti-VEGF and DEX-implants show VA gains of up to +20 letters. Analysis of the data from these observational studies suggests that the gain in VA is greater with DEX-implant than with anti-VEGF. Indeed, the mean maximum gain obtained after DEX-implant IVI (+9.6 letters) is higher than the mean gain obtained after anti-VEGF (+ 4.7 letters). This increased gain was obtained with a smaller number of IVI in the DEX group compared to the anti-VEGF group. In addition, by setting a gain threshold of 5 letters, the majority of real-life DEX studies achieved increases above this value, in contrast to the majority of anti-VEGF studies (Figure 5).
However, no difference was found in final visual acuity, which is around 62 letters for both groups. This result, which could contradict the observed gains in VA, can be partially explained by the BVA. Indeed, the latter is lower in the DEX studies (BVA 51.5 letters) compared to the anti-VEGF studies (BVA 57.3 letters). This lower level of VA in the DEX studies may relate to the fact that DEX-implant remains a second-line treatment in routine practice [8], thus explaining that the patients included in these studies are mostly non-naïve patients, with a long duration of DME, and therefore lower VA. Regarding the studies selected here, 9/32 anti-VEGF studies only concern non-naïve patients [16, 20, 21, 23, 29, 31, 32, 38, 42] with a mean duration of DME of 30.5 months, compared to 19/31 for DEX studies [13, 26, 46, 47, 49–57, 64, 69–73] with a similar duration of DME (26.8 months). Nevertheless, the mean numbers of treatments are quite different between the anti-VEGF studies and DEX studies with a mean number of 10.7 and 5.4 treatments before switching, respectively, which could constitute a bias.
Thus, in the RELDEX study [67], which is the observational study with the largest number of DME patients included, with a long-term follow-up of 3 years, we found a mean duration of DME of 24.7 months, with only 26.5% naïve patients. This is mainly explained by the fact that the DEX-implant obtained its EMA label later, and the patients included were mostly previously treated with anti-VEGF IVI, focal laser, or triamcinolone IVI. Although non-naïve patients switched to DEX-implants have a gain greater than one ETDRS line, naïve patients treated with this same molecule seem to have much higher gains compared to anti-VEGF, above two ETDRS lines. This difference between naïve and non-naïve patients seems less obvious in anti-VEGF studies. Thus, in the observational studies with anti-VEGF, there do not seem to be any clinically significant differences between naïve and non-naïve patients, with an average gain of + 5 letters for 5.2 IVI and + 4.8 letters for 6.2 IVI.
All these data argue in favor of the earlier use of DEX-implant, either as first-line therapy in naïve patients or more quickly as a second-line therapy. Indeed, in the literature, the response to anti-VEGF seems predictable after 3 to 6 injections [87] and not dependent on the number of injections [88].
Moreover, our analysis of the observational studies shows that patients with low BVA can potentially gain a substantial number of letters. Indeed, Figure 5 also highlights the fact that despite low BVA there are still gains in visual acuity, which are sometimes very significant, and this is especially true in the DEX studies. Indeed, Esen et al. report a maximum gain of + 10 letters in a population whose BVA was 36.5(±9.3) letters [55]. These data show that a functional benefit can be expected even in advanced DME with low BVA. Nevertheless, final VA is ultimately related to baseline VA, which is why it is important to initiate treatment as early as possible: the higher the baseline VA, the higher the final VA (Figures 2 and 4). However, BVA is not the only biomarker and other predictors exist. Indeed, it is clear from Figure 5 that the gains are variable for the same level of BVA. BVA in anti-VEGF and DEX-implant studies is therefore probably not the only explanation for the superior VA gains found in the DEX-implant studies. This assumption is illustrated in Figure 6 which represents the visual acuity gain for each population segment in relation to BVA (less than 50 letters, between 50 and 60 letters, and greater than 60 letters).
In the subgroup with low BVA (<50 letters), there is a marked difference between the anti-VEGF (+4.3 letters) and the DEX-implant (+10.5 letters) studies, whereas BVA is relatively similar (42.4 in anti -VEGF versus 39.4 in DEX-implant studies). This difference is also present in the BVA subgroups between 50 and 60 letters with a mean gain of +5.3 letters and +9.3 letters in the anti-VEGF studies (BVA 57.7 letters) and DEX-implant studies (BVA 54.1 letters), respectively. However, the greatest difference is in the subgroups with BVA of more than 60 letters with a mean gain of +3.1 letters (BVA 65.3 letters) and + 8.8 letters (BVA 68.4 letters), respectively.
Therefore, even taking into account the possibility of a ceiling effect, the difference in gain in favor of DEX-implant in this last subgroup shows that the mean VA gain, which seems better in real life with the DEX-implant, is not only due to the lower mean baseline VA for this molecule, as it persists in the high BVA subgroup (greater than 60 letters).
In comparison to the interventional studies, the observational DEX-implant studies appear to yield better results in terms of VA gain. Indeed, the MAGGIORE study reported a gain of + 2.5L for a mean of 2.9 IVI in the first year [84]. PLACID showed a gain of + 2.4L for 1.7 IVI in the first year [83]. This more limited visual gain in the interventional studies seems to be confirmed at 2 years and 3 years by the BEVORDEX studies (+ 6.9L for 5 IVI at 2 years) [85] and MEAD [82] (+ 2.6L for 4.1 IVI at 3 years). The possibility of retreating at an earlier stage in real life, as opposed to the fixed treatment regimens required for interventional studies (IVI every 6 months for MEAD [82], 5 months for MAGGIORE [84]), and also the large number of non-naïve patients in the pivotal study (duration of DME 23.6 months in MEAD and a low proportion of naïve patients 29.6% [82]) probably explain this difference in results.
On the other hand, the anti-VEGF studies show precisely the opposite, with better results obtained in the interventional studies compared to the observational studies. Indeed, the interventional studies report a higher gain both for studies using a frequent injection schema, such as RESOLVE (gain of + 10.3L for 10.2 IVI in the first year) [77], RISE / RIDE (+ 11.1 letters for 10.7 IVI) [9], or VIVID (gain of + 10.7 letters for the 8.7 IVI group 2q8) and VISTA (gain of + 10.7 letters for 8.4 IVI group 2q8) [10], and for studies using less frequent injection regimens, such as RESTORE (+ 6.8 letters for 7.4 IVI) [79]. This difference in visual acuity gain observed in the observational studies is probably directly related to the number of IVI. Indeed, the mean number of injections in real life is 4.7 in present review, whereas it exceeds a mean of 7 in randomized controlled trials. Indeed, in real life, it is more difficult to monitor and inject a diabetic patient on a monthly basis or more, especially given the well-known issues with compliance, notably during the first years of DME management. These data (VA gain directly related to the number of IVI) seem to be also confirmed for AMD [89–92] and retinal venous occlusions [93, 94]. These results underscore the fact that patients appear undertreated in real-life anti-VEGF studies, and these findings should be explained to the patient at the first consultation to encourage them to accept and schedule a sufficient, high number of injections over the first, and even the second, year of treatment.
Concerning side effects, cataract formation and increasing the intraocular pressure (IOP) are considered to be the main side effects of intravitreal corticosteroids and seem to be dose dependent [95, 96]. Boyer et al. reported in the pivotal interventional study that rates of cataract-related adverse events in phakic eyes were 67.9% and 20.4% for the 0.7 mg DEX-implant and sham groups, respectively. Increased IOP ≥ 25 mmHg was observed in 32.0% and 4.3% for the 0.7 mg DEX-implant and sham groups, respectively, and IOP ≥ 35 mmHg in 6.6% and 0.9% [82].
Some limitations have been also described with anti-VEGF, including the need for frequent injections, induction of resistance, and tachyphylaxis due to the long-term nature of the treatment [97]. Furthermore, sustained hypertension with anti-VGEF has been described since 2010 onwards. One of the reported risk factors is the number of injections [98]. Recently the DCR-net group reported a cumulative probability of sustained IOP elevation or of the initiation or extension of ocular hypotensive therapy by 3 years of 9.5% for the ranibizumab treatment group versus 3.4% for the sham injection treatment group in protocol I in 582 eyes (hazard ratio of 2.9) [99].
Concerning the real-life studies evaluated in present analysis, similar side effects have been reported in anti-VEGF and DEX studies (Table 1). Concerning the anti-VEGF studies, the incidence of cataract formation was between 0% and 15.4% [14, 20, 21, 24, 26, 28, 41, 42]. None of the studies reported statistically significant elevation of IOP in anti-VEGF studies, but the mean follow-up was only 15 months. However, only Blinder et al. reported an elevation over 10 mmHg in 5.1% of patients [14]. Regarding DEX studies, the incidence of cataract formation was between 0% and 50% [26, 46–74], depending on the number of injections [67]. Concerning IOP, an increase was reported between 0% and 29.5% of studies. The RELDEX study, analyzing 128 DME eyes, used a very strict definition (IOP > 25 mmHg) and found a rate of 10.2% [67]. Pareja-Rios et al., analyzing 113 DME eyes, found a rate of 4% (rise of IOP > 10 mmHg) [65]. Güler logically reported significantly higher IOP elevation in the DEX group than the anti-VEGF one (P<0.001) [26]. Only one patient in the 31 articles analyzed required filtering surgery (0.058%). The SAFODEX study [100] evaluated the tolerance of DEX-implant in 421 consecutives eyes which received 1,000 injections. Ocular hypertension was recorded for 28.5% (120 eyes) of injected eyes over a mean follow-up period of 16.8 months [3–55], but the risk of hypertension in DME eyes was statistically significantly inferior to that in uveitis and RVO eyes. Out of these 120 hypertonic eyes, intraocular pressure lowering medication was required for 31% of eyes, only three patients (affected by RVO) required filtering surgery, and, most importantly, topical treatment alone was sufficient for 97% of the cases. These results demonstrate that safety is easily manageable. Finally, the data are difficult to stack because the definitions of hypertension and the IOP threshold for drug prescription in the different studies are not the same (Table 2).
Table 2.
Summary of anti-VEGF and DEX-implant studies (VA: Visual Acuity, CSMT: central subfield macular thickness, ND: nondeclared, IVI: intravitreal injection, IOP: intraocular pressure).
| Study | Drugs | Study design | Patient status | Number (eyes) | Follow-up (months) | Mean Number IVI | Baseline VA (letters) | Final VA (letters) | Mean VA gain (letters) | IOP | Cataract progression/extraction | Others complications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blinder KJ et al. [14] | Anti-VEGF | Retrospective | Mixed | 156 | 36 | 14.2 | 59.0 | 64.5 | 5.5 | 7.7% IOP ≥ 25 mmHg, 1.3% IOP ≥35 mmHg, rise of IOP ≥10 mmHg in 5.1% | 15.4% | No endophthalmitis |
| Matsuda S et al. [15] | Anti-VEGF | Retrospective | Naïve | 124 | 12 | 5.8 | 57.5 | 64 | 6.5 | ND | ND | ND |
| Shah CP et al. [16] | Anti-VEGF | Prospective | Non-Naïve | 30 | 29.2 | 17.0 | 63.5 | 67 | 3.5 | ND | ND | ND |
| Shimizu N et al. [17] | Anti-VEGF | Retrospective | Mixed | 46 | 6 | 2.7 | 65.5 | 70 | 4.5 | ND | ND | ND |
| Wecker T, et al. [18] | Anti-VEGF | Retrospective | ND | 479 | 12 | 17.0 | 60.5 | 59.2 | -1.3 | ND | ND | ND |
| Yiu G et al. [19] | Anti-VEGF | Retrospective | Naïve | 33 | 6 | 2.7 | 60 | 64 | 3.85 | ND | ND | ND |
| Barhamy B et al. [20] | Aflibercept | Prospective | Non-Naïve | 43 | 6 | 5.0 | 67.8 | 71 | 3.2 | 0% (IOP ≥ 25mmHg or a rise of IOP ≥ 10mmHg) | 0% | None |
| Herbaut A. et al. [21] | Aflibercept | Retrospective | Non-Naïve | 29 | 6 | 3.0 | 57.1 | 65.1 | 8 | ND | ND | ND |
| Kaiho T et al. [22] | Aflibercept | ND | Non-Naïve | 51 | 12 | 3.8 | 65.5 | 70 | 4.5 | ND | ND | ND |
| Klein et al. [23] | Aflibercept | Retrospective | Non-Naïve | 11 | 6 | 4.7 | 54.8 | 62.3 | 7.5 | ND | ND | ND |
| Aksoy S et al. [24] | Bevacizumab | Prospective | Naïve | 20 | 6 | 6.0 | 51.0 | 55.5 | 4.5 | 10% (IOP > 21mmHg) | 2.50% | None |
| Fong DS et al. [25] | Bevacizumab | Retrospective | Mixed (65% Naïve, 30% Laser, 4% Steroïd) | 309 | 24 | 3.1 | 57 | 62.3 | 5.3 | ND | ND | ND |
| Güler E et al. [26] | Bevacizumab | Prospective | ND | 20 | 9 | 6.0 | 38 | 42 | 4 | ND | 0% | None |
| Hanhart J et al. [27] | Bevacizumab | Retrospective | Naïve | 35 | 8 | 3.6 | 60.5 | 64 | 3.5 | ND | ND | ND |
| Koc C et al. [28] | Bevacizumab | Retrospective | Naïve | 90 | 24 | 4.9 | 45.2 | 48.7 | 3.5 | ND | 13.7% | 1 cerebrovascular accident |
| Kook D et al. [29] | Bevacizumab | Prospective | Non-Naïve | 126 | 12 | 2.7 | 40.3 | 45.4 | 5.1 | ND | ND | ND |
| Riazi-Esfahani M et al. [30] | Bevacizumab | ND | Naïve | 46 | 6 | 67.5 | 72.5 | 5 | 6.5% (IOP ≥ 21 mmHg) | 0 | ND | |
| Fechter BS et al. [31] | Bevacizumab | ND | Non-Naïve | 30 | 12 | 9.3 | 63.5 | 70.35 | 6.9 | ND | ND | None |
| Yuksel E et al. [32] | Bevacizumab | Retrospective | Non-Naïve | 71 | 9.8 | 2.0 | 41 | 45.5 | 4.5 | ND | ND | ND |
| Solaiman KAM et al. [33] | Bevacizumab | Prospective | ND | 22 | 14 | 3.3 | 56.3 | 64.3 | 8 | ND | 9.1% | Subconjunctival haemorrhage |
| Cheema HR et al. [34] | Bevacizumab (Diffuse) | Retrospective | ND | 28 | 6 | 1.3 | 44.0 | 45 | 1 | ND | ND | ND |
| Cheema HR et al. [34] | Bevacizumab (Focal) | Prospective | ND | 20 | 6 | 2.1 | 69.0 | 73.5 | 4.5 | ND | ND | ND |
| Cheema HR et al. [34] | Bevacizumab (NSD) | Retrospective | ND | 13 | 6 | 2.6 | 38.5 | 45.5 | 7 | ND | ND | ND |
| Mushtaq B et al. [35] | Bevacizumab CSMT<400 | ND | ND | 81 | 12 | 3.3 | 56 | 62.5 | 6 | ND | ND | ND |
| Mushtaq B et al. [35] | Bevacizumab CSMT>400 | ND | ND | 94 | 12 | 4.0 | 51.5 | 57.5 | 6 | ND | ND | ND |
| Crosson JN et al. [36] | Bevacizumab | Retrospective | ND | 102 | 60 | 5.5 | 50 | 55 | 5 | ND | ND | ND |
| Solaiman et al. [33] | Bevacizumab | Prospective | ND | 22 | 14 | 2.4 | 54.5 | 59.1 | 4.6 | ND | 9.1% | ND |
| Chatziralli I et al. [37] | Ranibizumab | Retrospective | Naïve | 332 | 12 | 6.7 | 56.4 | 64.4 | 8 | ND | ND | ND |
| Ciulla TA et al. [38] | Ranibizumab | Retrospective | Non-Naïve | 33 | 12 | 6.0 | 59.0 | 63 | 4 | ND | ND | ND |
| Egan C, et al. [39] | Ranibizumab | Retrospective | Mixed (49,6% Naïve) | 3103 | 24 | 5.4 | 51.1 | 52.5 | 1.4 | ND | ND | 1 endophthalmitis |
| Granstrom T et al. [40] | Ranibizumab | Retrospective | ND | 59 | 12 | 5.0 | 65 | 70.2 | 5.2 | ND | ND | ND |
| Hadzibegovic DH et al. [41] | Ranibizumab | Retrospective | Mixed (97% Naïve) | 566 | 48 | 13.5 | 64.8 | 67.1 | 2.3 | ND | 9.9% | 1 traumatic cataract |
| Katz G et al. [42] | Ranibizumab | ND | Non-Naïve | 40 | 16 | 8.4 | 65 | 66.5 | 1.5 | ND | 0 | None |
| Koc C et al. [28] | Ranibizumab | Retrospective | Naïve | 101 | 24 | 6.9 | 49.8 | 54.8 | 5 | ND | 7.1% | None |
| Koyanagi Y et al. [43] | Ranibizumab | Retrospective | Non-Naïve | 25 | 12 | 7.4 | 60 | 68.5 | 8.5 | ND | 0 | None |
| Mori Y et al. [44] | Ranibizumab | Retrospective | Non-Naïve | 68 | 12 | 6.4 | 72 | 77.5 | 5.5 | ND | ND | ND |
| Wilke RGH et al. [45] | Ranibizumab | Retrospective | ND | 335 | 36 | 10.0 | 59 | 63.8 | 4.8 | ND | ND | ND |
| Akincioglu D et al. [46] | DEX Implant | Retrospective | Non-Naïve | 57 | 12 | 1.3 | 51 | 58 | 7 | 28% (rise of IOP > 10 mmHg) | 21.5% | ND |
| Alshahrani ST et al. [47] | DEX Implant | Retrospective | Non-Naïve | 26 | 6 | 1.0 | 52 | 59 | 7 | 26% IOP > 21mmHg | 1.8% | ND |
| Bansal P et al. [48] | DEX Implant | Retrospective | Mixed | 67 | 6 | 1.0 | 44 | 56 | 12 | 12% (IOP > 21 mmHg) | 4.5% | Subconjunctival haemorrhage |
| Chatziral.li I et al. [49] | DEX Implant | Prospective | Non-Naïve | 54 | 12 | 2.1 | 52 | 5.2 | 5.6% (IOP > 20mmHg) | 4.3% | ND | |
| Chhablani J et al. [50] | DEX Implant | Retrospective | Non-Naïve | 64 | 7.67 | 1.3 | 52.5 | 61 | 8.5 | 7.6% (rise of IOP > 10 mmHg) | 2.5% | ND |
| Chhablani J et al. [50] | DEX Implant | Retrospective | Naïve | 15 | 11 | 1.3 | 56 | 63 | 7 | 7.6% (rise of IOP > 10 mmHg) | 2.5% | ND |
| Cicinelli MV et al. [51] | DEX Implant | Retrospective | Non-Naïve | 45 | 12 | 1.9 | 64.2 | 70 | 5.8 | 18.4% (IOP ≥ 20 mmHg) | 20% | ND |
| Degoumois A et al. [52] | DEX Implant | Retrospective | Non-Naïve | 42 | 20.6 | 1.6 | 55 | 61.4 | 6.4 | 8% (IOP > 25 mmHg), 2 % (IOP> 30 mmHg) | 4.8% | ND |
| Dutra Medeiros M et al. [53] | DEX Implant | Retrospective | Non-Naïve | 58 | 6 | 1.0 | 52 | 63 | 11 | No anecdotal IOP elevation | ND | None |
| Escobar-Barranco JJ et al. [54] | DEX Implant | Prospective | Non-Naïve | 40 | 6 | 1.9 | 51.3 | 59.4 | 8.1 | 7.9% (rise of IOP > 10 mmHg) | 2.6% | 3.9% Intravitreal haemorrhage |
| Escobar-Barranco JJ et al. [54] | DEX Implant | Prospective | Naïve | 36 | 6 | 1.9 | 59.6 | 73.6 | 14.1 | 7.9% (rise of IOP > 10 mmHg) | 2.6% | 3.9% Intravitreal haemorrhage |
| Esen E et al. [55] | DEX Implant | Retrospective | Non-Naïve | 25 | 6 | 1.0 | 36.5 | 46.5 | 10 | 16% (IOP > 21 mmHg) | 4% | None |
| Güler E et al. [56] | DEX Implant | Prospective | Non-Naïve | 15 | 6 | 49 | 62 | 13 | 20% | 0 | None | |
| Iglicki et al. [57] | DEX Implant | Retrospective | Non-Naïve | 59 | 24 | 3.1 | 54.5 | 63 | 8.5 | 7.1% | ND | ND |
| Iglicki et al. [57] | DEX Implant | Retrospective | Naïve | 71 | 24 | 3.9 | 55.5 | 66.8 | 11.3 | 11.4% | ND | ND |
| Kaldirim H et al. [58] | DEX Implant | Retrospective | Non-Naïve | 35 | 6 | 1.0 | 58 | 69.5 | 11.5 | 11.4% | 0% | ND |
| Lozano Lopez V et al. [59] | DEX Implant | Retrospective | ND | 36 | 6 | 10.9 | 29.5% (IOP > 23 mmHg) 1.1% filtering surgery | ND | None | |||
| Mastropasqua R et al. [60] | DEX Implant | Prospective | Naïve | 27 | 6 | 1.7 | 68.5 | 79.5 | 11 | 0% | 0% | ND |
| Matonti F et al. [61] | DEX Implant | Retrospective | Mixed | 23 | 12 | 2.1 | 49.6 | 60 | 10.4 | 11.7% (IOP> 25 mmHg) | 0% | 26% Subconjunctival haemorrhage |
| Moon BG et al. [62] | DEX Implant | Retrospective | Mixed | 186 | 6 | >1 | 55 | 60.5 | 5.5 | 4.3% (IOP > 30mmHg) | 23.2% | 1 Infectious endophthalmitis |
| Guigou S et al. [63] | DEX Implant | Retrospective | Naïve | 16 | 6 | 1.2 | 51.1 | 71.3 | 20.2 | 11.7% (IOP > 25 mmHg), 13.3% (rise of IOP > 10 mmHg) | 0% | 26% Subconjunctival haemorrhage, 8.6% Intravitreal haemorrhage |
| Guigou S et al. [63] | DEX Implant | Retrospective | Mixed (20,5% De Naïve) | 78 | 6 | 1.2 | 53.9 | 61.92 | 8 | 11.7% (IOP > 25 mmHg), 13.3% (rise of IOP > 10 mmHg) | 0% | 27% Subconjunctival haemorrhage, 2.6% Intravitreal haemorrhage |
| Aknin I et al. [64] | DEX Implant | Retrospective | Mixed | 29 | 18 | 1.5 | 51.7 | 68.2 | 16.5 | 6.9% (IOP > 25 mmHg) | 13.8% | None |
| Pacella E et al [65] | DEX Implant | ND | Non-Naïve | 20 | 6 | 1.0 | 18.8 | 28.15 | 9.4 | 5.8% (IOP >26 mmHg) | 0% | None |
| Pareja-Rios et al. [66] | DEX Implant | Retrospective | Naïve | 113 | 12 | 1.4 | 43.5 | 53.2 | 9.7 | 4% (rise of IOP > 10mmHg) | ND | None |
| Pareja-Rios et al. [66] | DEX Implant | Retrospective | Naïve | 11 | 12 | 1.4 | 56.5 | 65.3 | 8.8 | 4% (rise of IOP > 10mmHg) | ND | ND |
| Bellocq D et al. [67] | DEX Implant | Prospective | Mixed (73% Naïve) | 37 | 6 | 1.5 | 58.7 | 68.7 | 10.1 | 14% (IOP > 25 mmHg), 3% (IOP > 35mmHg) 8% (rise of IOP > 10 mmHg) | ND | Subconjunctival haemorrhage |
| Fine et al. [13] | DEX Implant | Prospective | Non-Naïve | 101 | 12 | 2.0 | 57.2 | 65.9 | 8.7 | 12.2% (IOP > 25 mmHg), 2.8% (IOP > 35mmHg) 12.8% (rise of IOP > 10 mmHg) | ND | Vitreous floaters (4.3%) |
| Mal.clès A et al. [68] | DEX Implant | Retrospective | Mixed (27% Naïve) | 128 | 36 | 3.6 | 50.5 | 60.6 | 9.5 | 10.2% (IOP > 25 mmHg), 2.3% (IOP > 35mmHg) 19% (rise of IOP > 10 mmHg) | ND | ND |
| Sacconi R et al. [69] | DEX Implant | Prospective | Mixed | 14 | 12 | 1.7 | 72.5 | 80 | 7.5 | 21% (IOP > 21 mmHg) | 0% | ND |
| Scaramuzzi M et al. [69] | DEX Implant | Retrospective | Mixed (7% Naïve) | 15 | 12 | 2.0 | 51.5 | 60 | 8.5 | 20% | 8.3% | ND |
| Totan Y et al. [70] | DEX Implant | Prospective | Non-Naïve | 30 | 6 | >1 | 57 | 64.5 | 7.5 | 13.3% (IOP > 21 mmHg) | 0% | ND |
| Unsal. E et al. [71] | DEX Implant | Retrospective | Non-Naïve | 46 | 6 | 1.1 | 41 | 57.5 | 16.5 | 17.4% (IOP > 25 mmHg) | 8.7% | 12% Subconjunctival haemorrhage |
| Yucel OE et al. [72] | DEX Implant | Retrospective | Non-Naïve | 30 | BVA (letters) | 1.0 | 51 | 57 | 6 | 16.7% (IOP > 23 mmHg) | 13% | None |
| Zhioua I et al. [73] | DEX Implant | Retrospective | Non-Naïve | 13 | 9 | 1.1 | 29.6 | 35 | 5.4 | 15.4% (IOP > 21 mmHg) | 7.9% | None |
| Yorgun MA et al. [75] | DEX Implant | Retrospective | Non-Naïve | 41 | 6 | 1.0 | 42.5 | 50.5 | 8 | 12% (IOP> 21 mmHg) | 0% | None |
Lastly, only one endophthalmitis has been reported in the 31 articles among 2897 injections that have been realized (0.03%).
Our analysis has several limitations including the fact that study data searches on PubMed are not always capable of identifying all relevant material. Moreover, it is methodologically imperfect to make indirect comparisons between studies with different numbers, even if this provides an overall vision of the real-life data. 6,842 eyes were included in the anti-VEGF studies versus 1,703 eyes with the dexamethasone implant. Moreover, we report definitions of gain in VA for anti-VEGF studies different from those reported for DEX-implant studies. Indeed, for the anti-VEGF studies, the VA or gain values used were the end-of-study data, but for the DEX-implant studies, the VA or gain values used were the maximum mean change in BCVA (best improvement) from baseline after each DEX injection. This criterion for DEX-implant was validated by the FDA [13], because when DEX-implants are administered, less injections are required, so if final VA is measured a long time after the last injection, it is more likely to be underestimated. In addition, cataracts may form with DEX-implants, and thus artificially reduce the patient's vision. Finally, the best VA criterion corresponds to the primary outcome measure for the vast majority of the DEX-implant studies analyzed. Lastly, the results for the three different anti-VEGFs were taken together as a whole. In some studies, such as protocol T [81], some differences in efficacy were found between the three drugs, which might influence or explain some of the data in the present study.
By definition, observational real-life studies have limitations with patients lost to follow-up and missing data. However, the way that missing data are handled is not always reported in real-life studies and is variable and heterogeneous across the different articles. Nevertheless, these studies are primordial because they complete the pivotal studies because the patients included in “real-life” studies are not subject to selection and correspond to the patients seen in our daily routine practice and the treatment regimen corresponds to “real-life” injection intervals and “real-life” follow-up.
An additional potential bias is the difference in the proportion of naïve and non-naïve eyes: one-fifth of the Ozurdex eyes were treatment naïve versus two-thirds of the anti-VEGF eyes.
In conclusion, pharmacological treatment with anti-VEGF and DEX-implant shows significant VA gains in observational studies. The DEX-implant results report clinically VA gains that appear to be better than real-life gains from anti-VEGF. This impression of greater efficacy may be due to the lighter treatment regimen for this molecule and also to its specific mode of action. The choice of treatment must, however, take into account each individual patient's characteristic in order to offer them personalized treatment.
Conflicts of Interest
Laurent Kodjikian is the Principal Investigator for trials sponsored by Novartis, Allergan, Bayer, Théa, and Alcon; has sat on advisory boards for Alcon, Alimera, Allergan, Bayer, Roche, and Novartis; and has received lecture fees from Alcon, Alimera, Allergan, Bayer, Horus, Novartis, and Théa. David Bellocq and Thibaud Mathis declared no conflicts of interest.
Authors' Contributions
Laurent Kodjikian, David Bellocq, and Thibaud Mathis were the principal investigators who conceived and designed the study. This manuscript was drafted by David Bellocq, revised by Laurent Kodjikian and Thibaud Mathis, and approved by all living authors.
References
- 1.Abràmoff M. D., Lou Y., Erginay A., et al. Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Investigative Ophthalmology & Visual Science. 2016;57(13):5200–5206. doi: 10.1167/iovs.16-19964. [DOI] [PubMed] [Google Scholar]
- 2.Holekamp N. M. Overview of diabetic macular edema. The American Journal of Managed Care. 2016;(10):284–291. [PubMed] [Google Scholar]
- 3.Klein R., Klein B. E. K., Moss S. E., Davis M. D., DeMets D. L. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464–1474. doi: 10.1016/S0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 4.Gu K., Cowie C. C., Harris M. I. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971–1993. Diabetes Care. 1998;21(7):1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen-Khoa B., Goehring E. L., Werther W., et al. Hospitalized cardiovascular events in patients with diabetic macular edema. BMC Ophthalmology. 2012;12(1) doi: 10.1186/1471-2415-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Photocoagulation for diabetic macular edema. Early treatment diabetic retinopathy study report number 1. Early treatment diabetic retinopathy study group. JAMA Ophtalmology. 1985;103(12):1796–1806. doi: 10.1001/archopht.1985.01050120030015. [DOI] [PubMed] [Google Scholar]
- 7.Maeshima K., Utsugi-Sutoh N., Otani T., Kishi S. Progressive enlargement of scattered photocoagulation scars in diabetic retinopathy. Retina. 2004;24(4):507–511. doi: 10.1097/00006982-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Erfurth U., Garcia-Arumi J., Bandello F., et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA) Ophthalmologica. 2017;237(4):185–222. doi: 10.1159/000458539. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen Q. D., Brown D. M., Marcus D. M., et al. Ranibizumab for diabetic macular edema: results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Korobelnik J.-F., Do D. V., Schmidt-Erfurth U., et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Herland K., Akselsen J.-P., Skjønsberg O. H., Bjermer L. How representative are clinical study patients with asthma or COPD for a larger “real life” population of patients with obstructive lung disease? Respiratory Medicine. 2005;99(1):11–19. doi: 10.1016/j.rmed.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 12.Saturni S., Bellini F., Braido F., et al. Randomized controlled trials and real life studies. Approaches and methodologies: A clinical point of view. Pulmonary Pharmacology and Therapeutics. 2014;27(2):129–138. doi: 10.1016/j.pupt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Singer M. A., Dugel P. U., Fine H. F., Capone A., Maltman J. Real-World Assessment of Dexamethasone Intravitreal Implant in DME: Findings of the Prospective, Multicenter REINFORCE Study. Ophthalmic Surgery, Lasers & Imaging Retina. 2018;49(6):425–435. doi: 10.3928/23258160-20180601-07. [DOI] [PubMed] [Google Scholar]
- 14.Blinder K. J., Dugel P. U., Chen S., et al. Anti-VEGF treatment of diabetic macular edema in clinical practice: Effectiveness and patterns of use (ECHO study report 1) Clinical Ophthalmology. 2017;11:393–401. doi: 10.2147/OPTH.S128509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda S., Tam T., Singh R. P., et al. The impact of metabolic parameters on clinical response to VEGF inhibitors for diabetic macular edema. Journal of Diabetes and its Complications. 2014;28(2):166–170. doi: 10.1016/j.jdiacomp.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Shah C. P., Heier J. S. Aflibercept for diabetic macular edema in eyes previously treated with ranibizumab and/or bevacizumab may further improve macular thickness. Ophthalmic Surgery, Lasers and Imaging Retina. 2016;47(9):836–839. doi: 10.3928/23258160-20160901-06. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu N., Oshitari T., Tatsumi T., et al. Comparisons of Efficacy of Intravitreal Aflibercept and Ranibizumab in Eyes with Diabetic Macular Edema. BioMed Research International. 2017;2017:7. doi: 10.1155/2017/1747108.1747108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wecker T., Ehlken C., Bühler A., et al. Five-year visual acuity outcomes and injection patterns in patients with pro-re-nata treatments for AMD, DME, RVO and myopic CNV. British Journal of Ophthalmology. 2017;101(3):353–359. doi: 10.1136/bjophthalmol-2016-308668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yiu G., Manjunath V., Chiu S. J., Farsiu S., Mahmoud T. H. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. American Journal of Ophthalmology. 2014;158(4):745–751.e2. doi: 10.1016/j.ajo.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahrami B., Hong T., Zhu M., Schlub T. E., Chang A. Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefe's Archive for Clinical and Experimental Ophthalmology. 2017;255(6):1133–1140. doi: 10.1007/s00417-017-3624-y. [DOI] [PubMed] [Google Scholar]
- 21.Herbaut A., Fajnkuchen F., Qu-Knafo L., Nghiem-Buffet S., Bodaghi B., Giocanti-Auregan A. Switching to Aflibercept in Diabetic Macular Edema Not Responding to Ranibizumab and/or Intravitreal Dexamethasone Implant. Journal of Ophthalmology. 2017;2017:8. doi: 10.1155/2017/8035013.8035013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiho T., Oshitari T., Tatsumi T., et al. Efficacy of One-Year Treatment with Aflibercept for Diabetic Macular Edema with Practical Protocol. BioMed Research International. 2017;2017:6. doi: 10.1155/2017/7879691.7879691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein K. A., Cleary T. S., Reichel E. Effect of intravitreal aflibercept on recalcitrant diabetic macular edema. International Journal of Retina and Vitreous. 2017;3(1) doi: 10.1186/s40942-017-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aksoy S., Yilmaz G., Akkoyun I., Yazici A. C. Comparison of intravitreal bevacizumab and triamcinolone acetonide theraphies for diffuse diabetic macular edema. International Journal of Ophthalmology. 2015;8(3):550–555. doi: 10.3980/j.issn.2222-3959.2015.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fong D. S., Luong T. Q., Contreras R., et al. Treatment patterns and 2-year vision outcomes with bevacizumab in diabetic macular edema: an analysis from a large u.s. integrated health care system. Retina. 2018;38(9):1830–1838. doi: 10.1097/IAE.0000000000001790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Güler E., Totan Y., Güragaç F. B. Intravitreal bevacizumab and dexamethasone implant for treatment of chronic diabetic macular edema. Cutaneous and Ocular Toxicology. 2017;36(2):180–184. doi: 10.3109/15569527.2015.1127254. [DOI] [PubMed] [Google Scholar]
- 27.Hanhart J., Tiosano L., Averbukh E., Banin E., Hemo I., Chowers I. Fellow eye effect of unilateral intravitreal bevacizumab injection in eyes with diabetic macular edema. Eye (Basingstoke) 2014;28(6):646–653. doi: 10.1038/eye.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koç I., Kadaylfçllar S., Eldem B. Real-World Results of Intravitreal Ranibizumab, Bevacizumab, or Triamcinolone for Diabetic Macular Edema. Ophthalmologica. 2017;239(2-3):85–93. doi: 10.1159/000481180. [DOI] [PubMed] [Google Scholar]
- 29.Kook D., Wolf A., Kreutzer T., et al. Long-term effect of intravitreal bevacizumab (avastin) in patients with chronic diffuse diabetic macular edema. Retina. 2008;28(8):1053–1060. doi: 10.1097/IAE.0b013e318176de48. [DOI] [PubMed] [Google Scholar]
- 30.Riazi-Esfahani M., Riazi-Esfahani H., Ahmadraji A., et al. Intravitreal bevacizumab alone or combined with 1 mg triamcinolone in diabetic macular edema: a randomized clinical trial. International Ophthalmology. 2017:1–14. doi: 10.1007/s10792-017-0496-4. [DOI] [PubMed] [Google Scholar]
- 31.Fechter C., Frazier H., Marcus W. B., Farooq A., Singh H., Marcus D. M. Ranibizumab 0.3 mg for persistent diabetic macular edema after recent, frequent, and chronic bevacizumab: The ROTATE trial. Ophthalmic Surgery, Lasers and Imaging Retina. 2016;47(11):1–18. doi: 10.3928/23258160-20161031-07. [DOI] [PubMed] [Google Scholar]
- 32.Yuksel E., Ozdek S., Yuksel N., Hasanreisoglu B. Intravitreal bevacizumab treatment for refractory diabetic macular edema. International Ophthalmology. 2013;33(6):659–663. doi: 10.1007/s10792-013-9758-y. [DOI] [PubMed] [Google Scholar]
- 33.Solaiman K. A., Diab M. M., Dabour S. A. Repeated intravitreal bevacizumab injection with and without macular grid photocoagulation for treatment of diffuse diabetic macular edema. Retina. 2013;33(8):1623–1629. doi: 10.1097/iae.0b013e318285c99d. [DOI] [PubMed] [Google Scholar]
- 34.Cheema H. R., Habash A. A., Al-Askar E. Improvement of visual acuity based on optical coherence tomography patterns following intravitreal bevacizumab treatment in patients with diabetic macular edema. International Journal of Ophthalmology. 2014;7(2):251–255. doi: 10.3980/j.issn.2222-3959.2014.02.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mushtaq B., Crosby N. J., Dimopoulos A. T., et al. Effect of initial retinal thickness on outcome of intravitreal bevacizumab therapy for diabetic macular edema. Clinical Ophthalmology. 2014;8:807–812. doi: 10.2147/OPTH.S56624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crosson J. N., Mason L., Mason J. O. The Role of Focal Laser in the Anti–Vascular Endothelial Growth Factor Era. Ophthalmology and Eye Diseases. 2017;9 doi: 10.1177/1179172117738240.1179172117738240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chatziralli I., Santarelli M., Patrao N., et al. Identification of time point to best define sub-optimal response' following intravitreal ranibizumab therapy for diabetic macular edema based on real-life data. Eye (Basingstoke) 2017;31(11):1594–1599. doi: 10.1038/eye.2017.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ciulla T. A., Hussain R. M., Ciulla L. M., Sink B., Harris A. Ranibizumab for diabetic macular edema refractory to multiple prior treatments. Retina. 2016;36(7):1292–1297. doi: 10.1097/IAE.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 39.Egan C., Zhu H., Lee A., et al. The United Kingdom Diabetic Retinopathy Electronic Medical Record Users Group, Report 1: Baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. British Journal of Ophthalmology. 2017;101(1):75–80. doi: 10.1136/bjophthalmol-2016-309313. [DOI] [PubMed] [Google Scholar]
- 40.Granström T., Forsman H., Lindholm Olinder A., et al. Patient-reported outcomes and visual acuity after 12 months of anti-VEGF-treatment for sight-threatening diabetic macular edema in a real world setting. Diabetes Research and Clinical Practice. 2016;121:157–165. doi: 10.1016/j.diabres.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Hodzic-Hadzibegovic D., Sander B. A., Monberg T. J., Larsen M., Lund-Andersen H. Diabetic macular oedema treated with intravitreal anti-vascular endothelial growth factor - 2-4 years follow-up of visual acuity and retinal thickness in 566 patients following Danish national guidelines. Acta Ophthalmologica. 2017 doi: 10.1111/aos.13638. [DOI] [PubMed] [Google Scholar]
- 42.Katz G., Moisseiev E., Goldenberg D., et al. Ranibizumab for persistent diabetic macular edema after bevacizumab treatment. European Journal of Ophthalmology. 2017;27(2):210–214. doi: 10.5301/ejo.5000838. [DOI] [PubMed] [Google Scholar]
- 43.Koyanagi Y., Yoshida S., Kobayashi Y., et al. Visual Outcomes Based on Early Response to Anti-Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema. Ophthalmologica. 2018;239(2-3):94–102. doi: 10.1159/000481711. [DOI] [PubMed] [Google Scholar]
- 44.Mori Y., Murakami T., Suzuma K., et al. Relation between macular morphology and treatment frequency during twelve months with ranibizumab for diabetic macular edema. PLoS ONE. 2017;12(4) doi: 10.1371/journal.pone.0175809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilke R. G. H., Finger R. P., Sachs H. G. Real-life Data on the Treatment of Diabetic Macular Oedema in Germany. Klin Monatsbl Augenheilkd. 2017;234(12):1502–1507. doi: 10.1055/s-0043-115901. [DOI] [PubMed] [Google Scholar]
- 46.Akıncıoğlu D., Küçükevcilioğlu M., Durukan A. H., Aykaş S., Ayyıldız Ö., Erdurman F. C. Outcomes of intravitreal dexamethasone implant in the treatment of recalcitrant diabetic macular edema. Türk Oftalmoloji Dergisi. 2017;47(5):274–278. doi: 10.4274/tjo.28863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alshahrani S. T., Dolz-Marco R., Gallego-Pinazo R., Diaz-Llopis M., Arevalo J. F. Intravitreal dexamethasone implant for the treatment of refractory macular edema in retinal vascular diseases : Results of the KKESH International Collaborative Retina study group. Retina. 2016;36(1):131–136. doi: 10.1097/IAE.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 48.Bansal P., Gupta V., Gupta A., Dogra M. R., Ram J. Efficacy of Ozurdex implant in recalcitrant diabetic macular edema-a single-center experience. International Ophthalmology. 2016;36(2):207–216. doi: 10.1007/s10792-015-0103-5. [DOI] [PubMed] [Google Scholar]
- 49.Chatziralli I., Theodossiadis P., Parikakis E., et al. Dexamethasone Intravitreal Implant in Diabetic Macular Edema: Real-Life Data from a Prospective Study and Predictive Factors for Visual Outcome. Diabetes Therapy. 2017;8(6):1393–1404. doi: 10.1007/s13300-017-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chhablani J., Bansal P., Veritti D., et al. Dexamethasone implant in diabetic macular edema in real-life situations. Eye (Basingstoke) 2016;30(3):426–430. doi: 10.1038/eye.2015.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cicinelli M. V., Cavalleri M., Querques L., Rabiolo A., Bandello F., Querques G. Early response to ranibizumab predictive of functional outcome after dexamethasone for unresponsive diabetic macular oedema. British Journal of Ophthalmology. 2017;101(12):1689–1693. doi: 10.1136/bjophthalmol-2017-310242. [DOI] [PubMed] [Google Scholar]
- 52.Degoumois A., Akesbi J., Laurens C., et al. Efficacy of intravitreal dexamethasone implants in macular edema excluding venous occlusions: Results for a cohort of 80 patients. Journal Français d'Ophtalmologie. 2015;38(2):126–133. doi: 10.1016/j.jfo.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Dutra Medeiros M., Postorino M., Navarro R., Garcia-Arumí J., Mateo C., Corcóstegui B. Dexamethasone intravitreal implant for treatment of patients with persistent diabetic macular edema. Ophthalmologica. 2014;231(3):141–146. doi: 10.1159/000356413. [DOI] [PubMed] [Google Scholar]
- 54.Escobar-Barranco J. J., Pina-Marín B., Fernández-Bonet M. Dexamethasone implants in patients with naïve or refractory diffuse diabetic macular edema. Ophthalmologica. 2015;233:176–185. doi: 10.1159/000371770. [DOI] [PubMed] [Google Scholar]
- 55.Esen E., Sizmaz S., Demircan N. Efficacy of dexamethasone intravitreal implant for the treatment of persistent diffuse diabetic macular edema. International Ophthalmology. 2017;37(1) doi: 10.1007/s10792-016-0219-2. [DOI] [PubMed] [Google Scholar]
- 56.Iglicki. 1910-B0414 Poster Board Number and Communication ARVO & Euretina 2017.
- 57.Kaldırım H., Yazgan S., Atalay K., Gurez C., Savur F. Intravitreal dexamethasone implantation in patients with different morphological diabetic macular edema having insufficient response to ranibizumab. Retina. 2017 doi: 10.1097/IAE.0000000000001648. [DOI] [PubMed] [Google Scholar]
- 58.Lozano López V., Serrano García M., Mantolán Sarmiento C., et al. A cost-effectiveness study of dexamethasone implants in macular edema. Archivos de la Sociedad Española de Oftalmología. 2015;90(1):14–21. doi: 10.1016/j.oftal.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 59.Mastropasqua R., Toto L., Borrelli E., et al. Morphology and function over a one-year follow up period after intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema. PLoS ONE. 2015;10(12) doi: 10.1371/journal.pone.0145663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matonti F., Pommier S., Meyer F., et al. Long-term efficacy and safety of intravitreal dexamethasone implant for the treatment of diabetic macular edema. European Journal of Ophthalmology. 2016;26(5):454–459. doi: 10.5301/ejo.5000787. [DOI] [PubMed] [Google Scholar]
- 61.Moon B. G., Lee J. Y., Yu H. G., et al. Efficacy and Safety of a Dexamethasone Implant in Patients with Diabetic Macular Edema at Tertiary Centers in Korea. Journal of Ophthalmology. 2016;2016:9. doi: 10.1155/2016/9810270.9810270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guigou S., Pommier S., Meyer F. Efficacy and safety of intravitreal dexamethasone implant in patients with diabetic macular edema. Ophthalmologica. 2015;233(3-4):169–175. doi: 10.1159/000381356. [DOI] [PubMed] [Google Scholar]
- 63.Aknin I., Melki L. Longitudinal study of sustained-release dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmologica. 2016;235(4):187–188. doi: 10.1159/000446194. [DOI] [PubMed] [Google Scholar]
- 64.Pacella E., Vestri A. R., Muscella R., et al. Preliminary results of an intravitreal dexamethasone implant (Ozurdex®) in patients with persistent diabetic macular edema. Clinical Ophthalmology. 2013;7:1423–1428. doi: 10.2147/OPTH.S48364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pareja-Ríos A., Ruiz-De la Fuente-Rodríguez P., Bonaque-González S., López-Gálvez M., Lozano-López V., Romero-Aroca P. Intravitreal dexamethasone implants for diabetic macular edema. International Journal of Ophthalmology. 2018;11(1):77–82. doi: 10.18240/ijo.2018.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bellocq D., Akesbi J., Matonti F., et al. The Pattern of Recurrence in Diabetic Macular Edema Treated by Dexamethasone Implant: The PREDIAMEX Study. Ophthalmology Retina. 2018;2(6):567–573. doi: 10.1016/j.oret.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 67.Malcles A., Dot C., Voirin N., et al. Real-life study in diabetic macular edema treated with dexamethasone implant: The reldex study. Retina. 2017;37(4):753–760. doi: 10.1097/IAE.0000000000001234. [DOI] [PubMed] [Google Scholar]
- 68.Sacconi R., Parodi M. B., Casati S., Lattanzio R., Marchini G., Bandello F. Dexamethasone Implants in Diabetic Macular Edema Patients with High Visual Acuity. Ophthalmic Research. 2017;58(3):125–130. doi: 10.1159/000477256. [DOI] [PubMed] [Google Scholar]
- 69.Totan Y., Güler E., Gürağaç F. B. Dexamethasone intravitreal implant for chronic diabetic macular edema resistant to intravitreal bevacizumab treatment. Current Eye Research. 2016;41(1):107–113. doi: 10.3109/02713683.2014.1002048. [DOI] [PubMed] [Google Scholar]
- 70.Unsal E., Eltutar K., Sultan P., Erkul S. O., Osmanbasoglu O. A. Efficacy and Safety of Intravitreal Dexamethasone Implants for Treatment of Refractory Diabetic Macular Edema. Korean Journal of Ophthalmology. 2017;31(2):p. 115. doi: 10.3341/kjo.2017.31.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yucel O. E., Can E., Ozturk H. E., Birinci H., Sullu Y. Dexamethasone Implant in Chronic Diabetic Macular Edema Resistant to Intravitreal Ranibizumab Treatment. Ophthalmic Research. 2017;57(3):161–165. doi: 10.1159/000452422. [DOI] [PubMed] [Google Scholar]
- 72.Zhioua I., Semoun O., Lalloum F., Souied E. H. Intravitreal dexamethasone implant in patients with ranibizumab persistent diabetic macular edema. Retina. 2015;35(7):1429–1435. doi: 10.1097/IAE.0000000000000490. [DOI] [PubMed] [Google Scholar]
- 73.Arıkan Yorgun M., Toklu Y., Mutlu M., Uysal B. S., Çakmak H. B. Efficacy of single-dose dexamethasone implantation in patients with persistent diabetic macular edema. International Ophthalmology. 2016;36(4):531–539. doi: 10.1007/s10792-015-0155-6. [DOI] [PubMed] [Google Scholar]
- 74.Scaramuzzi M., Querques G., Spina C. L., Lattanzio R., Bandello F. Repeated intravitreal dexamethasone implant (Ozurdex) for diabetic macular edema. Retina. 2015;35(6):1216–1222. doi: 10.1097/iae.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 75.Elman M. J., Aiello L. P., Beck R. W. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064.e35–1077.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Michaelides M., Kaines A., Hamilton R. D., et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–1086. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 77.Massin P., Bandello F., Garweg J. G., et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33(11):2399–2405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ishibashi T., Li X., Koh A., et al. The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in asian patients with diabetic macular edema. Ophthalmology. 2015;122(7):1402–1415. doi: 10.1016/j.ophtha.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 79.Mitchell P., Bandello F., Schmidt-Erfurth U., et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 80.Pearce I., Banerjee S., Burton B. J. L. Ranibizumab 0.5 mg for diabetic macular edema with bimonthly monitoring after a phase ofinitial treatment: 18-month, multicenter, phase IIIB RELIGHT study. Opthalmology. 2015;122(9):1811–1819. doi: 10.1016/j.ophtha.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 81.The Diabetic Retinopathy Clinical Research Network, Wells J. A., Glassman A. R., et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. The New England Journal of Medicine. 2015;372:1193–1203. doi: 10.1056/NEJMoa1414264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boyer D. S., Yoon Y. H., Belfort R., Jr., et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121:1904–1914. doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 83.Callanan D. G., Gupta S., Boyer D. S., et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120(9):1843–1851. doi: 10.1016/j.ophtha.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 84.Callanan D. G., Loewenstein A., Patel S. S., et al. A multicenter, 12-month randomized study comparing dexamethasone intravitreal implant with ranibizumab in patients with diabetic macular edema. Graefe's Archive for Clinical and Experimental Ophthalmology. 2017;255(3):463–473. doi: 10.1007/s00417-016-3472-1. [DOI] [PubMed] [Google Scholar]
- 85.Gillies M. C., Lim L. L., Campain A., et al. A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: The BEVORDEX study. Ophthalmology. 2014;121(12):2473–2481. doi: 10.1016/j.ophtha.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 86.Turner R., Holman R., Stratton I., et al. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. British Medical Journal. 1998;317(7160):703–713. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez V. H., Campbell J., Holekamp N. M., et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol i data. American Journal of Ophthalmology. 2016;172:72–79. doi: 10.1016/j.ajo.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 88.Schmidt-Erfurth U., Lang G. E., Holz F. G., et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology. 2014;121(5):1045–1053. doi: 10.1016/j.ophtha.2013.11.041. [DOI] [PubMed] [Google Scholar]
- 89.CATT Research Group, Martin D. F., Maguire M. G., et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. The New England Journal of Medicine. 2011;64(20):1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.IVAN Study Investigators, Chakravarthy U., Harding S. P., et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 91.Busbee B. G., Ho A. C., Brown D. M., et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046–1056. doi: 10.1016/j.ophtha.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 92.Rosenfeld P. J., Brown D. M., Heier J. S., et al. Ranibizumab for neovascular age-related macular degeneration. The New England Journal of Medicine. 2006;355(14):1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 93.Brown D. M., Campochiaro P. A., Bhisitkul R. B., et al. Sustained benefits from ranibizumab for macular edema following branch retinal vein occlusion: 12-month outcomes of a phase III study. Ophthalmology. 2011;118(8):1594–1602. doi: 10.1016/j.ophtha.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 94.Campochiaro P. A., Brown D. M., Awh C. C., et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: Twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041–2049. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 95.Audren F., Lecleire-Collet A., Erginay A., et al. Intravitreal Triamcinolone Acetonide for Diffuse Diabetic Macular Edema: Phase 2 Trial Comparing 4 mg vs 2 mg. American Journal of Ophthalmology. 2006;142(5):794–e8. doi: 10.1016/j.ajo.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 96.Lambiase A., Abdolrahimzadeh S., Recupero S. M. An update on intravitreal implants in use for eye disorders. Drugs of Today. 2014;50(3):239–249. doi: 10.1358/dot.2014.50.3.2103755. [DOI] [PubMed] [Google Scholar]
- 97.Stewart M. W. Critical appraisal of ranibizumab in the treatment of diabetic macular edema. Clinical Ophthalmology. 2013;7:1257–1267. doi: 10.2147/OPTH.S36443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Y., Zhou M., Xia S., Jing Q., Gao L. Sustained Elevation of Intraocular Pressure Associated with Intravitreal Administration of Anti-vascular Endothelial Growth Factor: A Systematic Review and Meta-Analysis. Scientific Reports. 2016;6(1) doi: 10.1038/srep39301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bressler S. B., Almukhtar T., Bhorade A., et al. Repeated intravitreous ranibizumab injections for diabetic macular edema and the risk of sustained elevation of intraocular pressure or the need for ocular hypotensive treatment. JAMA Ophthalmology. 2015;133(5):589–597. doi: 10.1001/jamaophthalmol.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Malclès A., Dot C., Voirin N., et al. SAFETY of INTRAVITREAL DEXAMETHASONE IMPLANT (OZURDEX): The SAFODEX study. Incidence and Risk Factors of Ocular Hypertension. Retina. 2017;37(7):1352–1359. doi: 10.1097/IAE.0000000000001369. [DOI] [PubMed] [Google Scholar]