Abstract
We devoted this short interview piece with Dr Shou-Ching Tang at Augusta University to feature some promising results from a clinical phase II trial on a novel brain-penetrating peptide-paclitaxel-conjugate, ANG1005, in treating brain metastatic breast cancer. These results were presented by Dr. Tang at the recent annual meeting of the European Society for Medical Oncology (ESMO 2016 Congress). This development heralds an important step forward towards the development of effective chemotherapeutic agents, which can cross the blood-brain-barrier and effectively treat and prevent the brain metastatic cancers.
Keywords: ANG1005, Brain metastasis, Breast cancer, LRP-1, The blood-brain-barrier
Brain metastasis is one of the most lethal forms of breast cancer. At least 20% of breast cancer patients eventually develop brain metastases during the natural course of the disease. This is partially because the human brain has a unique micro-vascular system, called the “blood-brain-barrier” (BBB), which insulates the brain parenchyma from toxins, including systemic chemotherapy. In many instances, tumor cells use the brain as a sanctuary during systemic chemotherapy, which can result in cranial tumor relapse. Thus, the successful treatment or prevention of the brain metastasis of breast cancer will rely on a unique strategy to ferry chemotherapeutic agents across the BBB. In an effort to establish such a strategy, a group of oncologists recently announced the results of a promising clinical trial on a novel brain-penetrating peptide-paclitaxel-conjugate, ANG1005, at the annual meeting of the European Society for Medical Oncology (ESMO 2016 Congress).1 To follow up on this encouraging news, Genes & Diseases held a quick Q&A session with one of the trial's leading oncologists, Dr. Shou-Ching Tang, Professor of Medicine and leader of the Breast Cancer Multidisciplinary Team at Augusta University Cancer Center.
Q: How was this clinical trial designed?
Dr. Tang: This was a multi-center, non-controlled, and open-label phase II trial. A total of 130 breast cancer patients with brain metastases were enrolled, who were further divided into two subgroups for safety (72 patients) and efficacy (58 patients) observations. The patients were given ANG1005 (600 mg/m3 intravenously) once every three weeks until the first sign of severe toxicity or disease progression.
Q: What is ANG1005? Why it can penetrate the BBB?
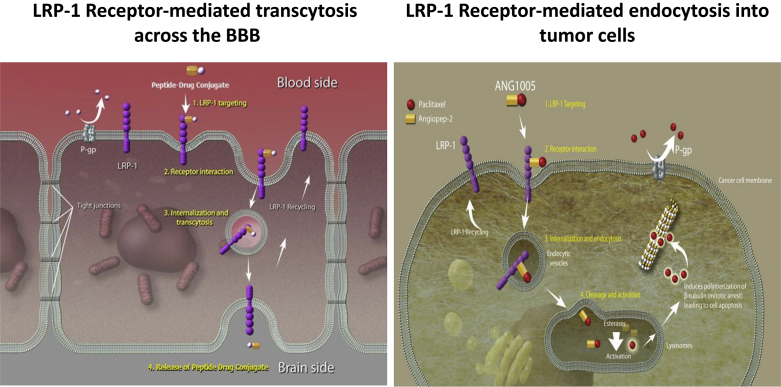
Dr. Tang: The BBB is made of vascular endothelium with tight cell-to-cell junctions, so large or electrically-charged metabolites and signaling molecules must be ferried across the cytoplasm of these endothelial cells through receptor-mediated and energy-dependent mechanisms. ANG1005, a conjugate of angiopep-2 and paclitaxel, binds to the low-density lipoprotein receptor-related protein (LRP)-1, which is abundantly expressed on the BBB and facilitates efficient transcytotic movement of ANG1005 across the BBB (Fig. 1). In a prior phase 1 clinical study (Protocol ANG1005-CLN-01 surgical sub-study), the intact ANG1005 and the paclitaxel released in tumors were quantified in patients with high-grade glioma who underwent debulking surgery for recurrent disease.2 Effective penetration of ANG1005 into brain tissue across the BBB has also been demonstrated in a breast cancer model (Ref. 3 Thomas et al., 2009).
Fig. 1.
ANG1005 crosses the blood-brain-barrier (BBB) and enters tumor cells through the LRP-1 receptor.
Q: What was the efficacy of ANG1005 in treating the brain metastasis of breast cancer in your phase II trial?
Dr. Tang: Of the 72 patients recruited for our study, efficacy data were available for 58 at the time of analysis. Ten (17%) patients showed a partial response and 31 (54%) were found to have stable disease (meaning “no progression of the disease”) according to radiological and neurological evaluations performed at four or more weeks post-ANG1005 therapy. This gave an overall patient benefit rate of 71%. The most encouraging finding is that among the 28 patients with leptomeningeal metastasis, a type of metastasis where cancer cells infiltrate the meninges, cerebrospinal fluid (CSF), or both, the overall survival rate at eight months post-ANG1005 treatment was 63%, which translates to a median overall survival time of eight months. This result compared favorably with the historical median survival time of four months with the standard treatment by other agents or radiation, and two months without any treatment.
Q: What are the adverse effects of ANG1005?
Dr. Tang: We observed toxicity and adverse effect profiles for ANG1005 that were similar to those reported for the conventional taxane drugs. This means that this novel peptide-drug conjugate did not result in any additional toxicity compared to paclitaxel alone. Surprisingly, we observed fewer symptoms of neuropathy among the trial patients receiving ANG1005, which was unexpected because paclitaxel (the conjugated drug) is a neurotoxic agent.
Q: What is the potential impact of ANG1005 on the treatment of the brain metastasis of breast and other cancers?
Dr. Tang: We were successful in the proof-of-concept stage, showing that it is possible to make an effective brain-penetrating taxane, which can directly treat brain metastases of many forms of taxane-sensitive cancers, including those originating from the breast and lung. Since we also found that ANG1005 is active both within and outside of the central nerve system (CNS), it will have the advantage of being able to cover the tumor sanctuary site in the CNS during the administration of systemic chemotherapy. That feature raises the possibility of preventing the occurrence of brain metastasis in the later course of these malignant diseases.
There are previously published observations that the brain metastasis of cancer may, to some extent, compromise the BBB barrier, thus making the brain cancer lesions more accessible to non-penetrating chemotherapy agents due to the “leakage” of these agents into the brain parenchyma. However, this has never been proven by a preclinical study or clinical trials. Even if this may be possible, we would argue that this leakage due to tumor-induced BBB interruption would only affect the treatment of established metastatic brain tumors, with no impact on the incidence of early intracranial metastasis in patients with an intact BBB. In this respect, we would hypothesize that ANG1005, or other agents with a similar brain-penetrating design, may play a crucial role in reducing the occurrence of metastasis by treating any intracranial micro-metastasis early in the course of disease. Further clinical and preclinical studies are warranted to ascertain whether this hypothesis is valid.
Importantly, most patients in our study were heavily pretreated, many with prior taxane-based therapy. Resistance to taxanes is caused by the multiple drug resistance (MDR) efflux pump. Unlike native paclitaxel, ANG1005 was not a substrate for the MDR efflux pump in in vitro studies, and has shown similar brain penetration in both MDR−/− mice and wild type mice.4 Indeed, 36 patients from our study were evaluable for an extracranial tumor response. We observed an 88% clinical benefit in this heavily-pretreated population, many of whom had received prior treatment with taxanes. In these patients, there was a 74% concordance between the intracranial and extracranial response following treatment with ANG1005. This is significant since it indicates that we can use ANG1005 to treat both peripheral metastatic breast cancer and the brain metastasis, even if the tumor has developed resistance to conventional taxanes. Furthermore, LRP-1 is overexpressed in many solid tumors, such as gliomas and head and neck, lung, and breast cancers,5 many of which are taxane-responsive. This suggests that ANG1005 can play a significant role in the management of these tumors.
Conflicts of interest
Dr. Tang declares no financial conflicts of interest.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Tang SC, Kumthekar P, Brenner AJ, et al. Ang1005, a novel peptide-paclitaxel conjugate crosses the BBB and shows activity in patients with recurrent CNS metastasis from breast cancer, results from a phase II clinical study. Abstract 3240. Presented at: European Society for Medical Oncology Congress; 2016; Oct. 7–11 Copenhagen, Denmark.
- 2.Drappatz J., Brenner A., Wong E.T. Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19:1567–1576. doi: 10.1158/1078-0432.CCR-12-2481. [DOI] [PubMed] [Google Scholar]
- 3.Thomas F.C., Taskar K., Rudraraju V. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Régina A., Demeule M., Ché C. Antitumour activity of ANG1005, a conjugate between paclitaxel and the new brain delivery vector Angiopep-2. Br J Pharmacol. 2008;155:185–197. doi: 10.1038/bjp.2008.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The human protein atlas, http://www.proteinatlas.org/ENSG00000123384-LRP1/cancer.