When oxygen is present, normal cells use mitochondria to oxidize glucose, but in the absence of oxygen, glucose is converted into lactate. About 90 years ago, the renowned German scientist Otto Warburg and co-workers made two landmark discoveries: (1) Cells from most cancers, regardless of the tissue of origin, exhibit an intense consumption of glucose, 10-fold or higher consumption than that of normal cells; (2) Within these cancerous cells, elevated generation of lactic acid occurs even in the presence of oxygen, thus leading them to coin the term “aerobic glycolysis”, in contrast to the conventional anaerobic glycolysis. From his observations, Warburg concluded that the mitochondria of tumor cells were dysfunctional. Although Warburg's assumption of a mitochondria defect was intriguing, the underlying molecular mechanisms and functions of the “Warburg effect” in tumorigenesis and tumor evolution remain largely unknown. Nevertheless, the “Warburg effect” or “aerobic glycolysis” are commonly used to describe the pivotal difference in energy production between normal and neoplastic cells.1, 2 In the past two decades, accumulating evidence has shown that different tumor types (and different cell subpopulations within a tumor) may have different bioenergetic alterations. Some tumor cells possess active and functional mitochondria for energy production, which is contrary to Warburg's theory of aerobic glycolysis. Moreover, metabolic flux analyses demonstrated that about 90% of the ATP in most healthy cells is derived from mitochondrial oxidative phosphorylation, while only about 10% comes from the metabolism of glucose to pyruvic acid. In contrast, cancer cells derive as much as 60% of their ATP from glycolysis via the “Warburg effect”, with the remaining 40% coming from mitochondrial oxidative phosphorylation, suggesting that a dynamic interplay exists between oxidative metabolism and glycolysis in cancer cells.1, 2, 3 Despite these revisions to Warburg's original theory, recent landmark discoveries in cancer research have uncovered that altered cancer metabolism not only allows rapid tumor cell proliferation via glycolysis, glutaminolysis, and increased nucleotide biosynthesis, but also renders certain bio-behaviors unique to cancer cells, such as cell survival within the ischemic and acidic tumor microenvironment, immune system evasion, and maintenance of the cancer stem cell state.1, 2, 3 In addition, nutrient-deprived epithelial cancer cells are able to survive these adverse conditions as a result of their metabolic plasticity, specifically their ability to undergo extensive metabolic reprogramming and exploit the metabolic capacities of surrounding cancer-associated fibroblasts (CAFs), the most abundant mesenchymal cell type present within the cancer microenvironment.1, 2, 3
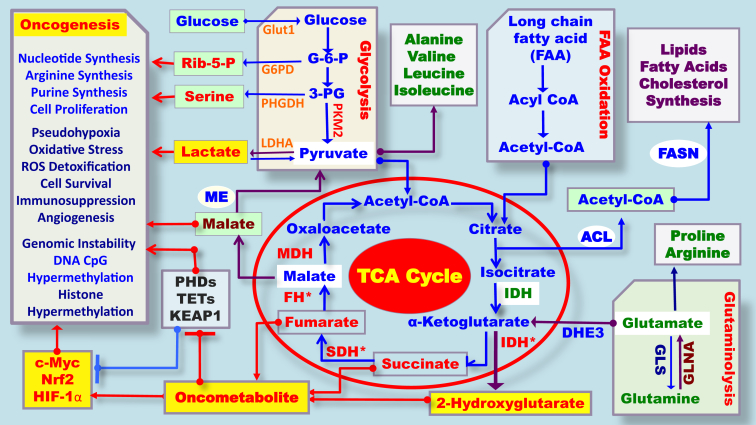
More recently, the discovery of specific gene mutations in metabolic enzymes and the observed accumulation of metabolites in cancer tissues explained at least some of the mechanisms underlying the Warburg effect or aerobic glycolysis in tumorigenesis (Fig. 1).3, 4, 5, 6, 7 For example, mutations in the mitochondrial tricarboxylic acid (TCA) cycle and respiratory chain component, succinate dehydrogenase (SDH), can cause pheochromocytoma and paraganglioma.3, 4 While inactivating mutations of fumarate hydratase (FH) are associated with hereditary leiomyomatosis and renal cell cancer, oncogenic gain-of-function mutations in isocitrate dehydrogenase 1/2 (IDH1/2) are associated with adult cases of glioblastoma and myeloid malignancies.5, 6, 7 In addition, mutations in these metabolic enzymes lead to the accumulation of so-called oncometabolites. Among these is 2-hydroxyglutarate (2HG), which behaves as a competitor for α-ketoglutarate (αKG)-dependent dioxygenases. These are involved in a broad spectrum of pathways such as the hypoxic response and epigenetic reprogramming.3, 7, 8, 9, 10, 11, 12 Due to its structural similarity to α-KG, 2HG competitively binds with α-KG-dependent dioxygenases, such as prolyl hydroxylases (PHDs), the ten eleven translocation family of 5-methylcytosine (3 mC) hydroxylases (TETs) and the Jumonji C domain-containing histone lysine demethylases (KDMs), promoting hypermethylation, which results in the suppression of genes involved in cell proliferation (Fig. 1).3, 11, 12, 13, 14 Loss of function mutations in two other TCA cycle enzymes, succinate dehydrogenase (SDH) and fumarate hydratase (FH), promotes the accumulation of succinate and fumarate, respectively. Succinate and fumarate are also considered to be oncometabolites. Both have functional and structural similarity to 2HG, and can promote epigenetic alterations by inhibiting the aforementioned group of α-KG-dependent enzymes, resulting in global histone and DNA hypermethylation (Fig. 1).11, 12, 13, 14 The recent identification of germline and somatic cancer-associated mutations in genes encoding key metabolic enzymes in cancer cells provided new support for Warburg's hypothesis that altered metabolism might have a role in cancer initiation, proliferation, and metastasis. Altered cancer metabolism can also serve as a biomarker for the diagnosis of disease and these molecules can be used for targeted therapies.9, 10, 11, 12, 13, 14, 15, 16, 17
Fig. 1.
Schematic representation of the metabolic pathways associated with oncometabolite accumulation and oncometabolite-driven carcinogenesis. *Gene mutation.
In addition to the genetic mutations of metabolic enzymes, changes in metabolic gene expression also appear to underlie aerobic glycolysis. Hypoxia-inducible factor 1 (HIF1) is stabilized under hypoxic conditions and induces the expression of many metabolic enzymes and transporters involved in central carbon metabolism, including the M2 isoform of pyruvate kinase (PKM2), pyruvate dehydrogenase kinase 1 (PDK1), glucose transporters (GLUTs) and lactate dehydrogenase A (LDHA), all of which contribute to the aerobic glycolysis phenotype.3, 4, 11, 15 Increased expression of PDK1, which phosphorylates and inhibits pyruvate dehydrogenase (PDH), also acts to limit glucose carbon flux into the TCA cycle. In contrast to most normal cells, cancer cells predominantly express PKM2, which limits the phosphoenolpyruvate (PEP)-to-pyruvate conversion and thus restricts overall glucose carbon flux into the TCA cycle.15 These latest landmark discoveries of TCA enzyme mutations and the emerging paradigm of oncometabolite-driven tumorigenesis have reignited the interest of cancer biologists in elucidating the links between metabolic dysfunctions and the altered gene expression in cancers. A new chapter has been started about cancer metabolism.1, 2, 3
In recognition of this emerging frontier and to promote domestic research on cancer metabolism, the Cancer Metabolism Subcommittee was formed under the auspice of the Chinese Society of Nutritional Oncology & Supportive Care (CSNOSC) of the CACA (China Anti-Cancer Association) on November 19, 2015. The first nationwide “Symposium on Cancer Metabolism”, organized by Dr. Y Liao, Chair of the Subcommittee, was held in Chongqing Medical University on November 20, 2015, following a closed-door panel meeting on the organization and infrastructure of the Cancer Metabolism Subcommittee of China. During that meeting, a dozen of the Subcommittee members presented their research on cancer metabolism or related fields.
Meanwhile, in recognition of the generous support from Chongqing Medical University, the Subcommittee sponsored the 2nd Annual Symposium on Cancer Metabolism in Chongqing in 2016. To cultivate local young investigators' interest in research on cancer metabolism, and to exchange ideas and advocate state-of-the-art metabolomics technologies, the Subcommittee, along with its subsidiary “Youth Subcommittee”, also hosted a two-day workshop on cancer metabolism before the commencement of the 2nd Symposium. This two-day workshop was designed specifically for graduate students or young investigators who wanted to learn about the latest progress in research on cancer metabolism. The lectures at the workshop were delivered by some of the panelists of the Subcommittee, and covered various aspects of cancer metabolism and modern metabolomics technologies. Based on the follow-up questionnaires provided by all participating students, there was high satisfaction with the contents of the workshop and a great appreciation of the high-quality lectures given by top-notch scientists in the field of cancer metabolism. In addition, some of these attendees also provided comments and suggestions for further improvement of the quality and contents of the workshop in the future.
In recognition of these two special events and the potential impact of the recent findings reported there, the panelists of the Subcommittee, along with the Editorial Board of Genes and Diseases, decided to sponsor a special issue on cancer metabolism to publish a series of reviews contributed by selected panel members of the Subcommittee. A meeting report and selected abstracts covering some of the topics presented at the 2nd Symposium are also included in this special issue.
Conflict of interest
The author declares no conflict of interest.
Acknowledgement
Research in Yong Liao's lab was supported in part by funds from the National Natural Science Foundation of China (NSFC-81172066, NSFC-81472858) and a major research project award from the NSFC (NSFC-915291003), A Start-up Fund (08-1286-001) and the Fund for “Innovation Team on Cancer Metabolism” (2209-16) from the 2nd Affiliated Hospital, Chongqing Medical University, Chongqing, P.R. China.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Potter M., Newport E., Morten K.J. The Warburg effect: 80 years on. Biochem Soc Trans. 2016 Oct 15;44(5):1499–1505. doi: 10.1042/BST20160094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee N., Kim D. Cancer metabolism: fueling more than just growth. Mol Cells. 2016 Dec;39(12):847–854. doi: 10.14348/molcells.2016.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao Y. Akt and glucose metabolism in cancer (Invited review) J Metabolism Nutr Cancer. 2014;1(2):80–88. [Google Scholar]
- 4.Baysal B.E., Ferrell R.E., Willett-Brozick J.E. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 5.Tomlinson I.P., Alam N.A., Rowan A.J., Multiple Leiomyoma Consortium Germline mutations in FH predispose to dominantly inherited uterine fibrosis, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 6.Waitkus M.S., Diplas B.H., Yan H. Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol. 2016 Jan;18(1):16–26. doi: 10.1093/neuonc/nov136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dang L., White D.W., Gross S. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009 Dec 10;462(7274):739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao S., Xu W., Jiang W. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010 Feb 19;327(5968):1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q., Zhang Y., Yang C. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010 Feb 19;327(5968):1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andronesi O.C., Rapalino O., Gerstner E. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J Clin Invest. 2013 Sep;123(9):3659–3663. doi: 10.1172/JCI67229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark O., Yen K., Mellinghoff I.K. Molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res. 2016 Apr 15;22(8):1837–1842. doi: 10.1158/1078-0432.CCR-13-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janke R., Dodson A.E., Rine J. Metabolism and epigenetics. Annu Rev Cell Dev Biol. 2015;31:473–496. doi: 10.1146/annurev-cellbio-100814-125544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowicki S., Gottlieb E. Oncometabolites: tailoring our genes. FEBS J. 2015 Aug;282(15):2796–2805. doi: 10.1111/febs.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morin A., Letouzé E., Gimenez-Roqueplo A.P., Favier J. Oncometabolites-driven tumorigenesis: from genetics to targeted therapy. Int J Cancer. 2014 Nov 15;135(10):2237–2248. doi: 10.1002/ijc.29080. [DOI] [PubMed] [Google Scholar]
- 15.Yang W., Lu Z. Pyruvate kinase M2 at a glance. J Cell Sci. 2015 May 1;128(9):1655–1660. doi: 10.1242/jcs.166629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beger R.D., Dunn W., Schmidt M.A., for “Precision Medicine and Pharmacometabolomics Task Group” – Metabolomics Society Initiative Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metabolomics. 2016;12(10):149. doi: 10.1007/s11306-016-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantae V., Krekels E.H., Esdonk M.J. Integration of pharmacometabolomics with pharmacokinetics and pharmacodynamics: towards personalized drug therapy. Metabolomics. 2017;13(1):9. doi: 10.1007/s11306-016-1143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]