Abstract
Human gene therapy has made significant advances in less than two decades. Within this short period of time, gene therapy has proceeded from the conceptual stage to technology development and laboratory research, and finally to clinical trials for the treatment of a variety of deadly diseases. Cardiovascular disease, cancer, and stroke are leading causes of death worldwide. Despite advances in medical, interventional, radiation and surgical treatments, the mortality rate remains high, and the need for novel therapies is great. Gene therapy provides an efficient approach to disease treatment. Notable advances in gene therapy have been made for genetic disorders, including severe combined immune deficiency, chronic granulomatus disorder, hemophilia and blindness, as well as for acquired diseases, including cancer and neurodegenerative and cardiovascular diseases. However, lack of an efficient delivery system to target cells as well as the difficulty of sustained expression of transgenes has hindered advancements in gene therapy. Ultrasound targeted microbubble destruction (UTMD) is a promising approach for target-specific gene delivery, and it has been successfully investigated for the treatment of many diseases in the past decade. In this paper, we review UTMD-mediated gene delivery for the treatment of cardiovascular diseases, cancer and stroke.
Keywords: Gene therapy, Cardiovascular diseases, Cancer, Stroke, UTMD
Gene therapy for genetic disorders
Gene therapy has been used successfully for the correction of abnormalities of genes, which result in clinical disorders. These treatments have corrected the disorders and clinical symptoms as well as improved the quality of patient lives. For example, in (1) severe combined immune deficiency (SCID), commonly known as the bubble boy disease, children are born without an effective immune system and will succumb to infections outside of the bubble without a bone marrow transplantation from a matched donor. A milestone study representing the first case of a gene therapy “cure”, or at least a long-term correction, for patients with this deadly genetic disorder was conducted by investigators in Italy. The therapeutic gene called adenosine deaminase (ADA) was introduced into the bone marrow cells of SCID patients, followed by transplantation of the genetically-corrected cells back into the same patients. The immune system was reconstituted in all treated patients without noticeable side effects, and these children now live normal lives with their families without the need for further treatment.1 (2) Chronic granulomatus disorder (CGD) is a genetic disease that weakens the immune system resulting in susceptibility to bacterial and fungal infections that can be fatal. Using gene transfer technologies similar to those employed in the SCID clinical trial, investigators in Germany treated two patients with CGD. They detected substantial gene transfer in both individuals' neutrophils which allowed for the development of a large number of functionally-corrected phagocytes and notable clinical improvement.2 (3) In hemophilia, patients are not able to produce blood clots and thus suffer from external and internal bleeding that can be life threatening. In a clinical trial conducted in the United States, the therapeutic gene Factor IX was introduced into the liver of hemophilia patients and restored their ability to form normal blood clots. The therapeutic effect, however, was short-lived because the genetically-corrected liver cells were likely recognized as foreign and rejected by the healthy immune systems of the patients.3 This immune-mediated rejection is similar to that which occurs after organ transplantation, and a curative outcome by gene therapy might be achievable with immune suppression or alternative gene delivery strategies currently being tested in phase I/II clinical trials. (4) Leber's congenital amaurosis (LCA) is a rare inherited retinal disease that causes severe visual impairment in infancy or early childhood with an incidence of approximately 1 in 80,000 people. LCA is characterized by nystagmus, sluggish or absent pupillary responses, and severe vision loss or blindness. Researchers at Moorfields Eye Hospital and University College London carried out the world's first gene therapy clinical trial for patients with RPE65 LCA and demonstrated that the experimental treatment is safe and can improve sight. These findings represent a landmark for gene therapy technology and could have significant impact on future treatments for eye disease.4
Gene therapy for acquired diseases
It is known that the onset of clinical disorders may be related to genetic alterations. With the development of new technologies, these genetic abnormalities have been identified and have become new targets of gene therapy. It has been demonstrated that some tumor formation are highly correlated with genetic mutation or alteration. (1) Cancer researchers have developed several different strategies for utilizing gene therapy in the treatment of a wide variety of cancers, including suicide gene therapy, oncolytic virotherapy, anti-angiogenesis and therapeutic gene vaccines. In fact, two-thirds of all gene therapy trials are cancer based and many of these trials are entering an advanced investigational stage, including a Phase III trial of Ad.p53 for head and neck cancer5 and a Phase III gene vaccine trial for prostate cancer.6 In the latter trial, use of sipuleucel-T immunotherapy prolonged overall survival in men with metastatic castration-resistant prostate cancer. Additionally, numerous Phase I and Phase II clinical trials for cancers in other organs are being conducted in academic medical centers and biotechnology companies using novel technologies and therapeutics developed on-site. (2) Stroke is the fifth leading cause of death and disability in developed countries.7 The morbidity and mortality associated with stroke result in severe social and economic burden on patients and their family members. Gene therapy could be applicable to the treatment of severe stroke, and several experimental studies have revealed the usefulness of gene therapy in the protection of neurons against ischemia, reduction of infarct size, and improvement of function.8, 9, 10, 11 (3) Cardiovascular diseases include coronary artery disease, heart failure, and cardiac arrhythmias. Nabel et al were the first to carry out successful gene therapy through the transfer of endothelial cells for expression of recombinant genes in the cardiovascular system in 1989.12 Following this achievement, gene therapy for cardiovascular diseases has been performed worldwide. However, the effectiveness of gene therapy for cardiovascular diseases has been modest because of the lack of gene delivery techniques to provide an adequate dose of a therapeutic gene to specific targets.13
In the early phase of gene therapy, naked DNA such as plasmids was directly injected into tissue. Because of lower transfection efficiency, the effectiveness of these treatments has been questioned. To improve transfection, most gene therapy trials have relied on adenoviral-associated platforms for gene delivery.14 Transfection efficiency has been improved significantly; however, the safety of viral vectors and the need for repeated catheterization raises significant safety issues for clinical trials. Because of these concerns, clinical gene therapy is currently limited to a few highly specialized Institutes. Plasmid therapy, by comparison, is a universally acceptable platform for safe gene delivery. However, DNA instability and the inability to deliver plasmids to specific sites have hindered the application of this approach. A novel gene delivery system is needed in gene therapy. Ultrasound targeted microbubble destruction (UTMD) permits precise, non-invasive gene delivery to target sites and increases transfection efficiency as well as limits off-target transfection.15, 16, 17, 18, 19 In this article, we review the most notable advancements in gene therapy and the use of the UTMD for gene delivery.
MBs and UTMD gene delivery technique
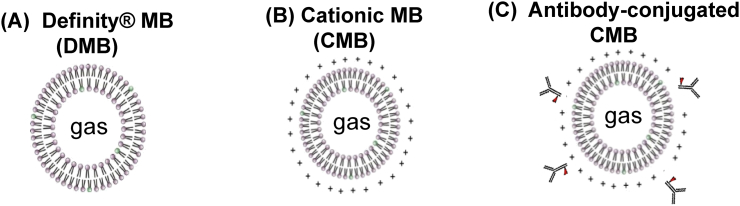
Medical ultrasound is a commonly used non-invasive technique for clinical imaging. Ultrasound imaging uses high-frequency sound waves to view real-time tissue or organs inside the human body. It can also capture blood flowing through blood vessels. Recently, microbubbles (MBs) have been used in the medical field to enhance ultrasound imaging to monitor blood flow in tissue or organs. MBs are small (less than 5 μm) gas-filled spherical voids that are generally stabilized by a material coating composed of a phospholipid or synthetic polymer (Fig. 1). The MBs act as an ultrasound enhancer. In ultrasound examination, a transducer (or probe) is placed directly on top of the targeted tissue or organ. The transducer will produce a frequency (usually 3.5 MHz) and receive an ultrasound signal at 7 MHz, which will specifically show MBs. This technique will reduce the signal from surrounding tissue, generating a higher signal-to-noise ratio and improving imaging significantly.
Fig. 1.
Schematics of three types of microbubbles (MBs). (A) Definity® MB (DMB). (B) Positively-charged cationic MB (CMB) to increase gene carrying capacity. (C) Antibody-conjugated CMB to increase targeting capacity.
Due to recent advances in the preparation technology of MBs and the innovations in ultrasound imaging, ultrasound is no longer confined to the detection of tissue perfusion, but extends to molecular imaging and targeted gene therapy. The diagnostic and therapeutic potential of MBs can be exploited using ultrasound. MBs are efficient reflectors of ultrasound and excellent contrast agents for ultrasonic imaging. MBs can also be used as carriers of therapeutic genes. An ultrasound transducer can be placed on the targeted tissue or organ for treatment. The ultrasound is able to visualize MBs in the tissue or organ, and ultrasound energy can be employed to disrupt MBs, facilitating the targeted release of therapeutic genes. The use of MBs as gene vectors is based on the hypothesis that destruction of DNA-loaded MBs by an ultrasound beam during their microvascular transit through the target area will result in localized transduction upon disruption of the MB shell while sparing non-targeted areas. UTMD has a number of advantages as it is target specific, highly effective, repeatable, non-invasive, relatively low-cost, and does not require radiation. It has been used to deliver genes to cells in vitro and more recently has been used to deliver genes in vivo to treat diabetes, cancer and cardiovascular diseases in experimental animal models.15, 20, 21, 22, 23, 24, 25 UTMD provides a non-invasive and non-viral method to effectively deliver plasmids to targeted organs.26, 27
Recent advances in MBs and UTMD gene delivery technique
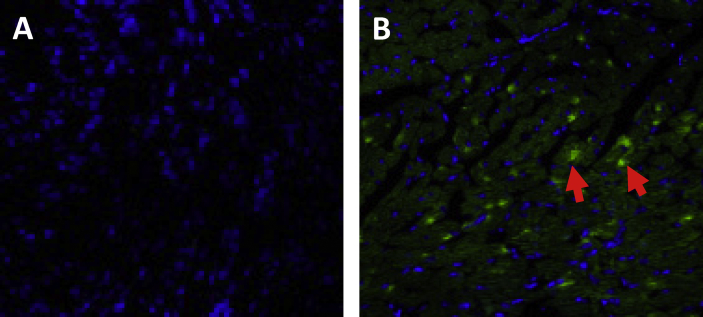
Because of the visualization in vivo of targeted tissue or organs and the ability to carry DNA, MBs in combination with ultrasound have been hypothesized to be useful as a targeted delivery system and therapy. Commercially available Definity MBs were first used as a carrier for therapeutic genes in vivo, and were then combined with ultrasound MB destruction to deliver therapeutic genes to the infarcted heart. To investigate the feasibility of UTMD for gene delivery to the ischemic myocardium, we bound plasmid DNA containing the green fluorescent protein (GFP) gene to Definity MBs and used ultrasound to target the delivery of the gene to the mouse myocardium. GFP gene expression was observed in the targeted myocardium (Fig. 2), but expression was not observed in other organs. Other groups also used this MB to deliver genes to the salivary glands and liver,28, 29 suggesting that this technique is an effective system for the delivery of therapeutic genes to targeted tissue.
Fig. 2.
Confocal microphotograph of mouse myocardial tissue after UTMD treatment to show the efficiency of gene transfection. Five days after UTMD treatment, mouse myocardial tissue samples were immunostained with an antibody against green fluorescent protein (GFP, green) with nuclear staining (Hoechst, blue). (A) Control mice were injected with empty plasmids. (B) GFP mice were injected with plasmids expressing GFP (arrows). Magnification = 600×.
Another important factor affecting the ability of MBs to provide targeted gene delivery is their carrying capacity. One of the limitations of commercially available Definity MBs is their weak cationic charge which limits their capacity for carrying negatively-charged agents, such as plasmid DNA. This has been confirmed by in vivo studies demonstrating modest gene transfection efficiency. To improve the efficacy of UTMD, our group designed and synthesized a novel cationic MB (CMB). By changing the composition of the membrane material of the MBs, we increased the cationic charge of the new CMBs.18 Characterization of this CMB revealed a much higher zeta potential than the Definity MB. The CMB demonstrated both a greater binding capacity and gene transfection efficiency compared to the Definity MB. For instance, the CMB bound 70% more plasmid DNA than the Definity MB as evaluated in in vitro studies.18 In the in vivo investigation, UTMD-mediated gene delivery with the CMB enhanced both transfection efficiency and gene expression in the heart after ischemic injury. The therapeutic effect of the CMBs was greater because of better gene delivery.18
The targeting technique for UTMD is based on the transducer (or probe) targeting the tissue or organ, which is precise and straightforward. However, an important factor influencing the quantity of therapeutic gene delivery is the amount of MBs with agents being delivered to the area of interest. Another limitation of the UTMD gene delivery technique is the wide distribution of lipid-shelled MBs in the body which contributes to lower gene delivery efficacy as the concentration of MBs in the area of interest is not high enough to achieve biological effects.30, 31 Additionally, ultrasound-facilitated gene delivery to injured tissue constitutes passive targeting. The specificity of MB delivery could be enhanced by adding tissue-specific antibodies to induce active targeting. Targeted MBs can be created by binding target molecules such as a specific antibody or ligand to the surface of MBs to enable specific tissue targeting. This will result in accumulation of MBs at targeted tissue and ultrasound destruction will increase the regional level of the therapeutic molecules. To achieve this, our group successfully conjugated tissue-specific antibodies in pathological conditions. For example, after myocardial ischemia-reperfusion injury, the injured myocardium will express matrix metalloproteinase 2 (MMP2), which is involved in matrix modulation and ventricular reconstruction. The regional elevation of MMP2 could be a target of tissue therapy. We have conjugated an antibody against MMP2 to the CMB and synthesized a new CMBMMP2 for targeted delivery. A thiolated MMP2 antibody to the PEG chains on the CMB surface was confirmed by fluorescence microscopy. Our in vivo evaluation demonstrated that the CMBMMP2 improved MB accumulation in the rat myocardial infarct region, with 57% more contrast intensity compared to the non-conjugated CMB. This technique has generated greater gene transfection in injured tissue, and the findings suggest that double targeting therapy using tissue-specific MBs is feasible and has significant potential.19
Gene therapy for cardiovascular diseases
Congestive heart failure (CHF) is a major health care concern with a rising incidence, particularly in older patients.32, 33, 34 Despite advances in medical, interventional, and surgical treatments, the mortality rate remains high and is primarily the result of maladaptive cardiac remodeling that initiates progressive ventricular dilation, wall thinning, and cardiac decompensation.35, 36 These deleterious processes are accelerated in the growing number of aged patients making the discovery of new therapies an urgent necessity. In preclinical studies, gene and cell therapies were found to restore cardiac function after an extensive myocardial infarction, but initial clinical trials were disappointing.37, 38, 39, 40, 41 Low gene transfection efficiency and low cell survival, especially in aged patients, limited the beneficial effects of this therapy. Improved methods of gene delivery and augmented cell survival are required to make gene therapy for CHF a viable treatment option for the aging population.
Our understanding of the molecular biology of the heart has expanded dramatically in recent years. The mechanisms involved in gene expression (mRNA), repression (miRNA), and transcriptional regulation have been elucidated and correlated with cardiac disease and/or recovery of cardiac function following injury. Early clinical attempts at gene therapy were focused on myocardial protection and salvage using vascular endothelial growth factor (VEGF). The goal was to stimulate endogenous angiogenesis to rescue the ischemic zone. The results were initially positive, demonstrating improved short-term perfusion, but failed to deliver long-term neovascularization for sustainable perfusion or prolonged functional cardiac improvement.42, 43, 44, 45 For example, it has been postulated that VEGF promotes a mitogenic response in the endothelium to sprout and form new tubes; however, angiogenic tip-cell penetration requires concomitant increases in extracellular matrix (ECM)-degrading enzymes.46 In addition, newly formed microvessels fail to mature with VEGF stimulation and do not adequately incorporate the pericyte and smooth muscle investiture required for vessel stability because this process requires low VEGF levels and an increase in angiopoietin and stromal derived factor.47 Recently, other gene therapy studies have been initiated to examine the delivery of sarcoendoplasmic reticulum calcium ATPase 2a (SERCA2a),48, 49 hepatocyte growth factor (HGF),50, 51 or a combination of VEGF and basic fibroblast growth factor (bFGF).52 A combinatorial approach to successful gene therapy appears to be inevitable. Research findings suggest that gene therapy is a beneficial and effective treatment; however, efficacy of the therapy fully relies on targeted gene delivery, proper gene expression in the therapeutic tissue, and persistence of biological function.
UTMD gene delivery technique for cardiovascular diseases
For cardiovascular diseases, UTMD provides a non-invasive and non-viral method to effectively deliver plasmids to targeted areas.26, 27 In addition, the limited endothelial injury induced by MB destruction facilitates gene delivery to the myocardium. Therefore, this non-invasive, repeatable and targeted technique is an effective means of delivering therapeutic genes to the ischemic myocardium.
We have shown that the VEGF gene can be effectively transfected into the ischemic myocardium by UTMD and that this gene therapy improved regional perfusion. VEGF gene transfection also stimulated cytokine production and enhanced cardiac regeneration after injury.15 These results suggest that UTMD can successfully deliver genes to the ischemic heart and improve myocardial perfusion and ventricular function. We also investigated the safety and optimal conditions for repeated UTMD-mediated delivery of therapeutic genes to the rat heart. Definity MBs were used to repeatedly deliver stem cell–mobilizing genes after myocardial infarction. Repeated delivery enhanced gene expression with minimal myocardial tissue injury and resulted in better restoration of ventricular function compared with a single treatment.16 Kobulnik et al compared the effectiveness of UTMD-mediated versus direct intramuscular injection gene delivery for therapeutic angiogenesis. They found that both intramuscular injection and UTMD-mediated delivery of the VEGF(165) gene produced significant increases in microvascular blood volume and microvascular blood flow. Microvascular blood flow was even greater in UTMD-treated than intramuscular injection-treated animals.53 Another group also tested a new gene delivery strategy using direct intramyocardial injection of the HGF gene in conjunction with MBs and UTMD. This delivery method enhanced gene expression by more than 10-fold in dogs with myocardial infarction.17
We subsequently carried out in vivo studies to assess the ability of the CMB to deliver the therapeutic AKT gene to the ischemic rat myocardium. AKT transfection with the CMB increased stem cell homing and enhanced tissue repair and regeneration, which resulted in reduced infarct size, increased infarct thickness, reduced apoptosis, increased vascular density, and improved cardiac perfusion and function compared to the Definity MB.18 Lee et al also used CMBs to investigate anti-apoptotic gene therapy with UTMD-mediated plasmid delivery of survivin, an inhibitor of an apoptosis protein, to prevent apoptosis and to attenuate left ventricular (LV) systolic dysfunction in a model of heart failure induced by doxorubicin. They demonstrated that LV fractional shortening (%) assessed by echocardiography and systolic function assessed by pressure-volume loops were greater in animals treated with survivin than those treated with an empty vector or control animals. They also showed that relative to the other experimental groups, survivin-treated animals had reduced apoptosis as measured staining for TUNEL, caspase activity and interstitial fibrosis.54 Thus, UTMD therapy using the CMB provides an efficient platform for the enhanced delivery of factors required to regenerate the ischemic heart and preserve cardiac function.
To enhance the ability of this gene delivery method to specifically target a region of interest, UTMD-mediated CMBMMP2 delivery of the metalloproteinase 3 (Timp3) gene was employed in a rat ischemia/reperfusion injury model. This study demonstrated significantly increased TIMP3 protein levels in the infarct scar and border zones 3 days post-UTMD gene delivery compared to levels found following delivery by the non-conjugated CMB. Increased levels of TIMP3 protein caused a reduction in MMP2 and MMP9 activities which resulted in smaller and thicker infarcts and improved cardiac function.19 Recently, Deng and colleagues developed a target MB (TMB) carrying an anti-intercellular adhesion molecule-1 (ICAM-1) antibody to selectively adhere to the ischemic vascular endothelium.55 Using this TMB, they investigated the feasibility and efficacy of UTMD-mediated delivery of the angiopoietin-1 (Ang-1) gene to the infarcted myocardium. They demonstrated that the ICAM-1 TMBs selectively adhered to the ischemic vascular endothelium in the infarct area of rabbits with acute myocardial infarction. Ventricular function was better and myocardial perfusion and microvascular density in the infarct area were greater in the TMB group two weeks after delivery of the Ang-1 gene compared with the non-TMB group. UTMD therapy with TMBs provides an efficient platform for the specific targeted delivery of factors intended to enhance angiogenesis and improve cardiac function after ischemic injury. This may provide a novel strategy for future gene therapy.
Gene therapy for cancer
Current therapies for cancer include surgery, radiation therapy, chemotherapy, immunotherapy, and gene therapy. Radiation therapy can cause side effects as it not only kills or slows the growth of cancer cells, but can also adversely affect nearby healthy cells. Chemotherapy is known to have widespread systemic side effects because in addition to killing fast-growing cancer cells, it also kills or slows the growth of healthy cells that grow and divide quickly. Immunotherapy including the use of monoclonal antibodies has the potential for systemic and specific killing of tumor cells. However, if the antibody is specific to a single tumor antigen, tumor evasion can still occur by down-regulation of that antigen.
Gene therapies have been shown to be effective for certain cancers.56, 57 Suicide gene therapy is one of the most attractive anti-cancer strategies. The most commonly used suicide gene, herpes simplex virus–1 thymidine kinase (HSVtK), in conjunction with ganciclovir (GCV), has been studied for use in gene therapy for cancer in several clinical trials.58, 59 Although gene therapy provides an alternative strategy for the treatment of cancer, its application in the clinic remains limited primarily due to the lack of a safe and efficient gene delivery system.60 For example, previous gene therapy studies required surgical access to inject gene vectors directly into the tumor, which is not always technically feasible and limits the possibility of repeated treatments. Currently, viral vectors are the favored clinical approach, but transduction of non-target tissues may occur, resulting in adverse side effects.61 Furthermore, viral vectors can elicit an immune response that could limit their effectiveness and prevent repeated delivery.62 Thus, an ideal gene therapy method for cancer treatment would be non-viral and capable of specifically targeting and destroying tumor cells after delivery while leaving healthy tissue and organs unaffected.
UTMD gene delivery technique for cancer
UTMD is emerging as a new gene therapy technique that may overcome some of the limitations of the viral gene delivery systems.20, 21 Because this non-invasive, site-specific, non-viral approach to gene delivery could have significant value for treating tumors, researchers have begun to evaluate it using in vivo tumor models. This research has focused on the suicide gene HSVtK,22, 63, 64, 65 short antisense oligodeoxynucleotide targeting the human androgen receptor to treat prostate tumours,66 gene therapy targeting apoptosis,67, 68 and anti-angiogenic gene therapy to suppress neovascularization in tumors.69, 70
Carson et al were among the first to report the use of intravenous injection of plasmid-loaded MBs and UTMD to treat malignant tumors. They tested the hypothesis that UTMD would specifically transduce tumor tissue and slow tumor growth when treated with HSVtK and GCV. As a first step, they demonstrated that UTMD-mediated delivery of reporter genes resulted in tumor expression of luciferase and GFP in perivascular areas and individual tumor cells. Then they showed that the doubling time of HSVtK-treated tumors was longer than GFP-treated control tumors and accompanied by increased apoptosis and more areas of cellular drop-out. Their data indicate that UTMD gene therapy can transduce solid tumors and mediate a therapeutic effect. Therefore, UTMD is a promising non-viral method of targeted gene therapy that may be useful in a spectrum of tumors.22
Recently, our group evaluated gene therapy targeting hepatocellular carcinoma (HCC) using the HSVtk/GCV suicide gene system. We further investigated the synergistic antitumor effect of co-delivery of the angiogenesis inhibitor of tissue inhibitor of metalloproteinase 3 (Timp3) gene. We demonstrated that the cell viability of cancer cells transfected with the HSVtk or Timp3 gene was reduced by >40% compared cancer cells transfected with the vector control. Cell viability was further inhibited by over 50% with co-transfection of the two genes. UTMD-mediated delivery of HSVtk or Timp3 suppressed tumor growth by >45% and increased the survival of tumor-bearing animals. Co-delivery of these two genes resulted in a further 30% improvement in tumor suppression and significant extension of animal survival. Furthermore, UTMD gene delivery increased the number of apoptotic cells and decreased the vascular density of tumors. Thus, targeted co-delivery of these two genes synergistically improved their antitumor effects and could provide an effective therapy for HCC.23
Besides its use for the delivery of DNA for cancer gene therapy, UTMD could also be used to deliver gene silencing factors to tumors. Fujii et al performed UTMD of vascular endothelial growth factor receptor-2 (VEGFR2) short hairpin (sh)RNA plasmid in an heterotopic mammary adenocarcinoma model in rats. They evaluated pulsing intervals (PIs) of 2, 5, 10, and 20 s. They demonstrated that UTMD with a PI of 10 s resulted in the greatest knockdown of VEGFR2 evaluated by PCR, immunostaining and Western blotting. This knockdown resulted in smaller tumor volumes and perfused areas and less tumor microvascular blood volume and flow than the scrambled-plasmid control. They concluded that for anti-VEGFR2 cancer gene therapy with UTMD, a PI of 10 s results in greater target knockdown and a more pronounced anti-angiogenic effect.24 Carson and colleagues also investigated the effect of UTMD to enhance the delivery of EGF receptor (EGFR)-directed siRNA to murine squamous cell carcinomas. In in vitro analyses, UTMD-mediated delivery of MBs loaded with EGFR-directed siRNA reduced EGFR expression and EGF-dependent squamous carcinoma cell growth. In the in vivo studies, serial UTMD-mediated delivery of EGFR siRNA to squamous cell carcinomas decreased EGFR expression and increased tumor doubling time. These results offer preclinical proof-of-concept for the use of UTMD to deliver gene-targeted siRNA for cancer therapy.25
Gene therapy for stroke
The current clinical management for stroke includes thrombolytic therapy, percutaneous intravascular interventions, behavioral rehabilitation strategies, and medication such as aspirin. The application of thrombolytic therapy is limited by a narrow time window (within 3 h after acute stroke onset) and there is a risk of serious hemorrhagic complications.71, 72, 73 Emergency intravascular interventions are also associated with a series of relative risks. Despite these therapies, many patients who have suffered a stroke remain disabled and require rehabilitation. The increased rate of morbidity and disability from stroke has prompted clinicians and researchers to explore more effective and safer treatments, especially for those patients for which thrombolytic therapy and percutaneous intravascular interventions are unsuitable. A novel approach to the treatment of stroke using gene transfer techniques has potential advantages over classical pharmacologic therapy.74
Choosing the appropriate genes for treating stroke is a great challenge for researchers. The following discusses the various genes that have been tested for the experimental treatment of stroke. The first are growth factor genes such as brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), and nerve growth factor (NGF). BDNF is a neurotrophin that promotes the survival and growth of developing neurons in vitro,75, 76 and improves motor neuronal function in animal neurological injury models.77, 78 In rats subjected to transient forebrain ischemia, BDNF was neuroprotective and attenuated ischemic neuronal injury.79 GDNF has been shown to affect striatal neurogenesis after rat stroke,8 and acts via the extracellular glycosylphospatidylinositol-linked receptor, GDNF family co-receptor a1 and the transmembrane tyrosine kinase, c-Ret or through a c-Ret-independent mechanism.80 Other groups also reported that GDNF receptor expression was upregulated in the penumbral areas following cerebral ischemia in rats, and short-term neuronal survival was improved by GDNF delivery.81 NGF, as the name implies, is a neuropeptide primarily involved in the regulation of growth, maintenance, proliferation, and survival of certain target neurons. In a rat stroke model, Andsberg et al showed that intrastriatal delivery of NGF could moderately mitigate neuronal death following stroke, which led to detectable functional sparing.82
Anti-apoptosis genes such as Bcl-2 and BCL-w have also been investigated. Shimazaki et al transferred the Bcl-2 gene into the CA1 pyramidal cell layer of the hippocampus and found that the application of Bcl-2 both before ischemia and after ischemia prevented DNA fragmentation in CA1 neurons.83 Similarly, Okada et al showed that post-ischemic delivery of the Bcl-2 gene conferred neuroprotection in gerbil hippocampus.9 Sun et al found that local intracerebral administration of BCL-w to rat cerebral cortex and striatum three weeks before stroke resulted in increased expression of BCL-w in cerebral cortex and striatal neurons, as well as in astroglia and endothelial cells. Recipients of BCL-w showed a 30% reduction in infarct size and a 33–40% improvement in neurological function compared to control groups.84 These results suggest that anti-apoptosis gene intervention may be a rational and effective therapeutic strategy for stroke.
The third group of genes that have been explored are angiogenesis genes, including VEGF. Many pre-clinical studies have suggested that VEGF administration following brain ischemia significantly alleviates neurological deficits and decreases infarct volume.10, 85 For example, Bellomo et al injected VEGF into the lateral ventricle of gerbils six or 12 days before ischemia and reported that VEGF gene therapy significantly improved animal survival and post-ischemic learning ability as well as delayed neuronal death in the CA1 area of the hippocampus.86
Lastly, there are also other genes such as Interleukin-1, neuroglobin and AIP (apoptotic protease activating factor-1 interacting protein) that have been reported to significantly reduce infarct size and improve functional outcome in focal cerebral ischemia models by different protective mechanisms after transduction which are still not defined.87, 88, 89 However, the therapeutic effects of these genes on stroke have been reported by only a few research studies and more experimental evidence is necessary to validate their efficacy.
UTMD gene delivery technique for stroke
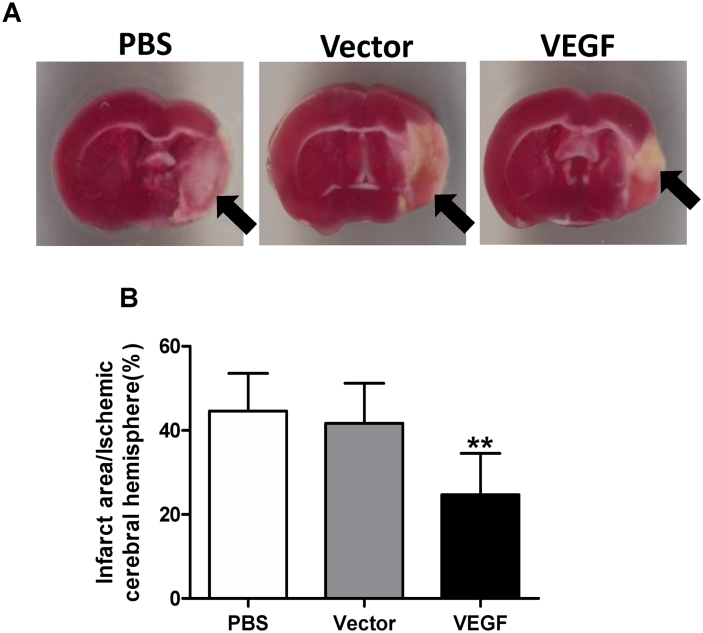
UTMD-mediated gene therapy (including delivery of the VEGF gene) targeting the heart improved myocardial perfusion and cardiac function after myocardial infarction in mice and rats.15, 16 Ultrasound destruction of gene-loaded MBs in the ischemic region released the genes into the area. We therefore hypothesized that delivery of the VEGF plasmid to the peri-ischemic region of the brain after infarction could be accomplished with UTMD. We showed that a transient disruption in the blood–brain barrier (BBB) was sufficient to permit transfection of cerebral tissue. We next evaluated VEGF delivery with UTMD for the treatment of ischemic stroke in a murine transient middle cerebral artery occlusion (tMCAO) model.11 Our study demonstrated that transcranial UTMD is a safe and effective technique for gene delivery and brain cell transfection after tMCAO in mice. A single transcranial UTMD treatment resulted in a transient increase in BBB permeability with no lasting adverse histological or functional effects. Bioluminescence imaging after delivery of the luciferase reporter gene confirmed that gene expression was confined to the targeted region of the brain, and immunostaining for GFP showed that transfected cells were located in the peri-infarct region. Our data were corroborated by other research groups who have also demonstrated gene transfection of normal brain tissue mediated by UTMD.90, 91 However, our findings specifically show the beneficial effect of this technique in a mouse stroke model.11 Overexpression of the VEGF gene after transcranial UTMD increased microvessel density, reduced apoptosis, decreased infarct size, and attenuated the impairment of neurologic function in mice with ischemic stroke (Fig. 3). Therefore, this minimally invasive methodology has significant potential for clinical application.
Fig. 3.
VEGF gene delivery by transcranial UTMD. (A) Staining with 2,3,5-triphenyltetrazolium 7 days after UTMD delineated the infarct area as white (arrows). (B) Mice in the vascular endothelial growth factor (VEGF) group had significantly smaller infarct areas compared with mice in the phosphate buffered saline (PBS) and empty vector groups (**p < 0.01).
Safety issues and limitations of the UTMD technique
Medical ultrasound is a commonly used non-invasive technique for clinical imaging. Following extensive pre-clinical studies and clinical trials, the Food and Drug Administration (FDA) approved using MBs in the medical field to enhance ultrasound imaging to see the lining of the left ventricle of the heart more clearly and monitor blood flow in tissue or organs. Ultrasound is generally considered a safe imaging modality.92 However, ultrasound should not be performed without a medical indication as previous studies have raised some safety issues. For example, a study at the Yale School of Medicine found a small but significant correlation between prolonged and frequent use of ultrasound and abnormal neuronal migration in mice.93 Another clinical study has linked the subtle effects of neurological damage to ultrasound by showing an increased incidence of left-handedness in boys (a marker for brain problems when not hereditary) and speech delays.94, 95, 96 Although these findings were not confirmed in a later follow-up,97 a recent study with a larger sample of 8865 children established a statistically significant, though weak association between ultrasound exposure and being non-right handed later in life.98 Diagnostic and therapeutic ultrasound equipment is thus regulated in the United States by the FDA, and worldwide by other national regulatory agencies. The main regulated parameters are the mechanical index, a parameter associated with the cavitation bio-effect, and the thermal index, a parameter associated with the tissue heating bio-effect. The FDA requires that ultrasound equipment not exceed established limits to ensure safety.
In addition to the safety issues mentioned above, UTMD has the following limitations: (a) MBs usually do not last long in circulation and have low circulation residence times. Therefore, UTMD should be performed at an early stage after MB delivery, and continuous MB infusion is better than intravenous bolus injection99; (b) Ultrasound produces more heat with increased frequency. For example, ultrasound of 3 MHz produced both vigorous heating (at 3.4 min) and an absolute temperature of 40 °C (at 4 min) at a depth of 2.5 cm, and it was more effective in heating muscle at this depth than 1 MHz ultrasound.100 Thus the ultrasonic frequency must be carefully selected and side effects should be monitored; (c) UTMD could cause local microvasculature ruptures and hemolysis. When the UTMD technique is used for tissue repair and regeneration, the nature of the tissue must be considered. For example, soft tissue like that of the brain should be treated using different parameters than harder tissue such as heart muscle or skeletal muscle.101 The advantage of UTMD for gene or molecule delivery is repeatability. However, repeated UTMD could result in tissue damage as multiple UTMD was associated with brain tissue hemorrhage and a mild elevation in cardiac troponin I protein release11, 16; and (d) Despite using targeted MBs, the adhesion efficiency is still low which means a small fraction of injected MBs bind to the area of interest limiting clinical applicability.102 One of the strategies to improve MB adhesion is to use dual targeting. Ferrante et al have used dual agents, mAb MVCAM.A to target VCAM-1 and a sialyl Lewisx polymer (PAA-sLex) to target P-selectin, for the purpose of molecular imaging of atherosclerotic plaques. Dual-targeted MBs adhered almost twice as efficiently as single-targeted MBs.103
Hence, the benefits and risks of UTMD should be carefully considered for clinical trials to assess therapeutic gene delivery. Some circumstances might require more gene transfers, and the relationship between number of treatments (UTMD) and the degree of microvascular injury will need to be established. Different MBs may demand different ultrasonic parameters during gene or molecule transfection. MBs combined with high energy ultrasound have been reported to damage cells and tissue.104, 105 Therefore, ultrasound energy has to be carefully monitored. Future studies will be required to define the optimal ultrasound time, frequency, duration and energy to deliver genes while minimizing tissue damage.
Conclusions
In summary, gene therapy has the potential to become an effective weapon to help fight diseases such as cardiovascular disease, cancer and stroke. UTMD gene therapy can transduce ischemic myocardium, solid tumors and the brain to mediate a therapeutic effect. With further development of technology and research, UTMD could be a promising non-viral method for targeted gene therapy that may be useful in across a broad spectrum of human diseases.
Conflicts of interest
None.
Acknowledgements
We thank Dr. Leigh Botly for help with the preparation and editing of this review article. The research reported in this article was supported by a Canadian Institutes of Health Research Foundation grant (332652) awarded to R-K.L.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Aiuti A., Cattaneo F., Galimberti S. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 2.Ott M.G., Schmidt M., Schwarzwaelder K. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12(4):401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 3.Herzog R.W. Hemophilia gene therapy: caught between a cure and an immune response. Mol Ther. 2015;23(9):1411–1412. doi: 10.1038/mt.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bainbridge J.W.B., Smith A.J., Barker S.S. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 5.INGN 201: Ad-p53, Ad5CMV-p53, Adenoviral p53, INGN 101, p53 gene therapy–Introgen, RPR/INGN 201. BioDrugs. 2003;17(3):216–222. doi: 10.2165/00063030-200317030-00010. [DOI] [PubMed] [Google Scholar]
- 6.Kantoff P.W., Higano C.S., Shore N.D. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T., Ahlenius H., Thored P., Kobayashi R., Kokaia Z., Lindvall O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke. 2006;37(9):2361–2367. doi: 10.1161/01.STR.0000236025.44089.e1. [DOI] [PubMed] [Google Scholar]
- 9.Okada T., Shimazaki K., Nomoto T. Adeno-associated viral vector-mediated gene therapy of ischemia-induced neuronal death. Methods Enzymol. 2002;346:378–393. doi: 10.1016/s0076-6879(02)46067-3. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y.-Q., Guo X., Qiu M.-H., Feng X.-Y., Sun F.-Y. VEGF overexpression enhances striatal neurogenesis in brain of adult rat after a transient middle cerebral artery occlusion. J Neurosci Res. 2007;85(1):73–82. doi: 10.1002/jnr.21091. [DOI] [PubMed] [Google Scholar]
- 11.Wang H.-B., Yang L., Wu J. Reduced ischemic injury after stroke in mice by angiogenic gene delivery via ultrasound-targeted microbubble destruction. J Neuropathol Exp Neurol. 2014;73(6):548–558. doi: 10.1097/NEN.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 12.Nabel E.G., Plautz G., Boyce F.M., Stanley J.C., Nabel G.J. Recombinant gene expression in vivo within endothelial cells of the arterial wall. Science. 1989;244(4910):1342–1344. doi: 10.1126/science.2499928. [DOI] [PubMed] [Google Scholar]
- 13.Fishbein I., Chorny M., Levy R.J. Site-specific gene therapy for cardiovascular disease. Curr Opin Drug Discov Devel. 2010;13(2):203–213. [PMC free article] [PubMed] [Google Scholar]
- 14.Daya S., Berns K.I. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(4):583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujii H., Sun Z., Li S.-H. Ultrasound-targeted gene delivery induces angiogenesis after a myocardial infarction in mice. JACC Cardiovasc Imaging. 2009;2(7):869–879. doi: 10.1016/j.jcmg.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Fujii H., Li S.-H., Wu J. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur Heart J. 2011;32(16):2075–2084. doi: 10.1093/eurheartj/ehq475. [DOI] [PubMed] [Google Scholar]
- 17.Yuan Q.-Y., Huang J., Chu B.-C., Li X.-S., Si L.-Y. A visible, targeted high-efficiency gene delivery and transfection strategy. BMC Biotechnol. 2011;11:56. doi: 10.1186/1472-6750-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun L., Huang C.-W., Wu J. The use of cationic microbubbles to improve ultrasound-targeted gene delivery to the ischemic myocardium. Biomaterials. 2013;34(8):2107–2116. doi: 10.1016/j.biomaterials.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 19.Yan P., Chen K.-J., Wu J. The use of MMP2 antibody-conjugated cationic microbubble to target the ischemic myocardium, enhance Timp3 gene transfection and improve cardiac function. Biomaterials. 2014;35(3):1063–1073. doi: 10.1016/j.biomaterials.2013.10.043. [DOI] [PubMed] [Google Scholar]
- 20.Chen S., Shimoda M., Wang M.-Y. Regeneration of pancreatic islets in vivo by ultrasound-targeted gene therapy. Gene Ther. 2010;17(11):1411–1420. doi: 10.1038/gt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villanueva F.S. Ultrasound mediated destruction of DNA-loaded microbubbles for enhancement of cell-based therapies: new promise amidst a confluence of uncertainties? JACC Cardiovasc Imaging. 2009;2(7):880–882. doi: 10.1016/j.jcmg.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 22.Carson A.R., McTiernan C.F., Lavery L. Gene therapy of carcinoma using ultrasound-targeted microbubble destruction. Ultrasound Med Biol. 2011;37(3):393–402. doi: 10.1016/j.ultrasmedbio.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu B.-F., Wu J., Zhang Y., Sung H.-W., Xie J., Li R.-K. Ultrasound-targeted HSVtk and Timp3 gene delivery for synergistically enhanced antitumor effects in hepatoma. Cancer Gene Ther. 2013;20(5):290–297. doi: 10.1038/cgt.2013.19. [DOI] [PubMed] [Google Scholar]
- 24.Fujii H., Matkar P., Liao C. Optimization of ultrasound-mediated anti-angiogenic Cancer Gene Therapy. Mol Ther Nucleic Acids. 2013;2:e94. doi: 10.1038/mtna.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carson A.R., McTiernan C.F., Lavery L. Ultrasound-targeted microbubble destruction to deliver siRNA cancer therapy. Cancer Res. 2012;72(23):6191–6199. doi: 10.1158/0008-5472.CAN-11-4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bekeredjian R., Chen S., Frenkel P.A., Grayburn P.A., Shohet R.V. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108(8):1022–1026. doi: 10.1161/01.CIR.0000084535.35435.AE. [DOI] [PubMed] [Google Scholar]
- 27.Bekeredjian R., Grayburn P.A., Shohet R.V. Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J Am Coll Cardiol. 2005;45(3):329–335. doi: 10.1016/j.jacc.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 28.Passineau M.J., Zourelias L., Machen L., Edwards P.C., Benza R.L. Ultrasound-assisted non-viral gene transfer to the salivary glands. Gene Ther. 2010;17(11):1318–1324. doi: 10.1038/gt.2010.86. [DOI] [PubMed] [Google Scholar]
- 29.Song S., Shen Z., Chen L., Brayman A.A., Miao C.H. Explorations of high-intensity therapeutic ultrasound and microbubble-mediated gene delivery in mouse liver. Gene Ther. 2011;18(10):1006–1014. doi: 10.1038/gt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki R., Takizawa T., Negishi Y. Gene delivery by combination of novel liposomal bubbles with perfluoropropane and ultrasound. J Control Release. 2007;117(1):130–136. doi: 10.1016/j.jconrel.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Browning R.J., Mulvana H., Tang M., Hajnal J.V., Wells D.J., Eckersley R.J. Influence of needle gauge on in vivo ultrasound and microbubble-mediated gene transfection. Ultrasound Med Biol. 2011;37(9):1531–1537. doi: 10.1016/j.ultrasmedbio.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Lefkowitz R.J., Willerson J.T. Prospects for cardiovascular research. JAMA. 2001;285(5):581–587. doi: 10.1001/jama.285.5.581. [DOI] [PubMed] [Google Scholar]
- 33.Lloyd-Jones D.M., Larson M.G., Leip E.P. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106(24):3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 34.Krumholz H.M., Chen Y.T., Wang Y., Vaccarino V., Radford M.J., Horwitz R.I. Predictors of readmission among elderly survivors of admission with heart failure. Am Heart J. 2000;139(1 Pt 1):72–77. doi: 10.1016/s0002-8703(00)90311-9. [DOI] [PubMed] [Google Scholar]
- 35.Cohn J.N., Ferrari R., Sharpe N. Cardiac remodeling–concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35(3):569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 36.Kurrelmeyer K., Kalra D., Bozkurt B. Cardiac remodeling as a consequence and cause of progressive heart failure. Clin Cardiol. 1998;21(12 suppl 1):I14–I19. doi: 10.1002/clc.4960211304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cleland J.G.F., Freemantle N., Coletta A.P., Clark A.L. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail. 2006;8(1):105–110. doi: 10.1016/j.ejheart.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Fazel S., Tang G.H.L., Angoulvant D. Current status of cellular therapy for ischemic heart disease. Ann Thorac Surg. 2005;79(6):S2238–S2247. doi: 10.1016/j.athoracsur.2005.02.085. [DOI] [PubMed] [Google Scholar]
- 39.Lee M.S., Makkar R.R. Stem-cell transplantation in myocardial infarction: a status report. Ann Intern Med. 2004;140(9):729–737. doi: 10.7326/0003-4819-140-9-200405040-00013. [DOI] [PubMed] [Google Scholar]
- 40.Weisel R.D., Fazel S., Fedak P.W.M., Li R.-K. Cardiac restoration by cell transplantation. Int J Cardiol. 2004;95(suppl 1):S5–S7. doi: 10.1016/s0167-5273(04)90002-2. [DOI] [PubMed] [Google Scholar]
- 41.Wollert K.C., Drexler H. Cell-based therapy for heart failure. Curr Opin Cardiol. 2006;21(3):234–239. doi: 10.1097/01.hco.0000221586.94490.d2. [DOI] [PubMed] [Google Scholar]
- 42.Gyöngyösi M., Khorsand A., Zamini S. NOGA-guided analysis of regional myocardial perfusion abnormalities treated with intramyocardial injections of plasmid encoding vascular endothelial growth factor A-165 in patients with chronic myocardial ischemia: subanalysis of the EUROINJECT-ONE multicenter double-blind randomized study. Circulation. 2005;112(9 suppl):I157–I165. doi: 10.1161/01.CIRCULATIONAHA.105.525782. [DOI] [PubMed] [Google Scholar]
- 43.Ripa R.S., Wang Y., Jørgensen E., Johnsen H.E., Hesse B., Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. Eur Heart J. 2006;27(15):1785–1792. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 44.Stewart D.J., Hilton J.D., Arnold J.M.O. Angiogenic gene therapy in patients with nonrevascularizable ischemic heart disease: a phase 2 randomized, controlled trial of AdVEGF(121) (AdVEGF121) versus maximum medical treatment. Gene Ther. 2006;13(21):1503–1511. doi: 10.1038/sj.gt.3302802. [DOI] [PubMed] [Google Scholar]
- 45.Stewart D.J., Kutryk M.J.B., Fitchett D. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17(6):1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Smet F., Segura I., De Bock K., Hohensinner P.J., Carmeliet P. Mechanisms of vessel branching: filopodia on endothelial tip cells lead the way. Arterioscler Thromb Vasc Biol. 2009;29(5):639–649. doi: 10.1161/ATVBAHA.109.185165. [DOI] [PubMed] [Google Scholar]
- 47.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473(7347):298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hajjar R.J., Zsebo K., Deckelbaum L. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008;14(5):355–367. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 49.Jaski B.E., Jessup M.L., Mancini D.M. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15(3):171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perin E.C., Silva G.V., Vela D.C. Human hepatocyte growth factor (VM202) gene therapy via transendocardial injection in a pig model of chronic myocardial ischemia. J Card Fail. 2011;17(7):601–611. doi: 10.1016/j.cardfail.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Yang Z.-J., Xu S.-L., Chen B. Hepatocyte growth factor plays a critical role in the regulation of cytokine production and induction of endothelial progenitor cell mobilization: a pilot gene therapy study in patients with coronary heart disease. Clin Exp Pharmacol Physiol. 2009;36(8):790–796. doi: 10.1111/j.1440-1681.2009.05151.x. [DOI] [PubMed] [Google Scholar]
- 52.Kukuła K., Chojnowska L., Dąbrowski M. Intramyocardial plasmid-encoding human vascular endothelial growth factor A165/basic fibroblast growth factor therapy using percutaneous transcatheter approach in patients with refractory coronary artery disease (VIF-CAD) Am Heart J. 2011;161(3):581–589. doi: 10.1016/j.ahj.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Kobulnik J., Kuliszewski M.A., Stewart D.J., Lindner J.R., Leong-Poi H. Comparison of gene delivery techniques for therapeutic angiogenesis ultrasound-mediated destruction of carrier microbubbles versus direct intramuscular injection. J Am Coll Cardiol. 2009;54(18):1735–1742. doi: 10.1016/j.jacc.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 54.Lee P.J.H., Rudenko D., Kuliszewski M.A. Survivin gene therapy attenuates left ventricular systolic dysfunction in doxorubicin cardiomyopathy by reducing apoptosis and fibrosis. Cardiovasc Res. 2014;101(3):423–433. doi: 10.1093/cvr/cvu001. [DOI] [PubMed] [Google Scholar]
- 55.Deng Q., Hu B., Cao S., Song H.-N., Chen J.-L., Zhou Q. Improving the efficacy of therapeutic angiogenesis by UTMD-mediated Ang-1 gene delivery to the infarcted myocardium. Int J Mol Med. 2015;36(2):335–344. doi: 10.3892/ijmm.2015.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardlik R., Behuliak M., Palffy R., Celec P., Li C.J. Gene therapy for cancer: bacteria-mediated anti-angiogenesis therapy. Gene Ther. 2011;18(5):425–431. doi: 10.1038/gt.2010.176. [DOI] [PubMed] [Google Scholar]
- 57.Schenk E., Essand M., Bangma C.H. Clinical adenoviral gene therapy for prostate cancer. Hum Gene Ther. 2010;21(7):807–813. doi: 10.1089/hum.2009.206. [DOI] [PubMed] [Google Scholar]
- 58.Rainov N.G. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11(17):2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 59.Shand N., Weber F., Mariani L. A phase 1–2 clinical trial of gene therapy for recurrent glioblastoma multiforme by tumor transduction with the herpes simplex thymidine kinase gene followed by ganciclovir. GLI328 European-Canadian Study Group. Hum Gene Ther. 1999;10(14):2325–2335. doi: 10.1089/10430349950016979. [DOI] [PubMed] [Google Scholar]
- 60.Chen Z., Liang K., Liu J. Enhancement of survivin gene downregulation and cell apoptosis by a novel combination: liposome microbubbles and ultrasound exposure. Med Oncol. 2009;26(4):491–500. doi: 10.1007/s12032-008-9161-0. [DOI] [PubMed] [Google Scholar]
- 61.Parry J.J., Sharma V., Andrews R., Moros E.G., Piwnica-Worms D., Rogers B.E. PET imaging of heat-inducible suicide gene expression in mice bearing head and neck squamous cell carcinoma xenografts. Cancer Gene Ther. 2009;16(2):161–170. doi: 10.1038/cgt.2008.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nayak S., Herzog R.W. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nie F., Xu H.-X., Lu M.-D., Wang Y., Tang Q. Anti-angiogenic gene therapy for hepatocellular carcinoma mediated by microbubble-enhanced ultrasound exposure: an in vivo experimental study. J Drug Target. 2008;16(5):389–395. doi: 10.1080/10611860802088846. [DOI] [PubMed] [Google Scholar]
- 64.Zhou S., Li S., Liu Z. Ultrasound-targeted microbubble destruction mediated herpes simplex virus-thymidine kinase gene treats hepatoma in mice. J Exp Clin Cancer Res. 2010;29:170. doi: 10.1186/1756-9966-29-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aoi A., Watanabe Y., Mori S., Takahashi M., Vassaux G., Kodama T. Herpes simplex virus thymidine kinase-mediated suicide gene therapy using nano/microbubbles and ultrasound. Ultrasound Med Biol. 2008;34(3):425–434. doi: 10.1016/j.ultrasmedbio.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 66.Haag P., Frauscher F., Gradl J. Microbubble-enhanced ultrasound to deliver an antisense oligodeoxynucleotide targeting the human androgen receptor into prostate tumours. J Steroid Biochem Mol Biol. 2006;102(1–5):103–113. doi: 10.1016/j.jsbmb.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 67.Chen Z.-Y., Liang K., Xie M.-X., Wang X.-F., Lü Q., Zhang J. Induced apoptosis with ultrasound-mediated microbubble destruction and shRNA targeting survivin in transplanted tumors. Adv Ther. 2009;26(1):99–106. doi: 10.1007/s12325-008-0129-4. [DOI] [PubMed] [Google Scholar]
- 68.Greco A., Di Benedetto A., Howard C.M. Eradication of therapy-resistant human prostate tumors using an ultrasound-guided site-specific cancer terminator virus delivery approach. Mol Ther. 2010;18(2):295–306. doi: 10.1038/mt.2009.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liao Z.-K., Tsai K.-C., Wang H.-T. Sonoporation-mediated anti-angiogenic gene transfer into muscle effectively regresses distant orthotopic tumors. Cancer Gene Ther. 2012;19(3):171–180. doi: 10.1038/cgt.2011.73. [DOI] [PubMed] [Google Scholar]
- 70.Duvshani-Eshet M., Benny O., Morgenstern A., Machluf M. Therapeutic ultrasound facilitates antiangiogenic gene delivery and inhibits prostate tumor growth. Mol Cancer Ther. 2007;6(8):2371–2382. doi: 10.1158/1535-7163.MCT-07-0019. [DOI] [PubMed] [Google Scholar]
- 71.Sandercock P., Berge E., Dennis M. Cost-effectiveness of thrombolysis with recombinant tissue plasminogen activator for acute ischemic stroke assessed by a model based on UK NHS costs. Stroke. 2004;35(6):1490–1497. doi: 10.1161/01.STR.0000126871.98801.6E. [DOI] [PubMed] [Google Scholar]
- 72.Thomalla G., Sobesky J., Köhrmann M. Two tales: hemorrhagic transformation but not parenchymal hemorrhage after thrombolysis is related to severity and duration of ischemia: MRI study of acute stroke patients treated with intravenous tissue plasminogen activator within 6 hours. Stroke. 2007;38(2):313–318. doi: 10.1161/01.STR.0000254565.51807.22. [DOI] [PubMed] [Google Scholar]
- 73.Tissue plasminogen activator for acute ischemic stroke The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 74.Ooboshi H., Ibayashi S., Takada J., Kumai Y., Iida M. Brain ischemia as a potential target of gene therapy. Exp Gerontol. 2003;38(1–2):183–187. doi: 10.1016/s0531-5565(02)00150-x. [DOI] [PubMed] [Google Scholar]
- 75.Henderson C.E., Camu W., Mettling C. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363(6426):266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 76.Roussa E., Krieglstein K. GDNF promotes neuronal differentiation and dopaminergic development of mouse mesencephalic neurospheres. Neurosci Lett. 2004;361(1–3):52–55. doi: 10.1016/j.neulet.2003.12.106. [DOI] [PubMed] [Google Scholar]
- 77.Mitsumoto H., Ikeda K., Klinkosz B., Cedarbaum J.M., Wong V., Lindsay R.M. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. 1994;265(5175):1107–1110. doi: 10.1126/science.8066451. [DOI] [PubMed] [Google Scholar]
- 78.Yan Q., Elliott J., Snider W.D. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992;360(6406):753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]
- 79.Tsukahara T., Yonekawa Y., Tanaka K. The role of brain-derived neurotrophic factor in transient forebrain ischemia in the rat brain. Neurosurgery. 1994;34(2):323–331. doi: 10.1227/00006123-199402000-00016. [DOI] [PubMed] [Google Scholar]
- 80.Airaksinen M.S., Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- 81.Wang Y., Lin S.Z., Chiou A.L., Williams L.R., Hoffer B.J. Glial cell line-derived neurotrophic factor protects against ischemia-induced injury in the cerebral cortex. J Neurosci. 1997;17(11):4341–4348. doi: 10.1523/JNEUROSCI.17-11-04341.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andsberg G., Kokaia Z., Klein R.L., Muzyczka N., Lindvall O., Mandel R.J. Neuropathological and behavioral consequences of adeno-associated viral vector-mediated continuous intrastriatal neurotrophin delivery in a focal ischemia model in rats. Neurobiol Dis. 2002;9(2):187–204. doi: 10.1006/nbdi.2001.0456. [DOI] [PubMed] [Google Scholar]
- 83.Shimazaki K., Urabe M., Monahan J., Ozawa K., Kawai N. Adeno-associated virus vector-mediated bcl-2 gene transfer into post-ischemic gerbil brain in vivo: prospects for gene therapy of ischemia-induced neuronal death. Gene Ther. 2000;7(14):1244–1249. doi: 10.1038/sj.gt.3301211. [DOI] [PubMed] [Google Scholar]
- 84.Sun Y., Jin K., Clark K.R. Adeno-associated virus-mediated delivery of BCL-w gene improves outcome after transient focal cerebral ischemia. Gene Ther. 2003;10(2):115–122. doi: 10.1038/sj.gt.3301868. [DOI] [PubMed] [Google Scholar]
- 85.Kaya D., Gürsoy-Ozdemir Y., Yemisci M., Tuncer N., Aktan S., Dalkara T. VEGF protects brain against focal ischemia without increasing blood–brain permeability when administered intracerebroventricularly. J Cereb Blood Flow Metab. 2005;25(9):1111–1118. doi: 10.1038/sj.jcbfm.9600109. [DOI] [PubMed] [Google Scholar]
- 86.Bellomo M., Adamo E.B., Deodato B. Enhancement of expression of vascular endothelial growth factor after adeno-associated virus gene transfer is associated with improvement of brain ischemia injury in the gerbil. Pharmacol Res. 2003;48(3):309–317. doi: 10.1016/s1043-6618(03)00128-2. [DOI] [PubMed] [Google Scholar]
- 87.Tsai T.-H., Chen S.-L., Xiao X. Gene treatment of cerebral stroke by rAAV vector delivering IL-1ra in a rat model. Neuroreport. 2003;14(6):803–807. doi: 10.1097/00001756-200305060-00005. [DOI] [PubMed] [Google Scholar]
- 88.Sun Y., Jin K., Peel A., Mao X.O., Xie L., Greenberg D.A. Neuroglobin protects the brain from experimental stroke in vivo. Proc Natl Acad Sci U. S. A. 2003;100(6):3497–3500. doi: 10.1073/pnas.0637726100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cao G., Xiao M., Sun F. Cloning of a novel Apaf-1-interacting protein: a potent suppressor of apoptosis and ischemic neuronal cell death. J Neurosci. 2004;24(27):6189–6201. doi: 10.1523/JNEUROSCI.1426-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang Q., Deng J., Wang F. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp Neurol. 2012;233(1):350–356. doi: 10.1016/j.expneurol.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 91.Huang Q., Deng J., Xie Z. Effective gene transfer into central nervous system following ultrasound-microbubbles-induced opening of the blood-brain barrier. Ultrasound Med Biol. 2012;38(7):1234–1243. doi: 10.1016/j.ultrasmedbio.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 92.Merritt C.R. Ultrasound safety: what are the issues? Radiology. 1989;173(2):304–306. doi: 10.1148/radiology.173.2.2678243. [DOI] [PubMed] [Google Scholar]
- 93.Ang E.S.B.C., Gluncic V., Duque A., Schafer M.E., Rakic P. Prenatal exposure to ultrasound waves impacts neuronal migration in mice. Proc Natl Acad Sci U. S. A. 2006;103(34):12903–12910. doi: 10.1073/pnas.0605294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kieler H., Cnattingius S., Haglund B., Palmgren J., Axelsson O. Sinistrality–a side-effect of prenatal sonography: a comparative study of young men. Epidemiol Camb Mass. 2001;12(6):618–623. doi: 10.1097/00001648-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Salvesen K.A., Vatten L.J., Eik-Nes S.H., Hugdahl K., Bakketeig L.S. Routine ultrasonography in utero and subsequent handedness and neurological development. BMJ. 1993;307(6897):159–164. doi: 10.1136/bmj.307.6897.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kieler H., Axelsson O., Haglund B., Nilsson S., Salvesen K.A. Routine ultrasound screening in pregnancy and the children's subsequent handedness. Early Hum Dev. 1998;50(2):233–245. doi: 10.1016/s0378-3782(97)00097-2. [DOI] [PubMed] [Google Scholar]
- 97.Heikkilä K., Vuoksimaa E., Oksava K., Saari-Kemppainen A., Iivanainen M. Handedness in the helsinki ultrasound trial. Ultrasound Obstet Gynecol. 2011;37(6):638–642. doi: 10.1002/uog.8962. [DOI] [PubMed] [Google Scholar]
- 98.Salvesen K.Å. Ultrasound in pregnancy and non-right handedness: meta-analysis of randomized trials. Ultrasound Obstet Gynecol. 2011;38(3):267–271. doi: 10.1002/uog.9055. [DOI] [PubMed] [Google Scholar]
- 99.Klibanov A.L. Targeted delivery of gas-filled microspheres, contrast agents for ultrasound imaging. Adv Drug Deliv Rev. 1999;37(1-3):139–157. doi: 10.1016/s0169-409x(98)00104-5. [DOI] [PubMed] [Google Scholar]
- 100.Hayes B.T., Merrick M.A., Sandrey M.A., Cordova M.L. Three-MHz ultrasound heats deeper into the tissues than originally theorized. J Athl Train. 2004;39(3):230–234. [PMC free article] [PubMed] [Google Scholar]
- 101.Klibanov A.L. Ligand-carrying gas-filled microbubbles: ultrasound contrast agents for targeted molecular imaging. Bioconjug Chem. 2005;16(1):9–17. doi: 10.1021/bc049898y. [DOI] [PubMed] [Google Scholar]
- 102.Takalkar A.M., Klibanov A.L., Rychak J.J., Lindner J.R., Ley K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J Control Release. 2004;96(3):473–482. doi: 10.1016/j.jconrel.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Ferrante E.A., Pickard J.E., Rychak J., Klibanov A., Ley K. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J Control Release. 2009;140(2):100–107. doi: 10.1016/j.jconrel.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krasovitski B., Kimmel E. Gas bubble pulsation in a semiconfined space subjected to ultrasound. J Acoust Soc Am. 2001;109(3):891–898. doi: 10.1121/1.1346683. [DOI] [PubMed] [Google Scholar]
- 105.Ward M., Wu J., Chiu J.F. Experimental study of the effects of Optison concentration on sonoporation in vitro. Ultrasound Med Biol. 2000;26(7):1169–1175. doi: 10.1016/s0301-5629(00)00260-x. [DOI] [PubMed] [Google Scholar]