Abstract
The recent emerging field of regenerative medicine is to present solutions for chronic diseases which cannot be sufficiently repaired by the body's own mechanisms. Stem cells are undifferentiated biological cells and have the potential to develop into many different cell types in the body during early life and growth. Self renewal and totipotency are the characteristic features of stem cells and it holds a promising result for treating various diseases like diabetic foot ulcer, heart diseases, lung diseases, Autism, Skin diseases, arthritis including eye disease. Failure of complete recovery of eye diseases and complications that follow conventional treatments have shifted search to a new form of regenerative medicine using Stem cells. The ocular progenitor cells are remarkable in stem cell biology and replenishing degenerated cells despite being present in low quantity and quiescence in our body has a high therapeutic value. In this paper we have review the applications on ocular progenitor stem cells in treatment of human eye diseases and address the strategies that have been exploited in an effort to regain visual function in the advance treatment of stem cells without any side effects and also present the significance in advance stem cell research.
Keywords: Eye diseases, Glaucoma, Macular degeneration, Ocular progenitor cells, Regenerative medicine, Stem cells
Introduction
Stem cell research is a potential and beneficial area in biology and medicine. These stem cells have the potential to become any type of cell in the body. One of the main characteristics of stem cells is their ability to self-renew or multiply while maintaining the potential to develop into other types of cells. There are different sources of stem cells but all types of stem cells have the same capacity to develop into multiple types of cells such as multipotent, pluripotent, and totipotent (Fig. 1). These cells can become cells of the blood, heart, lung, bones, skin, muscles, brain etc.1 In the last few years, it has been recognized that the systemic and local stem cell therapy has been used to treat various diseases like diabetes, eye diseases, foot ulcer, cancer, lung diseases, arthritis, Parkinson's diseases, Alzheimer's diseases, Osteoporosis etc., with better results. In 2000, India (31.7 million) topped the world with the highest number of people with diabetes mellitus followed by China (20.8 million) with the United States (17.7 million) in second and third place respectively.2 The eye diseases are the major problem and incurable diseases in India and developing countries because of the current scenario of leading diabetes.3 Eye diseases (retinopathy) are a possible complication of diabetes, known as diabetic retinopathy. It generally has no early warning signs and may surface suddenly. Sometimes, the person affected will have blurred vision, which deteriorates and improves during the course of a day.
Fig. 1.
Hierarchy of stem cells.
“Retinopathy” is a medical term describing the damage to the tiny blood vessels (capillaries) that nourish the retina. The retina is located at the back of the eye and it captures light and relays the information to the brain. The tiny blood vessels are adversely affected by high blood sugar associated with diabetes. The stem cell-based therapy represents newly emerging potential therapeutic approaches for the treatment for the degenerative eye diseases. The eye is a complex organ (Fig. 2) with highly specialized constituent tissues derived from different primordial cell lineages. The retina, for example, develops from neuroectoderm via the optic vesicle; the corneal epithelium is descended from surface ectoderm, while the iris and collagen-rich stroma of the cornea have a neural crest origin. The potential of ocular cells have been used as therapies for specific diseases because of its relative immunological privilege, surgical accessibility, and its being a self-contained system. In order to harness the potential of stem cell-based therapy to provide and restore sight in blind patients, the safety of the cells needs to be studied in detail. For the successful utilization of stem cells for therapeutic purposes, small molecules can be incorporated with or conjugated to them before transplantation to promote specific differentiation pathways.4 These cells serve to replace damaged cells and produce cytokines, growth factors, and other trophic molecules.5 Blindness or loss of visual function can be caused by failure of the light path to reach the retina or failure of the retina to capture and convert light to an electrochemical signal before transmission to the brain via optic nerve.6 The major causes contributing to blindness include age-related macular degeneration (ARMD), diabetic retinopathy, cataracts, and glaucoma7, 8, 9 which are genetically linked10 and associated with multiple risk factors including diet,11 hypertension,12 pregnancy13 and smoking.14
Fig. 2.
The normal cross section of human eye and applications of ocular stem cells.
(The original source of figure with the permission from Dhamodaran et al. Stem Cell Research & Therapy 2014, 5:56)
The eye also has many potential target diseases amenable to stem cell-based treatment, such as corneal limbal stem cell deficiency, glaucoma, age-related macular degeneration (AMD), and retinitis pigmentosa (RP). The corneal epithelium is a unique non-keratinised epithelial cell in an orderly arrangement, which is crucial to the maintenance of corneal transparency.15 It is widely accepted that the cornea is a self-renewing tissue maintained by limbal stem cells (LSCs) located at the limbus.16, 17 LSC deficiency (LSCD) is a major cause of blindness worldwide.18 In LSCD, the conjunctival epithelium migrates across the limbus, resulting in corneal opacity and vascularization. Current treatments have aimed at protecting vision and preventing visual impairment by early diagnosis using various methods of intervention such as surgery, ionising radiation, laser, or drug treatments.19, 20, 21 Despite the efficiencies of these treatment modalities, they do not provide a complete solution to stop the progression to blindness. More recent findings claims that stem cells have the capacity to revive degenerated cells or replace cells in many major diseases including ocular disorders.22, 23, 24, 25
Glaucoma
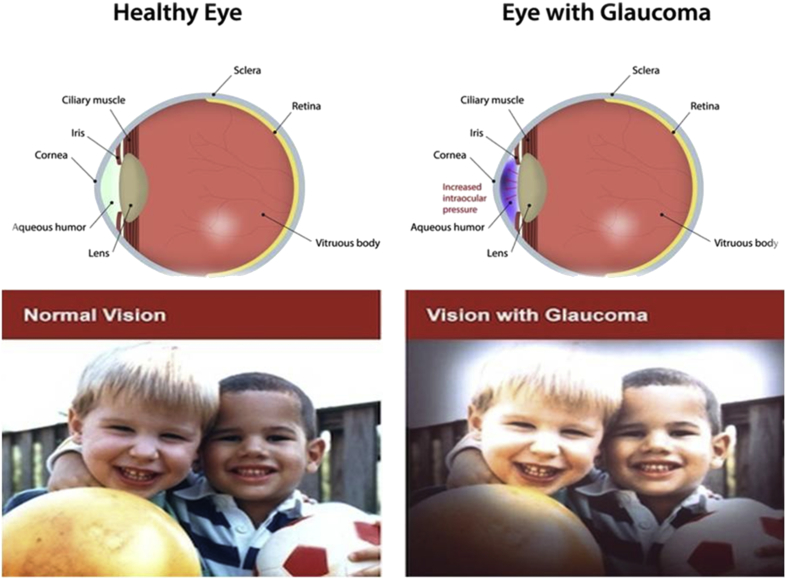
It is a group of eye diseases which result in damage to the optic nerve of the eye causing visual vision loss (Fig. 3). The visual loss in glaucoma is usually due to optic nerve damage caused by increased eye pressure. Prevalence models predict an increase of glaucoma incidence to 79.6 million by 2020 worldwide, a jump from 60.5 million in 2010 and it is the second leading cause of blindness worldwide.26 Risk factors for glaucoma include increased pressure in the eye, a family history of the condition, migraines, high blood pressure and obesity.
Fig. 3.
The normal range of vision and vision with glaucoma.
The two main types of glaucoma are open-angle glaucoma, which has several variants and is a long duration (chronic) condition, and angle-closure glaucoma, which may be either a sudden (acute) condition or a chronic disease. Although glaucoma cannot be cured, early diagnosis and treatment can minimize or prevent optic nerve damage and limited loss of vision. Blindness is a serious complication, so it can be prevented by regular eye examination and better treatment by stem cells therapy for glaucoma.27 Human stem cells have shown promise and deserve attention, not just in the laboratory, but in the clinical setting as well. This article provides an overview of stem cells for the treatment of eye diseases glaucoma via neuroprotection, neuroenhancement, and possibly cell replacement strategies (Fig. 4) explain the neurotrophic factors may be secreted by stem cells or other modified cell lines that can either be safely injected directly into the eye and, in order to be functional must establish working connections with specific parts of the brain or placed in a semipermeable capsule. These neurotrophic factors may have neuroprotective and/or neuroenhancing effects on retinal ganglion cells (RGCs), thus preserving vision and perhaps improving cellular function in patients with severe glaucoma and not cause any serious side effects.28, 29
Fig. 4.
Cell-based neuroprotection/neuroenhancement therapy.
Macular degeneration
Macular Degeneration is considered as an incurable eye disease and it is caused by the deterioration of the central portion of the retina (Fig. 5), the inside back layer of the eye that records the images we see and sends them via the optic nerve from the eye to the brain. The retina's central portion, known as the macula, is responsible for focusing central vision in the eye, and it controls our ability to read, drive a car, recognize faces or colours, and see objects in fine detail.
Fig. 5.
The normal range of vision and vision with macular degeneration.
Age-related macular degeneration (AMD or ARMD) is the most common cause of visual impairment and blindness in the elderly people. In 2000, more than nine million individuals were estimated to have AMD in the United States.30 Its prevalence is predicted to double by 2020.31 AMD is classified into two main forms: non-neovascular (also known as “dry” or “non-exudative”) or neovascular (also known as “wet” or “exudative”). The clinical hallmark of non-neovascular AMD is drusen, which are yellowish deposits at the level of the retinal pigment epithelium (RPE) which lies just under the neurosensory retina. This process is also associated with both hyperpigmentation and hypopigmentation of the retina due to morphological changes.32 The high risk factors of AMD is over 35% by the age of 75, and is increased by the family history of the disease or environmental factors such as smoking, nutritional deficiency, excessive sunlight exposure and hypertension.33
One of the major inherited ocular disorders is Retinitis Pigmentosa (RP). It is characterized by progressive degeneration of photoreceptors in the retina.34 Complete blindness in most cases proves that humans lack a homeostatic mechanism to replace lost photoreceptors.35 The earliest interventions used autologous tissue resident stem cells such as RPE cell suspensions or RPE-choroid sheets to improve vision of patients affected by age-related macular degeneration via sub retinal translocation.36 Other sources of stem or progenitors cells from extraocular tissues such as hematopoietic stem cells (HSCs),37 dental pulp stem cells (DPSCs),38 hair follicle stem cells (HFSCs),39 mesenchymal stem cells (MSCs),40 and induced pluripotent stem cells (iPSCs)41 have been explored for regenerating retinal neurons, corneal or conjunctival epithelial cells, and the RPE. The reason for using these stem cells is their capability to form neural progenitor cells or mature optic cells and the release of trophic factors important for reparative mechanism. The manipulation of these cells raises less debate over moral and ethical issues than the use of ESCs42 and fetal stem cells.43
Ocular progenitor cells
The Progenitor cells are proliferative cells with a limited capacity of self-renewal and are often unipotent some time oligopotent.44 The difference between stem cells and progenitor cells is that stem cells can replicate indefinitely but the progenitor cells can divide only a limited number of times.45 The functions of the progenitor cells are lie dormant or possess little activity in the tissue and exhibit slow growth which replace cell lost by normal attrition. The major markers proposed for epithelial stem cells in ocular or non-ocular tissues in the past decade can be categorized into at least three groups:
-
a)
Nuclear proteins such as the transcription factor p63.
-
b)
Cell membrane or transmembrane proteins including integrins (integrin β1, α6, α9), receptors (epidermal growth factor receptor [EGFR], transferrin receptor (CD71), and drug resistance transporters (ABCG-2).
-
c)
Cytoplasmic proteins such as cytokeratins (CK) (cytokeratin 19), nestin, and α enolase. In addition, a variety of differentiation markers have also been proposed to distinguish the stem cells from differentiated cells. These include cytokeratins K3 and K12, involucrin, intercellular adhesive molecule E-cadherin, and gap junction protein connexin 43, etc.46
The human ocular surface epithelium includes the corneal, limbal, and conjunctival stratified epithelia. Several recent lines of evidence have revealed that the corneal epithelial stem cells (CESCs) are localized at the basal cell layer of the peripheral cornea, and particularly at the limbus within the limbal epithelial crypts. The limbal CESCs, which express several markers, including p63, ABCG2, α9 and β1-integrins, EGFR, K19, α-enolase, and CD71, possess the ability to reconstitute an intact and functional corneal epithelium in in-vivo.47, 48 A small population of mitotic quiescent neural stem cells has also been identified in the ciliary epithelium (CE) region adjacent to the retina in adult mammalian eyes, which may proliferate in response to retinal injury in-vivo or after treatment with specific exogenous growth factors in in-vitro. These multipotent CESCs also designated retinal stem cells (RSCs), which are able to self-renew, express several specific stem cell markers, including telomerase, neural markers such as nestin, and retinal progenitor markers such as Pax 6.49 RSCs in CE may differentiate in vitro into distinct adult retinal progenitor populations, including retinal ganglion cells, as well as rod photoreceptors, bipolar cells, and Mueller glia cells, which are derived from early and late stages of retinal histogenesis, respectively. The proliferation and/or differentiation of RSCs in CE may also be regulated through the activation of other mitogenic and differentiation signalling, such as hedgehog, KIT, and Notch signalling pathways, which are also known to be important regulators of neurogenesis.50
Current clinical trails of stem cells for the treatment of eye disease
There are currently many clinical trials in progress which aims to test the safety and efficacy of stem cell transplantation in the eye (Table 1). These trails were focused on some the potential stem cells or progenitors cells from extraocular tissues such as hematopoietic stem cells (HSCs), dental pulp stem cells (DPSCs), hair follicle stem cells (HFSCs), mesenchymal stem cells (MSCs), and induced pluripotent stem cells (iPSCs) have been explored for regenerating retinal neurons, corneal or conjunctival epithelial cells, and the RPE. Stem cell-derived tissue replacement therapy for other retinal degenerative diseases is already in human clinical trials. In September 2014, a Japanese trial at RIKEN made history with the first human iPSC-derived tissue transplantation ever, which took place in the eye. An autologous iPSC-derived sheet of retinal pigment epithelial cells was surgically implanted in a patient with age-related macular degeneration. An update on two cell replacement trials for patients with Stargardt disease and age-related macular degeneration was published recently.65, 66 The reason for using these stem cells is their capability to form neural progenitor cells or mature optic cells and the release of trophic factors important for reparative mechanism.
Table 1.
Some of the Current clinical trails of extraocular Stem cell for the treatment of Ocular Disorders.
| Stem cells | Experimental design/research or disease model | Route of injection | Research outcomes | References/sources |
|---|---|---|---|---|
| Hematopoietic stem cells (HSCs) | Chemically damaged retinal neuron in mice | Intravenous injection | Fusion with ganglion, amacrine, and Muller glial cells, heterokaryons reprogramming, and dedifferentiation into neuroectodermal lineage | Sanges et al51 |
| Delivery of granulocyte-colony stimulating factor in rats with retina ischemia | Intravenous injection | Apoptosis of retinal cells was reduced and improved visual function. Localization of HSCs in the retinal layer | Lin et al52 | |
| Transplantation of human HSCs in mice with acute retinal ischemia-reperfusion injury | Intravenous injection | HSC-treated group of mice showed improved retinal histopathology. However there was no significant difference compared to control mice. No intraocular tumor and no abnormal proliferation of human cells in major organs | Park et al53 | |
| Transplantation in retinal degenerative conditions (atrophic ARMD, Retinitis Pigmentosa) or retinal vascular disease (diabetes, vein occlusion) | Intravenous injection | Clinical trial to measure primary outcome on adverse events is still ongoing | NCT01736059 (ClinicalTrials.gov) | |
| Induced pluripotent stem cells (iPSCs) | Injection of mouse fibroblast iPSC-conditioned medium | Intravenous injection | Maintenance of retina integrity and function by reducing apoptosis of retinal neurons following photodamage | Chang et al54 |
| Swine iPSCs-derived photoreceptors | Subretinal injection | Integration of photoreceptors was observed in chemically damaged retina | Zhou et al41 | |
| Generation of 3-dimensional neural retina sheet derived from mouse iPSCs and ESCs for subretinal transplantation into retinal degenerative mice | Subretinal injection | Development into outer nuclear layer (ONL) with completely structured inner and outer segments of photoreceptor | Assawachananont et al55 | |
| Generation of photoreceptor cell from adult mouse dermal fibroblast-derived iPSCs for subretinal transplantation into retinal degenerative mice | Subretinal injection | Development of functional photoreceptor in mice | Tucker et al56 | |
| Generation of RPE sheets from human iPSCs for transplantation into wet ARMD patients | Subretinal injection | Pilot safety study involving six patients is currently ongoing. RPE were observed to be retained in patients | Kamao et al57 | |
| Embryonic stem cells (ESCs) | In vitro differentiation of rostral neural progenitors into retinal neuron cells | Not available | Increased cell expression of CRX, S-opsin, and Rho/Rcvrn in hypoxic culture condition, indicating differentiation | Garita-Hernandez et al42 |
| Treatment of patients affected by Stargard'’s macular dystrophy and atrophic ARMD with human ESCs-derived RPE suspension | Submacular injection | Improved visual function. No signs of hyperproliferation, tumorigenicity, ectopic tissue formation, and immune rejection were observed | NCT01344993, NCT01345006 (ClinicalTrials.gov) | |
| Treatment of patients affected by wet ARMD with human ESCs-derived RPE sheets | Intraocular injection | Clinical trial is still ongoing. This method of delivery is hoped to overcome the disadvantages of using ESC-derived RPE suspension | NCT01691261 (ClinicalTrials.gov) | |
| Mesenchymal stem cells (MSCs) | Injection of bone marrow-derived MSCs into a laser-induced ocular hypertensive glaucoma of rat model | Intravitreal injection | Increase in retina ganglion cell (RGC) axon survival and significant decrease in the rate of RGC axon loss normalized to cumulative intraocular pressure exposure | Johnson et al28 |
| Transplantation of bone marrow-derived MSCs into Retinopathy of Prematurity (ROP) rat model | Not available | Reduced apoptosis in retinal cells with higher expression of neurotrophin-3 and CNTF in ROP rats | Zhao et al58 | |
| Direct topical application of MSCs or MSCs conditioned medium on cornea for2 hs | Corneal surface | Reduced inflammation, opacity, and neovascularization in chemically burned cornea | Oh et al40 | |
| Transplantation of bone marrow-derived MSCs in rats following optic nerve crush | Intravitreal injection | Rescued degeneration of retinal ganglion cells and axon regeneration | Mesentier-Louro et al59 | |
| Transplantation of bone marrow-derived MSCs in alkali-induced oxidative stress rabbit corneas | Corneal surface | Reduced apoptosis in corneal epithelial cells, vascularization, and infiltration of macrophages | Cejkova et al60 | |
| In vitro differentiation of adult human bone marrow stem cells with retinal pigmented epithelium cells | Coculture experiment | Differentiated cells expressed neuronal and photoreceptor phenotypes | Chiou et al61 | |
| In vivo delivery of human umbilical cord-derived MSCs to early retinal degenerative rat model | Not available | Inhibition of neovascularization and MSCs adopted RPE phenotypes | Liu et al62 | |
| Delivery of human adipose-derived MSCs to light-induced in vitro and in vivo models | Intravitreal injection | Inhibition of photoreceptor degeneration and retinal dysfunction | Sugitani et al63 | |
| Transplantation of human umbilical cord blood-derived MSCs to neurodegenerative rat model | Intraperitoneal injection | Promotion of regeneration and protection of damaged retinal ganglion cells | Zwart et al64 | |
| Adipose-derived stem cells | Injection of BMSCs in patients with advanced ARMD (atrophic or neovascular) | Intravitreal injection | Clinical trial to measure primary outcome on visual acuity is still ongoing | NCT01518127 (ClinicalTrials.gov) |
| Bone marrow stem cells (BMSCs) | Unilateral ocular transplantation into patients with advanced atrophic AMD | Subretinal injection | Clinical trial to measure primary outcome on adverse events is still ongoing | NCT01632527 (ClinicalTrials.gov) |
| Central nervous system stem cells | Unilateral ocular transplantation into patients with advanced atrophic AMD | Subretinal injection | Clinical trial to measure primary outcome on adverse events is still ongoing | NCT01632527 (ClinicalTrials.gov) |
Therapeutic potential of retinal stem cells and clinical applications
Recently, embryonic stem cell-derived retinal pigment epithelium has been used for treating patients with Stargardts disease and age-related macular degeneration. Overall, the different stem cells residing in different components of the eye have shown some success in clinical and animal studies in the field of regenerative medicine. Stem cell-based therapy holds an extraordinary prospective in improving the lives of people who suffer from visual disorders. Research in this area will continue to grow to develop new remedies in treating and preventing the problem of vision loss.67 The ideal stem cell source for feasible, wide-range therapeutic applications that could be standardized for use in a global scale would have the following characteristics:
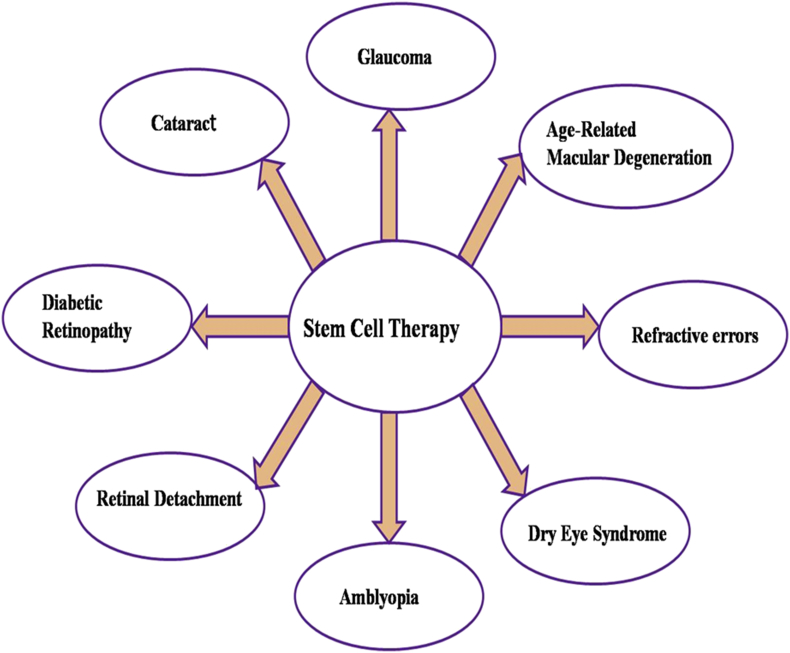
(1) Unlimited/renewable source; (2) high efficiency of differentiation into the cells of interest; (3) no immunogenic or tumorigenic risk; (4) across-the-board range of application; and (5) no ethical corollaries. Therefore, even though we focused primarily on strategies for cell replacement, the potential of the different stem cell sources for their use in therapeutic procedures will be discussed within this broader context. Stem cell-based therapy has been applied in many diseases with encouraging results. Stem cell therapy has demonstrated beneficial effects on several eye diseases (Fig. 6) including Refractive errors, Glaucoma, Cataract, Age-Related Macular Degeneration, Amblyopia, Diabetic retinopathy, Retinal detachment or Tear, Dry eye syndrome. The retinal degeneration fall into two broad categories: stem cells from (1), sources exogenous to the retina including mesenchymal stem cells (MSC) neural stem cells (NSCs) and embryonic/ induced pluripotent stem cells (ESCs/iPSCs); and (2), endogenous retinal stem cells such as Muller glia cells,68 Ciliary epithelia-derived stem cells69 and Retinal Pigment Epithelial (RPE) stem cells. The retinal pigmented epithelium (RPE) and neural retina (NR) are developed from outer and inner layer of optic cup, while the optic nerve is developed from optic stalk.70 Muller glial cells are the most abundant non-neuronal cells in the retina, providing structural and metabolic support for neural and vascular cells by extending their cell body vertically throughout the retina.71
Fig. 6.
Potential stem cell therapy for major eye diseases.
In retinal diseases, degeneration can be slowed by intraocular injection of soluble growth/ survival factors including acidic and basic fibroblast growth factor, brain-derived neurotrophic factor, ciliary neurotrophic factor, leukaemia inhibitory factor, interleukin-1β, and pigment epithelium-derived factor. There is evidence that at least some of these protective effects may be mediated indirectly via other retinal cells.72, 73 The use of physiologically intact retinal cells to replace damaged tissue is crucial when developing new therapies for retinal degenerations. Therefore, two main sources are considered: 1) the use of RPE cells to rescue photoreceptors, and 2) the use of photoreceptor precursors to repair the degenerating neural retina. RPE cell replacement in AMD has been pursued since then and its feasibility has been demonstrated as reports of human trials for wet as well as dry AMD indicate. Results by Binder et al,74 showed that patients undergoing CNV removal plus autologous RPE transplantation reached significant better reading acuity and higher multifocal electroretinogram response density than patients who underwent membrane excision alone. Retinal stem cells (RSC) have been successfully isolated from the pigmented ciliary bodies of several mammalian species, including humans.75, 76 By modulating time and environment in vitro these cells can yield significant numbers of lineage-specific cells like photoreceptors. Transplantation of these cells in normal and degenerative rodent retina was only minimally successful due to the limited ability of the cells to invade and integrate into the host retina.77
CD34 hemopoietic progenitor cell and clinical applications
Hematopoietic progenitor cell antigen CD34 also known as CD34 antigen is a protein that in humans is encoded by the CD34 gene and used as a marker of hematopoietic stem cells (HSC) and hematopoietic progenitor cells. It plays an important role in mediating cell–cell adhesion; therefore, it is believed that CD34 mediated adhesion regulates cell differentiation and proliferation. The CD34+ cells are derived from blood, bone marrow and umbilical cord blood and it proved to be an effective source for transplantation and treatment for the patients suffering from hematopoietic disorders or blood cell cancer treatment for many diseases including acute and chronic ischemic heart failure, spinal cord injury, liver cirrhosis, and peripheral vascular diseases and also proven to be excellent targets for gene therapy.78, 79 Because of the therapeutic potential, there is a need to identify easily accessible and reliable source of CD34+ cells, which are a primary focus for future translational application. The CD34, which was first detected in hemopoietic and lymphopoietic progenitors, is a heavily glycosylated type I transmembrane protein that does not share any significant similarity with other transmembrane proteins. Several monoclonal antibodies were raised against CD34, and at least 4 different epitopes could be recognized.80 In humans the first study to report successful engraftment following bone marrow transplantation with selected CD34+ positive cells was published in 1991.81 Nine patients with advanced breast cancer or neuroblastoma unresponsive to conventional therapy underwent myeloablative therapy followed by infusion of CD34+ cells separated by immunoabsorption with the CD34+ antibody. The purity of the CD34+ fraction ranged from 35 to 92% with a recovery of 42 ± 13%. Based on the current pace of stem cell research and the development of improved strategies for enhancing efficiency, there is hope that stem cell therapies may change the future of medical modalities. However, embryonic derived CD34+ progenitors have not been tested in a clinical setting. By comparison to retinal pigment epithelium progenitor cells and neuronal progenitor cells those are in clinical trials.82 The CD34+ cells were explored in animal models as potential therapy for degenerative or ischemic retinal conditions since they are multipotent and can have local trophic effects. Intravitreally injected CD34+ cells migrate into the retina and home into the damaged retinal vasculature or neuronal tissue. These human cells are detected in the mouse retina as long as 6 months following injection with no associated safety issues.53, 83, 84 This report describes the preliminary observations of the first clinical trial exploring the safety and feasibility of using intravitreally injected autologous bone marrow CD34+ cells to treat degenerative or ischemic retinal conditions. p63, a transcriptional factor involved in morphogenesis, has been proposed to identify keratinocyte stem cells at the limbus.85 Zhao et al86 have recently reported that limbal epithelial cells cultured in the presence of mitogens express neural progenitor markers, specifically nestin. They suggested that the adult corneal epithelium may serve as a model for characterising neural potential of heterologous stem cells or progenitors.
Recently, there is also research effort in developing a new mode of delivery of stem cells through direct application of contact lenses on the ocular surface. Observation of successful stratified epithelization on a corneal wound bed in a rabbit model of limbal stem cell deficiency following application of modified-contact lens (with plasma polymer with high acid functional group) cultured with limbal cells has high clinical indications, suggesting that surgery for corneal transplant may not be needed in the future.87 The Markers like Keratin 14 is used to map the distribution of precursor cells of cornea and suggested for corneal renewal with stem cells for alternative regenerative therapy.88 New research focussed on biodegradable polymers, poly-L-lactic (PLLA) and poly-DL-lactic-co-glycolic acid (85:15) (PLGA) (both of molecular weight 105 kd) were the biomaterials used with retinal pigment epithelial (RPE) and corneal endothelial cells for transplantation of the eye. The successful culture of retinal pigment epithelial and corneal endothelial monolayers on these substrates may have potential for transplanting cell monolayers in the eye to improve vision.89 Another study on polyglycolic acid (PGA) scaffold bearing an adherent corneal stromal cell insert are integrated into the ultrastructure of rabbit corneal stroma without compromising tissue transparency. Intrastromal implantation of PGA fiber scaffold implants bearing corneal stromal cells is a useful procedure for corneal stromal tissue reconstruction because, over an 8-week period, the implants become progressively more transparent. Marked losses of translucence during this period combined with restoration of ultrastructure indicate that the implants provide the functions needed for deturgescing initially swollen stroma.90 Choroidal neovascularization (CNV) is the most severe form of age-related macular degeneration (AMD), which causes rapid visual loss. Transplantation of cultured retinal pigment epithelium (RPE) cell sheet by tissue engineering is a possible approach to the treatment of CNV. The possibility of using magnetite nanoparticles and magnetic force to construct and deliver RPE cell sheets in vitro was investigated. This novel methodology, termed “magnetic force-based tissue engineering” (Mag-TE), is a possible approach for CNV treatment.91 Recent studies are focusing on using biomaterial scaffolds in combination with stem cells. Biomaterial scaffolds provide 3 dimensional structures resembling extracellular matrix environment in vivo.92 Several studies observe promising outcomes of using biomaterial scaffolds in association with induction medium to promote MSCS differentiation into hepatocyte like cells. Alginate scaffold is derived from natural polysaccharide based biomaterials that provide extracellular matrix structure allowing for cells adhesion. Lin et al showed the supportive effect of alginate scaffold on hepatic differentiation of rat BM-MSCs. The differentiated cells displayed hepatocytes phenotype and function including albumin secretion, urea production, glycogen storage and liver specific markers expression.93 However, the variability of materials between lots to lot is still a major disadvantage of natural biomaterials as compared to other materials. In addition to alginate scaffold, nanofibrous scaffold is synthetic polymer-based biomaterials that are widely used for stem cells culture. These scaffolds are made from defined chemical materials allowing easy control the quality and reproducibility of product.
Conclusion
This review article is a study of current stem cell therapy for the treatment of eye diseases that could improve prognosis and retard the pathogenesis of the eye disease is also being discussed. We have provided a comprehensive detail on the localization of ocular stem cells and explain the therapeutic potential of each stem cell. Stem cell-based therapy holds an extraordinary prospective in improving the lives of people who suffer from visual disorders. Ocular diseases can be classified into vascular defects, anatomical defects and neurodegenerative defects. Identification of the proper sources of stem cells is the first step towards this, followed by their isolation and characterization. Research in this area will continue to grow to develop new remedies in treating and helping in problems related to vision loss. Interestingly, stem cell-based therapy is not a one-stop general remedy; however, it carries a promising future in producing new biological elements used to treat vision loss. Further understanding of the retinal regeneration phenomenon will shed light on the cellular basis of retinal regeneration and expand the horizon for cell therapy for many intractable retinal degenerative diseases.
Conflicts of interest
The authors have no conflict of interest.
Acknowledgements
The author's wishes to acknowledge the generous support extended from Dr. Charles H. Newcomer and Tomiko Newcomer for the successful completion of this review article.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Fortier L.A. Stem cells: classifications, controversies, and clinical applications. Vet Surg. 2005;34(5):415–423. doi: 10.1111/j.1532-950X.2005.00063.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) WHO; Geneva: 2016. Country and Regional Data on Diabetes. [Google Scholar]
- 3.Sicree R., Shaw J., Zimmet P. Prevalence and projections. In: Gan D., editor. Diabetes Atlas International Diabetes Federation. 3rd ed. International Diabetes Federation; Brussels, Belgium: 2006. pp. 16–104. [Google Scholar]
- 4.Romano A.C., Espana E.M., Yoo S.H., Budak M.T., Wolosin J.M., Tseng S.C. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci. 2003;44:5125–5129. doi: 10.1167/iovs.03-0628. [DOI] [PubMed] [Google Scholar]
- 5.Sengupta N., Caballero S., Sullivan S.M. Regulation of adult hematopoietic stem cells fate for enhanced tissue-specific repair. Mol Ther. 2009;17:1594–1604. doi: 10.1038/mt.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shichida Y., Matsuyama T. Evolution of opsins and photo transduction. Philos Trans R Soc B Biol Sci. 2009;364(1531):2881–2895. doi: 10.1098/rstb.2009.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas J.B., George R., Asokan R. Prevalence and causes of vision loss in central and south Asia: 1990–2010. Br J Ophthalmol. 2014;98(5):592–598. doi: 10.1136/bjophthalmol-2013-303998. [DOI] [PubMed] [Google Scholar]
- 8.Bourne R.R.A., Jonas J.B., Flaxman S.R. Prevalence and causes of vision loss in high-income countries and in Eastern and Central Europe: 1990–2010. Br J Ophthalmol. 2014;98(5):629–638. doi: 10.1136/bjophthalmol-2013-304033. [DOI] [PubMed] [Google Scholar]
- 9.Vassilev Z.P., Ruigomez A., Soriano-Gabarro M., Rodrıguez L.A.G. Diabetes, cardiovascular morbidity, and risk of age related macular degeneration in a primary care population. Investig Ophthalmol Vis Sci. 2015;56(3):1585–1592. doi: 10.1167/iovs.14-16271. [DOI] [PubMed] [Google Scholar]
- 10.Cheng C.Y., Yamashiro K., Chen L.J. New loci and coding variants confer risk for age-related macular degeneration in East Asians. Nat Commun. 2015;6:6063. doi: 10.1038/ncomms7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akhtar S., Ahmed A., Randhawa M.A. Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies. J Health Popul Nutr. 2013;31(4):413–423. doi: 10.3329/jhpn.v31i4.19975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu X., Lyu D., Dong X., He J., Yao K. Hypertension and risk of cataract a meta-analysis. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114012. Article ID e114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sayin N., Kara N., Pekel G. Ocular complications of diabetes mellitus. World J Diabetes. 2015;6(1):92–108. doi: 10.4239/wjd.v6.i1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye J., He J., Wang C. Smoking and risk of age-related cataract: a meta-analysis. Investig Ophthalmol Vis Sci. 2012;53(7):3885–3895. doi: 10.1167/iovs.12-9820. [DOI] [PubMed] [Google Scholar]
- 15.Land M.F., Fernald R.D. The evolution of eyes. Annu Rev Neurosci. 1992;15:1–29. doi: 10.1146/annurev.ne.15.030192.000245. [DOI] [PubMed] [Google Scholar]
- 16.Cotsarelis G., Cheng S.Z., Dong G., Sun T.T., Lavker R.M. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- 17.Davanger M., Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. doi: 10.1038/229560a0. [DOI] [PubMed] [Google Scholar]
- 18.Dua H.S., Joseph A., Shanmuganathan V.A., Jones R.E. Stem cell differentiation and the effects of deficiency. Eye. 2003;17:877–885. doi: 10.1038/sj.eye.6700573. [DOI] [PubMed] [Google Scholar]
- 19.Jonas J.B., Kamppeter B.A., Harder B., Vossmerbaeumer U., Sauder G., Spandau U.H.M. Intravitreal triamcinolone acetonide for diabetic macular edema: a prospective, randomised study. J Ocular Pharmacol Ther. 2006;22(3):200–207. doi: 10.1089/jop.2006.22.200. [DOI] [PubMed] [Google Scholar]
- 20.Hou H.Y., Liang H.L., Wang Y.S. A therapeutic strategy for choroidal neovascularization based on recruitment of mesenchymal stem cells to the sites of lesions. Mol Ther. 2010;18(10):1837–1845. doi: 10.1038/mt.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stahl A., Smith L.E.H. An eye for discovery. J Clin Investig. 2010;120(9):3008–3011. doi: 10.1172/JCI44158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mok P.L., Leong C.F., Cheong S.K. Cellular mechanisms of emerging applications of mesenchymal stem cells. Malays J Pathol. 2013;35(1):17–32. [PubMed] [Google Scholar]
- 23.Achyut B.R., Varma N.R., Arbab A.S. Application of umbilical cord blood derived stem cells in diseases of the nervous system. J Stem Cell Res Ther. 2014;4:202. doi: 10.4172/2157-7633.1000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar S.S., Alarfaj A.A., Munusamy M.A. Recent developments in β-cell differentiation of pluripotent stem cells induced by small and large molecules. Int J Mol Sci. 2014;15(12):23418–23447. doi: 10.3390/ijms151223418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T., Wang F., Wu M., Wang Z.Z. Development of hematopoietic stem and progenitor cells from human pluripotent stem cells. J Cell Biochem. 2015;116(7):1179–1189. doi: 10.1002/jcb.25097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quigley H., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantravadi A.V., Vadhar N. Glaucoma. Prim Care. 2015;42(3):437–449. doi: 10.1016/j.pop.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Johnson T.V., Bull N.D., Hunt D.P. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:2051–2059. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng T.K., Fortino V.R., Palaez D. Progress of mesenchymal stem cell therapy for neural and retinal diseases. World J Stem Cells. 2014;6(2):111–119. doi: 10.4252/wjsc.v6.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman D.S., O'Colmain B.J., Munoz B. Prevalence of age-related macular degeneration in the United States. Arc Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 31.WHO. Prevention of Blindness and Visual Impairment; Priority Eye Diseases. Available online: http://www.who.int/blindness/causes/priority/en/index8.html Accessed 30 June 2014.
- 32.NHS Choices [homepage on the internet]. Epub: Cited February 2012 http://www.nhs.uk/conditions/maculardegeneration/Pages/Introduction.aspx.
- 33.VRMNY. Dry (Atrophic) Macular Degeneration. Epub: Cited January 2012. http://www.vrmny.com/pe/amd/dmd.html.
- 34.Fernandez-San Jose P.M., Corton F., Blanco-Kelly Targeted next-generation sequencing improves the diagnosis of autosomal dominant retinitis pigmentosa in Spanish patients. Investig Ophthalmol Vis Sci. 2015;56(4):2173–2182. doi: 10.1167/iovs.14-16178. [DOI] [PubMed] [Google Scholar]
- 35.Fliegauf M., Benzing T., Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8(11):880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 36.Falkner-Radler C.I., Krebs I., Glittenberg C. Human retinal pigment epithelium (RPE) transplantation: outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br J Ophthalmol. 2011;95(3):370–375. doi: 10.1136/bjo.2009.176305. [DOI] [PubMed] [Google Scholar]
- 37.Siqueira R.C., Messias A., Voltarelli J.C., Messias K., Arcieri R.S., Jorge R. Resolution of macular oedema associated with retinitis pigmentosa after intravitreal use of autologous BM-derived hematopoietic stem cell transplantation. Bone Marrow Transplant. 2013;48(4):612–613. doi: 10.1038/bmt.2012.185. [DOI] [PubMed] [Google Scholar]
- 38.Roozafzoon R., Lashay A., Vasei M. Dental pulp stem cells differentiation into retinal ganglion-like cells in a three dimensional network. Biochem Biophys Res Commun. 2015;457(2):154–160. doi: 10.1016/j.bbrc.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 39.Blazejewska E.A., Schlotzer-Schrehardt U., Zenkel M. Corneal limbal microenvironment can induce transdifferentiation of hair follicle stem cells into corneal epithelial-like cells. Stem Cells. 2009;27(3):642–652. doi: 10.1634/stemcells.2008-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oh J.Y., Kim M.K., Shin M.S. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26(4):1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L., Wang W., Liu Y. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Cells. 2011;29(6):972–980. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garita-Hernandez M., Diaz-Corrales F., Lukovic D. Hypoxia increases the yield of photoreceptors differentiating from mouse embryonic stem cells and improves the modelling of retinogenesis in vitro. Stem Cells. 2013;31(5):966–978. doi: 10.1002/stem.1339. [DOI] [PubMed] [Google Scholar]
- 43.Kelley M.W., Turner J.K., Reh T.A. Regulation of proliferation and photoreceptor differentiation in fetal human retinal cell cultures. Investig Ophthalmol Vis Sci. 1995;36(7):1280–1289. [PubMed] [Google Scholar]
- 44.Weiss S., Reynolds B.A., Vescovi A.L., Morshead C., Craig C.G., van der Kooy D. Is there a neural stem cell in the mammalian forebrain? Trends Neurosci. 1996;19:387–393. doi: 10.1016/s0166-2236(96)10035-7. [DOI] [PubMed] [Google Scholar]
- 45.Seaberg R.M., Van Der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci. 2003;26(3):125–131. doi: 10.1016/S0166-2236(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 46.Chen Z., de Paiva C.S., Luo L. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lavker R.M., Tseng S.C., Sun T.T. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004;78:433–446. doi: 10.1016/j.exer.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 48.Dua H.S., Shanmuganathan V.A., Powell-Richards A.O. Limbal epithelial crypts: a novel anatomical structure and a putative limbal stem cell niche. Br J Ophthalmol. 2005;89:529–532. doi: 10.1136/bjo.2004.049742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad I., Das A.V., James J. Neural stem cells in the mammalian eye: types and regulation. Semin Cell Dev Biol. 2004;15:53–62. doi: 10.1016/j.semcdb.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Moshiri A., McGuire C.R., Reh T.A. Sonic hedgehog regulates proliferation of the retinal ciliary marginal zone in post hatch chicks. Dev Dyn. 2005;233:66–75. doi: 10.1002/dvdy.20299. [DOI] [PubMed] [Google Scholar]
- 51.Sanges D., Romo N., Simonte G. Wnt/β-catenin signalling triggers neuron reprogramming and regeneration in the mouse retina. Cell Rep. 2013;4(2):271–286. doi: 10.1016/j.celrep.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Lin P.K., Ke C.Y., Khor C.N., Cai Y.J., Lee Y.J. Involvement of SDF1a and STAT3 in granulocyte colony stimulating factor rescues optic ischemia-induced retinal function loss by mobilizing hematopoietic stem cells. Investig Ophthalmol Vis Sci. 2013;54(3):1920–1930. doi: 10.1167/iovs.12-10499. [DOI] [PubMed] [Google Scholar]
- 53.Park S.S., Caballero S., Bauer G. Long-term effects of intravitreal injection of GMP-grade bone-marrow-derived CD34+ cells in NOD-SCID mice with acute ischemia-reperfusion injury. Investig Ophthalmol Vis Sci. 2012;53(2):986–994. doi: 10.1167/iovs.11-8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang H.M., Hung K.H., Hsu C.C., Lin T.C., Chen S.Y. Using induced pluripotent stem cell-derived conditional medium to attenuate the light-induced photo damaged retina of rats. J Chin Med Assoc. 2015;78(3):169–176. doi: 10.1016/j.jcma.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 55.Assawachananont J., Mandai M., Okamoto S. Transplantation of embryonic and induced pluripotent stem cell derived 3D retinal sheets into retinal degenerative mice. Stem Cell Rep. 2014;2(5):662–674. doi: 10.1016/j.stemcr.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tucker B.A., Park I.H., Qi S.D. Transplantation of adult mouse iPS cell-derived photoreceptor precursors restores retinal structure and function in degenerative mice. PLoS One. 2011;6(4) doi: 10.1371/journal.pone.0018992. Article ID e18992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamao H., Mandai M., Okamoto S. Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Rep. 2014;2(2):205–218. doi: 10.1016/j.stemcr.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y.S., Zhao K.X., Wang X.L., Chen Y.X., Wang L., Mu Q.J. Effects of bone marrow mesenchymal stem cell transplantation on retinal cell apoptosis in premature rats with retinopathy. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14(12):971–975. [PubMed] [Google Scholar]
- 59.Mesentier-Louro L.A., Zaverucha-do-Valle C., da Silva A.J. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0110722. Article ID e110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cejkova J., Trosan P., Cejka C. Suppression of alkali induced oxidative injury in the cornea by mesenchymal stem cells growing on nanofiber scaffolds and transferred onto the damaged corneal surface. Exp Eye Res. 2013;116:312–323. doi: 10.1016/j.exer.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Chiou S.H., Kao C.L., Peng C.H. A novel in vitro retinal differentiation model by co-culturing adult human bone marrow stem cells with retinal pigmented epithelium cells. Biochem Biophys Res Commun. 2005;326(3):578–585. doi: 10.1016/j.bbrc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 62.Liu J.T., Chen Y.L., Chen W.C. Role of pigment epithelium-derived factor in stem/progenitor cell-associated neovascularization. J Biomed Biotechnol. 2012;2012:10. doi: 10.1155/2012/871272. Article ID871272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugitani S., Tsuruma K., Ohno Y. The potential neuroprotective effect of human adipose stem cells conditioned medium against light-induced retinal damage. Exp Eye Res. 2013;116:254–264. doi: 10.1016/j.exer.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 64.Zwart I., Hill A.J., Al-Allaf F. Umbilical cord blood mesenchymal stromal cells are neuroprotective and promote regeneration in a rat optic tract model. Exp Neurol. 2009;216(2):439–448. doi: 10.1016/j.expneurol.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 65.Ocata Therapeutics. Safety and tolerability of sub-retinal transplantation of hESC derived RPE (MA09-hRPE) cells in patients with advanced dry age related macular degeneration (dry AMD): NCT01344993. Clinical trial. gov. https://clinicaltrials.gov/ct2/show/NCT01344993?term=NCT01344993&rank=1.Updated November 3, 2014. Accessed 25 March 2015.
- 66.Schwartz S.D., Regillo C.D., Lam B.I. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–516. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 67.Padma Priya Sivan, Sakinah Syed, Pooi-Ling Mok. Stem cell therapy for treatment of ocular disorders. Stem Cells Int. 2016:1–18. doi: 10.1155/2016/8304879. 8304879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ooto S., Akagi T., Kageyama R. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci U S A. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahmad I., Tang L., Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–521. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- 70.Junqueira L.C.U., Mescher A.L. 12th ed. McGraw-HillMedical; New York, NY, USA: 2010. Junqueira's Basic Histology. Text and Atlas. [Google Scholar]
- 71.Turner D.L., Cepko C.L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- 72.Chaum E. Retinal neuroprotection by growth factors: a mechanistic perspective. J Cell Biochem. 2003;88(1):57–75. doi: 10.1002/jcb.10354. [DOI] [PubMed] [Google Scholar]
- 73.Wahlin K.J., Campochiaro P.A., Zack D.J. Neurotrophic factors cause activation of intracellular signalling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Investig Ophthalmol Vis Sci. 2000;41(3):927–936. [PubMed] [Google Scholar]
- 74.Binder S., Krebs I., Hilgers R.D. Outcome of transplantation of autologous retinal pigment epithelium in age-related macular degeneration: a prospective trial. Investig Ophthalmol Vis Sci. 2004;45(11):4151–4160. doi: 10.1167/iovs.04-0118. [DOI] [PubMed] [Google Scholar]
- 75.Tropepe V., Coles B.L., Chiasson B.J. Retinal stem cells in the adult mammalian eye. Science. 2000;287(5460):2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 76.Coles B.L., Angenieux B., Inoue T. Facile isolation and the characterization of human retinal stem cells. Proc Natl Acad Sci U S A. 2004;101(44):15772–15777. doi: 10.1073/pnas.0401596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ahmad I. Stem cells: new opportunities to treat eye diseases. Investig Ophthalmol Vis Sci. 2001;42(12):2743–2748. [PubMed] [Google Scholar]
- 78.Shi Qiang, VandeBerg John L. Experimental approaches to derive CD34+ progenitors from human and nonhuman primate embryonic stem cells. Am J Stem Cells. 2015;4(1):32–37. [PMC free article] [PubMed] [Google Scholar]
- 79.Kekre N., Antin J.H. Hematopoietic stem cell transplantation donor sources in the 21st century: choosing the ideal donor when a perfect match does not exist. Blood. 2014;124(3):334–343. doi: 10.1182/blood-2014-02-514760. [DOI] [PubMed] [Google Scholar]
- 80.Hadjadj F., Debre P., Merle-Beral H. Immunochemical characterization of the CD34+ workshop antibodies. Tissue Antigens. 1993;42:374a. [Google Scholar]
- 81.Berenson R.J., Bensinger W.I., Hill R.S. Engraftment after infusion of CD34+ marrow cells on patients with breast cancer or neuroblastoma. Blood. 1991;77:1717–1722. [PubMed] [Google Scholar]
- 82.Schwartz S.D., Hubschman J.P., Heilwell G. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012;379:713–720. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 83.Caballero S., Sengupta N., Afzal A. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes. 2007;56:960–967. doi: 10.2337/db06-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calzi L., Kent D.L., Change K.H. Labeling of stem cells with monocrystalline iron oxide for tracking and localization by magnetic resonance imaging. Microvasc Res. 2009;78:132–139. doi: 10.1016/j.mvr.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pellegrini G., Dellambra E., Golisano O. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao X., Das A.V., Thoreson W.B. Adult corneal limbal epithelium: a model for studying neural potential of non-neural stem cells/progenitors. Dev Biol. 2002;250:317–331. [PubMed] [Google Scholar]
- 87.Brown K.D., Low S., Mariappan I. Plasma polymer coated contact lenses for the culture and transfer of corneal epithelial cells in the treatment of limbal stem cell deficiency. Tissue Eng Part A. 2014;20(3–4):646–655. doi: 10.1089/ten.tea.2013.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dhamodaran K., Subramani M., Matalia H., Jayadev C., Shetty R., Das D. One for all: a standardized protocol for ex-vivo culture of limbal, conjunctival and oral mucosal epithelial cells into corneal lineage. Cytotherapy. 2016;18(4):546–561. doi: 10.1016/j.jcyt.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Hadlock T., Singh S., Vacanti J.P., Mclaughlin B.J. Ocular cell monolayers cultured on biodegradable substrates. Tissue Eng. 1999;5(3):187–196. doi: 10.1089/ten.1999.5.187. [DOI] [PubMed] [Google Scholar]
- 90.Hu X., Lui W., Cui L. Tissue engineering of nearly transparent corneal stroma. Tissue Eng. 2005;11(11-12):1710–1717. doi: 10.1089/ten.2005.11.1710. [DOI] [PubMed] [Google Scholar]
- 91.Ito A., Hibino E., Kobayashi C. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng. 2005;11(3-4):489–496. doi: 10.1089/ten.2005.11.489. [DOI] [PubMed] [Google Scholar]
- 92.Lin N., Lin J., Bo L., Weidong P., Chen S., Xu R. Differentiation of bone marrow-derived mesenchymal stem cells into hepatocyte-like cells in an alginate scaffold. Cell Prolif. 2010;43:427–434. doi: 10.1111/j.1365-2184.2010.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yi T., Song S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch Pharmacol Res. 2012;35(2):213–221. doi: 10.1007/s12272-012-0202-z. [DOI] [PubMed] [Google Scholar]