Abstract
Global biodiversity is affected by manifold drivers, yet the extent to which environmental changes contribute to changes in local diversity is poorly understood. We investigated biodiversity changes in a meta-analysis of 39 European re-survey studies in temperate forests (3,988 vegetation records in total, 17-75 years between the two surveys) by assessing the importance of coarse-resolution (i.e. among-sites) and fine-resolution (i.e. within-sites) environmental differences, and also the importance of changing environmental conditions between surveys.
We contribute to clarifying the mechanisms underlying the direction and magnitude of local-scale biodiversity changes. While not detecting any net diversity loss, we observed considerable among-site variation, partly explained by temporal changes in light availability (a local driver) and game density (a regional driver). Furthermore, strong evidence was found that pre-survey levels of nitrogen deposition determined subsequent diversity changes. We conclude that models forecasting future biodiversity changes should consider coarse-resolution environmental changes, account for differences in baseline environmental conditions and for local changes in fine-resolution environmental conditions.
Keywords: atmospheric nitrogen deposition, evenness, forestREplot, forest management, game browsing, Shannon diversity, spatio-temporal re-survey data, species richness
Introduction
The way in which environmental factors influence the composition and diversity of plant communities is one of the key questions in current ecological research (Sutherland et al., 2013). Over the past decades, the impact of anthropogenic drivers, such as changing management, deposition of air pollutants, biotic invasion, or changing climate have been linked to shifts in community structure and composition (e.g. Bertrand et al., 2011, Grimm et al., 2013). Pereira et al. (2012) ranked habitat loss, overexploitation, invasive species, climate change, and pollution as the main drivers of current changes in community composition and biodiversity. While these general patterns emerge from observations and experiments at a local scale, covering a broad range of different taxonomic groups, biomes and environmental factors, there is limited knowledge on how long-term changes in local communities contribute to and whether they mirror global patterns (Dornelas et al., 2014).
Recently, several meta-analyses addressing this knowledge gap have found evidence of compositional changes in local communities, even though mean change in local species diversity was not significantly different from zero. Across marine, freshwater and terrestrial biomes, Dornelas et al. (2014) found no systematic temporal changes in species richness, but significant species turnover. Assemblages were undergoing biodiversity changes, but not systematic diversity losses, as lost species were likely replaced by immigrating species; a result in accordance with studies of terrestrial animal communities (Supp & Ernest, 2014). Amongst terrestrial plant communities, Vellend et al. (2013) also observed no systematic loss or gain in species numbers and evenness over time.
These last two studies, however, also emphasized the considerable variation in biodiversity change among the reported drivers of vegetation change and habitats, some of which was highly context dependent. Context dependency has been reported previously; e.g. contrasting effects of nitrogen deposition on diversity are contingent on soil acid status and vegetation cover (Johnston et al., 1986). Murphy and Romanuk (2014) found, in a comparison of data from several terrestrial biomes (boreal, northern temperate forests, tropics) and several functional groups (producers, ectotherms, and endotherms), differences among functional groups (highest diversity loss for endotherms) and among biomes (highest decline in the tropics). There were strong differences between the environmental drivers related to the loss in species numbers; while habitat loss was important in the tropics, it was not relevant in boreal or temperate forests.
All abovementioned studies explored global patterns by relating temporal changes in biodiversity to differences in environmental conditions such as anthropogenic drivers. The novelty of this study is that we explicitly link observed temporal changes in biodiversity with observed broad-scale and local-scale changes in environmental conditions during the survey period across multiple single studies in a meta-analysis. We focus on temporal changes in understory vascular plant diversity across European temperate forests. In temperate forest ecosystems – alongside habitat loss and fragmentation – atmospheric pollution, management change, climate change, and disturbances such as browsing by game, biotic invasions, and windthrows are known to drive changes in vegetation composition and diversity (Bernhardt-Römermann et al., 2007, Bobbink et al., 2010, De Frenne et al., 2013, Gilliam, 2006, Lenoir et al., 2010, Paillet et al., 2010, Royo et al., 2010, Smart et al., 2014, Verheyen et al., 2012). However, each of these drivers acts on different spatial scales (cf. extent and resolution). For example, macroclimate environmental factors differ between sites across large spatial extents (e.g. continents) and have coarse spatial resolution (≥ 1 km2: cf. gridded data), while, for instance, light availability varies across smaller spatial extents (e.g. forest stands) involving finer spatial resolutions (< 1000 m2: cf. plot data). Our study aims to solve this issue by using a spatio-temporal approach. We analyze temporal diversity changes at different spatial scales involving continental to regional (extent of single study areas) spatial extents and coarse (gridded data) to fine (plot data) spatial resolutions to unravel which environmental factors drive changes in diversity. The temporal scale comprises several decades, over which changes in species composition were recorded using re-surveys of semi-permanent and permanent vegetation plots. Long-term re-survey studies are particularly important for communities with relatively slow dynamics, such as herb layers in temperate forests (Gilliam, 2007). Furthermore, the herb layer typically comprises a large fraction of vascular plant diversity and is sensitive to various global-change drivers, including atmospheric deposition and forest management (Gilliam, 2007). Another advantage of analyzing re-survey plots is that we account for initial environmental conditions (cf. the baseline) as well as changes in environmental conditions over time. Consequently, we characterize ecological factors at the beginning and ending of the surveys related to herbivory pressure, forest management, climate and atmospheric depositions, as not only changes but also the baseline of these factors could influence changes in diversity (Dayton et al., 1998, Pauly, 1995).
We used a network of 39 individual re-survey studies representing temperate forest ecosystems of Europe to address two objectives: (i) Based on previous studies, we expect no net diversity changes at the continental extent, but considerable across-site variation. We will explicitly test for patterns of diversity change across all 39 studies and determine which coarse-grained environmental drivers (e.g. climate, atmospheric pollution) and site-level habitat conditions (e.g., herbivory pressure, silvicultural management) contribute most to temporal changes in diversity across study sites. (ii) At the spatial extent of each of the selected studies, we expect plot-specific environmental conditions like nutrient availability and forest management to drive local changes in biodiversity. Thus, we tested which local environmental drivers are associated with temporal change in local biodiversity.
Materials and Methods
Datasets
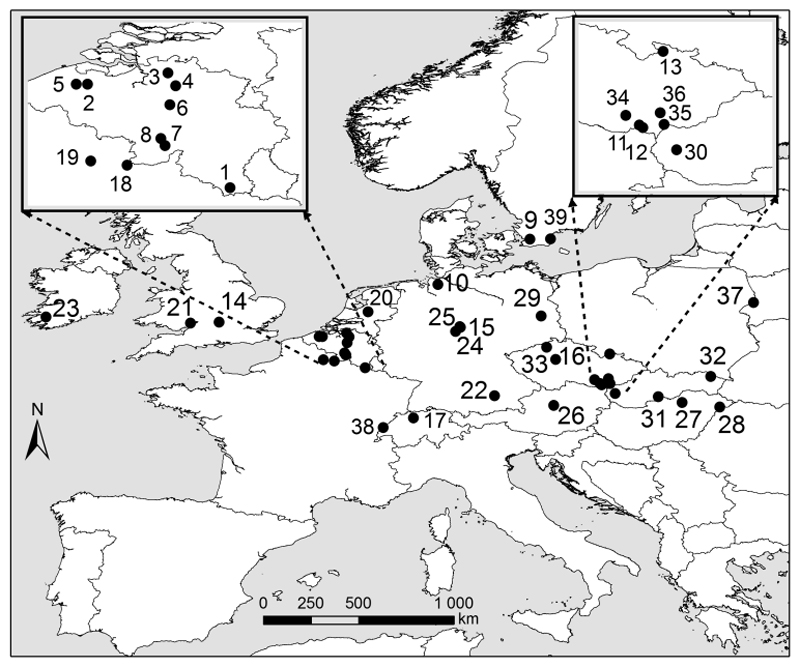
We used 39 independent forest understory re-survey studies (hereafter referred to as datasets), collected in semi-natural temperate forests across 13 European countries ranging from Switzerland and Hungary to Sweden (south–north) and from Ireland to Poland (west–east). Twenty-two and twenty-three of these datasets were previously analyzed in Verheyen et al. (2012) and De Frenne et al. (2013), respectively. We extended these data by adding 16 datasets to increase coverage of temperate understory plant communities across Europe (Fig. 1, see Table S1 in Supporting Information). All datasets describe the vegetation of ancient forest sites (sensu Peterken, 1996) in which no stand-replacing disturbances (e.g. clear-cuttings followed by replanting with conifers) have taken place since the date of the first survey (hereafter called baseline survey). However, management system changes could have taken place without significant changes in tree species composition (e.g. transformation from coppicing to mature forest). Each dataset is composed of multiple permanent or semi-permanent vegetation plots (mean number was 51 plots with a range from 11 to 164; plot sizes ranged between 1 m2 and 1000 m2, with most between 100 m2 and 500 m2). For each of the 1,994 plots, a complete inventory of all understory plant species was available for two time periods (for the Stenshuvud dataset spring ephemerals were not recorded). Baseline surveys were carried out between 1930 and 1993 and the most recent re-surveys between 1987 and 2012 (hereafter called re-survey). Time intervals between the two surveys ranged between 17 and 75 years (37 years on average). Cover of all vascular species in the understory layer for both survey dates was recorded in percent. Where available (33 of 39 datasets), plot-level cover data for the shrub and tree layers were included as well. All datasets are also included in a global database of forest herb layer re-survey plots from temperate forests (www.forestreplot.ugent.be). For further details see Table S1.
Fig. 1.
Map showing the locations of the 39 datasets included in this study (numbers correspond to Table S1).
Response variables
We used three measures of biodiversity differing in the weight given to species abundances:
Species richness (S), the number of species present within a vegetation plot, gives equal weight to all species;
Shannon diversity, a combined measure of species richness and evenness, which weights species by their abundance. We used the exponent of the Shannon index as a true diversity index (referred to henceforth as H);
Species evenness (E) is the relative percentage of species distributed within one plot. It is independent from species richness and ranges from zero to one, with one representing a perfectly equal distribution of all occurring species. Evenness was calculated following Smith and Wilson (1996). See Appendix S2 for more information.
Explanatory variables
To explain temporal changes in diversity across all 39 datasets (covering a continental extent) we used coarse-grained (≥ 1 km2) variables related to climate, soil conditions, and deposition of pollutants from the air, as well as site-level (study area) variables related to herbivory pressure and management intensity. To explain changes in diversity between plots within a given dataset (covering a regional extent) and separately for each dataset, we used plot-level (< 1000 m2) variables related to light availability at the forest floor, nutrient cycling and total herb layer cover, which may reflect competition among herb layer species. All these variables were used to characterize the environmental conditions at (i) the beginning or end of the observation and (ii) changes during the time of observations (Table S3).
Explanatory variables for diversity changes among datasets (hereafter referred to as coarse-grained variables): We used mean annual temperature (MAT), mean annual precipitation (MAP), and seasonality in precipitation (coefficient of variation derived from monthly precipitation values) to characterize climatic conditions. Climate data were derived from the Climatic Research Unit at a spatial resolution of 0.5° latitude/longitude grid cells covering monthly means for the period 1901-2013 (Harris et al., 2014). We calculated the 10-year means for each of the three aforementioned climate variables before the baseline survey, and the ten years before the re-surveys for each dataset. Current climatic conditions were characterized using the data derived for the re-survey; changes in climatic conditions were characterized by subtracting the values for the baseline survey from those of the re-survey. Nitrogen deposition was quantified using the EMEP database at a 50-km spatial resolution. The accumulated rate of atmospheric N-deposition between the two surveys and the amount of nitrogen already deposited at the time of the baseline survey were calculated based on the methods described in Appendix S2. Soil conditions were characterized by topsoil acidity (pH) and bulk density (which may reflect rooting conditions and water and nutrient retention capacity), all derived from the Harmonized World Soil Database at a 1-km spatial resolution (FAO/IIASA/ISRIC/ISSCAS/JRC, 2009). As the variability in soil conditions may be very large within one square-kilometer, we used these variables to place the datasets along the continental gradient in soil conditions only. We assume that soil conditions remained stable over time. Game density was estimated as the sum of locally occurring large herbivores (per 100 ha; in most cases roe deer, fallow deer, red deer). Additionally, for each dataset it was estimated whether game numbers increased, decreased, or remained stable between the two vegetation surveys (game changes). These variables were based on the local knowledge available to the data contributors. Game densities were log-transformed prior to analysis. Management intensity was estimated by data contributors in three classes (‘no management’; ‘low intensity management’, referring to removal of small fractions of the canopy trees due to low-frequency selection cutting, i.e. less than 1x per 10 years; and ‘high intensity management’, referring to removal of significant fractions of the canopy trees at higher frequency, i.e. more than 1x per 10 years) and change in management intensity (management changes) as either increase, decrease or stable. Year of the baseline survey, inter-census intervals, and size of the study area were used as covariates to account for the potential effects of site and survey-specific conditions on diversity change.
Explanatory variables for diversity changes within datasets (hereafter referred to as plot-level variables). All plot-level variables were calculated for the baseline survey and the re-survey separately. We used two measures likely to be linked to light availability at the forest floor, both reflecting local changes in management of the woody overstory. Total cover of all tree and shrub layer species (TSL) was calculated based on species-specific cover values (Fischer, 2015, formula in Appendix S2). The shade casting ability (SCA) of the tree species was calculated as a cover-weighted average of the shade casting ability index scores listed in Baeten et al. (2009); these range between 1 (low shade casting ability) and 5 (high shade casting ability) (also see approach in Van Calster et al., 2007, Verheyen et al., 2012). Two local-scale variables refer to plot-specific nutrient cycling and availability. The litter quality (LQ) of the occurring tree species was calculated as the cover-weighted average litter quality index scores listed in Baeten et al. (2009). LQ scores range between 1 (slow decomposition rate) and 5 (high decomposition rate) (see also approach in Van Calster et al., 2007, Verheyen et al., 2012). As measures for plot-specific nutrient availability, we used a proxy for humus quality and the turnover rates of organic matter (Hms) (calculated as the product of the species cover weighted Ellenberg indicator values for N x R, Ellenberg et al., 2001, Rogister, 1978). Ellenberg indicator values for N and R classify plant species according to the position of their realized ecological niche along a nitrogen and soil reaction gradient, respectively (Ellenberg et al., 2001). Furthermore, total cover of the herb layer (HL), which may reflect competition among herb layer species, was calculated from species-specific cover values following Fischer (2015, formula in Appendix S2).
Statistical analyses
Diversity changes among datasets
For each dataset, temporal changes were characterized by calculating response ratios (study-level RR) for the three diversity measures. These were calculated per study as the mean log-ratios between the re-survey and the baseline survey (Hedges et al., 1999). RR were log-transformed prior to analyses, following Hedges et al. (1999) so that RR larger than zero means increases and RR below zero indicates decreases over time. We used meta-regression (random-effects model, for technical details see Viechtbauer, 2010) to test whether RR for each diversity measure differed significantly from zero. All statistics were performed in R 3.1.2 (R Development Core Team, 2014). RR were calculated using the escalc-function (method: ROM), meta-regression analyses were done using the rma.uni-function (Sidik-Jonkman estimator) of the metafor-package (Viechtbauer, 2010).
A similar approach was used to identify which species changed significantly in terms of percent cover over time. For each dataset, species-specific Cohen’s d effect-sizes and their pooled within-group variances were calculated based on cover estimations. Cohen’s d effect-sizes were chosen, in contrast to RR, because it can accommodate cover values of zero. These effect-sizes were determined by taking the difference between the means of the baseline and the re-survey divided by the pooled standard deviation (Cohen, 1988). For each species, meta-regression analysis was performed to test whether changes across datasets were significantly different from zero.
To link coarse-grained environmental variables to dataset-level changes in diversity (represented by study-level RR), we used meta-regression models including moderator variables (mixed-effects model according to Viechtbauer, 2010). Moderators (study-level variables) may account for parts of the heterogeneity in the overall effects. All abovementioned coarse-grained environmental variables were used as moderators. Full models with 2-way interactions were simplified via backward selection of the least significant variables until the final minimal adequate model obtained a minimal Akaike Information Criterion (Crawley, 2007). Prior to analysis, all continuous moderators were standardized to make the explanatory variables that were measured on different scales comparable (Schielzeth, 2010). All standardized variables were checked for possible inter-correlations (no significant correlations observed).
Diversity changes within datasets
Prior to analyses on the plot-level, temporal changes in all response (S, H, E) and explanatory plot-level variables were calculated as log-transformed response ratios (RR). To test for the influence of plot-level variables on diversity changes between the baseline and re-surveys, we used path analyses based on the plot-level RR of each diversity index (S, H, E). The RR of the plot-level explanatory variables included are linked as shown in the a priori path model in Fig. 2 (hereafter called the light & nutrient model) and as described in detail in Table 1. This path model assesses the relative importance of simultaneously operating environmental drivers on diversity (Arkle et al., 2014, Grace et al., 2014). It was not possible to fit the light & nutrient model for all single datasets, so we built an alternative path model not including the total cover of tree and shrub layer species (TSL) and shade casting ability of tree species (SCA), hereafter called the nutrient model. We fitted both models to each dataset (33 in total, as for six datasets no information about the tree layer was available), and chose for further analyses the best fitting model based on the χ2-value, its p-value, and the Root Mean Square Error of Approximation (RMSEA).
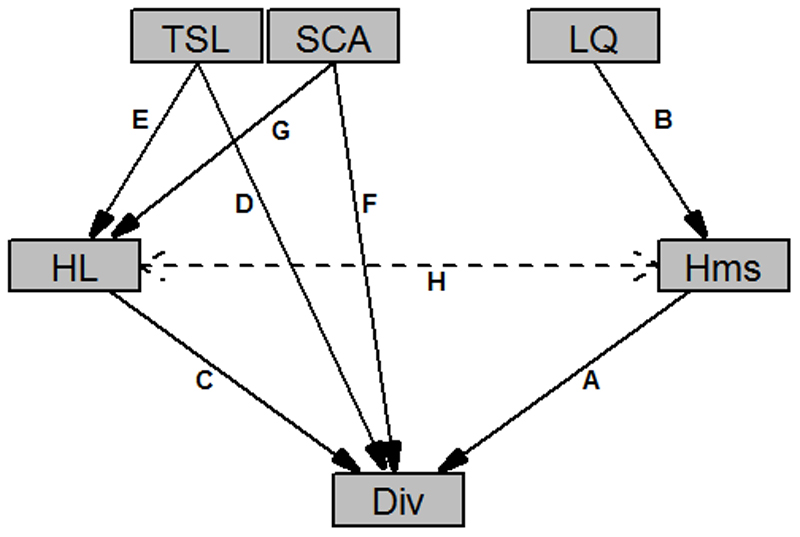
Fig. 2.
A priori developed path model used to analyze the influence of temporal changes (RR) in local environmental drivers on changes in different measures of biodiversity (Div, here species richness, Shannon diversity, evenness) in herbaceous plant communities. Abbreviations refer to plot-level response ratios between the baseline and the re-survey: TSL: Total cover of tree and shrub layer species; SCA: Shade casting ability of the tree species; LQ: Litter quality of the tree species; HL: Total cover of herb layer species; Hms: Humus quality. Capital letters are described in Table 1.
Table 1.
Pathways and ecological processes associated with the a priori path model shown in Fig. 2; abbreviations according to Fig. 2.
| Pathway | Code | Process | Mechanistic examples |
|---|---|---|---|
| Hms → Div | A | Influence of humus quality (nutrient availability) on biodiversity | Changes towards higher nutrient availability favor nutrient demanding species and suppress oligotrophic species |
| LQ → Hms | B | Influence of litter quality (decomposition rate) on humus quality (nutrient availability) | Changes towards faster decomposition of tree leaf litter lead to better humus quality (higher nutrient availability) |
| HL → Div | C | Influence of competition (plant individual density) on biodiversity | Changes towards higher total cover of the herb layer are related to changes in abundance of dominating species |
| TSL → Div | D | Influence of light availability (total cover of tree and shrub layer species) on diversity | Changes towards a more closed canopy of the overstory results in less available light at the forest floor and may lead to decreased abundance of light demanding species |
| TSL → HL | E | Influence of light availability (total cover of tree and shrub layer species) on interspecific competition | Changes towards a more open canopy of the overstory allow a higher total cover of the herb layer due to higher light availability |
| SCA → Div | F | Influence of shade casting ability of the tree layer species on diversity | Changes towards canopy species with higher shade casting ability (late successional species) lead to a decrease in the abundance of light demanding herbaceous species |
| SCA → HL | G | Influence of shade casting ability of the tree layer species on competition | Changes towards species with higher shade casting ability (late successional species) allow a lower total cover of the herb layer due to a lower availability of the resource light |
| Hms ↔ HL | H | Co-variation between humus quality and interspecific competition of herb layer species | Changes towards better humus quality (higher nutrient availability) allow high nutrient-demanding species to increase in cover and consequently lead to a higher total cover of the herb layer; tall nutrient demanding species have better decomposability of litter and lead to better humus quality |
For each dataset, we extracted the standardized path coefficients and their variances from the best fitting model. Distinguishing between the two model types (light & nutrient or nutrient), we fitted separate meta-regressions for each model path (using the path coefficients and variances derived for each single dataset) to summarize its path coefficient and to test for overall differences from zero. Path analyses were conducted using the sem-function (maximum likelihood estimation with robust standard errors and a Satorra-Bentler scaled test statistic) of the lavaan package (Rosseel, 2012).
The two types of path models included different sets of environmental factors related to understory light and nutrient availability (light & nutrient vs. nutrient). As we were interested in whether the better fit of a dataset into one of these two model types was related to potential differences in coarse-grained environmental factors, we tested whether coarse-grained environmental factors differ between the dataset described best by the light & nutrient model or the nutrient model, using a t-test for continuous or a χ2-test for categorical variables.
Results
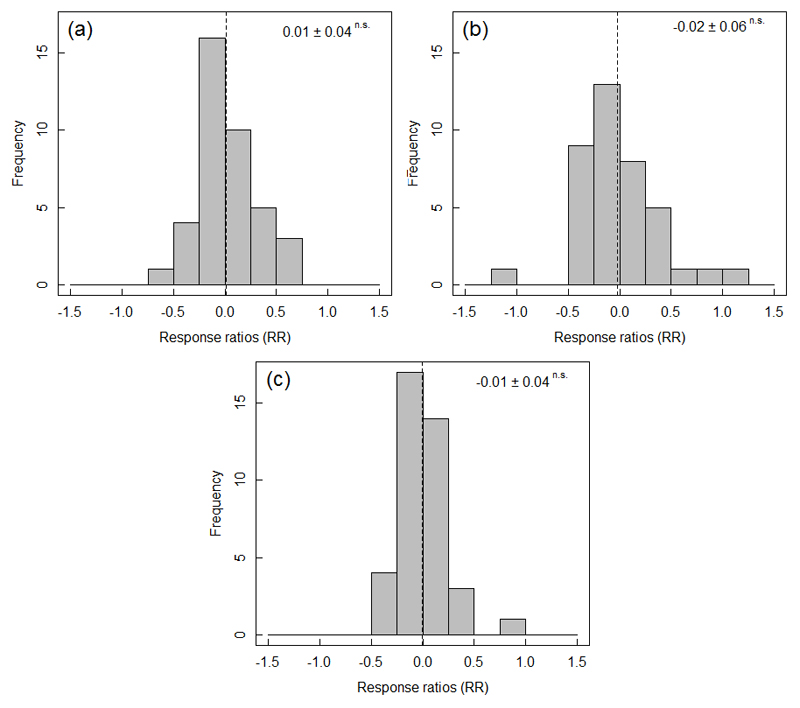
For all three measures of diversity, average temporal changes between the baseline survey and the re-survey were not significantly different from zero but there was high variability among datasets (Fig. 3). While the range in values of response ratios was between -0.56 and 0.69, -1.07 and 1.09, -0.40 and 0.79, for S, H, or E respectively, tests for skewness were insignificant (results not shown). An overview on the species with significant cover changes over time is provided in Table S4. We observed 39 species decreasing in cover vs. 12 species increasing in cover. Based on autecological knowledge of the species, species that decreased in cover tended to be associated with open, thermophilous forests on mostly nutrient-poor soils (e.g. Carex montana, Iris variegata, Melampyrum pratense, Tanacetum corymbosum), or acidic topsoil conditions (e.g. Festuca ovina, Genista germanica, Luzula luzuloides). In contrast, species that increased in cover between surveys are associated with shaded and moist conditions often associated with increased nutrient availability (e.g. Carex remota, Dryopteris carthusiana, Dryopteris dilatata, Impatiens parviflora, Poa trivialis) or are light demanding and associated with human disturbance (e.g. Galeopsis tetrahit, Lactuca serriola, Plantago major, Senecio viscosus).
Fig. 3.
Temporal changes in understory herbaceous plant biodiversity. Histograms for all datasets (N = 39) of the change in different aspects of biodiversity (A: species richness; B: Shannon diversity; C: evenness) between the first and recent vegetation sampling estimated using response ratios. Dashed lines denote the mean RR. Mean RR ± SE and statistical significance of the change are presented in the upper right corner.
All three diversity measures decreased less if the accumulated nitrogen deposition at the time of the baseline survey was high, i.e. if the initial levels of atmospheric nitrogen deposition were high, diversity changes were lower (Table 2). We found that S and H increased when game density declined between the two surveys, while stable or increasing game densities lead to a decrease in species richness. Furthermore, S and H decreased when browsing pressure was high. Surprisingly, none of the most parsimonious models included significant effects of climatic variables, accumulated N-deposition between the surveys, soil variables, or management factors.
Table 2.
Effects of large-scale factors on changes in forest understory diversity for 39 sites across Europe. Summary statistics of the mixed-effect meta-regression models are shown. The table includes estimates (i.e. RR), standard errors (SE), t- and corresponding p-values for all environmental variables included in the most parsimonious’ models. τ2 is an estimate of residual amount of heterogeneity. Significant results are marked as bold.
| Estimate | SE | t-value | p-value | |
|---|---|---|---|---|
| Species richness (overall p-value<0.001, τ2= 0.04, r2= 0.488) | ||||
| Intercept [decreasing game density] | 0.278 | 0.09 | 3.02 | 0.006 |
| Game density | -0.217 | 0.05 | -4.43 | 0.000 |
| Changes in game density [stable] | -0.281 | 0.12 | -2.33 | 0.029 |
| Changes in game density [increase] | -0.316 | 0.11 | -2.75 | 0.012 |
| Accumulated N-deposition at baseline survey | -0.157 | 0.04 | -3.86 | 0.001 |
| Changes in mean annual temperature | -0.074 | 0.04 | -1.83 | 0.081 |
| Shannon diversity (overall p-value= 0.001, τ2= 0.09, r2= 0.436) | ||||
| Intercept [decreasing game density] | 0.331 | 0.13 | 2.49 | 0.020 |
| Game density | -0.302 | 0.07 | -4.36 | 0.000 |
| Changes in game density [stable] | -0.372 | 0.17 | -2.15 | 0.042 |
| Changes in game density [increase] | -0.373 | 0.16 | -2.29 | 0.031 |
| Accumulated N-deposition at baseline survey | -0.191 | 0.06 | -3.28 | 0.003 |
| Evenness (overall p-value= 0.048, τ2= 0.04, r2= 0.080) | ||||
| Intercept | -0.004 | 0.03 | -0.12 | 0.904 |
| Accumulated N-deposition at baseline survey | -0.070 | 0.03 | -2.05 | 0.048 |
To test for the influence of local overstory to understory relationships on long-term diversity changes, two alternative path models (light & nutrient, and nutrient model) fitted the data well for all datasets (non-significant χ2-values and RMSEA < 0.08). Sixteen and 17 datasets were best described by the light & nutrient and the nutrient model, respectively. For each dataset, the same model type was fit for each diversity measure. Datasets described best by the light & nutrient model had a significantly higher amount of cumulative N-deposition at the baseline survey (525 kg/ha) than the nutrient model datasets (324 kg/ha) (Table S5). Although not significantly different, nutrient model datasets were first surveyed earlier than the light & nutrient model datasets (1963 vs. 1971). In the light & nutrient model datasets, the total cover of the tree and shrub layer significantly decreased between the two surveys (RR: -0.15), but it was more stable at the nutrient model datasets (RR: 0.05).
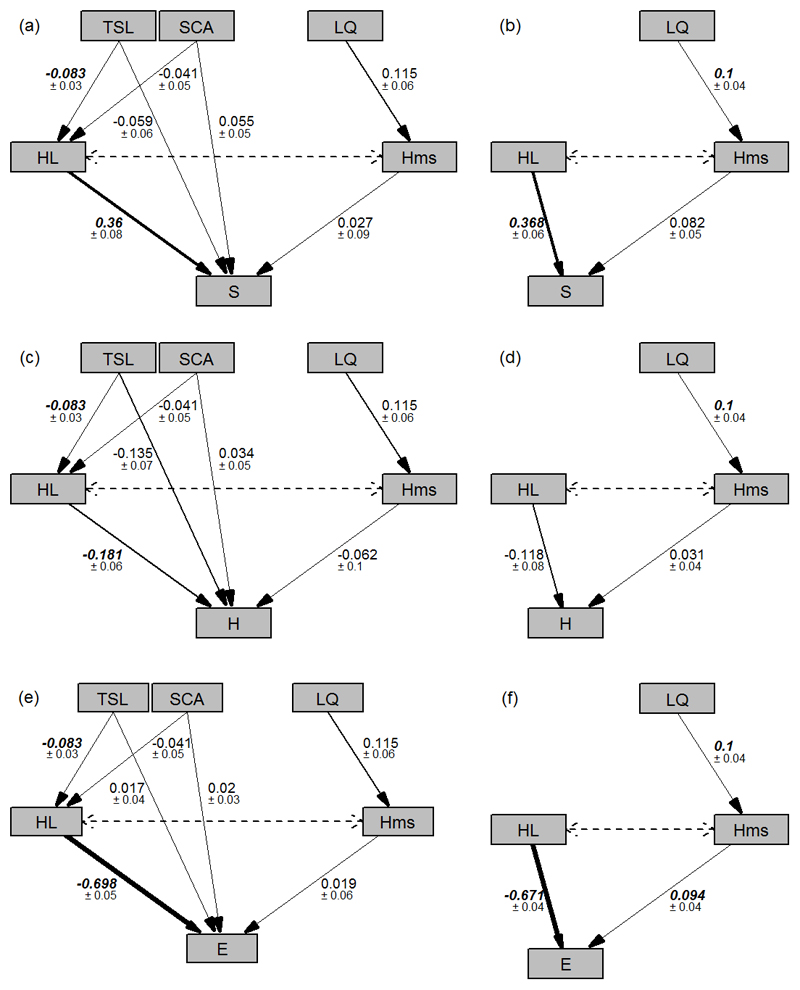
With regards to the light & nutrient model (Fig. 4), total cover of the herb layer (HL) mediated the influence of stand characteristics on diversity. However, only increasing total tree and shrub layer cover (TSL) had a significantly negative influence on HL. The influence of HL on diversity varied in magnitude and direction across diversity measures. For example, the path coefficient was strongly negative for E, i.e. when more weight was given to plant cover in the calculation of the diversity measure. The differences in the path coefficient of the causal relationship between HL and diversity for all diversity measures became more apparent when considering the combined effect of TSL and HL on diversity: for S, the negative effect of TSL on HL was reduced by the positive relationship between HL and S, while for E it was strengthened by the negative relation between HL and E.
Fig. 4.
Results of the path analyses revealing the influence of local environmental drivers on different aspects of biodiversity (S: Species richness, H: Shannon diversity, E: Evenness). Fig. A, C, E show the light & nutrient model results, B, D, F show those of the nutrient model results. Standardized path coefficients ± SE, derived from the meta-regression analyses, are shown and are the summaries of dataset-level individual path analyses (Table S6). Significant path coefficients are shown in bold; abbreviations follow Fig. 2.
For the light & nutrient model, litter (LQ) and humus quality (Hms) did not contribute significantly to changes in understory diversity. In contrast, for the nutrient model we observed that changes towards better litter quality were generally associated with better humus quality. The influence of humus quality on diversity was only significant for E, which slightly increased with increasing humus quality.
Discussion
The comprehensive analysis of 39 European forest datasets did not reveal systematic changes in local species diversity (richness, Shannon, evenness) with time, in line with our expectations and previous meta-analyses on biodiversity changes in temperate forests of Europe (Verheyen et al., 2012). The selected datasets include only ancient forests, so these results do not apply to forest communities subjected to direct impacts of habitat loss and land conversion, which are major drivers of global biodiversity loss (Foley et al., 2005, Pereira et al., 2012, Vellend et al., 2013). We focused on mostly intact habitats in order to disentangle subtle changes in diversity patterns that are not as obvious as when a forest has been, for instance, converted into agricultural land. Given the focus on temporally stable forest habitats, our results do not contradict the findings of global losses in diversity.
Influence of broad-scale environmental factors
Overall changes in local diversity were not significantly different from zero, but in most datasets species diversity either increased or decreased with time. We observed, echoing Dirnböck et al. (2014), a shift towards dominance of more nutrient-demanding forest species, and, echoing Paillet et al. (2010) a shift in favor of disturbance-tolerant species (ruderals).
Decreases in all three diversity measures of diversity were related to high game densities. Similar patterns were observed by Katona et al. (2013) and Jenkins et al. (2014), who attributed decreases in species richness at high browsing pressure to selective damage of plants preferred by game for feeding. Such selective feeding may result in increased cover of ferns, a pattern in accordance with our results. Jenkins et al. (2014) predicted a recovery in species richness when game density declines, which is also consistent with our findings.
We did not observe significant impacts of climatic variables, accumulated N-deposition between surveys, soil variables, or management on species diversity. We explain this unexpected pattern in two ways: (1) Changing environmental conditions do not necessarily causes predictable directional changes in the forest floor environment. For instance, in a forest with an already dense overstory, a decrease in forest management may not alter the light regime at the forest floor. Thus, the influence of this factor might not affect forest floor species diversity. In contrast, in a forest with an open canopy, a decrease in forest management may lead to canopy closure lower light availability in the forest floor, and, consequently, an increase/decrease in species diversity of the forest understory. We argue that temporal changes in biodiversity due to forest management are highly context dependent. (2) In several cases, the resolution of the available environmental data may not be sufficient to detect impacts of environment on diversity. For example, we observed a strong influence of shrub and tree cover on diversity, which may be used as a proxy for management intensity (Verheyen et al., 2012). However, management intensity, a categorical variable, did not affect any diversity measure in this meta-analysis. We conclude that one limitation of the present study was that the quantification of certain environmental drivers (e.g., management intensity, topsoil acidity (pH), climatic variables) was too coarse to capture temporal changes in biodiversity. Future research should try to include more detailed characterizations of environment, especially those intended to reflect anthropogenic drivers.
One of our most striking results was that larger changes in all three diversity indices occurred in regions where less accumulated nitrogen deposited at the time of the baseline survey. Thus, the datasets in our study had different baselines (e.g. Dayton et al., 1998, Isbell et al., 2014, Pauly, 1995); for datasets with low diversity changes over time, but having experienced high amounts of already deposited nitrogen at the time of the baseline survey, community changes induced by nitrogen may already have taken place before the baseline survey. Surprisingly, diversity changes were only weakly related to the year of the baseline survey. An explanation may be that the year of the baseline survey acts as a proxy for different environmental conditions at the start of the observation period. When identifying appropriate baselines for observational studies, we should rely on more direct measures of environmental conditions, such as levels of pre-existing deposited nitrogen, rather than indirect measures, such as year.
Bobbink et al. (2010) proposed deposition rates of 20 kg/ha*year, a value exceeded by most of our datasets, as a threshold below which the amount of annual nitrogen deposition in forest ecosystems may not lead to compositional changes in understory vegetation in temperate forests (critical load). Focusing on species diversity only, we observed that N-deposition did not influence any measure of diversity, which contradicts the expectations by the concept of critical load, but is in accordance with the study by Verheyen et al. (2012) who postulated that dense forest canopies may buffer the effect of enhanced nitrogen availability. To explain the missing influence of N-deposition on species richness, there are two potential interpretations: (i) changes in stand characteristics towards a denser canopy cover may delay diversity changes in the understory until the stands are opened again (Keith et al., 2009, Verheyen et al., 2012) or (ii) due to different baselines at the time of the initial survey, nitrogen-induced changes had already occurred.
Influence of local-scale environmental factors
We tested these two hypotheses with a path analysis approach. The datasets were subdivided into two groups: the first (the light & nutrient model sites) included all datasets where vertical stand characteristics were important (mainly total sum of the tree and shrub layer) and the second (the nutrient model datasets) included all datasets where nutrient cycling and availability (litter and humus quality) were related to changes in diversity indices. If the ‘different baseline’ hypothesis is true, we would assume that nutrient-related changes mainly occur in datasets with low levels of pre-existing nitrogen deposition (Gilliam, 2006). Conversely, if diversity changes were delayed due to changes in stand characteristics towards a denser tree canopy, we should find high diversity changes in datasets where total canopy cover decreases (Verheyen et al., 2012). Both hypotheses appear valid. On one hand, in datasets with low pre-existing nitrogen deposition, nutrient-related environmental factors controlled biodiversity changes. On the other hand, for the datasets best described by the light & nutrient model, the total sum of tree and shrub layer cover decreased between the two surveys. This environmental driver, related to light availability at the forest floor, was the strongest driver of diversity changes. Both processes should be considered to fully understand the processes underlying temporal changes in diversity. Indeed, it is important to incorporate potential differences in baselines of environmental drivers (here the already accumulated nitrogen deposition at the time of the baseline survey, but one may think of other factors not incorporated in this study), but, also local changes in stand structures related to forest management may delay plant community responses to increased nitrogen deposition (e.g. environmental factors that change within the observation period).
The applied path analysis approach clearly demonstrates that the magnitude of plot-specific biodiversity changes is due to the combined effect of simultaneous environmental drivers. It is noteworthy that, direct and mediated effects of overstory stand characteristics both impacted changes in herb layer biodiversity patterns. The observed direct effect of decreasing light availability at the forest floor (increasing tree layer cover) resulted in declines in species richness and Shannon diversity, which coincides with recent studies (Bernhardt-Römermann et al., 2010, Decocq et al., 2004, Kopecký et al., 2013, Plue et al., 2013). Persistent shading results in local extinctions by reducing establishment and survival of light-demanding species (Valverde & Silvertown, 1997). Interestingly, understory-overstory relationships included strong mediating effects, which either diluted or strengthened overstory effects on understory diversity. We observed a decline in total cover of the herb layer when light availability at the forest floor decreased over time. Reduced light availability at the forest floor slows down mineralization of organic matter and triggers the buildup of thicker litter layers (Van Calster et al., 2007). Consequently, herb layer cover co-varied with humus quality. However, the impact of herb layer cover on diversity differed for each diversity measure. Species richness increased with herb layer cover, which may be explained by two complementary factors: (i) the co-occurrence of shade-tolerant and heliophilous species at higher levels of light availability may lead to increased total cover of the herb layer, and (ii) the spread of nutrient-demanding species often builds a dense herb layer. The species originally present would likely decrease in cover (possibly leading to local extinctions in the long-term), but in the short term they would still contribute to species richness. The first of these processes would also lead to the observed increase in evenness where total herb layer cover was lower. Where light availability was reduced, shade-tolerant species likely exhibited relatively high survival, yet as weak competitors, evenness would increase as none of these species dominated the community. In contrast with increased humus quality, evenness would decrease as species promoted by higher nutrient availability will dominate communities (Bernhardt-Römermann et al., 2010, Gilliam, 2006).
Conclusion
In our study we found evidence that three complementary processes are needed to explain dataset-specific changes in biodiversity. First, potential differences in baselines of environmental drivers, such as the accumulated nitrogen deposition at the time of the baseline survey, may lead to differences in diversity responses because nitrogen-induced changes may already have taken place before the baseline survey started. Second, temporal changes in local stand structure governing light availability at the forest floor may delay vegetation responses to increased nitrogen deposition. Third, increasing game density was associated with decreases in species diversity across the study areas, suggesting that the selective feeding preferences of wild animals may strongly affect regional biodiversity changes.
Surprisingly we observed that changes in coarse-grained environmental conditions (like climate) had no significant effect on diversity changes (but see marginal influence of changes in mean annual temperature on species richness). We conclude that models forecasting future biodiversity changes should not only account for changes in coarse-grained environmental conditions (cf. future climate change and land-use change scenarios) but also incorporate both coarse-grained baseline environmental conditions (e.g., baseline nitrogen deposition) and local fine-resolution changes in environmental conditions to improve the accuracy of their predictions.
Supplementary Material
Additional Supporting Information may be found in the online version of this article:
Acknowledgements
The research of RH, MC, MK, FM, MM, PP, OV has received funding from the European Research Council under the European Union’s Seventh Framework Program (FP7/2007-2013) – ERC Grant agreement no. 278065, long-term research development project no. RVO 67985939, and grant no. CZ.1.07/2.3.00/20.0267 of the Ministry of Education of the Czech Republic. The research of KV is supported by the ERC Consolidator Grant 614839 – PASTFORWARD. The research of DLK, FJGM and MN has been supported by the National Parks & Wildlife Service of the Government of Ireland. DC was supported by the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Science Foundation (FZT 118). We thank Germund Tyler and Johnny Cornelis for providing data.
References
- Arkle RS, Pilliod DS, Hanser SE, et al. Quantifying restoration effectiveness using multi-scale habitat models: implications for sage-grouse in the Great Basin. Ecosphere. 2014;5 art31. [Google Scholar]
- Baeten L, Bauwens B, De Schrijver A, et al. Herb layer changes (1954-2000) related to the conversion of coppice-with-standards forest and soil acidification. Applied Vegetation Science. 2009;12:187–197. [Google Scholar]
- Bernhardt-Römermann M, Kudernatsch T, Pfadenhauer J, Kirchner M, Jakobi G, Fischer A. Long-term effects of nitrogen-deposition on vegetation in a deciduous forest near Munich, Germany. Applied Vegetation Science. 2007;10:399–406. [Google Scholar]
- Bernhardt-Römermann M, Römermann C, Pillar VD, Kudernatsch T, Fischer A. High functional diversity is related to high nitrogen availability in a deciduous forest – Evidence from a functional trait approach. Folia Geobotanica. 2010;45:111–124. [Google Scholar]
- Bertrand R, Lenoir J, Piedallu C, et al. Changes in plant community composition lag behind climate warming in lowland forests. Nature. 2011;479:517–520. doi: 10.1038/nature10548. [DOI] [PubMed] [Google Scholar]
- Bobbink R, Hicks K, Galloway J, et al. Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications. 2010;20:30–59. doi: 10.1890/08-1140.1. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Ed. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Crawley MJ. The R Book. Chichester: Wiley & Sons; 2007. [Google Scholar]
- Dayton PK, Tegner MJ, Edwards PB, Riser KL. Sliding baselines, ghosts, and reduced expectations in kelp forest communities. Ecological Applications. 1998;8:309–322. [Google Scholar]
- De Frenne P, Rodriguez-Sanchez F, Coomes DA, et al. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18561–18565. doi: 10.1073/pnas.1311190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decocq G, Aubert M, Dupont F, et al. Plant diversity in a managed temperate deciduous forest: understorey response to two silvicultural systems. Journal of Applied Ecology. 2004;41:1065–1079. [Google Scholar]
- Dirnböck T, Grandin U, Bernhardt-Römermann M, et al. Forest floor vegetation response to nitrogen deposition in Europe. Global Change Biology. 2014;20:429–440. doi: 10.1111/gcb.12440. [DOI] [PubMed] [Google Scholar]
- Dornelas M, Gotelli NJ, Mcgill B, Shimadzu H, Moyes F, Sievers C, Magurran AE. Assemblage Time Series Reveal Biodiversity Change but Not Systematic Loss. Science. 2014;344:296–299. doi: 10.1126/science.1248484. [DOI] [PubMed] [Google Scholar]
- Ellenberg H, Weber HE, Düll R, Wirth V, Werner W. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica. (3 ed) 2001;18:1–262. [Google Scholar]
- Fao/Iiasa/Isric/Isscas/Jrc. Harmonized World Soil Database (version 1.1) FAO; Rome, Italy and IIASA, Laxenburg, Austria: 2009. [Google Scholar]
- Fischer HS. On the combination of species cover values from different vegetation layers. Applied Vegetation Science. 2015;18:169–170. [Google Scholar]
- Foley JA, Defries R, Asner GP, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- Gilliam FS. Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. Journal of Ecology. 2006;94:1176–1191. [Google Scholar]
- Gilliam FS. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience. 2007;57:845–858. [Google Scholar]
- Grace JB, Adler PB, Harpole WS, Borer ET, Seabloom EW. Causal networks clarify productivity-richness interrelations, bivariate plots do not. Functional Ecology. 2014;28:787–798. [Google Scholar]
- Grimm NB, Staudinger MD, Staudt A, et al. Climate-change impacts on ecological systems: introduction to a US assessment. Frontiers in Ecology and the Environment. 2013;11:456–464. [Google Scholar]
- Harris I, Jones PD, Osborn TJ, Lister DH. Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 Dataset. International Journal of Climatology. 2014;34:623–642. [Google Scholar]
- Hedges LV, Gurevitch J, Curtis PS. The meta-analysis of response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- Isbell F, Tilman D, Polasky S, Loreau M. The biodiversity-dependent ecosystem service debt. Ecology Letters. 2014 doi: 10.1111/ele.12393. [DOI] [PubMed] [Google Scholar]
- Jenkins LH, Jenkins MA, Webster CR, Zollner PA, Shields JM. Herbaceous layer response to 17 years of controlled deer hunting in forested natural areas. Biological Conservation. 2014;175:119–128. [Google Scholar]
- Johnston AE, Goulding KWT, Poulton PR. Soil acidification during more than 100 years under permanent grassland and woodland at Rothamsted. Soil Use and Management. 1986;2:3–10. [Google Scholar]
- Katona K, Kiss M, Bleier N, et al. Ungulate browsing shapes climate change impacts on forest biodiversity in Hungary. Biodiversity and Conservation. 2013;22:1167–1180. [Google Scholar]
- Keith SA, Newton AC, Morecroft MD, Bealey CE, Bullock JM. Taxonomic homogenization of woodland plant communities over 70 years. Proceedings of the Royal Society B-Biological Sciences. 2009;276:3539–3544. doi: 10.1098/rspb.2009.0938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecký M, Hédl R, Szabó P. Non-random extinctions dominate plant community changes in abandoned coppices. Journal of Applied Ecology. 2013;50:79–87. doi: 10.1111/1365-2664.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir J, Gegout JC, Dupouey JL, Bert D, Svenning JC. Forest plant community changes during 1989-2007 in response to climate warming in the Jura Mountains (France and Switzerland) Journal of Vegetation Science. 2010;21:949–964. [Google Scholar]
- Murphy GEP, Romanuk TN. A meta-analysis of declines in local species richness from human disturbances. Ecology and Evolution. 2014;4:91–103. doi: 10.1002/ece3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillet Y, Bergès L, Hjältén J, et al. Does biodiversity differ between managed and unmanaged forests? A meta-analysis on species richness in Europe. Conservation Biology. 2010;24:101–112. doi: 10.1111/j.1523-1739.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Pauly D. Anecdotes and the shifting baseline syndrome of fisheries. Trends in Ecology & Evolution. 1995;10:430. doi: 10.1016/s0169-5347(00)89171-5. [DOI] [PubMed] [Google Scholar]
- Pereira HM, Navarro LM, Martins IS. Global Biodiversity Change: The Bad, the Good, and the Unknown. Annual Review of Environment and Resources. 2012;37:25–50. [Google Scholar]
- Peterken GF. Natural Woodland. Ecology and Conservation in Northern TemperateRegions. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Plue J, Van Gils B, De Schrijver A, Peppler-Lisbach C, Verheyen K, Hermy M. Forest herb layer response to long-term light deficit along a forest developmental series. Acta Oecologica. 2013;53:63–72. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- Rogister JE. De ekologische mR- en mN-waarden van de kruidlaag en de humuskwaliteit van bosplantengezelschappen. Groenendaal-Hoeilaart: Proefstation van Waters en Bossen; 1978. [Google Scholar]
- Rosseel Y. lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software. 2012;48:1–36. [Google Scholar]
- Royo AA, Collins R, Adams MB, Kirschbaum C, Carson WP. Pervasive interactions between ungulate browsers and disturbance regimes promote temperate forest herbaceous diversity. Ecology. 2010;91:93–105. doi: 10.1890/08-1680.1. [DOI] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution. 2010;1:103–113. [Google Scholar]
- Smart SM, Ellison AM, Bunce RGH, et al. Quantifying the impact of an extreme climate event on species diversity in fragmented temperate forests: the effect of the October 1987 storm on British broadleaved woodlands. Journal of Ecology. 2014;102:1273–1287. [Google Scholar]
- Smith B, Wilson JB. A consumer's guide to evenness indices. Oikos. 1996;76:70–82. [Google Scholar]
- Supp SR, Ernest SKM. Species-level and community-level responses to disturbance: a cross-community analysis. Ecology. 2014;95:1717–1723. doi: 10.1890/13-2250.1. [DOI] [PubMed] [Google Scholar]
- Sutherland WJ, Freckleton RP, Godfray HCJ, et al. Identification of 100 fundamental ecological questions. Journal of Ecology. 2013;101:58–67. [Google Scholar]
- Valverde T, Silvertown J. An integrated model of demography, patch dynamics and seed dispersal in a woodland herb, Primula vulgaris. Oikos. 1997;80:67–77. [Google Scholar]
- Van Calster H, Baeten L, De Schrijver A, De Keersmaeker L, Rogister JE, Verheyen K, Hermy M. Management driven changes (1967-2005) in soil acidity and the understorey plant community following conversion of a coppice-with-standards forest. Forest Ecology and Management. 2007;241:258–271. [Google Scholar]
- Vellend M, Baeten L, Myers-Smith IH, et al. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19456–19459. doi: 10.1073/pnas.1312779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheyen K, Baeten L, De Frenne P, et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. Journal of Ecology. 2012;100:352–365. [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.