Abstract
More and more ecologists have started to resurvey communities sampled in earlier decades to determine long-term shifts in community composition and infer the likely drivers of the ecological changes observed. However, to assess the relative importance of, and interactions among, multiple drivers joint analyses of resurvey data from many regions spanning large environmental gradients are needed. In this paper we illustrate how combining resurvey data from multiple regions can increase the likelihood of driver-orthogonality within the design and show that repeatedly surveying across multiple regions provides higher representativeness and comprehensiveness, allowing us to answer more completely a broader range of questions. We provide general guidelines to aid implementation of multi-region resurvey databases. In so doing, we aim to encourage resurvey database development across other community types and biomes to advance global environmental change research.
Keywords: legacy data, (quasi-)permanent plots, community ecology, ground layer vegetation, temperate forest
1. Introduction
Increasing human impacts on the environment have large and pervasive effects on the composition and functioning of ecosystems (Millenium Ecosystem Assessment 2005). This makes it important to document and understand how ecosystems and communities are changing and to determine how the multiple drivers of global change interact. Without such knowledge, we are unable to develop appropriate strategies for the effective conservation and restoration of biodiversity and to maintain desired ecosystem functions.
To improve our understanding of how multiple global change drivers affect ecosystems, we should combine different methods (Luo et al. 2011). Quantifying how ecosystems and communities vary along environmental gradients is an important source of information in this respect (e.g. Newbold et al. 2015), complementing knowledge gained from experiments and modelling studies (cf. Luo et al. 2011). Environmental gradient studies can give information on ecosystem responses to multiple drivers across space, and can also be used to infer how ecosystems may potentially respond to temporally varying drivers. However, such space-for-time approaches rely on many assumptions (e.g. Walker et al. 2010). Repeat observations of the same community over time to quantify how communities are changing are therefore invaluable additional sources of information (e.g. Tingley & Beissinger 2009, Dornelas et al. 2012), particularly when data extend to several decades or longer, as more reliable and informative signals to estimate the nature and rates of change can be obtained (cf. Magnuson 1990; Pauly 1995).
More and more ecologists have started to resurvey communities sampled in earlier decades to determine long-term shifts in community composition and infer the likely drivers of the ecological changes observed. Plant ecologists now use vegetation data from early to mid-20th century vegetation descriptions to examine long-term changes in these communities (see e.g. Bakker et al. 1996 for an earlier discussion on the topic). Many examples from other communities exist as well (e.g., birds: Tingley and Beissinger 2013; butterflies: Nieto-Sánchez et al. 2015; small-mammal communities: Moritz et al. 2008; zoobenthos: Olsson et al. 2013).
However, most resurvey studies have worked with data collected in single regions and their utility is limited if we are to understand the importance of the multiple, often interacting, global-change drivers that affect plant and animal communities. These drivers vary at multiple spatial and temporal scales and often covary in space and time. Proper assessments of the relative importance of multiple drivers and of the interactions among them require us to analyse resurvey data from multiple regions, spanning large environmental gradients and multiple geographic regions.
In this paper we provide arguments as to how pooling resurvey data from multiple regions realises the potential to make major contributions to the understanding of community dynamics and response to various interacting environmental changes. We illustrate our arguments with published results from long-term resurveys of temperate forest ground layer vegetation, and share lessons to enable database development and data retention in other community types and biomes. Our approach serves as an example of data sharing and collaboration (Wolkovich et al. 2012, Mills et al. 2015) and furthermore provides an example of how to make best use of heritage datasets, which are often abandoned and at risk of being lost (see also Vellend et al. 2013).
2. The added value of multi-region community resurvey data: representativeness, comprehensiveness and orthogonality
Well-reasoned criteria for dataset inclusion are needed to turn a collection of datasets into a powerful ecological research platform. In this section we therefore start by defining the main features of resurvey datasets suitable for inclusion in a multi-region analysis and then compare how a collection of resurvey datasets performs compared to multi-region experiments and a priori designed community monitoring networks.
We define a resurvey data set as a collection of community surveys sampled at multiple locations within a defined region and across at least two points in time. The two time points typically span a period of at least several decades in order to obtain a true long-term perspective on environmental and community change; i.e. the unique, invaluable feature offered by heritage datasets. A region is defined here as a geographic entity with more or less similar site conditions, including climate, major soil types and levels of atmospheric nitrogen deposition. Regions are defined this way since the main objective of multi-region resurvey data analyses is to quantify the (interactive) effects of multiple drivers which often vary at different scales. For instance, climate change generally plays out at larger spatial scales, whereas management changes can vary among locations within a single region. However, the combined outcome of both drivers will ultimately determine changes in the local microclimate and the resulting changes in community composition (see Fig. 1 for an example). A combination of multiple regions with multiple resurveyed locations within each region is therefore a key design feature of a research platform that aims at understanding long-term community changes. Besides these general criteria, also specific criteria for the inclusion of datasets in the research platform need to be defined so that the platform resembles a priori community monitoring networks with a standardized design (Table 1).
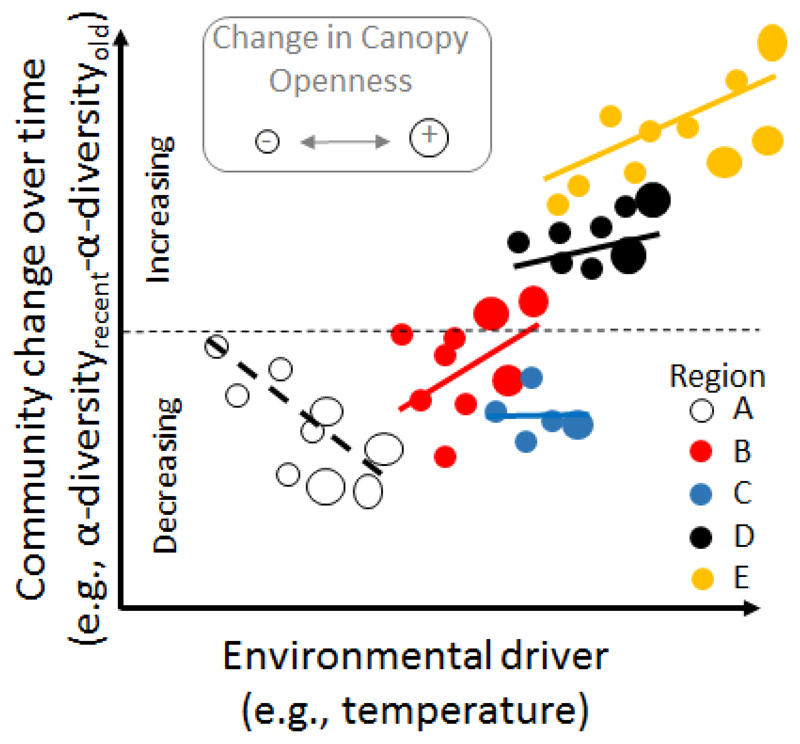
Figure 1.
Collecting data across multiple regions will generate insights that cannot be obtained from single-region studies. In this hypothetical example for forests, nevertheless inspired by De Frenne et al. (2013), alpha-diversity losses and gains over time are observed in colder and warmer regions, respectively. The within-region microclimatic variation caused by closing or opening tree canopies between the two surveys respectively attenuates or reinforces this general trend in alpha-diversity change across the macroclimatic gradient. Only sampling a few locations from each region would show a simplistic relationship and likely lead to incorrect inference.
Table 1.
Overview of criteria used to decide on the inclusion of datasets in multiregion community resurvey studies, illustrated with the decisions taken to feed the ‘forestREplot’ network with datasets.
| Dataset inclusion criteria | forestREplot | |
|---|---|---|
| Criteria | Rationale | |
| General criteria | ||
| Suitable for the scientific goals and questions at hand? | Forest ground layer resurveyed at multiple locations within a region with more or less similar site conditions, including climate, major soil types and levels of atmospheric deposition | This type of data structure is needed to isolate the effects of drivers acting at larger scales, such as changing climate or levels atmospheric pollutant deposition from effects of drivers acting at a more local scale, such as management changes (see also Fig. 1) |
| Specific criteria | ||
| Relevant geographic region? | Temperate forest as defined by Olsen et al. (2001) | Ground layer in temperate forest comprises the majority of plant diversity and has an important impact on ecosystem functioning |
| Relevant system characteristics? | Natural and semi-natural forests according to Peterken (1996). Both are composed of locally native trees and shrubs which often derive from natural regeneration or coppicing rather than planting (in case of semi-natural forests) or have not been managed at all (in case of natural forest) | Management actions such as soil working and fertilization may completely override effects of other global change drivers |
| Between the two surveys, no human-induced conversion to stand types no longer in line with the natural or semi-natural forest criteria has taken place | ||
| Relevant study design? | (Quasi-)permanent plots | Minimizes so-called pseudo-turnover |
| At least 20 plots which can be treated as independent observations (i.e. distributed over a sufficiently large area) per dataset | Sufficient replicates within single regions are needed | |
| At least 20 years between the oldest and most recent survey | Forest ground layer vegetation often shows delayed responses to environmental changes | |
| Plot size varies between 1 m2 and 1000 m2 | Plots falling within this size range are expected to present a representative picture of the ground layer vegetation community | |
| Relevant response variables? | Presence/absence or cover data of all vascular plants in the ground layer community | Needed to get a complete view on community change |
Such a multi-region network of community resurvey data scores well for all three fundamental design criteria for ecological research platforms, notably comprehensiveness, representativeness and orthogonality (Nadrowski et al. 2010, Baeten et al. 2013; Fig. 2). "Comprehensiveness" in this paper relates to the spectrum of ecological questions that can be addressed with a particular research platform. "Representativeness" refers to the relevance of analysed results for sites that were not included in the investigation. Finally, the "orthogonality" of the platform refers to its ability to disentangle the separate effects of each environmental driver on the response variable(s) under study. Most obviously, the representativeness generally increases when multiple regions are incorporated, because sites not initially investigated will more likely fit within the investigated environmental gradients when these gradients are large. This should lead to more reliable inference. The spatiotemporal replication of community data (i.e. resurveys in multiple locations in multiple regions) strongly increases the likelihood of orthogonality within the design. Similarly, repeatedly surveying broadly across multiple landscapes or regions results in high comprehensiveness, allowing us to more completely answer a broader range of questions, and potentially unanticipated ones.
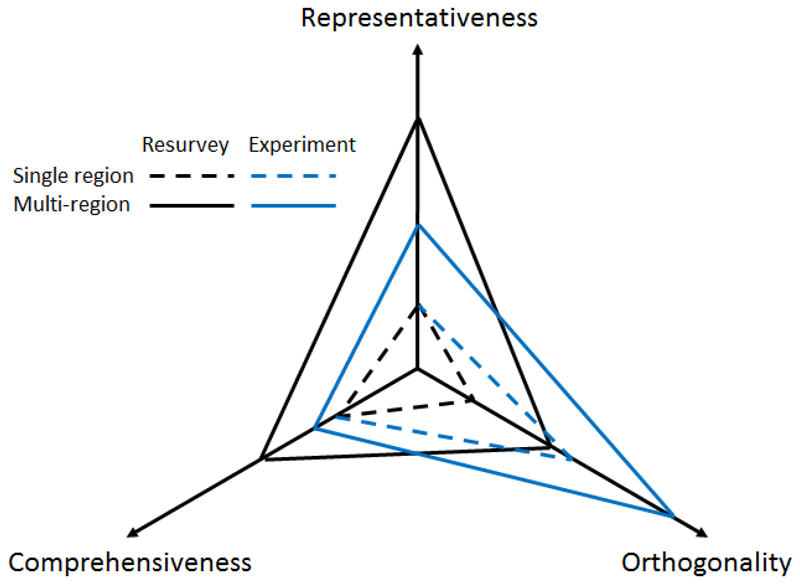
Figure 2.
Comprehensiveness, representativeness, and orthogonality of single-region vs. multiple-region resurveys and experiments. Experiments are more orthogonal than observatories and the combination of multiple regions typically creates higher orthogonality and generates more comprehensive and representative results than from a single region.
In addition, long-term multi-region resurveys have the ability to complement the outcomes of globally distributed experiments with environmental manipulations, such as nitrogen addition (cf. Fraser et al. 2013, Borer et al. 2014; Fig. 2). Although experiments typically score higher on the orthogonality axis, they reduce representativeness and often comprehensiveness by using simplified communities and often extreme (‘shock’) treatments (e.g. a sudden shift from low to high temperature regimes) with a limited number of treatment levels. Furthermore, treatment responses are rarely monitored for more than a few years. These elements constrain the spectrum of questions that can be addressed with experiments and hence their comprehensiveness. Making best use of long-term resurveys from multiple sites as a complement to experimental approaches therefore responds to calls for more integrated approaches to better understand the effects of global changes on complex ecological communities and ecosystem functions (Luo et al. 2011, De Frenne et al. 2013) (see Frerker et al. 2014 for a good example). In that respect, multi-region resurvey studies are in fact analogous to the ‘Phase IV clinical trials’ in medical research, with the experiments covering the earlier phases (see Box 1).
Box 1. Multi-region resurvey studies as ‘Phase IV clinical trials’ in global change biology.
We argue that long-term resurveys from multiple regions can complement experimental approaches to understand the effects of global changes on complex ecological communities and ecosystem functions. This is because controlled experiments in ecology increase orthogonality at the expense of representativeness and comprehensiveness, by monitoring often simplified communities exposed to a restricted number of treatments over a limited time. As such, there are several clear analogies with clinical trials performed in medical research.
Clinical trials are prospective medical research studies on human participants that are designed to answer specific questions about medical interventions, including new treatments (e.g. the development of a novel drug), which warrant further safety and efficacy assessment and comparison (Chow and Liu 2004). They are implemented following four consecutive steps: three prior to commercialisation, but after successful preclinical trials; the fourth one during the commercial life of the drug. This Phase IV, corresponding to drug use in real life instead of under controlled conditions, monitors the efficacy and safety of a drug when it is used in a broad range of patients with various pathologies, concomitantly with other potentially interacting drugs, over the long term. Similarly to phase IV clinical trials, resurvey studies in ecology allow to follow up the effects of a given factor subjected to a broad range of ecosystems with various abiotic and biotic conditions, concomitantly with other potentially interacting factors, over the long term. In Table Box 1, we make a parallel between clinical trials and ecological studies.
Table Box 1. Different phases in clinical trials and their analogues in ecological research.
| Phase | Objective in clinical research | Analogue in ecology |
|---|---|---|
| Preclinical | Safety tests on lab animals | Greenhouse assays (e.g. glyphosate resistance in Koger and Reddy 2005) |
| Pre-commercial | Controlled experiments on humans | Controlled field experiments |
| Phase I | Screening for safety, pharmaco-kinetics and -dynamics | Effect of a given input (e.g. ozone in Velikova et al. 2005) |
| Phase II | Establishing the drug efficacy against a placebo, and the dose-effect relationship | Analysis of the response-effect relationship in plant communities (e.g. nitrogen in Fan et al. 2014) |
| Phase III | Comparing the drug efficacy/safety to an alternative treatment | Comparing the separated and combined effect of factors on vegetation (e.g. light and nitrogen in De Frenne et al. 2015) |
| Commercial life | Monitoring effects in the general public | Monitoring long-term effects in situ |
| Phase IV | Epidemiological studies | Multi-region resurvey studies, e.g. Dirnböck et al. (2014) or Bernhardt-Römermann et al. (2015) |
Likewise to medical research, a fundamental difference in ecology is the distinction between randomized controlled experiments (phases I to III) and monitoring studies (phase IV; called epidemiologic studies in medical research), with the former aiding mechanistic explanation. In monitoring studies, however, the investigators seek associations (correlations) to infer causal relationships between a given factor experienced by participants (e.g. plant communities) and their health status (e.g. species richness). Like in case-control studies, resurveys allow comparing two or more existing groups differing in outcome (cases and controls) with respect to some supposed causal attributes that occurred before or between the two surveys.
Parallel to the rise of globally distributed experiments, also more and more a priori designed community monitoring networks across large environmental gradients are being established. They include top-down designed networks such as the European Level I and II monitoring networks of air pollution effects on forests (http://icp-forests.net/) and the UK Countryside Survey (http://www.countrysidesurvey.org.uk). Multi-region community resurvey networks with a more bottom-up approach, in which regions participate on a voluntary basis, have emerged as well. The GLORIA-network (Pauli et al. 2015) can serve as a prime example. The network applies a highly standardized ‘Multi-Summit Approach’ to survey alpine biodiversity and vegetation patterns on four mountain summits per target region. The results of this observation network help us to better understand the response of alpine biota to climate change (see e.g. Pauli et al. 2012). The first plots were established in 2001 and have been resurveyed at regular intervals since then. Although these multi-region monitoring networks have already produced very valuable results and will certainly continue do so in the future, they have rarely been established more than one or two decades ago and therefore well after the rise in many anthropogenic pressures. Since insights in longer term changes are badly needed (cf. Pauly 1995), attempts should be made to make best use of archived community survey data collected in a more distant past.
In the next section, we illustrate how to put together a network using heritage community resurvey data by introducing forestREplot. In addition, we synthesize already published results from forestREplot to show how new insights can be developed and more general conclusions reached.
3. Putting long-term multi-region resurveys into practice: the forestREplot network as an example
Resurveys of long-term (quasi-)permanent plots are particularly appropriate for communities that exhibit slow dynamics, such as ground layer communities in forests. These plant communities often show delayed responses to environmental changes. The long life span of many ground layer species (Ehrlén and Lehtilä 2002) promotes remnant populations and extinction debts (Eriksson 1996, Vellend et al. 2006), while slow immigration rates can lead to colonization credits (Verheyen et al. 2003). Since the ground layer in temperate forests comprises the majority of plant diversity in these systems and has an important impact on their functioning (Gilliam 2007), it is important to document the long-term changes in the ground layer composition and diversity and to understand the drivers that underlie these changes. Changes documented in forest understories may also serve as early warnings of impacts to even slower canopy dynamics.
The ‘forestREplot’ network (www.forestreplot.ugent.be) brings together standardized ground layer vegetation resurvey plots collected in natural or semi-natural forests in different regions across Europe and North America (Verheyen et al. 2012, De Frenne et al. 2013, Baeten et al. 2014, Bernhardt-Römermann et al. 2015). Table 1 gives an overview of the criteria used for dataset inclusion in forestREplot. The database currently consists of 55 datasets and nearly 3000 plot-pairs with a mean inter-census interval of 35.7 years (see Depauw and Maes 2015 for more info and Appendix I for an overview).
The network aims to: (i) collect and archive datasets of resurveyed vegetation plots in temperate forests worldwide and (ii) perform analyses across multiple sites to answer novel research questions in ecology, with a specific focus on the ground layer and the impacts that various, often interacting, global change drivers have on this layer. In many respects, the design and management of the forestREplot network adheres to the guidelines for globally distributed experiments outlined by Fraser et al. (2013) and Borer et al. (2014).
Here we illustrate with forestREplot how multiple resurvey datasets can address a broad spectrum of ecological questions (i.e. the comprehensiveness), with results being representative for real-world changes in temperate forest communities (i.e. the increased representativeness). Furthermore, we show how the approach may disentangle the relative importance of multiple drivers of change in the ground layer of forests (i.e. the increase in orthogonality).
3.1. Comprehensiveness
To quantify the spectrum of ecological questions that can be addressed with multi-site resurvey data, here shown using the forestREplot example, we performed a two-step survey among 32 participants of the first forestREplot workshop organised in December 2014 in Ghent, Belgium. All participants to the workshop were data contributors to the forestREplot-database. Prior to the meeting, the workshop organizers (K.V., L.B., L.D.P., M.B.-R., P.D.F., R.H. and S.M.) quantitatively assessed which of the current 100 fundamental questions in ecology (as listed by Sutherland et al. 2013) could be answered with the forestREplot database by attributing a score between one (not suitable) to three (very suitable) to all questions. This resulted in a subset of 42 fundamental questions of the original Sutherland et al. 2013 list with a score ≥2. Next, we asked the workshop participants to score the potential of the forestREplot database to answer these 42 questions. The top ten questions which had the highest probability of being scored very suitable can be found in Table 2. The full list with question and scores can be found in Appendix II.
Table 2.
Top ten of the most important ecological questions following Sutherland et al. (2013) that can be addressed with the multi-site ground layer resurvey data incorporated in the forestREplot-database.
| Rank | Question $ | Category $ | Prob[rank=’very suitable’]* |
|---|---|---|---|
| 1 | Can we predict the responses of ecosystems to environmental change based on the traits of species? | Ecosystems and functioning | 0.67 |
| 2 | How do spatial and temporal environmental heterogeneity influence diversity at different scales? | Communities and diversity | 0.64 |
| 3 | What is the magnitude of the extinction debt following the loss and fragmentation of natural habitats, and when will it be paid? | Human impacts and global change | 0.58 |
| 4 | Which ecosystems and what properties are most sensitive to changes in community composition? | Ecosystems and functioning | 0.51 |
| 5 | To what extent is local species composition and diversity controlled by dispersal limitation and the regional species pool? | Communities and diversity | 0.50 |
| 6 | How well can community properties and responses to environmental change be predicted from the distribution of simple synoptic traits, e.g. body size, leaf area? | Communities and diversity | 0.48 |
| 7 | What are the indirect effects of harvesting on ecosystem structure and dynamics? | Human impacts and global change | 0.48 |
| 8 | How do natural communities respond to increased frequencies of extreme weather events predicted under global climate change? | Human impacts and global change | 0.40 |
| 9 | What are the most appropriate baselines for determining the magnitude and direction of ecological changes? | Methods | 0.39 |
| 10 | In the face of rapid environmental change, what determines whether species adapt, shift their ranges or go extinct? | Human impacts and global change | 0.37 |
taken from the list of Sutherland et al. (2013)
We fitted cumulative link models, which are regression models for ordinal data (clm in the R package ordinal; Christensen 2015, R Core Team 2015). Results show the estimated probability that a question was rated as ‘very suitable’ across the 32 respondents.
3.2. Representativeness
As we amass resurvey data from more sites, spread over larger regions, we gain a clearer picture of which changes are local or idiosyncratic to a few locations and which reflect more general and widespread changes (Fig. 2). However, results from any given database are clearly bounded by the variation within the set of species, communities, and environmental conditions present within the database. Resurvey data included in forestREplot, for instance, only come from semi-natural and natural forests (see Depauw and Maes 2015). Furthermore, forestREplot is merely a collection of datasets and not a designed monitoring program based on probabilistic sampling, such as National Forest Inventories (NFI), which reduces the representativeness and makes the statistical analyses more complicated. For instance, many of the first surveys were made for phytosociological purposes, meaning that plot locations are not entirely randomly chosen. These limitations have to be acknowledged when using the data (cf. Holeksa and Woźniak 2005, Michalcova et al. 2011). On the other hand, most monitoring programs designed to be representative do not (yet) span long time periods. Furthermore, the spatial sampling resolution in these monitoring programs is often rather low so that smaller scale changes risk going undetected.
3.3. Orthogonality
Single-region studies have shown that ground-layer vegetation in temperate forests responds sensitively to global change drivers, including forest management, atmospheric nitrogen deposition, and climate change (Table 3). However, these studies often do not show consistent responses, as exemplified for species richness in Table 3. Furthermore, community responses may not be monotonic over longer environmental gradients. To analyse the orthogonal and interacting effects of these drivers on biodiversity, it is necessary to either include many sites and studied factors within a single, large-scale, study, or to combine results from several single studies in joint analyses.
Table 3.
Impact of selected environmental drivers on changes in ground layer species richness in temperate forests. Shown are exemplarily single-region studies and, per environmental driver, its estimated general importance based on multi-region resurvey studies.
| Driver | Single-region vegetation resurveys (examples) | Direction of effect on species richness | Multi-region analyses |
|---|---|---|---|
| Increased forest management intensity |
Økland et al. (2003) Li and Waller (2015) Kirby and Thomas (2000) Brunet et al. (1996) Decocq et al. (2004) Schmidt (2005) Van Calster et al. (2008) Hédl et al. (2010) Kopecký et al. (2013) |
Negative Negative No effect Positive Positive Positive Positive Positive Positive |
The most important factor driving understory vegetation composition (Paillet et al. 2010), may mask the effects of climate change (De Frenne et al. 2013), or nutrient deposition (Verheyen et al. 2012) |
| Increased N-deposition |
Hédl (2004) Skrindo and Økland (2002) Bernhardt-Römermann et al. (2007) |
Negative No effect Positive |
Pre-survey levels of N deposition determine subsequent changes in biodiversity (Bernhardt-Römermann et al. 2015); actual N-deposition is less important than forest management (Verheyen et al. 2012); exceedance of critical loads favors N demanding species (Dirnböck et al. 2014) |
| Climate warming |
Kirby et al. (2005) Heinrichs et al. (2012) Naaf and Wulf (2010, 2011) Savage and Vellend (2015) |
Negative No effect Positive Positive |
Buffering effects of canopy closure on increased dominance of warm-adapted species as a result of climate warming (De Frenne et al. 2013) |
For instance, Verheyen et al. (2012) presented a meta-analysis of 23 local-scale resurveys from across Europe that focused on the contribution of atmospheric N deposition versus changes in forest management to explain changes in herb layer composition. Shifts in vegetation composition seemed mainly related to management-related alterations in the canopy structure and composition, independent of the N deposition.
An additional study exploring the mechanisms driving temporal changes in biodiversity was performed by Bernhardt-Römermann et al. (2015). Using 39 data sets of resurvey data on forest understory communities across Europe, temporal changes in species richness were related to environmental data at multiple spatial scales (continental, regional, local). These joint analyses were designed to relate temporal changes in species richness with i) across-site variation in environmental conditions at the time of the initial vegetation survey (i.e. baselines) and ii) temporal changes in environmental conditions between vegetation surveys. No significant and directional changes in local biodiversity were found, although there was considerable across-site variation, corroborating earlier findings (Verheyen et al. 2012, Vellend et al. 2013). This across-site variation was determined by both local and regional scale drivers (temporal changes in local stand structure and game density). Most excitingly, strong evidence was found that pre-survey levels of N deposition determined subsequent changes in biodiversity. Recently, Simkin et al. (2016) confirmed the existence of context-dependent effects of N-deposition on plant diversity using a large dataset from the US.
Thirdly, increased dominance of warm-adapted plant species (so-called ‘thermophilization’) as a result of climate warming has been identified across several ecosystems (Bertrand et al. 2011, Gottfried et al. 2012). However, De Frenne et al. (2013) found that this thermophilization was lowest in forests that had become denser over time across Europe and North America, suggesting that reducing management intensity to increase shading can buffer the impacts of global warming (cf. also De Frenne et al. 2015).
These three examples show how multi-region analyses can increase orthogonality compared to single-region studies.
4. Challenges associated with resurvey data
Despite the great potential that combining long-term resurvey data from multiple regions holds, some important challenges remain, both at the level of the individual resurvey studies and when trying to combine them.
Sources of unwanted variability or bias in resurvey studies have received considerable attention in the scientific literature (e.g. Tingley and Beissinger, 2009). Taking the example of vegetation resurveys, studies have been performed to quantify the level of bias introduced due to (1) relocation errors (e.g. Fischer & Stöcklin 1997, Kopecký and Macek 2015); (2) species detectability, observer effects and sampling exhaustiveness (Archaux et al. 2006, Vittoz and Guisan 2007, Milberg et al. 2008); (3) taxonomic inconsistencies (Jansen and Dengler 2010); (4) and to differences in recording dates (Van Calster et al. 2008). Recently, Semboli et al. (2014) highlighted a new source of bias, notably a changing vegetation composition after multiple resurvey visits due to, among others, trampling effects. Many of these biases are not easy to solve, particularly when the first surveyors are no longer around. Hence the need for a robust archiving of survey data so that at least future generations of researchers are not confronted with these issues (see Box 2).
Box 2. Maintaining the resource. Towards a publicly accessible data and metadata archive for resurveys.
Addressing important ecological questions on ecosystem responses to environmental change through the use of long term data requires the data to exist in the first place, necessitating support for long term ecological research infrastructure and its integration e.g. the European Platform for Biodiversity Research Strategy, the International Long Term Ecological Research Network. It then requires indefinite survival of these high quality data and, equally important, their accompanying metadata. In the past, ecological researchers tended to maintain their own records, passing on data and its context to a relay of successors. However, relay batons have been dropped, successors have not emerged, records have consequently been lost or destroyed, and ‘information entropy’ has ensued (Michener et al. 1997). To avoid unnecessary data loss, well documented procedures to preserve data with accompanying metadata are required (Figure I). Of fundamental importance is preservation of the metadata – defined as representing the higher level information or instructions that describe the content, context, quality (e.g. data anomalies / missing data), structure and accessibility of a specific dataset (Michener et al. 1997). In the context of vegetation resurveys, for example, this includes detailed descriptions of cover estimation to enable spatial and temporal comparisons. Without metadata, understanding and analyses would be impossible (see also Borer et al. 2014).
Fundamental ecological research questions will be most effectively answered through knowing what data are available where. While networks such as forestREplot have grown informally and identified separate datasets that have been manually integrated to allow synthetic analyses (e.g. Verheyen et al. 2012, De Frenne et al. 2013), more efficient global solutions for data manipulation, integration and retrieval may be realised through the support of ecoinformatics (Michener and Jones 2012). Efforts in this field (e.g. Madin et al. 2007, 2008, Leinfelder et al. 2010, Bendix et al. 2012) will be in vain however without the required archiving of resurvey data and metadata, together with education to make scientists aware of ecoinformatic networking possibilities (Michener et al. 2012).
Ultimately, archiving may be best incentivised for scientists through publication of the data (in “data papers” rather than typical research articles) using established channels of automated and semi-automated data checking culminating in peer review (Costello et al. 2013). Organisations such as the Global Biodiversity Information Facility can aid this publication and archiving endeavour. Otherwise the contemporary situation (where 80% of scientists want to access data created by others but only 20% have actually shared their data) may continue to persist and valuable opportunities to answer fundamental ecological questions may be lost as time-poor scientists prioritize publication over making data available (Costello et al. 2013).
Not all data can or need be accessible to all. However, arguments exist that we will only get solutions to environmental issues if data are made easily accessible to, and understood by, a broad audience (Peters 2010). Records of data existence would be invaluable for researchers, as would instructions for how interested parties can access them with associated rights of use – through for example the distributed system of national and international funded data platforms as proposed by the World Data System of the International Council of Science (Bendix et al. 2012). In addition to electronic data, records that need to be kept according to rigorous procedures include field notes, samples, photographs, and maps.
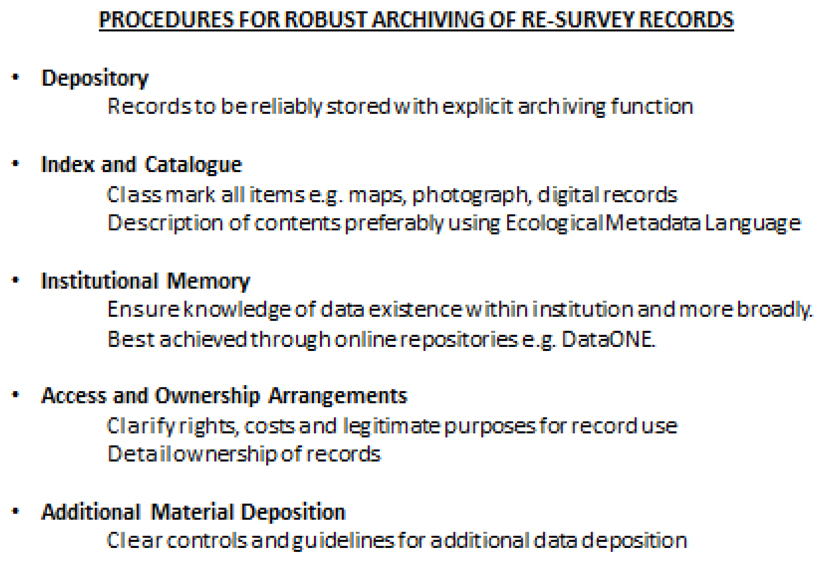
Figure Box 2.
Procedures for the robust archiving of resurvey records. Data may not need to be made available to all but it is crucial that its existence not be forgotten given the opportunity they provide to answer fundamental ecological questions. Given the pressure for scientists to publish, archiving may ultimately be best incentivised through credit for data publication. In the meantime, and while barriers to this outcome are still present, it is imperative that metadata and the records themselves are robustly archived with researchers able to find out about their existence through online search tools e.g. DataONE.
When multiple datasets are combined, additional challenges arise that relate to differences in baselines (e.g. due to historical land use or air pollution legacies), in the time interval between surveys and in sampling protocols. For instance, if there is covariation of plot sizes or the time interval between the surveys with environmental changes of interest, then the observed community changes might be principally caused by species-area or temporal effects. These issues require serious attention, for instance, by setting strict inclusion criteria for resurvey datasets with deviating baseline conditions, resurvey time intervals, sampling unit properties or internal heterogeneity. However, options exist to statistically control for the potentially distorting effects, e.g. by using response ratios of old and recent plot values which enable the comparison between datasets with different plot sites, by including resurvey time intervals or the year of the first survey as covariates in statistical models (e.g. Verheyen et al. 2012, Bernhardt-Römermann et al. 2015)
5. Outlook
The challenges described above should not discourage researchers from seeking to recover historical legacy data, from working to properly document and archive the data (Box 2), and from doing the matched resurveys necessary to document long-term ecological change. Many valuable historical community descriptions exist that can be used to generate and test novel insights into ecological change. Furthermore, insights will be deeper and more general when we can combine data from multiple regions and analyse the results in a comparative context. In this paper, we used the forestREplot network as an example of the power that long-term resurvey data have for addressing how communities are responding to a broad range of environmental factors. However, we should bear in mind that forestREplot focuses only on forest ground layer communities in natural and semi-natural temperate forests. We therefore encourage the development of more multi-region resurvey databases for other community types and biomes, as well as new modes of (trait-based) analysis. These will increase the number and nature of the comparisons we can make, allowing, in turn, to test a wider range of hypotheses and reach more general conclusions. Over time, such tests, performed on replicated sets of regions across many distinct biomes, will allow to more fully assess the several, often interacting, effects of forces driving ecological change.
Acknowledgements
The ideas for this paper developed at a workshop held in Ghent (BE, December 2014). Financial support was provided by the European Research Council through the PASTFORWARD project [ERC Consolidator Grant 614839) attributed to KV.
Footnotes
None of the authors has a conflict of interest.
Contributor Information
Kris Verheyen, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium.
Pieter De Frenne, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium, Pieter.Defrenne@UGent.be.
Lander Baeten, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium, Lander.Baeten@UGent.be.
Donald M. Waller, Lab/department: Botany Department, Institute: University of Wisconsin–Madison, Postal address: 430 Lincoln Dr., Madison, WI 53706, USA, dmwaller@wisc.edu
Radim Hédl, Lab/department: Department of Vegetation Ecology, Institute: Institute of Botany, The Czech Academy of Sciences, Postal address: Lidická 25/27, Brno, 60200, Czech Republic, radim.hedl@ibot.cas.cz; Lab/department: Department of Botany, Institute: Palacky University in Olomouc, Postal address: Slechtitelu 27, Olomouc, 78371, Czech Republic.
Michael P Perring, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium, michael.perring@ugent.be; Lab/department: Ecosystem Restoration and Intervention Ecology Research Group; School of Plant Biology, Institute: The University of Western Australia, Postal address: 35, Stirling Highway, Crawley WA 6009, AUSTRALIA, michael.perring@uwa.edu.au.
Haben Blondeel, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium, Haben.Blondeel@UGent.be.
Jörg Brunet, Lab/department: Southern Swedish Forest Research Centre, Institute: Swedish University of Agricultural Sciences, Postal address: PO Box 49, 230 53 Alnarp, Sweden, jorg.brunet@slu.se.
Markéeta Chudomelova, Lab/department: Department of Vegetation Ecology, Institute: Institute of Botany, The Czech Academy of Sciences, Postal address: Lidická 25/27, Brno, 60200, Czech Republic; Lab/department: Department of Botany and Zoology, Institute: Faculty of Sciences, Masaryk University, Postal address: Kotlářská 2, Brno CZ-60200, Czech Republic, marketachudomelova@seznam.cz.
Guillaume Decocq, Lab/department: UR “Ecologie et Dynamique des Systèmes Anthropisés” (EDYSAN, FRE 3498 CNRS-UPJV), Institute: Jules Verne University of Picardy, Postal address: 1, rue des Louvels, 80037 Amiens Cédex, FRANCE, guillaume.decocq@u-picardie.fr.
Emiel De Lombaerde, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium, Emiel.Delombaerde@UGent.be.
Leen Depauw, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium, Leen.Depauw@UGent.be.
Thomas Dirnböck, Lab/department: Department for Ecosystem Research, Institute: Environment Agency Austria, Postal address: Spittelauer Lände 5, 1090 Vienna, Austria, Thomas.dirnboeck@umweltbundesamt.at.
Tomasz Durak, Lab/department: Department of Botany, Institute: University of Rzeszów, Postal address: Zelwerowicza 4, Rzeszów PL-35-601, Poland, tdurak@univ.rzeszow.pl.
Ove Eriksson, Lab/department: Department of Ecology, Environment and Plant Sciences, Institute: Stockholm University, Postal address: SE – 106 91 Stockholm, Sweden, ove.eriksson@su.se.
Frank S Gilliam, Lab/department: Department of Biological Sciences, Institute: Marshall University, Postal address: 1 John Marshall Drive, Huntington, WV 25755-2510, USA, gilliam@marshall.edu.
Thilo Heinken, Lab/department: Biodiversity Research / Systematic Botany, Institute: Institute for Biochemistry and Biology, University of Potsdam, Postal address: Maulbeerallee 1, 14469 Potsdam, Germany, heinken@uni-potsdam.de.
Steffi Heinrichs, Lab/department: Department Silviculture & Forest Ecology of the Temperate Zones, Institute: Georg-August-University Göttingen, Burckhardt Institute, Postal address: Büsgenweg 1, 37077 Göttingen, Germany, sheinri@gwdg.de.
Martin Hermy, Lab/department: Dept Earth & Environmental Sciences, Institute: University of Leuven (KU Leuven), Postal address: Celestijnenlaan 200E, Heverlee 3001, Belgium, Martin.hermy@ees.kuleuven.be.
Bogdan Jaroszewicz, Lab/department: Białowieża Geobotanical Station, Institute: University of Warsaw, Faculty of Biology, Postal address: Sportowa 19, Białowieża, 17-230, Poland b.jaroszewicz@uw.edu.pl.
Michael A. Jenkins, Lab/department: Department of Forestry and Natural Resources, Institute: Purdue University, Postal address: 715 West State Street, West Lafayette, IN 47907-2061, USA, jenkinma@purdue.edu
Sarah E Johnson, Lab/department: Department of Natural Resources and Biology, Institute: Northland College, Postal address: 1411 Ellis Avenue, Ashland, Wisconsin 54806, USA, sjohnson@northland.edu.
Keith J. Kirby, Lab/department: Department of Plant Sciences, Institute: Oxford University, Postal address: South Parks Road, Oxford OX1 3RB, UK, Keith.kirby@bnc.oxon.org
Martin Kopecký, Lab/department: Department of Vegetation Ecology, Institute: Institute of Botany, The Czech Academy of Sciences, Postal address: Lidická 25/27, Brno, CZ-602 00, Czech Republic, ma.kopecky@gmail.com, Lab/department: Department of Forest Ecology, Institute: Faculty of Forestry and Wood Sciences, Czech University of Life Sciences Prague, Postal address: Kamýcká 129, CZ-165 21, Prague 6 - Suchdol, Czech Republic.
Dries Landuyt, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium, Dries.Landuyt@UGent.be.
Jonathan Lenoir, Lab/department: UR “Ecologie et dynamique des systems anthropisés” (EDYSAN, FRE 3498 CNRS-UPJV), Institute: Université de Picardie Jules Verne, Postal address: 1 Rue des Louvels, 80000 Amiens, France, jonathan.lenoir@u-picardie.fr.
Daijiang Li, Lab/department: Department of Botany, Institute: University of Wisconsin – Madison, Postal address: 430 Lincoln Drive, Madison, WI 53706, USA, daijianglee@gmail.com.
Martin Macek, Lab/department: Dept. of GIS and RS, Institute: Institute of Botany of the Czech Academy of Sciences, Postal address: Zámek 1, Průhonice 252 43, Czech Republic, martin.macek@ibot.cas.cz.
Sybryn Maes, Lab/department: Forest & Nature Lab, Institute: Department of Forest & Water Management, Ghent University, Postal address: Geraardsbergsesteenweg 267, 9090 Melle-Gontrode, Belgium, Sybryn.maes@ugent.be.
Frantisek Máliš, Lab/department: Department of Phytology, Faculty of Forestry, Institute: Technical University in Zvolen, Postal address: T. G. Masaryka 24, 960 53 Zvolen, Slovakia, malis@tuzvo.sk.
Fraser J.G. Mitchell, Lab/department: School of Natural Sciences, Trinity, Institute: College Dublin, Postal address: Dublin 2, Ireland, fraser.mitchell@tcd.ie
Tobias Naaf, Lab/department: Institute of Land Use Systems, Institute: Leibniz Centre for Agricultural Landscape Research (ZALF), Postal address: Eberswalder Straße 84, 15374 Müncheberg, Germany, naaf@zalf.de.
George Peterken, Postal address: Beechwood House, St Briavels Common, Lydney GL15 6SL, UK, gfpeterken@tiscali.co.uk.
Petr Petřík, Lab/department: Department of GIS and Remote Sensing, Institute: Institute of Botany, Czech Academy of Sciences, Postal address: Zámek 1, Průhonice 25243, Czech Republic, petrik@ibot.cas.cz.
Kamila Reczyńska, Lab/department: Wrocław University, Institute: Museum of Natural History, Postal address: Sienkiewicza 21, Wrocław 50-335, Poland, kamila.reczynska@gmail.com.
David A Rogers, Lab/department: Biological Sciences, Institute: University of Wisconsin – Parkside, Postal address: 900 Wood Rd., Kenosha, Wisconsin 53141, USA, rogersd@uwp.edu.
Fride Hoistad Schei, Lab/department: Forestry and Forest Resources, Institute: Norwegian Institute of Bioeconomy Research, Postal address: Fanaflaten 4, 5244 Fana, Norway, hof@nibio.no.
Wolfgang Schmidt, Lab/department: Silviculture and Forest Ecology of the Temperate Zones, Institute: Faculty of Forestry and Forest Ecology, Georg-August-University Göttingen, Postal address: Büsgenweg 1, 37077 Göttingen, Germany, wschmid1@gwdg.de.
Tibor Standovár, Lab/department: Dept. Plant Systematics, Ecology and Theoretical Biology, Institute: Eötvös Loránd University, Postal address: Pázmány sétány 1/C, H-1117 Budapest, Hungary, standy@caesar.elte.hu.
Krzystof Świerkosz, Lab/department: Wrocław University, Institute: Museum of Natural History, Postal address: Sienkiewicza 21, Wrocław 50-335, Poland, krzysztof.swierkosz@life.pl.
Karol Ujházy, Lab/department: Department of Phytology, Institute: Technical University in Zvolen, Postal address: T. G. Masaryka 24, SK-960 53 Zvolen, Slovakia, karol.ujhazy@tuzvo.sk.
Hans Van Calster, Lab/department: Biometry & Quality Assurance, Institute: Research Institute for Nature and Forest, Postal address: Kliniekstraat 25, Brussels, 1070, Belgium, Hans.vancalster@inbo.be.
Mark Vellend, Lab/department: Département de biologie, Institute: Université de Sherbrooke, Postal address: 2500 boulevard de l’Université, Sherbrooke, Québec J1K 2R1, Canada, mark.vellend@usherbrooke.ca.
Ondřej Vild, Lab/department: Department of Vegetation Ecology, Institute: Institute of Botany, The Czech Academy of Sciences, Postal address: Lidická 25/27, Brno, 60200, Czech Republic, Lab/department: Department of Botany and Zoology, Institute: Faculty of Sciences, Masaryk University, Postal address: Kotlářská 2, Brno CZ-60200, Czech Republic, ondrej.vild@ibot.cas.cz.
Kerry Woods, Lab/department: Natural Sciences, Institute: Bennington College, Postal address: 1 College Drive, Bennington, VT 05201, USA, kwoods@bennington.edu.
Monika Wulf, Lab/department: Leibniz Centre for Agricultural Landscape Research (ZALF), Institute: Institute of Land Use Systems, Postal address: Eberswalder Straße 84, Müncheberg, 15374, Germany, mwulf@zalf.de.
Markus Bernhard-Römermann, Lab/department: Institute of Ecology, Institute: Friedrich Schiller University Jena, Postal address: Dornburger Str. 159, 07743 Jena, Germany, markus.bernhardt@uni-jena.de.
References
- Archaux F, Gosselin F, Bergès L, Chevalier R. Effects of sampling time, species richness and observer on the exhaustiveness of plant censuses. Journal of Vegetation Science. 2006;17:299–306. [Google Scholar]
- Baeten L, et al. A model-based approach to studying changes in compositional heterogeneity. Methods in Ecology and Evolution. 2014;5:156–164. [Google Scholar]
- Baeten L, et al. A novel comparative research platform designed to determine the functional significance of tree species diversity in European forests. Perspectives in Plant Ecology, Evolution and Systematics. 2013;15:281–291. [Google Scholar]
- Bakker JP, Olff H, Willems JH, Zobel M. Why do we need permanent plots in the study of long-term vegetation dynamics? Journal of Vegetation Science. 1996;7:147–155. [Google Scholar]
- Bendix J, Nieschulze J, Michener WK. Data platforms in integrative biodiversity research. Ecological Informatics. 2012;11:1–4. [Google Scholar]
- Bernhardt-Römermann M, et al. Drivers of temporal changes in temperate forest plant diversity vary across spatial scales. Global Change Biology. 2015;21:3726–3737. doi: 10.1111/gcb.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt-Römermann M, Kudernatsch T, Pfadenhauer J, Kirchner M, Jakobi G, Fischer A. Long-term effects of nitrogen deposition on vegetation in a deciduous forest near Munich, Germany. Applied Vegetation Science. 2007;10:399–406. [Google Scholar]
- Bertrand R, et al. Changes in plant community composition lag behind climate warming in lowland forests. Nature. 2011;479:517–20. doi: 10.1038/nature10548. [DOI] [PubMed] [Google Scholar]
- Borer ET, Harpole WS, Adler PB, Lind EM, Orrock JL, Seabloom EW, Smith MD. Finding generality in ecology: a model for globally distributed experiments. Methods in Ecology and Evolution. 2014;5:65–73. [Google Scholar]
- Brunet J, Falkengren-Grerup U, Tyler G. Herb layer vegetation of south Swedish beech and oak forests - Effects of management and soil acidity during one decade. Forest Ecology and Management. 1996;88:259–272. [Google Scholar]
- Chow SC, Liu JP. Design and Analysis of Clinical Trials: Concepts and Methodologies. John Wiley and Sons, Inc; 2004. [Google Scholar]
- Christensen RHB. ordinal - Regression Models for Ordinal Data. 2015. pp. 6–28. R package version 2015. [Google Scholar]
- Costello MJ, Michener WK, Gahegan M, Zhang Z-Q, Bourne PE. Biodiversity data should be published, cited, and peer reviewed. Trends in Ecology and Evolution. 2013;28:454–461. doi: 10.1016/j.tree.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Decocq G, Aubert M, Dupont F, Alard D, Saguez R, Wattez-Franger A, Foucault BDE, Delelis-Dusollier A, Bardat J. Plant diversity in a managed temperate deciduous forest: understorey response to two silvicultural systems. Journal of Applied Ecology. 2004;41:1065–1079. [Google Scholar]
- De Frenne P, et al. Microclimate moderates plant responses to macroclimate warming. Proceedings of the National Academy of Sciences. 2013;110:18561–18565. doi: 10.1073/pnas.1311190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Frenne P, Rodríguez-Sánchez F, De Schrijver A, Coomes DA, Hermy M, Vangansbeke P, Verheyen K. Light accelerates plant responses to warming. Nature Plants. 2015;1:15110. doi: 10.1038/nplants.2015.110. [DOI] [PubMed] [Google Scholar]
- Depauw L, Maes S. forestREplot: a global database of temperate forest herb layer resurvey plots. British Ecological Society Bulletin. 2015;46:31–34. [Google Scholar]
- Dirnböck T, et al. Forest floor vegetation response to nitrogen deposition in Europe. Global Change Biology. 2014;20:429–440. doi: 10.1111/gcb.12440. [DOI] [PubMed] [Google Scholar]
- Dornelas M, et al. Quantifying temporal change in biodiversity: challenges and opportunities. Proceedings of the Royal Society B. 2012;280:1–10. doi: 10.1098/rspb.2012.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlén J, Lehtilä K. How perennial are perennial plants? Oikos. 2002;98:308–322. [Google Scholar]
- Eriksson O. Regional dynamics of plants : a review of evidence for remnant, source-sink and metapopulations. Oikos. 1996;77:248–258. [Google Scholar]
- Fan H, Wu J, Liu W, Yuan Y, Huang R, Liao Y, Li Y. Nitrogen deposition promotes ecosystem carbon accumulation by reducing soil carbon emission in a subtropical forest. Plant and Soil. 2014;379:361–371. [Google Scholar]
- Fischer M, Stöcklin J. Local extinctions of plants in remnants of extensively used calcareous grasslands 1950-1985. Conservation Biology. 1997;11:727–737. [Google Scholar]
- Fraser LH, et al. Coordinated distributed experiments: An emerging tool for testing global hypotheses in ecology and environmental science. Frontiers in Ecology and the Environment. 2013;11:147–155. [Google Scholar]
- Frerker K, Sabo A, Waller D. Long-term regional shifts in plant community composition are largely explained by local deer impact experiments. PloS one. 2014;9:1–17. doi: 10.1371/journal.pone.0115843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam FS. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience. 2007;57:845–858. [Google Scholar]
- Gottfried M, et al. Continent-wide response of mountain vegetation to climate change. Nature Clim Change. 2012;2:111–115. [Google Scholar]
- Hédl R. Vegetation of beech forests in the Rychlebské Mountains, Czech Republic, re-inspected after 60 years with assessment of environmental changes. Plant Ecology. 2004;170:243–265. [Google Scholar]
- Hédl R, Kopecký M, Komárek J. Half a century of succession in a temperate oakwood: From species-rich community to mesic forest. Diversity and Distributions. 2010;16:267–276. [Google Scholar]
- Heinrichs S, Winterhoff W, Schmidt W. Vegetation dynamics of beech forests on limestone in central Germany over half a century – effects of climate change, forest management, eutrophication or game browsing? Biodiversity and Ecology. 2012;4:49–61. [Google Scholar]
- Holeksa J, Woźniak G. Biased vegetation patterns and detection of vegetation changes using phytosociological databases. A case study in the forests of the Babia Góra National Park (the West Carpathians, Poland) Phytocoenologia. 2005;35:1–18. [Google Scholar]
- Jansen F, Dengler J. Plant names in vegetation databases - a neglected source of bias. Journal of Vegetation Science. 2010;21:1179–1186. [Google Scholar]
- Kirby K, Smart S, Black H, Bunce R, Corney P, Smithers R. Long term ecological change in British Woodland (1971-2001) English Nature. 2005 Research Report no. 653. [Google Scholar]
- Kirby KJ, Thomas RC. Changes in the ground flora in Wytham Woods, southern England from 1974 to 1991–implications for nature conservation. Journal of Vegetation Science. 2000;11:871–880. [Google Scholar]
- Koger CH, Reddy KN. Role of absorption and translocation in the mechanism of glyphosate resistance in horseweed (Conyza canadensis) Weed Science. 2005;53:84–89. [Google Scholar]
- Kopecký M, Hédl R, Szabó P. Non-random extinctions dominate plant community changes in abandoned coppices. Journal of Applied Ecology. 2013;50:79–87. doi: 10.1111/1365-2664.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecký M, Macek M. Vegetation resurvey is robust to plot location uncertainty. Diversity and Distributions. 2015;21:322–330. doi: 10.1111/ddi.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinfelder B, Tao J, Costa D, Jones MB, Servilla M, O’Brien M, Burt C. A metadata-driven approach to loading and querying heterogeneous scientific data. Ecological Informatics. 2010;5:3–8. [Google Scholar]
- Li D, Waller D. Drivers of observed biotic homogenization in pine barrens of Central Wisconsin. Ecology. 2015;96:1030–1041. doi: 10.1890/14-0893.1. [DOI] [PubMed] [Google Scholar]
- Luo Y, et al. Coordinated approaches to quantify long-term ecosystem dynamics in response to global change. Global Change Biology. 2011;17:843–854. [Google Scholar]
- Madin JS, Bowers S, Schildhauer M, Krivov S, Pennington D, Villa F. An ontology for describing and synthesizing ecological observation data. Ecological Informatics. 2007;2:279–296. [Google Scholar]
- Madin JS, Bowers S, Schildhauer MP, Jones MB. Advancing ecological research with ontologies. Trends in Ecology and Evolution. 2008;23:159–168. doi: 10.1016/j.tree.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Magnuson J. Long-term ecological research and the invisible present. BioScience. 1990;40:495–501. [Google Scholar]
- Michalcova D, Lvoncik S, Chytry M, Hajek O. Bias in vegetation databases? A comparison of stratified-random and preferential sampling. Journal of Vegetation Science. 2011;22:281–291. [Google Scholar]
- Michener WK, et al. Participatory design of DataONE—enabling cyberinfrastructure for the biological and environmental sciences. Ecological Informatics. 2012;11:5–15. [Google Scholar]
- Michener WK, Brunt JW, Helly JJ, Kirchner TB, Stafford SG. Nongeospatial metadata for the ecological sciences. Ecological Applications. 1997;7:330–342. [Google Scholar]
- Michener WK, Jones MB. Ecoinformatics: supporting ecology as a data-intensive science. Trends in Ecology and Evolution. 2012;27:85–93. doi: 10.1016/j.tree.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Milberg P, Bergstedt J, Fridman J, Odell G, Westerberg L. Observer bias and random variation in vegetation monitoring data. Journal of Vegetation Science. 2008;19:633–644. [Google Scholar]
- Millenium Ecosystem Assessment. Ecosystems and Human Well-being: Biodiversity Synthesis. 2005 [Google Scholar]
- Mills JA, et al. Archiving Primary Data: Solutions for Long-Term Studies. Trends in Ecology and Evolution. 2015;30:581–589. doi: 10.1016/j.tree.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Moritz C, Patton JL, Conroy CJ, Parra JL, White GC, Beissinger SR. Impact of a century of climate change on small-mammal communities in Yosemite National Park, USA. Science. 2008;322:261–264. doi: 10.1126/science.1163428. [DOI] [PubMed] [Google Scholar]
- Naaf T, Wulf M. Habitat specialists and generalists drive homogenization and differentiation of temperate forest plant communities at the regional scale. Biological Conservation. 2010;143:848–855. [Google Scholar]
- Naaf T, Wulf M. Traits of winner and loser species indicate drivers of herb layer changes over two decades in forests of NW Germany. Journal of Vegetation Science. 2011;22:516–527. [Google Scholar]
- Nadrowski K, Wirth C, Scherer-Lorenzen M. Is forest diversity driving ecosystem function and service? Current Opinion in Environmental Sustainability. 2010;2:75–79. [Google Scholar]
- Newbold T, et al. Global effects of land use on local terrestrial biodiversity. Nature. 2015;520:45–50. doi: 10.1038/nature14324. [DOI] [PubMed] [Google Scholar]
- Nieto-Sánchez S, Gutiérrez D, Wilson RJ. Long-term change and spatial variation in butterfly communities over an elevational gradient: driven by climate, buffered by habitat. Diversity and Distributions. 2015;21:950–961. [Google Scholar]
- Økland T, Rydgren K, Økland RH, Storaunet KO, Rolstad J. Variation in environmental conditions, understorey species number, abundance and composition among natural and managed Picea abies forest stands. Forest Ecology and Management. 2003;177:17–37. [Google Scholar]
- Olsson J, Bergstrom L, Gardmark A. Top-down regulation, climate and multi-decadal changes in coastal zoobenthos communities in two Baltic sea areas. PLoS ONE. 2013;8:1–13. doi: 10.1371/journal.pone.0064767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillet Y, et al. Biodiversity differences between managed and unmanaged forests: meta-analysis of species richness in Europe. Conservation Biology. 2010;24:101–112. doi: 10.1111/j.1523-1739.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- Pauli H, et al. Recent plant diversity changes on Europe’s mountain summits. Science. 2012;336:353–355. doi: 10.1126/science.1219033. [DOI] [PubMed] [Google Scholar]
- Pauli H, Gottfried M, Lamprecht A, Niessner S, Rumpf S, Winkler M, Steinbauer K, Grabherr H. GLORIA-Coordination: Austrian Academy of Sciences & University of Natural Resources and Life Sciences; 2015. The GLORIA field manual – standard Multi-Summit approach, supplementary methods and extra approaches. [Google Scholar]
- Pauly D. Anecdotes and the shifting baseline syndrome of fisheries. Trends in Ecology and Evolution. 1995;10:430. doi: 10.1016/s0169-5347(00)89171-5. [DOI] [PubMed] [Google Scholar]
- Peterken G. Natural Woodland: Ecology and Conservation in Northern Temperate Regions. Cambridge University Press; 1996. [Google Scholar]
- Peters DPC. Accessible ecology: synthesis of the long, deep, and broad. Trends in Ecology and Evolution. 2010;25:592–601. doi: 10.1016/j.tree.2010.07.005. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. 2015 [Google Scholar]
- Savage J, Vellend M. Elevational shifts, biotic homogenization and time lags in vegetation change during 40 years of climate warming. Ecography. 2015;38:546–555. [Google Scholar]
- Schmidt W. Herb layer species as indicators of biodiversity of managed and unmanaged beech forests. Forest, Snow and Landscape Research. 2005;79:111–125. [Google Scholar]
- Semboli O, Beina D, Closset-Kopp D, Gourlet-Fleury S, Decocq G. Does long-term monitoring of tropical forests using permanent plots provide unbiased results? Applied Vegetation Science. 2014;17:737–743. [Google Scholar]
- Simkin SM, et al. Proceedings of the National Academy of Sciences of the United States of America: In press; 2016. Conditional vulnerability of plant diversity to atmospheric nitrogen deposition across the USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrindo A, Økland RH. Effects of fertilization on understorey vegetation in a Norwegian Pinus sylvestris forest. Applied Vegetation Science. 2002;5:167–172. [Google Scholar]
- Sutherland WJ, et al. Identification of 100 fundamental ecological questions. Journal of Ecology. 2013;101:58–67. [Google Scholar]
- Tingley MW, Beissinger SR. Cryptic loss of montane avian richness and high community turnover over 100 years. Ecology. 2013;94:598–609. doi: 10.1890/12-0928.1. [DOI] [PubMed] [Google Scholar]
- Tingley MW, Beissinger SR. Detecting range shifts from historical species occurrences: new perspectives on old data. Trends in Ecology and Evolution. 2009;24:625–633. doi: 10.1016/j.tree.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Van Calster H, Baeten L, Verheyen K, De Keersmaeker L, Dekeyser S, Rogister JE, Hermy M. Diverging effects of overstorey conversion scenarios on the understorey vegetation in a former coppice-with-standards forest. Forest Ecology and Management. 2008;256:519–528. [Google Scholar]
- Velikova V, Tsonev T, Pinelli P, Alessio GA, Loreto F. Localized ozone fumigation system for studying ozone effects on photosynthesis, respiration, electron transport rate and isoprene emission in field-grown Mediterranean oak species. Tree Physiology. 2005;25:1523–1532. doi: 10.1093/treephys/25.12.1523. [DOI] [PubMed] [Google Scholar]
- Vellend M, Baeten L, Myers-Smith IH, Elmendorf SC, Beauséjour R, Brown CD, De Frenne P, Verheyen K, Wipf S. Global meta-analysis reveals no net change in local-scale plant biodiversity over time. Proceedings of the National Academy of Sciences. 2013;110:19456–19459. doi: 10.1073/pnas.1312779110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellend M, Verheyen K, Jacquemyn H, Kolb A, Van Calster H, Peterken G, Hermy M. Extiction debt of forest plants persists for more than a century following habitat fragmentation. Ecology. 2006;87:542–548. doi: 10.1890/05-1182. [DOI] [PubMed] [Google Scholar]
- Verheyen K, et al. Driving factors behind the eutrophication signal in understorey plant communities of deciduous temperate forests. Journal of Ecology. 2012;100:352–365. [Google Scholar]
- Verheyen K, Honnay O, Motzkin G, Hermy M, Foster DR. Response of forest plant species to land-use change: A life-history trait-based approach. Journal of Ecology. 2003;91:563–577. [Google Scholar]
- Vittoz P, Guisan A. How reliable is the monitoring of permanent vegetation plots? A test with multiple observers. Journal of Vegetation Science. 2007;18:413–422. [Google Scholar]
- Walker LR, Wardle DA, Bardgett RD, Clarkson BD. The use of chronosequences in studies of ecological succession and soil development. Journal of Ecology. 2010;98:725–736. [Google Scholar]
- Wolkovich EM, Regetz J, O’Connor MI. Advances in global change research require open science by individual researchers. Global Change Biology. 2012;18:2102–2110. [Google Scholar]