Abstract
Background:
The role of postoperative radiotherapy (PORT) in patients with clinical stage III-N2 (cIII-N2) non-small cell lung cancer (NSCLC) treated with induction chemotherapy and surgical resection with persistent ypN2 disease is not well-established.
Methods:
We retrospectively reviewed a prospectively maintained database for patients with cIII-N2 NSCLC who underwent induction chemotherapy followed by resection (2004–2016). Exclusion criteria included induction radiotherapy, non-biopsy-confirmed cN2 disease, incomplete resection, ypN0/1, and nonanatomic resection. The primary outcome was locoregional recurrence (LR); secondary outcomes were disease-free survival (DFS), lung cancer–specific death (LCSD), and overall survival (OS). Associations between variables and outcomes were assessed using Fine and Gray competing risk regression for LR/LCSD and Cox proportional hazard models for survival.
Results:
Of the 501 patients identified with cIII-N2 disease, 99 met the inclusion criteria. Median follow-up was 25 months (range, 3–137). Sixty-nine patients (70%) received PORT. Sixty (61%) developed a recurrence: 3 (5%) with an initial isolated LR and 57 (95%) with an initial distant recurrence. On multivariable analysis, PORT was not associated with LR (HR, 0.51 [95% CI, 0.22–1.21], P=0.13). PORT was also not associated with DFS (P=0.6) or LCSD (P=0.1). PORT was associated with improved 3-year OS (55% [95% CI, 42%−71%]) versus the no-PORT group (50% [95% CI, 34%−74%]) (P=0.04).
Conclusion:
PORT is not independently associated with decreased LR or improved DFS/LCSD in this patient population. Given that the predominant failure pattern was distant recurrence, future clinical trials should focus on adjuvant systemic therapies, which may decrease distant recurrences in ypN2 patients.
Introduction
Five-year overall survival (OS) for patients with pN2 non-small cell lung cancer (NSCLC) ranges from 30% to 49% (1, 2). Current evidence-based guidelines recommend patients with preoperatively identified N2 disease undergo either definitive chemoradiation or induction therapy followed by surgical resection (National Comprehensive Cancer Network 2017 Guidelines) (3). Some studies suggest surgical resection is associated with a decreased risk of recurrence in this population (3, 4). A prior study evaluating patients with clinical stage III-N2 (cIII-N2) NSCLC found that, even in patients with pathologic nodal downstaging (ypN0/N1) after induction chemotherapy and surgical resection, locoregional recurrence (LR) rates approached 30% (5). The risk of LR in patients with persistent ypN2 disease treated with PORT has been reported to be even higher, with >50% of patients experiencing LR after 49 months of follow-up (6).
Given the high recurrence rates in this population, postoperative radiotherapy (PORT) has been advocated. A previous meta-analysis demonstrated that, in all patients with surgically resected NSCLC, PORT was not associated with improved survival (7). Several subsequent studies demonstrated that PORT may improve survival and decrease LR in patients with pN2 disease (8–11). However, whether PORT can decrease LR and improve disease-free survival (DFS) or lung cancer–specific death (LCSD) in patients with cIII-N2 NSCLC treated with induction chemotherapy followed by surgical resection, with persistent ypN2 disease, has not been well established. We sought to clarify the relationship between PORT and LR, DFS, LCSD, and OS in this select population of patients.
Patients and Methods
Patient Characteristics
Following Institutional Review Board approval, we performed a retrospective review of a prospectively maintained database for patients with cIII-N2 NSCLC (according to the 8th Edition of the AJCC Cancer Staging Manual) who underwent induction therapy followed by R0 surgical resection, with or without PORT, at our institution between January 2004 and December 2016. We excluded patients who died within 90 days or had follow-up <90 days postsurgery, as these patients were unable to receive PORT. We also excluded patients with no pathologic confirmation of N2 disease before induction therapy, secondary to the high false-positive rate for PET (12). Additional factors that would favor either PORT (R1/R2 resection and/or nonanatomic resection) or no PORT (induction radiotherapy and/or ypN0–1) were also used as exclusion criteria (Fig. 1). Patient demographic characteristics, clinical and pathologic stage (AJCC 8th edition), operative approach, and therapy details (chemotherapy and/or PORT) were collected by chart review (13). At Memorial Sloan Kettering, we do not routinely restage the mediastinum before resection, provided the patient's disease is deemed resectable before induction therapy (12, 14). Dissection or sampling of nodal stations is performed during the initial part of the procedure in cases of induction therapy. For postinduction cases, we attempt to remove all disease, including performing complete lymphadenectomy (12, 14). Treatment effect was classified into two categories: (1) ≥90% treatment effect or major pathologic response and (2) <90% treatment effect or major pathologic response (15). Complete (R0) resection was defined as removal of all microscopic and macroscopic disease. Referral for PORT was made on the basis of provider assessment of intraoperative findings, the pathologic report, and tumor board discussions, or a combination of these.
Figure 1.
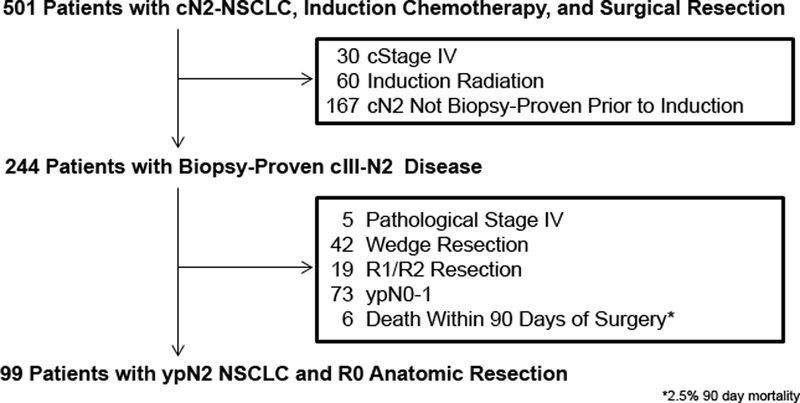
CONSORT diagram. NSCLC, non-small cell lung cancer.
Follow-up
Patient follow-up, including history, physical, and chest CT, was performed every 6 months for the first 2 years after surgery and yearly thereafter. Dates of follow-up and of death and/or recurrences, when applicable, were noted. LR was defined as a recurrence in the surgical margin, in the same lobe as the resection (for those with sublobar resections), or involvement of the ipsilateral hilar and/or ipsilateral mediastinal lymph nodes (16–18). Distant recurrence (DR) was defined as any recurrence outside the LR zone. Recurrences were identified radiographically (by PET or CT) and/or by pathologic confirmation. Metachronous lesions were differentiated from recurrences using the criteria established by Martini and Melamed (19) and, when available, by genomic profiling of tumors.
Statistical Analysis
Patient demographic and clinicopathologic characteristics were summarized and compared between groups using Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. Our primary objective was to evaluate the association between PORT status and the primary endpoint of LR. Secondary endpoints were DFS, LCSD, and OS. For descriptive purposes, all survival endpoints were calculated from the date of surgery to the event of interest or the last follow-up, depending on the endpoint (LR, LR; LCSD, LCSD; DFS, recurrence or death; OS, death).
As we analyzed a retrospective database, we considered the issue of “guaranteed survival time”: patients who received PORT had to have survived long enough to receive PORT, whereas patients who did not receive PORT did not have this survival requirement. Without addressing guaranteed survival time, the longer survival requirement to receive PORT may make the PORT group appear to have a better survival prognosis (20). To address this potential bias and to appropriately credit survival time to the treatment, two approaches have been proposed in the literature: (1) treating PORT status as a time-varying covariate and (2) using the landmark approach. We present results from the latter approach, using 90 days postsurgery as the landmark time from which to calculate survival. For sensitivity analyses, we repeated the analyses using the first approach (treating PORT status as a time-varying covariate)—we observed similar findings using this approach.
LR was analyzed using the competing-risk analysis framework. Death without LR was considered a competing event. The cumulative incidence of LR was estimated and compared between groups using Gray’s method (21). LCSD was analyzed similarly to LR, in which non-lung cancer specific death was considered a competing event and cumulative incidence of LCSD was estimated. Univariable and multivariable analysis for associations between LR and clinicopathologic factors, particularly PORT, were assessed using Fine and Gray competing risk regression (21). DFS and OS were estimated using the Kaplan-Meier approach (from the landmark of 90 days postsurgery) and compared between groups using the log-rank test. Univariable Cox proportional hazards models were stratified by pathologic stage where applicable. Variables with P≤0.1 from univariable models were considered for inclusion in multivariable models, along with PORT (time-varying covariate) and other clinically important factors for each endpoint. Two-sided P<0.05 was considered statistically significant. Statistical analyses were performed using Stata 13.0 (StataCorp, College Station, TX) and R 3.2.4. (R Development Core Team, Vienna, Austria).
Results
Patient Population
In total, 501 patients were identified in the initial query of our database: 99 met the final inclusion criteria (Fig. 1). Of these, 69 (70%) received PORT; 30 (30%) did not. Table 1 shows characteristics of the two cohorts. Overall, both groups were similar. Patients in the PORT group had a median tumor maximum standardized uptake value of 11 versus 9 in the no-PORT group (P=0.03) (Table 1). Although the difference was not statistically significant, patients in the no-PORT group were more likely to require a pneumonectomy (n=3 [10%]) than patients in the PORT group (n=1 [1%]) (P=0.08). All patients in this cohort had ypN2 disease; however, 3 patients (2 no-PORT and 1 PORT) had a complete pathologic tumor response (ypT0) despite persistent ypN2 disease.
Table 1.
Clinicopathologic Characteristics by PORT Status
| Variable | N (%) or Median (25th, 75th percentile) | P | |
|---|---|---|---|
| PORT (N=69, 70%) |
No PORT (N=30; 30%) |
||
| Age at surgery (years) | 67.0 (57, 71) | 65.0 (60, 76) | 0.5 |
| Ever smoker | |||
| No | 11 (16%) | 4 (13%) | 1.0 |
| Yes | 58 (84%) | 26 (87%) | |
| Charlson Comorbidity Index | 3.0 (2.0, 4.0) | 3.0 (2.0, 4.0) | 1.0 |
| Cardiac comorbidity | |||
| No | 36 (52%) | 18 (60%) | 0.5 |
| Yes | 33 (48%) | 12 (40%) | |
| Pulmonary comorbiditya | |||
| No | 50 (72%) | 19 (63%) | 0.5 |
| Yes | 19 (28%) | 11 (37%) | |
| Clinical stage before induction therapy | |||
| T1–2N2 | 57 (83%) | 27 (90%) | 0.5 |
| T3–4N2 | 12 (17%) | 3 (10%) | |
| Pre-induction tumor SUVmax (N=90) | 11.3 (6.7, 14.6) | 9.3 (5.7, 11.2) | 0.03 |
| Treatment effect by pathology | |||
| >90%b | 5 (7.2%) | 1 (3.3%) | 0.7 |
| <90% | 64 (93%) | 29 (97%) | |
| Pneumonectomy | |||
| No | 68 (98.6%) | 27 (90%) | 0.08 |
| Yes | 1 (1.4%) | 3 (10%) | |
| N2 nodal stations examined | 2 (2–3) | 2 (2–3) | 0.7 |
| Lymphovascular invasion (n=96) | |||
| No | 18 (26%) | 12 (43%) | 0.15 |
| Yes | 50 (74%) | 16 (57%) | |
| Number of ypN2 nodal stations involved | |||
| Single | 48 (70%) | 22 (73%) | 0.8 |
| Multiple | 21 (30%) | 8 (27%) | |
| Tumor pathologic subtype | |||
| Squamous, large, other | 12 (17%) | 6 (20%) | 0.8 |
| Adenocarcinoma | 57 (83%) | 24 (80%) | |
| Pathologic tumor stage (ypT) | |||
| 1a, 1b, 1c | 29 (42%) | 13 (43%) | 0.7 |
| 2a, 2b | 25 (36%) | 11 (37%) | |
| 3 | 11 (16%) | 3 (10%) | |
| 4 | 3 (4.3%) | 1 (3.3%) | |
| 0c | 1 (1.4%) | 2 (6.7%) | |
| Adjuvant systemic therapy | |||
| No | 60 (87%) | 21 (70%) | 0.05 |
| Yes | 9 (13%) | 9 (30%) | |
| Type of adjuvant systemic therapy (N=18) | 0.6 | ||
| Other systemic therapy (N=8) | 5 (56%) | 3 (33%) | |
| Tyrosine kinase inhibitor (Erlotinib) (N=10) | 4 (44%) | 6 (67%) | |
Pulmonary comorbidity includes history of tuberculosis, asthma, chronic obstructive pulmonary disease, and/or pulmonary fibrosis, as determined by pulmonary function tests.
Major pathologic response.
ypT0 tumors had complete response of the primary tumor despite persistent disease present in the N2 nodes.
PORT = postoperative radiotherapy; SUVmax = maximum standardized uptake value.
Chemotherapy and PORT
All patients received induction therapy: 98 (99%) received doublet-cisplatin-based chemotherapy, and 1 (1%) received isolated targeted therapy (crizotinib). The median number of cycles was 4 (IQR [25th, 75th percentile], 3–4 cycles). Eighteen patients (18%) also received adjuvant therapy: 56% (n=10) had adjuvant tyrosine kinase inhibitor (TKI) therapy, and 44% (n=8) had non-TKI-based systemic therapy.
Sixty-nine patients received PORT. Median time from surgery to PORT was 51 days (IQR, 43–63 days), and 96% of patients in the PORT group received PORT within 90 days of surgery. The median dose was 50.4 Gy (IQR, 5040–5400 cGy). Most patients received intensity modulated radiotherapy (n=39); however, 27 had radiation delivered via 3-dimensional conformal radiotherapy, and 3 had radiation delivered via 2-dimensional radiotherapy.
Patterns of Recurrence
The median follow-up was 25 months (range, 3–137 months). Overall, 60 patients (61%) experienced recurrence: 21 (70%) in the no-PORT group versus 39 (57%) in the PORT group (Supplemental Table 1). Twenty patients in the no-PORT group (67%) eventually developed distant metastases versus 39 in the PORT group (57%). Patterns of recurrence are listed in Supplemental Table 1.
Twenty-seven patients (27%) developed LR: 14 in the PORT group (20%) versus 13 in the no-PORT group (43%). Only 3 patients (3%) developed an isolated LR as their site of first recurrence, and of these only 1 (1%) never developed DR. This patient was in the no-PORT group and after 68 months of follow-up was still alive.
We first assessed the relationship between clinicopathologic factors, particularly use of PORT, and LR. PORT was associated with a decreased hazard of LR on univariable analysis (subdistribution hazard ratio [SHR], 0.46 [95% CI, 0.22–0.98]; P=0.045). Complete pathologic response of the primary tumor (ypT0) (protective) and Charlson Comorbidity Index (higher risk) were associated with LR on univariable competing risk regression (Table 2). All other factors, including multistation N2 disease, were not significantly associated with LR. Despite a numeric difference in the prevalence of LR in the PORT versus no-PORT groups, when we evaluated PORT, Charlson Comorbidity Index, and pneumonectomy using multivariable competing risk regression for LR, PORT was no longer significantly associated with LR (SHR, 0.51 [95% CI, 0.22–1.21]; P=0.13) (Table 2).
Table 2.
Univariable and Multivariable Competing Risk Regression for Locoregional Recurrence
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| SHR (95% CI) | P | SHR (95% CI) | P | |
| PORT | ||||
| No | 1.00 | 1.00 | — | |
| Yes | 0.46 (0.22, 0.98) | 0.045 | 0.51 (0.22, 1.21) | 0.13 |
| Age at surgery | 1.01 (0.98, 1.04) | 0.6 | ||
| History of smoking | ||||
| No | 1.00 | — | ||
| Yes | 2.62 (0.63, 10.93) | 0.2 | — | — |
| Charlson Comorbidity Index | 1.29 (1.00, 1.66) | 0.05 | 1.30 (0.99, 1.70) | 0.06 |
| Cardiac comorbidity | ||||
| No | 1.00 | — | ||
| Yes | 2.09 (0.96, 4.54) | 0.06 | — | — |
| Pulmonary comorbidity | ||||
| No | 1.00 | — | ||
| Yes | 1.10 (0.49, 2.47) | 0.8 | — | — |
| Preinduction SUVmax primary | 0.99 (0.96, 1.02) | 0.4 | — | — |
| Treatment effect on pathology | ||||
| >90% | 1.00 | — | ||
| <90% | 1.35 (0.16, 11.09) | 0.8 | — | — |
| Pneumonectomy | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 2.63 (0.42–16.42) | 0.3 | 2.08 (0.26, 16.54) | 0.5 |
| Lymphovascular invasion | ||||
| No | 1.00 | — | ||
| Yes | 0.69 (0.31, 1.52) | 0.4 | — | — |
| Tumor pathologic subtype | ||||
| Squamous, large, other | 1.00 | — | — | |
| Adenocarcinoma | 0.55 (0.21, 1.47) | 0.2 | — | |
| Adjuvant systemic therapy | ||||
| No | 1.00 | — | ||
| Yes | 0.94 (0.34, 2.60) | 0.9 | — | — |
| Pathologic tumor stage (ypT) | ||||
| 1a, 1b, 1c | 1.00 | — | ||
| 2a, 2b | 1.71 (0.75, 3.91) | 0.2 | — | — |
| 3 | 0.99 (0.28, 3.50) | 1.0 | — | — |
| 4 | 1.42 (0.15, 13.83) | 0.8 | — | — |
| 0 | 0.00 (0.00, 0.00) | <0.01 | — | — |
| Multistation ypN2 disease | ||||
| No | 1.00 | — | ||
| Yes | 0.82 (0.33, 2.07) | 0.7 | — | — |
Pulmonary comorbidity includes history of tuberculosis, asthma, chronic obstructive pulmonary disease, and/or pulmonary fibrosis, as determined by pulmonary function tests.
Major pathologic response.
PORT = postoperative radiotherapy; SHR = subdistribution hazard ratio; SUVmax = maximum standardized uptake value.
Relationship between PORT and DFS, LCSD, and OS
To investigate whether delayed onset of recurrence results in improved survival, we next evaluated DFS, LCSD, and OS by PORT status. We found no statistically significant differences in DFS between patients who received PORT and those who did not (Fig. 2A, log-rank test P=0.6; univariable SHR, 0.86 [95% CI, 0.49–1.49]; P=0.6). Three-year DFS was 42% (95% CI, 26%−68%) for the no-PORT group versus 25% (95% CI, 15%−41%) for the PORT group. Similarly, PORT was not associated with improved LCSD (Fig. 2B; Gray’s test P=0.1; univariable SHR, 0.57 [95% CI, 0.29–1.12]; P=0.10). However, we did find a significant difference in OS by PORT status. On univariable analysis, we found that PORT (protective), ypIIIB versus ypIIIA (higher risk), and multistation ypN2 disease (higher risk) were associated with OS. On multivariable analysis, only PORT (protective) and multistation ypN2 disease (higher risk) were associated with OS (Table 3). Three-year OS was 50% (95% CI, 34%−74%) in the no-PORT group versus 55% (95% CI, 42%−71%) in the PORT group (P=0.04, Fig. 2C). Supplemental Figure 1 shows 5-year outcomes.
Figure 2.
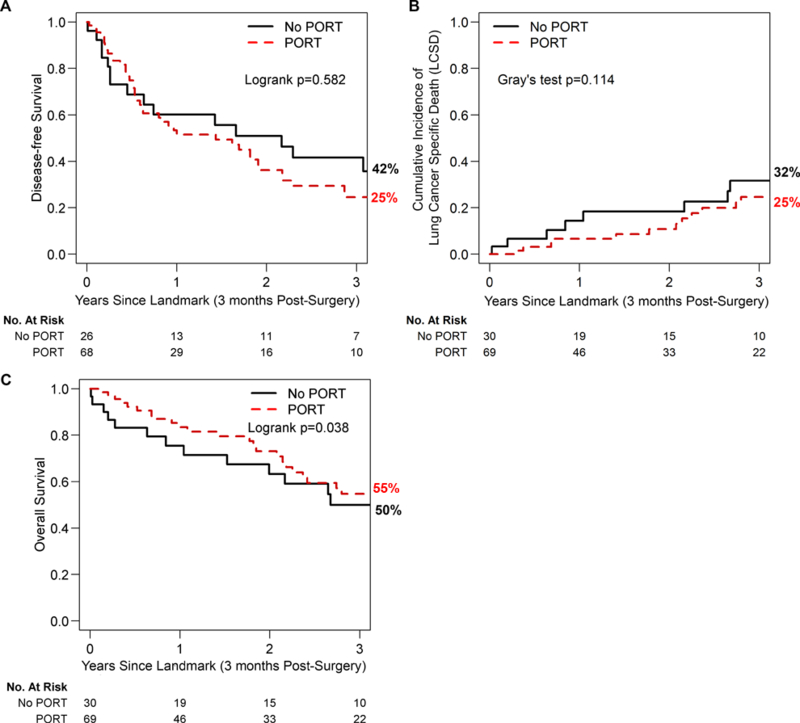
From landmark (90 days postsurgery), the (A) 3-year disease-free survival for postoperative radiotherapy (PORT; 25% [95% CI, 15%−41%]) versus no PORT (42% [95% CI, 26%−68%]), (B) lung cancer–specific death for PORT (25% [95% CI, 15%−41%]) versus no PORT (32% [95% CI, 17%−58%]), and (C) overall survival for PORT (55% [95% CI, 42%−71%]) versus no PORT (50% [95% CI, 34%−74%]).
Table 3.
Univariable and Multivariable Competing Risk Regression for Overall Survival
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| SHR (95% CI) | P | SHR (95% CI) | P | |
| PORT | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 0.54 (0.30, 0.97) | 0.04 | 0.46 (0.24, 0.88) | 0.02 |
| Age at surgery | 1.02 (0.99, 1.05) | 0.2 | 1.02 (0.99,1.05) | 0.3 |
| Charlson Comorbidity Index | 1.16 (0.91, 1.48) | 0.2 | 1.16 (0.90, 1.50) | 0.3 |
| Cardiac comorbidity | ||||
| No | 1.00 | — | — | — |
| Yes | 0.86 (0.49, 1.51) | 0.6 | ||
| Pulmonary comorbidity | ||||
| No | 1.00 | — | ||
| Yes | 0.89 (0.49, 1.63) | 0.7 | — | — |
| Preinduction tumor SUVmax | 0.99 (0.97, 1.01) | 0.4 | — | — |
| Pneumonectomy | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 3.32 (1.00, 11.01) | 0.05 | 1.88 (0.53, 6.61) | 0.3 |
| Lymphovascular invasion | ||||
| No | 1.00 | — | ||
| Yes | 1.20 (0.64, 2.26) | 0.6 | — | — |
| Tumor response grade | ||||
| <90% | 1.00 | — | ||
| >90% | 0.89 (0.21, 3.72) | 0.9 | — | — |
| Tumor pathologic subtype | ||||
| Squamous, large, other | 1.00 | — | ||
| Adenocarcinoma | 0.62 (0.27, 1.44) | 0.3 | — | — |
| Pathologic stage (yp) | ||||
| ypIIIA | 1.00 | — | 1.00 | — |
| ypIIIB | 1.80 (0.92, 3.52) | 0.087 | 1.64 (0.68, 3.92) | 0.3 |
| No. N2 nodal stations involved | ||||
| Single | 1.00 | — | 1.00 | — |
| Multiple | 2.37 (1.28, 4.37) | 0.006 | 2.77 (1.25, 6.13) | 0.01 |
| Adjuvant systemic therapy | ||||
| No | 1.00 | — | 1.00 | — |
| Yes | 0.76 (0.39, 1.51) | 0.4 | 0.55 (0.24,1.19) | 0.1 |
PORT = postoperative radiotherapy; SHR = subdistribution hazard ratio; SUVmax = maximum standardized uptake value.
Comment
The recent results of the randomized phase III clinical trial by the Swiss cooperative group SAKK demonstrated that the addition of radiation to induction chemotherapy for pN2 NSCLC does not improve DFS or OS (22). This suggests that one definitive local therapeutic modality plus induction chemotherapy is sufficient to treat resectable IIIA-N2 NSCLC. However, the role of PORT following induction chemotherapy for ypN2 disease is poorly studied, resulting in a knowledge gap on how to best treat these patients.
The results of our study in this selected cohort enriched for a high likelihood of LR and DR show that PORT is not associated with decreased LR on multivariable analysis. Previous studies that evaluated PORT in all patients with pN2 disease found that PORT may decrease hazard of LR and improve OS. A recent study evaluated 150 patients with biopsy-proven stage III-N2 NSCLC who underwent induction chemotherapy followed by surgical resection (6). The authors compared patients with ypN2 disease and/or R1 resection (23% of cohort) who received PORT against patients with ypN1 or N0 disease who did not receive PORT; they found no significant difference in LR as the first site of recurrence (37% for PORT vs. 45% for no-PORT; P=0.6), the cumulative development of LR (47% for PORT vs. 49% for no-PORT; P=0.95), or OS after 49 months of follow-up (6). In contrast, our study compared PORT following induction chemotherapy and resection only in ypN2 patients and, therefore, more directly compares the oncologic value of PORT in this population. In addition, all patients in our group had an R0 resection, compared with 77% of patients in the above study.
We found that PORT was not associated with a significant improvement in DFS. Conversely, we did find an association between PORT and improved OS, but not LCSD. Moreover, of the 60 patients who developed a recurrence, 59 (98%) had a DR at some point, which is the most likely driver of survival when compared with death from LR (0%). A retrospective review of the Surveillance, Epidemiology, and End Results database evaluated patients with stage II or III NSCLC and had similar results. In patients with pN2 disease (n=1987), 5-year OS was 27% with PORT versus 20% without PORT (P=0.004) (23). On multivariable analysis, PORT was associated with improved OS (SHR, 0.86 [95% CI, 0.762–0.959]; P=0.077) (23). This study did not directly evaluate DFS. An unintended, retrospective post-hoc analysis of 116 patients with pN2 disease enrolled in the Adjuvant Navelbine International Trialist Association randomized trial found PORT was associated with improved 5-year OS (21% versus 17%), but no multivariable analysis was performed (8). This study is also different from ours in that it examined the role of PORT following adjuvant—not induction—chemotherapy. Any improved OS in the PORT population in our study or others may be real. However, our results that DFS and LCSD are not significantly associated with improved outcomes with PORT suggest the difference in OS may be secondary to an inherent selection toward patients with better performance status receiving PORT.
An interesting finding in our study was that multistation N2 disease was not associated with an increased risk of LR on multivariable analysis; however, it was associated with worse OS. Previous studies have demonstrated poor outcomes in patients with multistation N2 disease. In one study of 613 patients with pN1–2 NSCLC, multistation N2 disease was associated with worse OS (24), but the authors did not specifically evaluate recurrence. In addition, patients who received induction therapy were excluded. A second study evaluated OS and recurrence-free survival in 287 patients with pN2 NSCLC and found multistation N2 disease was associated with worse 5-year OS (P=0.002) and DFS (P=0.007) (25). Specific evaluation for LR was not performed. These studies mirror our findings that multistation N2 disease is associated with poor OS in all patients with ypN2 disease. Whether patients with multistation pN2 benefit more from PORT than patients with single-station pN2 disease remains an open question.
Finally, we found that pathologic complete response of the primary tumor (ypT0) was associated with a decreased incidence of LR, despite persistent ypN2 disease. Previous studies have found that pathologic complete response is associated with a favorable prognosis. Mouillet et al. analyzed two phase III trials comprising 492 patients with stage IB-II NSCLC who underwent induction chemotherapy followed by surgical resection. Although the authors did not specifically evaluate LR, they did find that 8.3% of patients (n=41) had pathologic complete response, with a 5-year OS of 80% versus 55.8% for those who did not (P<0.001). In addition, patients with pathologic complete response had a 5-year DFS of 80% versus 45% for those who did not (P<0.001) (26).
Limitations of our study include its single-institution and retrospective design, which may limit generalizability. In addition, although the two groups appear to be similar, there may have been an inherent selection bias for patients with more-aggressive tumor biology to receive PORT or patients with poor performance status being ineligible for PORT. A third limitation is our sample size, which may have led to our study being underpowered to identify small associations between PORT and LR. However, this is also a strength of the study, as all patients had persistent ypN2 disease after induction chemotherapy, which suggests that these tumors are chemoresistant, more aggressive, and primed to develop recurrence. One could speculate that these patients may benefit by receiving adjuvant systemic, targeted, or immune therapies, instead of additional local therapy.
Currently, a phase III randomized trial, the Lung Adjuvant Radiotherapy Trial (clinicaltrials.gov, NCT00410683), is actively accruing patients with pN2 disease, but the results are not anticipated until 2022. Moreover, the primary endpoint is not to discern the role of PORT following induction chemotherapy, although those patients are not excluded from enrollment. However, the number of patients undergoing induction chemotherapy before resection is likely to be low, as most centers in Europe do not offer induction therapy before surgery for resectable N2 disease.
In conclusion, we found that, following induction chemotherapy and an R0 resection for IIIA-N2 disease, the use of PORT for ypN2 disease is not independently associated with a statistically significant lower incidence or LR or longer DFS. Although OS was improved in patients who received PORT, the LCSD was no different between PORT and no-PORT groups, suggesting that this association is likely secondary to an inherent selection bias.
Supplementary Material
Acknowledgments
Funding: NIH Cancer Center Support Grant P30 CA008748 and T32CA009501 (W.S.B.).
Abbreviations
- cIII-N2
clinical stage III-N2
- DFS
disease-free survival
- DR
distant recurrence
- LCSD
lung cancer–specific death
- LR
locoregional recurrence
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PORT
postoperative radiotherapy
- SHR
subdistribution hazard ratio
- SUVmax
maximum standardized uptake value
- TKI
tyrosine kinase inhibitor
Footnotes
Presented at: STSA 64th Annual Meeting, San Antonio, TX, November 8–11, 2017.
COI statement: All authors have no conflicts.
References
- 1.Asamura H, Chansky K, Crowley J et al. The international association for the study of lung cancer lung cancer staging project: Proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol 2015;10(12):1675–1684. [DOI] [PubMed] [Google Scholar]
- 2.Yang CF, Kumar A, Gulack BC et al. Long-term outcomes after lobectomy for non-small cell lung cancer when unsuspected pN2 disease is found: A National Cancer Data Base analysis. J Thorac Cardiovasc Surg 2016;151(5):1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramnath N, Dilling TJ, Harris LJ et al. Treatment of stage III non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143(5 Suppl):e314S–e340S. [DOI] [PubMed] [Google Scholar]
- 4.Albain KS, Swann RS, Rusch VW et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: A phase III randomised controlled trial. Lancet 2009;374(9687):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amini A, Lou F, Correa AM et al. Predictors for locoregional recurrence for clinical stageIII-N2 non-small cell lung cancer with nodal downstaging after induction chemotherapy and surgery. Ann Surg Oncol 2013;20(6):1934–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billiet C, Peeters S, Decaluwe H et al. Outcome after PORT in ypN2 or R1/R2 versus no PORTin ypN0 stage III-N2 nsclc after induction chemotherapy and resection. J Thorac Oncol 2016;11(11):1940–1953. [DOI] [PubMed] [Google Scholar]
- 7.Postoperative radiotherapy in non-small-cell lung cancer: Systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Port meta-analysis trialists group. Lancet 1998;352(9124):257–263. [PubMed] [Google Scholar]
- 8.Douillard JY, Rosell R, De Lena M et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: The Adjuvant Navelbine International Trialist Association (ANITA) randomized trial. Int J Radiat Oncol Biol Phys 2008;72(3):695–701. [DOI] [PubMed] [Google Scholar]
- 9.Mikell JL, Gillespie TW, Hall WA et al. Postoperative radiotherapy is associated with better survival in non-small cell lung cancer with involved N2 lymph nodes: Results of an analysis of the National Cancer Data Base. J Thorac Oncol 2015;10(3):462–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Billiet C, Decaluwe H, Peeters S et al. Modern post-operative radiotherapy for stage III non-small cell lung cancer may improve local control and survival: A meta-analysis. Radiother Oncol 2014;110(1):3–8. [DOI] [PubMed] [Google Scholar]
- 11.Martin LW, Correa AM, Hofstetter W et al. The evolution of treatment outcomes for resected stage IIIA non-small cell lung cancer over 16 years at a single institution. J Thorac Cardiovasc Surg 2005;130(6):1601–1610. [DOI] [PubMed] [Google Scholar]
- 12.Ripley RT, Suzuki K, Tan KS et al. Postinduction positron emission tomography assessment of N2 nodes is not associated with ypB2 disease or overall survival in stage IIIA non-small cell lung cancer. J Thorac Cardiovasc Surg 2016;151(4):969–977, 979 e961–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 14.Ripley RT, Rusch VW. Role of induction therapy: Surgical resection of non-small cell lung cancer after induction therapy. Thorac Surg Clin 2013;23(3):273–285. [DOI] [PubMed] [Google Scholar]
- 15.Pataer A, Kalhor N, Correa AM et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7(5):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelsey CR, Higgins KA, Peterson BL et al. Local recurrence after surgery for non-small cell lung cancer: A recursive partitioning analysis of multi-institutional data. J Thorac Cardiovasc Surg 2013;146(4):768–773 e761. [DOI] [PubMed] [Google Scholar]
- 17.Kelsey CR, Marks LB, Hollis D et al. Local recurrence after surgery for early stage lung cancer: An 11-year experience with 975 patients. Cancer 2009;115(22):5218–5227. [DOI] [PubMed] [Google Scholar]
- 18.Varlotto JM, Yao AN, DeCamp MM et al. Nodal stage of surgically resected non-small cell lung cancer and its effect on recurrence patterns and overall survival. Int J Radiat Oncol Biol Phys 2015;91(4):765–773. [DOI] [PubMed] [Google Scholar]
- 19.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70(4):606–612. [PubMed] [Google Scholar]
- 20.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol 1983;1(11):710–719. [DOI] [PubMed] [Google Scholar]
- 21.RJ G A class of k-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics 1988;16(3):1141–1154. [Google Scholar]
- 22.Pless M, Stupp R, Ris HB et al. Induction chemoradiation in stage IIIA/N2 non-small-cell lung cancer: A phase 3 randomised trial. Lancet 2015;386(9998):1049–1056. [DOI] [PubMed] [Google Scholar]
- 23.Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the Surveillance, Epidemiology, and End Results database. J Clin Oncol 2006;24(19):2998–3006. [DOI] [PubMed] [Google Scholar]
- 24.Legras A, Mordant P, Arame A et al. Long-term survival of patients with pN2 lung cancer according to the pattern of lymphatic spread. Ann Thorac Surg 2014;97(4):1156–1162. [DOI] [PubMed] [Google Scholar]
- 25.Uehara H, Nakao M, Mun M et al. Significant prognostic factors for completely resected pN2 non-small cell lung cancer without neoadjuvant therapy. Ann Thorac Cardiovasc Surg 2015;21(4):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mouillet G, Monnet E, Milleron B et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non-small-cell lung cancer: Combined analysis of two IFCT randomized trials. J Thorac Oncol 2012;7(5):841–849. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


