Abstract
Background:
Premature infants are vulnerable to destructive brain injury and disturbed neurological development. Prematurity may alter maturation of the central autonomic nervous system (ANS).
Aims:
To compare ANS function (using heart rate variability; HRV) between preterm infants with normal neuroimaging at term equivalent age and low-risk term controls.
Study design, subjects:
We performed a case-control study of preterm infants born ≤28 weeks gestational age that had normal brain imaging and archived continuous EKG data at term equivalent age. We documented other factors thought to influence ANS maturation (e.g. infection, ventilation days, and postnatal steroids). Controls were low-risk term gestational age newborns from uncomplicated pregnancies/deliveries. We characterized HRV metrics using frequency-(Welch periodogram) and time-domain (detrended fluctuation) analyses. Sympathetic tone was characterized by α1, root mean square analysis (RMS1 and RMS2), low-frequency (LF) power, and normalized LF (nLF) and parasympathetic tone was characterized by high-frequency (HF) power and normalized HF (nHF). α2 characterized ultraslow changes in heart rate. We used ANCOVA to compare HRV metrics between groups.
Outcome measures, results:
HRV from 26 preterm infants were compared to 55 controls. Analyzed HRV data for preterm infants were recorded at median (range) gestational age of 39 (36–39) weeks and for controls at 39 (37–41) weeks gestational age. α1, RMS2, LF and HF were significantly higher in control infants and remained significant after controlling for infection, ventilator days, and postnatal steroids (P<0.005).
Conclusions:
Autonomic maturation is impaired in a premature extrauterine environment. In the absence of destructive brain injury, our data suggest an important role for disturbed programming in this impaired autonomic development.
Keywords: heart rate variability, preterm newborn, autonomic nervous system, ANS, autonomic development, brain imaging
Introduction
In premature newborns, the central autonomic nervous system (ANS) is immature and may be inadequately prepared for the demands of cardiorespiratory transition at birth.(1) About 25% of premature infants develop brain injury(2) which may further impact ANS maturation and function.(3) The sympathetic system develops earlier than the parasympathetic system with the latter undergoing accelerated maturation at 25–30 weeks gestation, a time period when premature newborns may already be ex utero and undergoing transition.(4, 5) In addition to prematurity itself, multiple clinical and caregiving exposures in the neonatal intensive care unit (NICU) such as oxygenation disturbances, mechanical ventilation, hemodynamic instability, infections, pain, and surgery may impede normal maturation of the ANS. This premature engagement of the ANS under the condition of premature birth may result in “dysmaturation”, or a shift in the temporal program of ANS maturation due to aberrant programming.(6, 7)
ANS function can be measured in the infant non-invasively from physiologic signals of heart rate (HR), respiratory rate, and blood pressure. HR variability (HRV), or the fluctuation in the length of time between successive heart beats (R-R intervals), provides a measure of sympathetic and parasympathetic function and therefore ANS maturation.(8, 9) As the ANS matures, there should be increased parasympathetic function.(10) HRV can be measured by the frequency domain. High-frequency variability reflects parasympathetic function and is influenced by the respiratory rate, while low-frequency variability is due to a combination of sympathetic and parasympathetic inputs and baroreflex-induced changes in HR.(11) HRV may also be measured by time-domain analysis which evaluates short- and long-term variability in the HR. Long-term variability is influenced by both the sympathetic and parasympathetic nervous systems, meanwhile short-term variability is influenced more by the parasympathetic nervous system based on rapid ANS needs.(12)
HRV measures of autonomic tone are dampened in premature infants compared to infants born at term.(13) However, prior studies include infants with neurologic complications of prematurity such as brain injury (i.e. white matter injury or intraventricular hemorrhage [IVH]), which impact HRV and ANS maturation in premature infants.(3, 14, 15) To exclude the role of direct brain injury on ANS development, preterm infants included in this study had no brain injury by term-equivalent neuroimaging. The objective of this study was to evaluate ANS function through HRV analysis in preterm infants that have normal neuroimaging at term-equivalent age compared to term control infants.
MATERIALS AND METHODS
Participants
Premature infants with birth gestational age (GA) less than 29 weeks who survived to discharge from the NICU in 2014 at Children’s National Health System, Washington, D.C. and had quality archived physiologic data were studied retrospectively following Institutional Review Board approval. All infants had a normal or near-normal term-equivalent neuroimaging study (brain magnetic resonance imaging [MRI] and/or cranial ultrasound [US]) and no clinical neurologic problems including seizures during their NICU hospitalization. Some infants were part of a separate Institutional Review Board approved protocol and underwent an unsedated term-equivalent brain MRI prior to hospital discharge. Brain MRI findings considered “near-normal” included minimal susceptibility weighted change consistent with prior grade I IVH, minimal punctate area(s) of T1 hyper-intensity in the white matter, or small punctate area(s) of susceptibility weighted change in the cerebellum. Infants were excluded if they had grade II IVH or greater (on cranial US or MRI) or more significant brain MRI findings. The medical record was reviewed to determine other risk factors for abnormal ANS maturation such as infection, necrotizing enterocolitis (NEC), postnatal steroids, surgical ligation of patent ductus arteriosus, and duration of mechanical ventilator support.(15–17)
Low-risk controls were term newborns prospectively enrolled within three days of birth following Institutional Review Board approval and informed consent at Inova Women and Children’s Hospital, Fairfax, Virginia. Eligible newborns were from uncomplicated pregnancies and deliveries, without significant maternal illness, uncomplicated labor and delivery, ≥37 weeks GA at birth, with a normal birth weight (10th - 90th percentile for GA), and without postnatal infection. None of the low-risk controls had neuroimaging. All were discharged home from the birth hospital at the time of their mother.
Data Collection
The medical history of the infants was ascribed from the clinical database and included 1) gender, 2) GA at birth (born <26 weeks GA [0] or between 27–28 weeks GA [1] or born at term GA (37–41 weeks), [2], 3) any infection developed and if respiratory, blood, or urine culture was positive, 4) developed infection and/or NEC, 5) patent ductus arteriosus ligation, 6) number of days on assisted oxygen support, 7) receipt of postnatal steroids, and 8) caffeine treatment and date of discontinuation. All infants were off caffeine or other centrally-acting medications at the time of the retrieved electrocardiogram (EKG) data for at least 7 days prior to the HRV recording.
Within the preterm infant group, EKGs were retrieved from an institutional Research Data Export archive (IntelliVue Information Center, Philips Healthcare, Andover, MA, USA). We used data from the same hour (6 pm – 7 pm) at term-equivalent age from all infants except two, for which we used their data from 36 weeks GA due to timing of NICU discharge. The sampling rate of the EKG retrieved from Research Data Export was 125 Hz. For the term control infants, we obtained EKG data using the EGI physiological input box (Electrical Geodesics, Inc. EGI: www.egi.com, Eugene, OR, USA). The sampling rate of the EKG measured using EGI-physiological input box was 1000 Hz. The duration of the study for term infants was between 45 – 60 minutes.
EKG Processing
EKG was bandpass filtered between 0.5 – 70 Hz to attenuate the baseline drift and the R-wave (the wave with the dominant amplitude in each cardiac cycle) was identified using a recently proposed method.(18) Beat-to-beat interval (RRi) was calculated. Artifacts such as missed and/or extra beats were removed through an automatic approach.(19) The RRi were partitioned into non-overlapping 10-minute epochs. For spectral analysis, the RRi were converted into evenly sampled data using a cubic spline interpol2ation technique with a sampling rate of 5 Hz.
Detrended Fluctuation Analysis – Time Domain Characterization
Detrended fluctuation analysis is a modified root mean square (RMS) analysis approach.(20) This approach involves the following four steps: 1) remove the average value of the data and calculate the profile function as the cumulative sum of the data; 2) partition the profile into windows containing ‘s’ number of beats; 3) fit the profile inside each window using a 4th order polynomial and calculate the local fluctuation function as the RMS of the deviation of the profile from the best polynomial fits; 4) calculate the global fluctuation function as the median of all the local fluctuations. Repeat steps 2 to 4 for different values of ‘s’. Using the global function the following metrics were calculated: RMS1 as the maximum value of the global fluctuation function for ‘s’ between 15–50 beats; RMS2 was calculated as the maximum of the global fluctuation function for ‘s’ between 100–150 beats.(21) We also calculated α exponent from the slope of global fluctuation function versus ‘s’ in double logarithmic representation. α1was obtained from the region 15–30 beats (short time scale) and α2 was obtained from the region 35–150 beats (long time scale/ ultralow frequency).(21) The α metrics characterize the autocorrelation in the RRi whereas the RMS characterizes the variability in the RRi. The α metrics are dimensionless quantities. The RMS metrics are in units of seconds (sec).
Spectral Analysis
We used the Welch periodogram approach to estimate the power spectrum. In this approach the interpolated RRi were divided into 30 – second epochs. For RRi in each epoch, the periodogram was calculated as the square of the magnitude of the Fourier transform of the data. We estimated the power spectrum as the average of the periodograms over all epochs. Using the power spectrum, we estimated the powers in low-frequency (LF) and high-frequency (HF) as the median of the logarithm of the spectral power in 0.05 – 0.25 Hz and 0.3 – 1 Hz frequency bands, respectively. We also calculated normalized low-frequency (nLF) as the ratio of the sum of the powers in the 0.05 – 0.25 Hz band to the total power and normalized high-frequency (nHF) as the ratio of the powers in 0.3 – 1 Hz band to the total power. Total power was defined as the sum of the powers from 0.05 – 2 Hz. The normalized spectral powers (nLF and nHF) are dimensionless quantities. The absolute spectral powers are in unit of decibel (dB).
Statistical Analysis
The following metrics characterize the sympathetic tone: α1, RMS1 (sec), RMS2 (sec), whereas HF (dB) and nHF characterize the parasympathetic tone.(22) LF (dB) and nLF power reflect both sympathetic and parasympathetic mediated activity. α2 characterizes ultraslow changes in the heart rate, below the frequency of sympathetic tone. Every HRV metric was averaged over all 10-minute epochs to get a representative value for each infant. Receiver operating characteristic (ROC) analysis was performed for the unadjusted HRV metrics in time and frequency domains. An area under the ROC curve (AUC) > 0.7 was considered a meaningful separation.
The difference in the HRV metrics between controls and preterm infants was studied using ANOVA. The final comparison of HRV metrics was modeled using ANCOVA, adjusting for the covariates that were significantly different between the controls and the preterm infants. To deflate the Type-I error rate arising from multiple comparisons we have applied a multiple-comparison correction and considered P < .005 as statistically significant. The difference in the distribution of postconceptual GA at HRV analysis between preterm infants and controls was assessed by the Kolmogorov-Smirnov test.
RESULTS
Clinical
Twenty-six preterm infants with normal/near-normal term-equivalent neuroimaging had quality HRV data for analysis and were compared to 55 low-risk term control newborns. Clinical characteristics are reported in Table 1. Twelve (46%) of the preterm infants received postnatal steroids. Six of 9 preterm infants with clinical concern for infection had a positive respiratory, urine, or blood culture. HRV data was analyzed for the preterm infants at a median (range) postconceptual age of 39 (36–39) weeks which was similar to timing of 39 (37–41) weeks for the term control infants (P = .0540).
Table 1:
Clinical Characteristics of Preterm and Term Control Infants
| Preterm (n = 26) | Term Control (n = 55) | |
|---|---|---|
| Birth GA ≤ 26 weeks (n/%) | 7 (26%) | – |
| Birth GA 26 to 28 weeks (n/%) | 19 (74%) | – |
| Male (n/%) | 13 (50%) | 25 (46%) |
| Postnatal steroids (n/%) | 12 (46%) | – |
| Head US (n/%) | 26 (100%) | – |
| Brain MRI (n/%) | 18 (69%) | – |
| Infection (n/%) | 9 (35%) | – |
| NEC (n/%) | 3 (12%) | – |
| Patent ductus arteriosus ligation (n/%) | 5 (19%) | – |
| Ventilation days (mean ± SD) | 17.5 ± 14.9 | – |
| Postconceptual age (weeks) at HRV analysis (mean ± SD) | 38.3 ± 1.0 | 39.2 ± 0.7 |
n = number of cases
HRV Metrics
The ROC curves obtained for the comparison of time and frequency domain metrics are shown in Figure 1A and B, respectively. The AUC values were greater than 0.7 for the following metrics: α1, RMS1 (sec), RMS2 (sec), nLF, LF (dB), and HF (dB). This indicates that low-risk control infants display higher variability than preterm infants. The mean (standard deviation) values of the HRV metrics were as follows: α1: 1.2 (0.41), α2: 1.2 (0.16), RMS1 (sec): 0.024 (0.015), RMS2 (sec): 0.094 (0.054), LF: −2.97 (0.58), HF: −3.85 (0.48), nLF: 0.67 (0.13), nHF: 0.26 (0.094). ANOVA showed that the following metrics were higher in controls than preterm infants (P <.0001): α1, RMS1 (sec), RMS2 (sec), LF (dB), HF (dB), and nLF. There was no difference in α2 between controls and preterm infants. There were no differences in HRV metrics of preterm infants born at < 26 weeks GA and preterm infants born between 26 and 28 weeks GA (P > .05). Among the preterm infants, there was no difference in the HRV metrics for the preterm infants that developed NEC or had a culture positive for infection (n = 12) compared to those that did not develop NEC or have an infection (n = 14).
Figure 1: Time and Frequency Domain Receiver Operating Characteristic Curves (ROC).
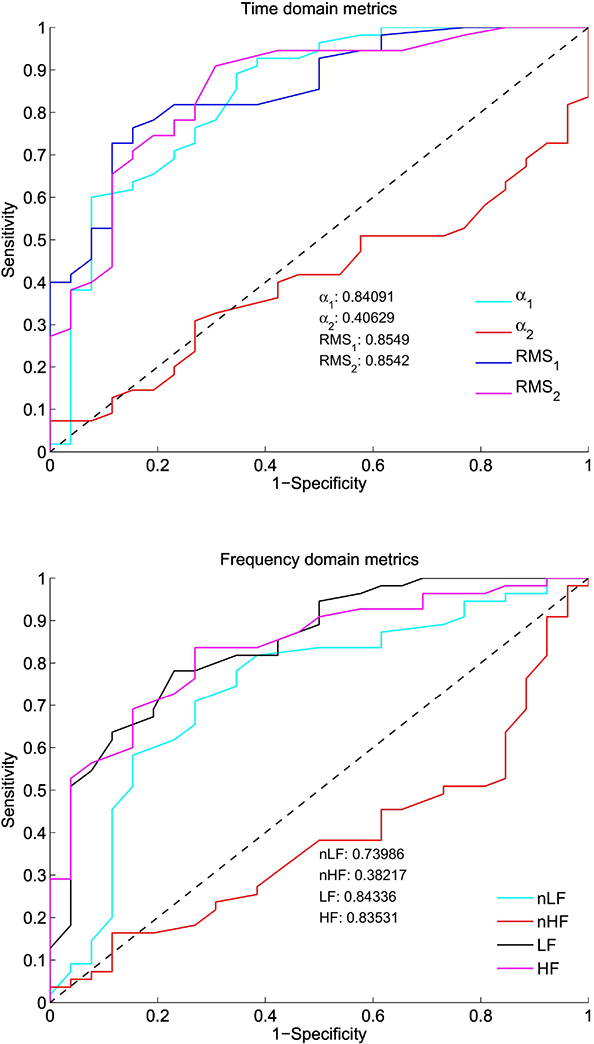
The ROC area under the curve was calculated for (A) time domain indices (α1, α2, RMS1 [sec], and RMS2 [sec]) and for (B) frequency domain indices (nLF, nHF, LF [dB], and HF [dB]). The curves show higher ANS tone in term control versus preterm infants at term-equivalent age in α1, RMS1, RMS2, nLF, LF, and HF. The diagonal dashed line indicates an area under the curve of 0.5.
Presence or absence of infection, the number of days on mechanical ventilation, and postnatal steroids were the only three variables that were significantly and independently different between controls and preterm infants. Hence, the final ANCOVA model used for comparing the HRV metrics of controls and preterm infants had the number of days on the ventilator, presence or absence of infection, and postnatal steroids as control variables (Table 2). The ANCOVA analysis showed that the following metrics were higher in controls than in preterm infants: α1 (P <.0001), RMS2 (P = .0004), LF (P = <.0001) and HF (P = .0017). Since we had multiple HRV metric comparisons in our data, to deflate the Type I error we used only those comparisons associated with a P <.005. Thus, except RMS1 and nLF, all of the metrics that were different between controls and preterm infants in the ANOVA model remained significantly different between the two groups even after adjusting for the control variables.
Table 2:
ANCOVA results controlling for infection, ventilator days, and postnatal steroids
| Dependent | F-Value | P-Value |
|---|---|---|
| α1 | 22.38 | <0.0001* |
| α2 | 0.4 | 0.53 |
| RMS1 (sec) | 0.11 | 0.74 |
| RMS2 (sec) | 13.97 | 0.0004* |
| nLF | 7.08 | 0.0095 |
| nHF | 0.58 | 0.45 |
| LF (dB) | 3.81 | <0.0001* |
| HF (dB) | 1.89 | 0.0017* |
indicates significant difference
DISCUSSION
This study evaluated ANS function by HRV analysis at the term-equivalent age in preterm infants, born at ≤ 28 weeks gestation, who had normal neuroimaging. Compared to low-risk term-born infants, we found that preterm infants have depressed autonomic tone around term corrected GA even without evidence of direct brain injury on term-equivalent neuroimaging. The decrease in ANS tone was found in both sympathetic and in parasympathetic indices. This finding indicates persistent immaturity or under-development of the sympathetic and parasympathetic arms of the ANS during extra-uterine development of neurologically normal premature infants in the NICU.
Our studies’ findings of under-development of the overall ANS tone in preterm infants prior to NICU discharge are in line with a longitudinal ANS study by Patural et al., in 39 preterm newborns (mean GA = 28 weeks), that found indices of HRV did not significantly increase or mature by term-equivalent age, compared to term newborns, suggesting possible arrest of ANS development during the ex-utero third trimester in premature newborns.(15) Twenty-six percent of patients in their study had evidence for prematurity-related brain injury before discharge.(15) Since prematurity is associated with a high incidence of brain injury, which may in turn directly affect autonomic development, we aimed in this study to exclude the role of destructive brain injury. The findings of our study thus provide confirmation of impaired autonomic development during premature extrauterine maturation, even in the absence of major prematurity-related brain lesions. Similarly, we also did not find a difference in the degree of prematurity and ANS tone at term among our preterm cohort, although all infants were ≤ 28 weeks GA. Autonomic maturation may, therefore, be altered early after premature birth and without significant “catch-up” development by term corrected age.
The immature ANS of the premature infant undergoes development in the context of a vastly different set of afferent signals than does its age-matched fetal counterpart. For example, the intensity of physiological fluctuations including ambient sensory stimuli, caregiving events, iatrogenic exposures to medications and ventilatory support, amongst others, are markedly different in the two environments. Although the exact mechanisms of dysmature third trimester ANS development in premature infants remain unclear at this time, it is likely that this mismatched developmental milieu plays a role in derailing the normal programming of the ANS.
Under normal in utero conditions and birth at term, the ANS shows maturation throughout the fetal period with continued maturation during infancy.(8, 23) ANS maturation/development in preterm newborns may however occur over a very protracted time course (months to years after NICU discharge), since at two months of age preterm infants still show decreased parasympathetic tone compared to term-born infants.(12) By age two years, ex-preterm-born children seem to have ANS tone that is close to that of children born at term, but still less overall.(24) Thus, preterm-born infants and young children may remain more vulnerable to cardiovascular compromise during illness throughout this period of prolonged ANS maturation.
The study aimed to evaluate co-morbid medical conditions in premature newborns that can have additive effects on impaired ANS function and maturation at term. Through the hypothalamic-pituitary-adrenal axis, the ANS is integrally involved in mounting the early cardiovascular response to infection/sepsis.(17, 25) Prior to clinical identification of NEC in preterm infants, there is a decrease in HRV due to reduced parasympathetic tone.(26) Our study however evaluated HRV indices only prior to discharge and not near the time of acute infection and/or NEC in preterm infants. HRV changes associated with acute infection/NEC may be transient and resolve after resolution of infection as we have recently shown in a study of preterm infants with NEC.(27) The central ANS is also involved in respiratory function and mechanical ventilation can affect ANS tone. Preterm infants with a failed initial extubation attempt had lower HRV compared to successfully extubated preterm infants.(28) Although ventilator days and infection were not present in the controls, among the preterm infants our results indicate that the depression in HRV is independent of this contribution. This finding was also similar for exposure to postnatal steroids. Early birth GA may therefore be the most critical factor affecting ANS function at term age.
The prolonged time course for ANS maturation in preterm infants, even in the absence of brain injury, may increase the risk for “dysmaturation,” which can have serious implications. For example, impaired cardiovascular control from ANS immaturity may contribute to risk of sudden infant death syndrome/apparent life threatening events (ALTE) in premature newborns after discharge from the NICU.(29) In a study of premature newborns that developed ALTE there was a paradoxical increase in parasympathetic tone and lower sympathetic tone at 36 weeks post conceptual age, likely restricting the ability of these infants to auto-resuscitate and increase HR and blood pressure during hypoxic episodes.(30) Immature ANS function may also increase risk for IVH, a potentially devastating complication of prematurity.(31) Furthermore, immature or abnormal development of the ANS in preterm infants has also been implicated in long-term behavioral and neuropsychological disorders(32) and cardiovascular health.(33)
This study is limited by the use of retrospective preterm infant cases. We did not have access to maternal data of fever or chorioamnionitis. Not all preterm infant cases had term-equivalent brain MRI in addition to cranial US and some included cases had mild imaging changes. It is likely that some of the eight infants that did not have brain MRI may have had similar changes which may be seen among preterm infants who complete term-equivalent brain MRI.(34) Some of the preterm infants had non-neurologic complications of prematurity including infection, PDA, and NEC; however these factors did not show difference in our analysis. None of the control infants had non-neurologic complications. There was a difference in sampling rates of ECG data between the preterm and term groups however based on our prior analysis experience (unpublished) this would not have impacted the results presented in this work. HRV metrics may be affected by sleep state,(8) which we tried to mitigate by averaging our metrics over a period of one hour. The study is however enhanced by comparison of HRV data in preterm infants to a low-risk term control cohort enrolled at a high-volume delivery hospital and studied close to birth.
Conclusion
Premature birth affects function of the ANS that does not normalize prior to discharge, even in the presence of normal neuroimaging. This study supports the need for investigation into ways to promote normal ANS maturation and development in the extra-uterine environment.
Highlights.
Autonomic nervous system maturation is impaired in premature infants.
Preterm infants have decreased autonomic tone at term gestational age.
The sympathetic and parasympathetic tones are both decreased in preterm infants.
Preterm newborns with no brain injury are at risk for impaired autonomic maturation.
Acknowledgments
Funding: This study was supported by the Children’s National Inova Collaborative (CNICA) Research Program, through institutional support from Children’s National Health System, Washington, DC and the Inova Health System, Fairfax, VA. Dr. Mulkey is supported by Award Numbers UL1TR001876 and KL2TR001877 from the NIH National center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National center for Advancing Translational Sciences or the National Institutes of Health.
Abbreviations:
- ANS
autonomic nervous system
- EKG
electrocardiogram
- GA
gestational age
- HF
high-frequency
- HR
heart rate
- HRV
heart rate variability
- IVH
intraventricular hemorrhage
- LF
low-frequency
- MRI
magnetic resonance imaging
- NEC
necrotizing enterocolitis
- NICU
neonatal intensive care unit
- nHF
normalized high-frequency
- nLF
normalized low-frequency
- RMS
root mean square
- US
ultrasound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Dr. Mulkey wrote the first draft of the manuscript. There was no honorarium, grant, or other form of payment to Dr. Mulkey or any of the co-authors to produce the manuscript.
References
- 1.Yiallourou SR, Witcombe NB, Sands SA, Walker AM, Horne RS. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum Dev. 2013;89(3):145–52. Epub 2012/10/13. [DOI] [PubMed] [Google Scholar]
- 2.Neubauer V, Djurdjevic T, Griesmaier E, Biermayr M, Gizewski ER, Kiechl-Kohlendorfer U. Routine Magnetic Resonance Imaging at Term-Equivalent Age Detects Brain Injury in 25% of a Contemporary Cohort of Very Preterm Infants. PloS one. 2017;12(1):e0169442 Epub 2017/01/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiriez G, Mougey C, Vermeylen D, Wermenbol V, Lanquart JP, Lin JS, et al. Altered autonomic control in preterm newborns with impaired neurological outcomes. Clin Auton Res. 2015;25(4):233–42. Epub 2015/08/09. [DOI] [PubMed] [Google Scholar]
- 4.Longin E, Gerstner T, Schaible T, Lenz T, Konig S. Maturation of the autonomic nervous system: differences in heart rate variability in premature vs. term infants. Journal of perinatal medicine. 2006;34(4):303–8. Epub 2006/07/22. [DOI] [PubMed] [Google Scholar]
- 5.P H B J P V M C T G D G R F. Birth prematurity determines prolonged autonomic nervous system immaturity. Clin Auton Res. 2004;14:391–5. [DOI] [PubMed] [Google Scholar]
- 6.Zouikr I, Bartholomeusz MD, Hodgson DM. Early life programming of pain: focus on neuroimmune to endocrine communication. Journal of translational medicine. 2016;14(1):123 Epub 2016/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulkey SB, du Plessis AJ. The critical role of the central autonomic nervous system in fetal-neonatal transition Seminars in Pediatric Neurology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fyfe KL, Yiallourou SR, Wong FY, Odoi A, Walker AM, Horne RS. The Effect of Gestational Age at Birth on Post-Term Maturation of Heart Rate Variability. Sleep. 2015. Epub 2015/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Electrophysiology TFotESoCtNASoP. Heart Rate Variability. Standards of Measurement, Physiological Interpretation, and Clinical Use. 1996;93(5):1043–65. [PubMed] [Google Scholar]
- 10.Fyfe KL, Yiallourou SR, Wong FY, Horne RS. The development of cardiovascular and cerebral vascular control in preterm infants. Sleep medicine reviews. 2014;18(4):299–310. Epub 2013/08/03. [DOI] [PubMed] [Google Scholar]
- 11.Malliani A, Lombardi F, Pagani M. Power spectrum analysis of heart rate variability: a tool to explore neural regulatory mechanisms. Br Heart J. 1994;71(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lucchini M, Fifer WP, Sahni R, Signorini MG. Novel heart rate parameters for the assessment of autonomic nervous system function in premature infants. Physiological measurement. 2016;37(9):1436–46. Epub 2016/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padhye NS, Verklan M, Brazdeikis A, Williams AL, Khattak AZ, Lasky RE. A comparison of fetal and neonatal heart rate variability at similar post-menstrual ages. Conference proceedings : Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual Conference. 2008;2008:2801–4. Epub 2009/01/24. [DOI] [PubMed]
- 14.Hanna BD, Nelson MN, White-Traut RC, Silvestri JM, Vasan U, Rey PM, et al. Heart rate variability in preterm brain-injured and very-low-birth-weight infants. Biology of the neonate. 2000;77(3):147–55. Epub 2000/03/24. [DOI] [PubMed] [Google Scholar]
- 15.Patural H, Pichot V, Jaziri F, Teyssier G, Gaspoz JM, Roche F, et al. Autonomic cardiac control of very preterm newborns: a prolonged dysfunction. Early Hum Dev. 2008;84(10):681–7. Epub 2008/06/17. [DOI] [PubMed] [Google Scholar]
- 16.Goudjil S, Imestouren F, Chazal C, Ghostine G, Wallois F, Leke A, et al. Patent ductus arteriosus in preterm infants is associated with cardiac autonomic alteration and predominant parasympathetic stimulation. Early Hum Dev. 2013;89(9):631–4. Epub 2013/05/15. [DOI] [PubMed] [Google Scholar]
- 17.Fairchild KD, O’Shea TM. Heart rate characteristics: physiomarkers for detection of late-onset neonatal sepsis. Clin Perinatol. 2010;37(3):581–98. Epub 2010/09/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kota S, Swisher CB, Al-Shargabi T, Andescavage N, du Plessis A, Govindan RB. Identification of QRS complex in non-stationary electrocardiogram of sick infants. Comput Biol Med. 2017;87:211–6. [DOI] [PubMed] [Google Scholar]
- 19.Govindan RB, Al-Shargabi T, Metzler M, Andescavage NN, Joshi R, Du Plessis A. A spike correction approach for variability analysis of heart rate in sick infants. Physica A. 2016;444:35–42. Epub 42. [Google Scholar]
- 20.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5(1):82–7. [DOI] [PubMed] [Google Scholar]
- 21.Govindan RB, Massaro AN, Al-Shargabi T, Andescavage NN, Chang T, Glass P, et al. Detrended fluctuation analysis of non-stationary cardiac beat-to-beat interval of sick infants. EPL (Europhysics Letters). 2014;108:40005–p1–p6. [Google Scholar]
- 22.Metzler M, Govindan R, Al-Shargabi T, Vezina G, Andescavage N, Wang Y, et al. Pattern of brain injury and depressed heart rate variability in newborns with hypoxic ischemic encephalopathy. Pediatr Res. 2017;82(3):438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karin J, Hirsch M, Akselrod S. An estimate of fetal autonomic state by spectral analysis of fetal heart rate fluctuations. Pediatr Res. 1993;34(2):134–8. [DOI] [PubMed] [Google Scholar]
- 24.De Rogalski Landrot I, Roche F, Pichot V, Teyssier G, Gaspoz JM, Barthelemy JC, et al. Autonomic nervous system activity in premature and full-term infants from theoretical term to 7 years. Autonomic neuroscience : basic & clinical. 2007;136(1–2):105–9. Epub 2007/06/09. [DOI] [PubMed] [Google Scholar]
- 25.Karrow NA. Activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system during inflammation and altered programming of the neuroendocrine-immune axis during fetal and neonatal development: lessons learned from the model inflammagen, lipopolysaccharide. Brain, behavior, and immunity. 2006;20(2):144–58. Epub 2005/07/19. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan BA, Grice SM, Lake DE, Moorman JR, Fairchild KD. Infection and other clinical correlates of abnormal heart rate characteristics in preterm infants. J Pediatr. 2014;164(4):775–80. Epub 2014/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Shargabi T, Reich D, Govindan RB, Shankar S, Metzler M, Cristante C, et al. Changes in Autonomic Tone in Premature Infants Developing Necrotizing Enterocolitis. American journal of perinatology. 2018. Epub 2018/04/03. [DOI] [PubMed] [Google Scholar]
- 28.Kaczmarek J, Chawla S, Marchica C, Dwaihy M, Grundy L, Sant’Anna GM. Heart rate variability and extubation readiness in extremely preterm infants. Neonatology. 2013;104(1):42–8. Epub 2013/05/29. [DOI] [PubMed] [Google Scholar]
- 29.Fyfe K, Odoi A, Yiallourou SR, Wong F, Walker AM, Horne RS. Preterm Infants Exhibit Greater Variability in Cerebrovascular Control than Term Infants. Sleep. 2015. Epub 2015/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nino G, Govindan RB, Al-Shargabi T, Metzler M, Massaro AN, Perez GF, et al. Premature Infants Rehospitalized because of an Apparent Life-Threatening Event Had Distinctive Autonomic Developmental Trajectories. American journal of respiratory and critical care medicine. 2016;194(3):379–81. Epub 2016/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tuzcu V, Nas S, Ulusar U, Ugur A, Kaiser JR. Altered heart rhythm dynamics in very low birth weight infants with impending intraventricular hemorrhage. Pediatrics. 2009;123(3):810–5. Epub 2009/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porges SW, Furman SA. The Early Development of the Autonomic Nervous System Provides a Neural Platform for Social Behavior: A Polyvagal Perspective. Infant and child development. 2011;20(1):106–18. Epub 2011/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen G, Vella S, Jeffery H, Lagercrantz H, Katz-Salamon M. Cardiovascular stress hyperreactivity in babies of smokers and in babies born preterm. Circulation. 2008;118(18):1848–53. Epub 2008/10/15. [DOI] [PubMed] [Google Scholar]
- 34.Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr. 2003;143(2):171–9. Epub 2003/09/13. [DOI] [PubMed] [Google Scholar]


