Abstract
Background:
Repetitive transcranial magnetic stimulation (rTMS) is an effective treatment for medication-refractory major depression, yet the mechanisms of action for this intervention are poorly understood. Here we investigate cerebral cortex thickness as a possible biomarker of rTMS treatment response.
Methods:
Longitudinal change in cortical thickness is evaluated relative to clinical outcomes across 48 participants in 2 cohorts undergoing left dorsolateral prefrontal cortex rTMS as a treatment for depression.
Results:
Our results reveal changes in thickness in a region of the left rostral anterior cingulate cortex that correlate with clinical response, with this region becoming thicker in patients who respond favorably to rTMS and thinner in patients with a less favorable response. Moreover, the baseline cortical thickness in this region correlates with rTMS treatment response – those patients with thinner cortex before treatment tended to have the most clinical improvement.
Conclusions:
This study is the first analysis of longitudinal cortical thickness change with rTMS as a treatment for depression with similar results across two cohorts. These results support further investigation into the use of structural MRI as a possible biomarker of rTMS treatment response.
Keywords: Cortical thickness, Freesurfer, Magnetic resonance imaging, Neuromodulation
Introduction
Repetitive transcranial magnetic stimulation (rTMS) is an effective treatment for medication-refractory major depressive disorder [1], yet the mechanisms of action for this intervention are poorly understood. Studies have shown that rTMS treatment for depression may be associated with changes in serum markers (e.g. BDNF) as well as functional and neurochemical changes in the brain, both at the site of stimulation and in remote regions [2]. These studies aim to identify potentially clinically useful bio-markers that would provide an objective measure of treatment response, provide mechanistic insight to rTMS, and, ideally, predict which patients are most likely to benefit from rTMS. To date there are relatively few studies investigating the structural correlates of rTMS treatment response, yet if useful, structural MRI has the potential to be incorporated into clinical practice more easily than other biomarkers under evaluation (e.g. resting state functional connectivity MRI or EEG) as it uses existing, largely automated analysis software packages and routine clinical imaging hardware.
In 2007, May et al. published a first demonstration that focal 1 Hz rTMS delivered daily for five days is associated with a local increase in gray matter at the site of stimulation in the temporal lobe of healthy adults [3]. For patients receiving rTMS to the dorsolateral prefrontal cortex (DLPFC) as a treatment for depression, longitudinal studies have shown no global change in brain volume [4], a significant reduction in left hippocampus volume that was driven by the non-responders [5], and a significant increase in hippocampus volume that did not correlate with clinical response [6]. A 2016 study showed a longitudinal increase in gray matter density in several brain areas over a course of rTMS for depression and a single region, the rostral anterior cingulate cortex (rACC) extending into medial prefrontal cortex, with increased gray matter density that correlated with clinical improvement [7].
In the present study we sought to evaluate whether cerebral cortex thickness may be a potential biomarker of rTMS treatment response in depression. Specifically, we examined whether there were regional changes in cerebral cortex thickness associated with clinical improvement. We hypothesized that cortical thickness would increase in the left DLPFC based on prior research showing regional structural changes at the stimulation site [3], along with increases in cortical thickness at the anterior cingulate in correspondence with clinical improvement, which may correspond to increased gray matter density reported previously [7]. In addition we measured hippocampus volume to evaluate whether nonresponders had decreased hippocampus volume with rTMS treatment, as was reported previously [5] versus hippocampal enlargement, which has been reported recently in association with rTMS treatment for depression [6] and with some consistency with electroconvulsive treatment of depression [8]. While we had specific anatomical hypotheses, our analyses included both region-of-interest (ROI) and vertex-wide analyses that were not constrained by pre-specified ROIs.
Methods and materials
48 patients with treatment-resistant major depression were evaluated and treated using rTMS at one of two sites, the Berenson Allen Center for Noninvasive Brain Stimulation, Beth Israel Deaconess Medical Center (BIDMC) (N = 21) or Weill Cornell Medical College (N = 27). Both datasets have been analyzed previously [9,10,31], including a voxel-based morphometry analysis of gray matter density with findings in the rostral anterior cingulate cortex as reported above [7]. Cerebral cortex thickness has not been evaluated previously in either dataset. Diagnosis was confirmed in both groups by a clinical interview performed by a psychiatrist. While major depression was the target population, three patients had prior episodes of hypomania and thus met criteria for bipolar II disorder.
TMS Treatment.
Each participant had a treatment course of rTMS applied to the left DLPFC according to the following protocol: 10 Hz rTMS in 4 s trains with 26 s intertrain interval, 3000 pulses, over 37.5 min. At BIDMC TMS was delivered using a NeuroStar TMS Therapy System (Neuronetics, Inc., Malvern, Pennsylvania) or Magstim Super Rapid stimulator (Magstim Company Ltd., UK) equipped with a 70-mm figure-of-eight coil and at Cornell TMS was delivered with the NeuroStar system. DLPFC targeting was 5.5 cm anterior to the motor cortex at BIDMC and via the beam F3 method at Cornell [11]. The number of rTMS sessions was 30–36 at BIDMC and 25 at Cornell. The primary measure of treatment response was the Beck Depression Inventory (BDI) [12] at BIDMC and the Hamilton Depression Rating Scale-24 Item (HamD) [13] at Cornell.
Imaging Acquisition.
An MRI was acquired within a 7-day window before and after the rTMS treatment course. At BIDMC the MRI was conducted using a GE 3T HDX scanner. High-resolution T1-weighted structural images were acquired via a 3D-turbo field echo sequence (TE = 2.9 ms, flip angle = 15°, 0.94 ×.94 × 1 mm resolution). At Cornell the MRI was acquired using a GE Signa Excite 3T scanner. High-resolution T1-weighted anatomical scans with 1 × 1 × 1 mm resolution were obtained with an 8-channel phase array head coil using a three dimensional spoiled gradient echo sequence with TR/TE/FA of 9 ms/3.5 ms/13°. Other details of this sample have been reported previously [7,9,10].
FreeSurfer Processing.
Structural MRI (T1\ MPRAGE sequences) data were processed using FreeSurfer, an automated software package that parcellates the brain using anatomical landmarks, including delineation of the white and pial surface to define cerebral cortex thickness across over a hundred thousand vertices [14]. The longitudinal processing stream of FreeSurfer was used to optimize detection of changes in cerebral cortex thickness in the same individual across two time points [15], before and after the course of rTMS, a timespan of 4–7 weeks. This resulted in a cortical thickness difference value from pre-to post-rTMS for each vertex in the cerebral cortex for each patient. There is a lack of consensus regarding the optimal smoothing kernel size for FreeSurfer so we report data for commonly used kernels of 10, 15 and 20 mm. 15 was used for the main analyses with other smoothing kernel data reported as supplementary material. FreeSurfer parcellation of the cerebral cortex boundaries was reviewed individually for each scan to ensure anatomical accuracy prior to performing any analyses. The data from three patients was excluded due to inadequate parcellation, likely secondary to motion artifact (2 patients, which included 1 patient with bipolar II) or marked atrophy at baseline (1 patient).
A Priori Regions of Interest.
To evaluate cortical thickness changes at the stimulation site each individual from the BIDMC cohort had the stimulation site identified using neuronavigation with Brainsight frameless stereotactic equipment. Patient-specific stimulation sites were recorded in stereotactic space and projected to the nearest brain surface position perpendicular to a plane tangential to the scalp. Sites were then transformed as a 20 mm diameter spherical ROI from MNI volume space to FreeSurfer surface space. For the Cornell cohort the site was estimated using a 20 mm diameter spherical ROI at the average F3 MNI coordinate −41.5, 41, 33 [16].
Hippocampus volume was traced manually using a previously published protocol [17]. Prior to tracing for this analysis reliability was demonstrated using both inter-rater (with original study data) and intra-rater intraclass correlations, at 0.958 (95% confidence interval 0.835–0.989) and 0.974 (95% confidence interval 0.894–0.993) respectively. The FreeSurfer-derived hippocampus volume was also evaluated.
ROI analyses, other than the stimulation site and the manual segmentation of the hippocampus described above, were conducted using FreeSurfer-derived regions from their standard atlas ROIs [18,19].
Statistical Analysis.
The main analysis examined the relationship between longitudinal cortical thickness changes with change in depression ratings using a general linear model in FreeSurfer’s QDEC program (www.surfer.nmr.mgh.harvard.edu). In order to combine depression ratings across different rating scales we rank-ordered the percent change in HamD and BDI separately and scaled it to a 100-point scale before combining data from the two groups into a single continuous scale. A statistical threshold of P < 0.001 uncorrected was selected a priori for the main analysis as the effect size was expected to be small, due to minute changes in cortical thickness in adults over a period of weeks and high inter-individual variability given the heterogeneity of depression and variable response to TMS. Given the lenient statistical threshold the analysis is considered exploratory. Institutional Review Boards approved the study prior to data collection at each site and informed consent was obtained from all patients.
Results
Demographic and Global Structural Data.
The average patient age was 48 ± 15 with 30 female participants. Depression symptoms improved from pre-to post-rTMS across the 45 patients included in the final analysis (40.1 ± 26.5% reduction; P < 0.001) with 19 patients (42%) meeting the pre-defined criteria for ‘responder’ set as at least a 50% reduction in HamD/BDI. Global morphometric data were first reviewed. There were no longitudinal changes in average cortical volume (pre-rTMS 447194 ± 52300 mm3, post-rTMS 446875 ± 52529 mm3, P = 0.90) or average thickness (pre-rTMS 2.42 ± 0.10 mm, post-rTMS 2.42 ± 0.11 mm, P = 0.92) across the entire cerebral cortex. The average longitudinal change in cortical volume and thickness also did not differ significantly in comparing data between sites (P = 0.25 and P =.24, respectively). There were no absolute significant regional changes in cerebral cortex thickness independent of treatment response at P < 0.001 using a vertex-wide analysis.
Main Analysis.
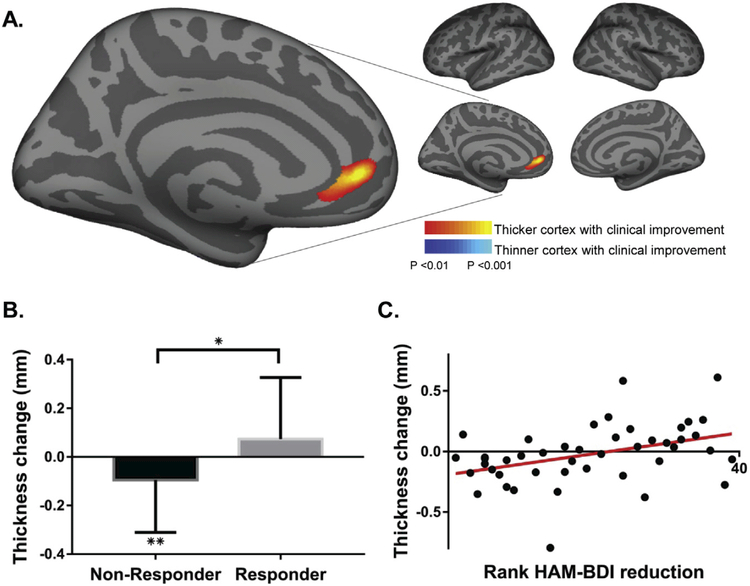
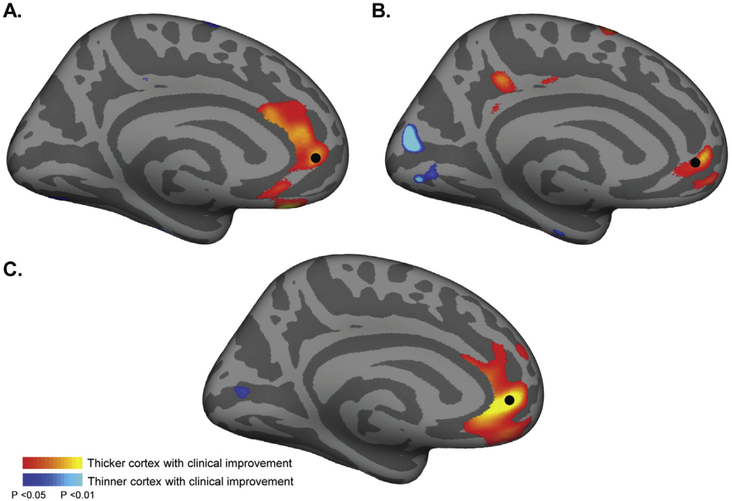
The primary vertex-wide analysis revealed a region of the left rostral anterior cingulate cortex (rACC) with increased cortical thickness in association with clinical improvement (Fig. 1A). The peak correlation was at the rostral anterior cingulate along the cingulate sulcus and bordering the medial prefrontal cortex (P < 0.001, vertex #33172 in FreeSurfer space and −5.5, 47, −3.3 in MNI space). This regional correlation was present regardless of smoothing kernel used (P < 0.001; Fig. S1) and also significant when excluding both bipolar II patients (P < 0.001; Fig. S2). Extracting the average cortical thickness change data from within this left rACC region allowed post-hoc descriptive statistical analyses. There was a significant difference in average thickness change in this region between responders and non-responders, with a trend towards increased thickness in the responders and a significant decrease in thickness in non-responders (Fig. 1B). A scatterplot shows the correlation of average cortical thickness change within this ROI with clinical improvement (r =0.4, P < 0.01; Fig. 1C). Although statistically significant, the absolute changes in thickness at this site were minimal at <0.1 mm on average. We also evaluated whether the rACC correlation was driven primarily by data from one site and the finding was present using data from each site independently (Fig. 2), though at a lower statistical threshold of P < 0.01 and with some variation noted in the spatial distribution.
Fig. 1. Cortical thickness change correlates with clinical improvement.
A. A cortex-wide analysis revealed a significant correlation between cortical thickness change in left rostral anterior cingulate cortex (rACC) and improvement in depression symptoms. The peak correlation is significant at P < 0.001 uncorrected. Findings are shown at P < 0.01 to display the extent of the spatial distribution of the correlation, also see Fig. S3 showing these results at a P < 0.001 threshold. B. Average cortical thickness changes within this rACC region differ between responders and non-responders (+0.074 and −0.095 mm, respectively; P < 0.05) with non-responders showing a significant reduction in cortical thickness (P < 0.01). C. A scatter plot shows the correlation between change in cortical thickness within this ROI and clinical response (r = 0.4, P < 0.01). *P < 0.05, **P < 0.01.
Fig. 2. Cross-cohort thickness data contributing to main finding.
Panels A and B show the results of the main analysis (Fig. 1) from the Cornell (A) and BIDMC (B) cohorts, respectively with the combined findings in C. For reference a black dot denotes the rACC region where the effect was strongest in the group analysis. Scatter plots showing the correlation of clinical response and cortical thickness from each site are displayed in Fig. S4. Note a lower statistical threshold was used here (P < 0.05) relative to the main analysis to account for the reduced power from a smaller sample size.
Region-of-Interest Analyses.
In addition to the main cortex-wide analysis that was unconstrained by a priori hypotheses, we also conducted ROI analyses to further evaluate the a priori hypotheses (Table 1). Regional cortical thickness in the left DLPFC in the regions most closely approximating the stimulation site (rostral and caudal middle frontal gyrus) showed no significant change in cortical thickness across time and no correlation with clinical improvement. However, there was a near significant difference in cortical thickness change amongst responders (increased thickness) and non-responders (decreased thickness) in the left rostral middle frontal gyrus (P = 0.053). There was no significant longitudinal change in cortical thickness at the individualized stimulation sites.
Table 1.
Region of interest results.
| Region | Mean thickness change (mm) | Non-Responder thickness change (mm) | Responder thickness change (mm) | Responder vs Non-responder T-test P value | Correlation (r, P); N = 45 |
|---|---|---|---|---|---|
| Left rostral middle frontal | 0.008 ± 0.062, P = 0.389 | −0.007 ± 0.067 | 0.029 ± 0.012 | 0.0530 | 0.218, 0.150 |
| Left caudal middle frontal | 0.004 ± 0.012, P = 0.605 | 0.008 ± 0.071 | −0.008 ± 0.083a | 0.604 | −0.019, 0.902 |
| Left rostral + caudal middle frontal | 0.010 ± 0.043, P = 0.823 | 0.001 ± 0.119 | 0.021 ± 0.123 | 0.527 | 0.030, 0.843 |
| Right rostral middle frontal | 0.004 ± 0.084, P = 0.737 | 0.004 ± 0.086 | 0.005 ± 0.085a | 0.896 | 0.012, 0.939 |
| Right caudal middle frontal | −0.005 ± 0.074, P = 0.638 | 0.001 ± 0.089a | −0.013 ± 0.046 | 0.221 | −0.096, 0.528 |
| Right rostral + caudal middle frontal | −.001 ± 0.133, P = 0.962 | 0.004 ± 0.149 | −0.008 ± 0.113a | 0.581 | −0.046, 0.765 |
| Left rostral anterior cingulate | −0.022 ± .0167, P = 0.378 | −0.067 ± 0.163 | 0.040 ± 0.114 | 0.042 | 0.411, 0.005 |
| Right rostral anterior cingulate | −0.147 ± 0.071, P = 0.936 | −0.036 ± 0.154 | 0.036 ± 0.216a | 0.276 | 0.218, 0.151 |
| Total rostral anterior cingulate | 0.010 ± 0.120, P = 0.591 | −0.104 ± 0.223 | 0.076 ± 0.225a | 0.052 | 0.395, 0.007 |
| Left subcallosal | −0.064 ± 0.193, P = 0.033 | −0.076 ± 0.217 | −0.045 ± 0.157 | 0.596 | 0.042, 0.782 |
| Right subcallosal | −0.030 ± 0.269, P = 0.459 | −0.044± 0.262 | −0.011 ± 0.286 | 0.686 | 0.215, 0.156 |
| Total subcallosal | −0.094 ± 0.384, P = 0.109 | −0.121 ± 0.404a | −0.056 ± 0.362 | 0.641 | 0.172, 0.258 |
| Stimulation Site (L-DLPFC) | −0.011 ± 0.114, P = 0.519 | −0.014 ± 0.135 | −0.007 ± 0.079 | 0.883 | 0.050, 0.747 |
| Mean volume change (mm3) | Non-Responder volume change (mm3) | Responder volume change (mm3) | Responder vs Non-responder T-test P value | Correlation (r, P); N = 45 | |
|---|---|---|---|---|---|
| FreeSurfer left hippocampus | −29.109 ± 128.302, P = 0.135 | −40.622 ± 129.319 | −11.839 ± 128.462 | 0.467 | 0.140, 0.360 |
| FreeSurfer right hippocampus | −23.3438 ± 111.382, P = 0.167 | −29.211 ± 97.684 | −14.528 ± 131.827 | 0.670 | −0.035, 0.821 |
| FreeSurfer total hippocampus | −52.447 ± 197.729, P = 0.082 | −69.833 ± 194.594 | −26.367 ± 205.128 | 0.476 | 0.071, 0.643 |
| Manually-traced left hippocampus | −36.127 ± 139.465, P = 0.089 | −57.139 ± 149.045 | −4.610 ± 120.891 | 0.220 | 0.102, 0.503 |
| Manually-traced right hippocampus | −46.816 ± 139.280, P = 0.029 | −69.284 ± 160.428a | −13.114 ± 94.033 | 0.188 | 0.202, 0.182 |
| Manually-traced total hippocampus | −82.943 ± 244.983, P = 0.028 | −126.423 ± 268.936a | −17.723 ± 192.910 | 0.147 | 0.173, 0.255 |
Indicates value significantly different than zero, Paired samples T test, P < 0.05; P values < 0.05 are in bold. Abbreviations include FS FreeSurfer; L-DLPFC left dorsolateral prefrontal cortex.
Results from the left rostral anterior cingulate cortex ROI derived from FreeSurfer closely resembled those derived from the vertex-wide analysis described above, with a significant difference in cortical thickness between responders and non-responders (P < 0.05), with thickness change significantly correlated to clinical improvement, such that better clinical response is associated with increased thickness (r = 0.41, P < 0.01). Results from the left subcallosal ACC, which most closely approximates the subgenual ACC, showed a significant decrease in cortical thickness (P < 0.05) following a course of rTMS that did not correlate with clinical improvement. Total bilateral hippocampus volume was significantly reduced following the course of rTMS (P < 0.05), which appeared to be driven by the non-responders, with responders having no significant change in hippocampal volume. The results were similar with FreeSurfer-derived hippocampal volume but failed to reach statistical significance (P =.08).
Does baseline scan relate to treatment response?
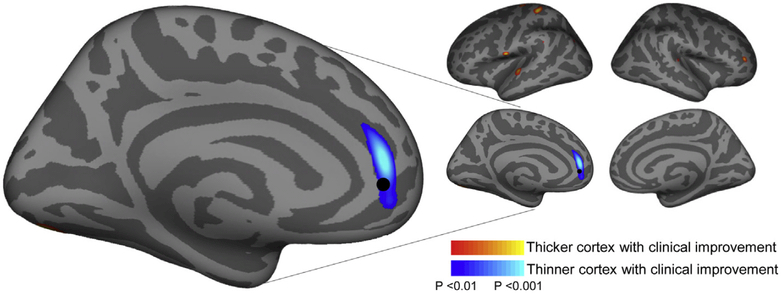
A secondary analysis examined the relationship between pre-treatment cortical thickness relative to eventual clinical response to rTMS treatment. This analysis revealed a cortical region in the same vicinity of the main analysis with a peak correlation slightly dorsal to the main finding that was significantly thinner in patients who would go on to have a better clinical response to rTMS (P < 0.001; Fig. 3). This finding was significant regardless of smoothing kernel used (Fig. S5). Cortical thickness within this region had a non-significant trend towards increasing post-treatment (mean thickness change: 0.077 mm ± 0.27 mm, P = 0.06). Notably, while pre-treatment cortical thickness in this region was correlated with eventual improvement it was not correlated with pre-treatment severity of depression.
Fig. 3. Pretreatment cortical thickness relates to eventual clinical response.
An analysis of pre-treatment MRI data revealed a region where baseline (pre-treatment) cortical thickness correlated with treatment response (P < 0.001); those individuals with thinner cortex in this region had the most clinical improvement with rTMS treatment. For reference a black dot denotes the rACC region where the effect from the main analysis was strongest (from Fig. 1).
Discussion
The current results suggest that cerebral cortex thickness changes in the rACC in relation to a patient’s clinical response to rTMS for depression. Moreover, the results offer preliminary support that pre-treatment cerebral cortex thickness correlates with eventual treatment response. These results are considered preliminary, but if borne out in additional larger samples the findings would add to a growing body of literature already supporting a role of the rostral anterior cingulate region in depression [32], including a large study of over 2000 depressed patients showing this region is thinner in association with depression [20] and may even be a structural marker of vulnerability to depression [21]. In addition, previous studies have highlighted changes in the rACC in association with rTMS treatment for depression [7,9,10]. Most pertinent to the current analysis is a prior voxel-based morphometry analysis using an overlapping cohort of 27 participants that demonstrated increased gray matter density of the rostral anterior cingulate over a treatment course that correlated with clinical improvement [7]. While both gray matter density and cortical thickness analyses are valid approaches for measuring cortical changes they often lead to discrepant findings within the same dataset [22–25], with FreeSurfer-derived cortical thickness being more sensitive to longitudinal changes in some studies that have used both measures [23,24]. The current study suggests that changes in gray matter density observed previously in this region [7] are related, at least in part, to changes in cortical thickness. This is significant because neural plasticity may be more reliably reflected by structural changes within cortical columns that share a common ontogenetic origin [26,27]. Another strength of the current analysis is that our findings are similar across two separate cohorts (Fig. 2).
In addition to structural changes there have also been functional changes noted in the rACC with rTMS treatment for depression. A PET study showed increased metabolism in the rACC and adjacent medial prefrontal cortex in depressed patients prior to rTMS, with higher metabolism predicting better response to rTMS [28], though this may not be unique to rTMS treatment [33]. Using functional connectivity MRI it was noted that the subgenual ACC is hyper-connected to the default mode network in depressed patients prior to rTMS treatment and the subgenual ACC functional connectivity to the anterior node of the default mode network (rACC and medial PFC) normalized in association with clinical improvement [10]. Moreover, GABA metabolites, measured with MR spectroscopy, increased in this region over a course of rTMS and this change correlated with clinical improvement [9]. Despite using different imaging modalities each of these studies has highlighted changes in the rACC that correlate with clinical response.
Some of the current findings are provocative and warrant further study, including the observed increased thickness of the left rostral middle frontal gyrus, decreased thickness of the subcallosal ACC, and overall decrease in hippocampus volume that occurred after the course of rTMS treatment. None of these findings correlated with clinical response, but it is possible the current analysis was underpowered to detect such relationships.
There are several limitations of the current analysis. First, we did not correct for multiple comparisons and future larger studies will be needed to further evaluate the current findings. In addition, the magnitude of change in cortical thickness was quite low and the ability to detect such changes in the sub-millimeter range relies on automated surface reconstruction with high resolution surface averaging techniques [14,29], which has received some experimental validation [30]. The current study did not include a sham-controlled arm or alternate treatment arm to evaluate whether cortical thickness changes may relate to changes in symptom severity unrelated to receiving rTMS. Finally, while our objective in this study was to explore cortical thickness as a possible biomarker of rTMS treatment response the current findings are unlikely to be useful for guiding treatment in individual patients using current technology. Many challenges remain such as interpreting results across scanners and institutions and it is not clear that the group level results shown here could be meaningfully applied to individuals to guide clinical decisions.
Future work will aim to extend the analysis to subcortical regions and cerebellum, and explore individual patterns of morpho-metric changes and whether these relate to changes in functional connectivity MRI, with the ultimate aim of defining a multimodal biomarker profile that predicts rTMS treatment response.
Supplementary Material
Acknowledgements
We thank Joel Bruss, Eric Axelson, and Peg Nopoulos for assistance with various aspects of FreeSurfer processing.
Funding
A.D.B. was supported by National Institutes of Health grant K12HD027748–24, K12NS098482, the Sidney R. Baer, Jr. Foundation, and the Roy J. Carver Trust. M.D.F. was supported by National Institutes of Health grant K23NS083741, R01 MH113929, the Nancy Lurie Marks Foundation, and the Mather’s Foundation, 29943. A.P.L. was supported in part by the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R01 MH100186; R01 HD069776; R01 NS073601; R21 MH099196; R21 NS085491; and R21 HD07616), and Harvard Catalyst j The Harvard Clinical and Translational Science Center (National Centres of Competence in Research and the National Center for Advancing Translation Sciences, National Institutes of Health, UL1 RR025758). M.J.D. was supported by a NARSAD Young Investigator Award and the Pritzker Neuropsychiatric Disorders Research Consortium. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Institutes of Health, or the Sidney R. Baer Jr. Foundation.
Abbreviations:
- BDI
Beck Depression Inventory
- BDNF
brain-derived neurotrophic factor
- BIDMC
Beth Israel Deaconess Medical Center
- DLPFC
dorsolateral prefrontal cortex
- EEG
electroencephalography
- GABA
gamma-Aminobutyric acid
- HamD
Hamilton Depression Rating Scale-24 Item
- MNI
Montreal Neurological Institute
- PET
positron emission tomography
- rACC
rostral anterior cingulate cortex
- ROI
Region-of-interest
- rTMS
Repetitive transcranial magnetic stimulation
Footnotes
Declarations of interest
The authors declare no financial interests relevant to this work. M.D.F. is listed as inventor on submitted or issued patents on guiding neurological interventions with fMRI. A.P.L. serves on the scientific advisory boards Magstim, Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, Axilum Robotics, Constant Therapy, and Neosync; and is listed as inventor in issued patents and patent applications on the real-time integration of transcranial magnetic stimulation with electroencephalography and magnetic resonance imaging. M.J.D. reports materials transfer to complete studies of rTMS for depression from Neuronetics and grant funding for a clinical trial of low field magnetic stimulation for major depression from TAL Medical; and has also received consulting fees from Myriad Genetics. A.D.B. is a consultant on a clinical trial data safety and monitoring board for Ekso Bionics. B.D.U, C.L., and M.J.L. have no conflicts to disclose.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.brs.2018.01.029.
References
- [1].Gaynes BN, Lloyd SW, Lux L, Gartlehner G, Hansen RA, Brode S, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J Clin Psychiatr 2014;75:477–89. 10.4088/JCP.13r08815. quiz 489. [DOI] [PubMed] [Google Scholar]
- [2].Fidalgo TM, Morales-Quezada JL, Muzy GSC, Chiavetta NM, Mendonca ME, Santana MVB, et al. Biological markers in noninvasive brain stimulation trials in major depressive disorder: a systematic review. J ECT 2014;30:47–61. 10.1097/YCT.0b013e31828b34d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].May a, Hajak G, Gänßbauer S, Steffens T, Langguth B, Kleinjung T, et al. Structual brain alterations following 5 days of intervention dynamic aspects of neuroplasticity.pdf. Cereb Cortex 2007;17:205–10. 10.1093/cercor/bhj138. [DOI] [PubMed] [Google Scholar]
- [4].Nahas Z, DeBrux C, Chandler V, Lorberbaum JP, Speer AM, Molloy MA, et al. Lack of significant changes on magnetic resonance scans before and after 2 weeks of daily left prefrontal repetitive transcranial magnetic stimulation for depression. J ECT 2000;16:380–90. [DOI] [PubMed] [Google Scholar]
- [5].Furtado CP, Hoy KE, Maller JJ, Savage G, Daskalakis ZJ, Fitzgerald PB. An investigation of medial temporal lobe changes and cognition following anti-depressant response: a prospective rTMS study. Brain Stimul 2013;6:346–54. 10.1016/j.brs.2012.06.006. [DOI] [PubMed] [Google Scholar]
- [6].Hayasaka S, Nakamura M, Noda Y, Izuno T, Saeki T, Iwanari H, et al. Lateralized hippocampal volume increase following high-frequency left prefrontal repetitive transcranial magnetic stimulation in patients with major depression. Psychiatr Clin Neurosci 2017. 10.1111/pcn.12547. [DOI] [PubMed] [Google Scholar]
- [7].Lan MJ, Chhetry BT, Liston C, Mann JJ, Dubin M. Transcranial magnetic stimulation of left dorsolateral prefrontal cortex induces brain morphological changes in regions associated with a treatment resistant major depressive episode: an exploratory analysis. Brain Stimul 2016;9:577–83. 10.1016/j.brs.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilkinson ST, Sanacora G, Bloch MH. Hippocampal volume changes following electroconvulsive Therapy: a systematic review and meta-analysis. Biol Psychiatry Cogn Neurosci Neuroimaging 2017;2:327–35. 10.1016/j.bpsc.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dubin MJ, Mao X, Banerjee S, Goodman Z, Lapidus KAB, Kang G, et al. Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci 2016;41:E37–45. 10.1503/jpn.150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, et al. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatr 2014;76:517–26. 10.1016/j.biopsych.2014.01.023.Default. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mir-Moghtadaei A, Caballero R, Fried P, Fox MD, Lee K, Giacobbe P, et al. Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul 2015;8:965–73. 10.1016/j.brs.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beck AT, Steer RA, Brown GK. Manual for the Beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- [13].Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 2000;97:11050–5. 10.1073/pnas.200033797200033797 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage 2012;61: 1402–18. 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fried PJ, Rushmore Iii RJ, Moss MB, Valero-Cabre A, Pascual-Leone A. Causal evidence supporting functional dissociation of verbal and spatial working memory in the human dorsolateral prefrontal cortex. Eur J Neurosci 2014;39: 1973–81. 10.1111/ejn.12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pantel J, O’Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, et al. A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus 2000;10: 752–8. . [DOI] [PubMed] [Google Scholar]
- [18].Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–80. 10.1016/j.neuroimage.2006.01.021. S1053-8119(06)00043-7 [pii]. [DOI] [PubMed] [Google Scholar]
- [19].Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010;53:1–15. 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA major depressive disorder working group. Mol Psychiatr 2017;22:900–9. 10.1038/mp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boes AD, McCormick LM, Coryell WH, Nopoulos P. Rostral anterior cingulate cortex volume correlates with depressed mood in normal healthy children. Biol Psychiatr 2008;63:391–7. 10.1016/j.bio-psych.2007.07.018. S0006-3223(07)00730-5[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kong L, Herold CJ, Zöllner F, Salat DH, Lässer MM, Schmid LA, et al. Comparison of grey matter volume and thickness for analysing cortical changes in chronic schizophrenia: a matter of surface area, grey/white matter intensity contrast, and curvature. Psychiatr Res 2015;231:176–83. 10.1016/j.pscychresns.2014.12.004. [DOI] [PubMed] [Google Scholar]
- [23].Rajagopalan V, Pioro EP. Disparate voxel based morphometry (VBM) results between SPM and FSL softwares in ALS patients with frontotemporal dementia: which VBM results to consider? BMC Neurol 2015;15:32 10.1186/s12883-015-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Clarkson MJ, Cardoso MJ, Ridgway GR, Modat M, Leung KK, Rohrer JD, et al. A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage 2011;57:856–65. 10.1016/j.neuroimage.2011.05.053. [DOI] [PubMed] [Google Scholar]
- [25].Li Q, Pardoe H, Lichter R, Werden E, Raffelt A, Cumming T, et al. Cortical thickness estimation in longitudinal stroke studies: a comparison of 3 measurement methods. NeuroImage Clin 2015;8:526–35. 10.1016/j.nicl.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Na K-S, Won E, Kang J, Chang HS, Yoon H-K, Tae WS, et al. Brain-derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Sci Rep 2016;6:21089 10.1038/srep21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rakic P. Specification of cerebral cortical areas. Science 1988;241:170–6. [DOI] [PubMed] [Google Scholar]
- [28].Li C-T, Wang S-J, Hirvonen J, Hsieh J-C, Bai Y-M, Hong C-J, et al. Antidepressant mechanism of add-on repetitive transcranial magnetic stimulation in medication-resistant depression using cerebral glucose metabolism. J Affect Disord 2010;127:219–29. 10.1016/j.jad.2010.05.028. [DOI] [PubMed] [Google Scholar]
- [29].Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage 1999;9: 195–207. 10.1006/nimg.1998.0396. S1053-8119(98)90396-2 [pii]. [DOI] [PubMed] [Google Scholar]
- [30].Cardinale F, Chinnici G, Bramerio M, Mai R, Sartori I, Cossu M, et al. Validation of freeSurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics 2014;12:535–42. 10.1007/s12021-014-9229-2. [DOI] [PubMed] [Google Scholar]
- [31].Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, et al. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol Psychiatry 2017:1–10. 10.1016/J.BIOPSYCH.2017.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mayberg HS. Limbic-Cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci 1997;9:471–81. 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- [33].Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport 1997;8:1057–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.