Abstract
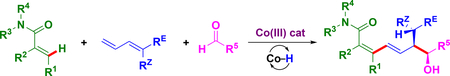
Two-component C–H bond additions to a large variety of coupling partners have been developed with applications towards materials, natural product and drug synthesis. Sequential three-component C–H bond addition across two different coupling partners potentially enables the convergent synthesis of complex molecular scaffolds from simple precursors. Here, we report three-component Co(III)-catalyzed C–H bond additions to dienes and aldehydes that proceeds with high regio- and stereoselectivity resulting in two new carbon-carbon σ-bonds and from four to six new stereocenters. The reaction relies on the synergistic reactivity of the diene and aldehyde with neither undergoing C–H bond addition alone. A detailed mechanism is supported by X-ray structural characterization of a Co(III)-allyl intermediate, observed transfer of stereochemical information, and kinetic isotope studies. The applicability of the method to biologically relevant molecules is exemplified by the rapid synthesis of the western fragment of the complex ionophore antibiotic lasalocid A.
Graphical Abstract
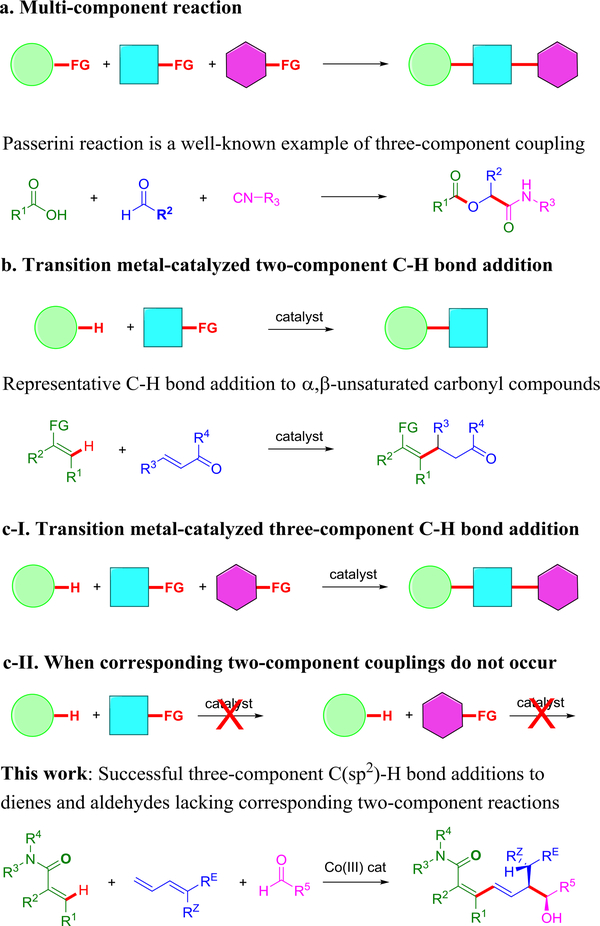
Multi-component reactions combine three or more coupling partners to enable the rapid generation of complex structures from simple precursors. The partners are combined in a precise way by reaction of specific functional groups on the different coupling partners (Figure 1a)1–4. Multi-component reactions as exemplified by the three-component Passerini reaction of aldehydes, isocyanides and carboxylic acids have been extensively studied and employed in synthesis (see Figure 1a)1. In transition metal-catalyzed C–H functionalization, the ubiquitous C–H bond rather than a functional group serves as the site for reaction. Many transition metal-catalyzed two-component additions of C–H bonds to a large variety of different coupling partners have been developed such as additions to α,β-unsaturated carbonyl compounds (Figure 1b)5–10. Sequential, three-component addition reactions of nonacidic C–H bonds to two different coupling partners could provide access to an enormous range of different products (Figure 1c-I), and in promising initial results we have recently reported the first examples11,12. For these reported transformations, at least two out of the three coupling partners are well-documented to undergo efficient two-component coupling with the metal catalyst used in the three-component coupling8. An even greater variety of useful structures might be achieved through synergistic three-component C–H bond addition. In this scenario, which is distinct from synergistic catalysis13, the individual coupling partners do not undergo two-component transformations (Figure 1c-II).
Figure 1: Three-component strategy for the rapid assembly of complex structures.
a. Multi-component reactions. b. Transition metal-catalyzed two-component C–H bond additions. c. Sequential three-component C–H bond additions.
Herein, we show that aromatic and α,β-unsaturated amides undergo Co(III)-catalyzed β-C–H bond addition across dienes and aldehydes to provide a carbon framework with high regio- and stereocontrol that incorporates two new C–C σ-bonds and four or more new stereocenters14,15. Notably, neither the diene or aldehyde partner individually undergoes Co(III)-catalyzed two-component C–H addition (vide infra). Moreover, use of the bulk chemical feedstock butadiene16,17 as the diene partner results in the introduction of vicinal α-methyl and hydroxyl asymmetric carbons that are commonly found in natural products.
Results
Optimization studies.
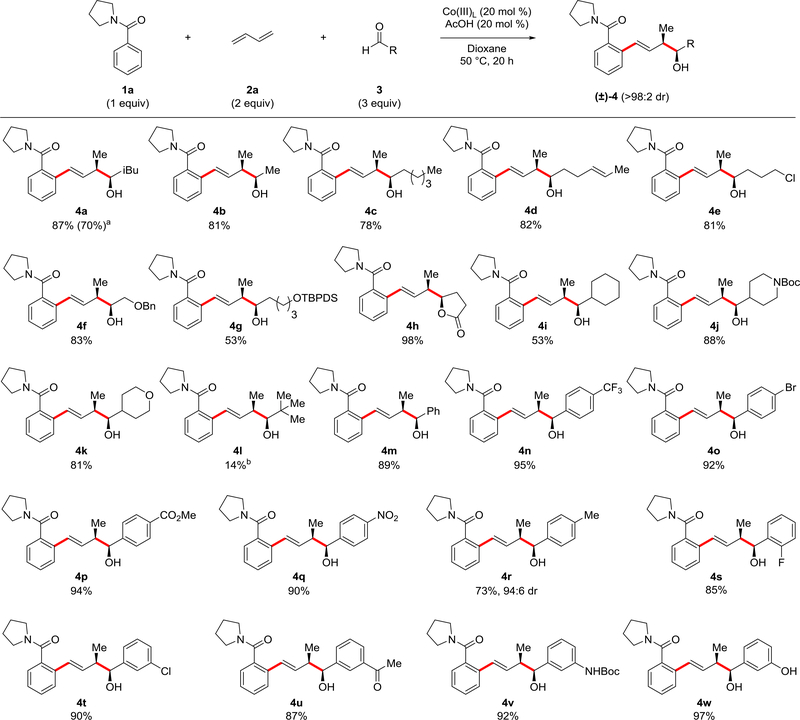
A thorough exploration of conditions was conducted to determine optimal reaction parameters for three-component coupling of benzamide 1a, butadiene (2a) and isovaleraldehyde (3a) to provide alcohol 4a in 87% yield (Figure 2; see Supplementary Table 2 in Supplementary Tables). The most effective catalyst was found to be a cationic earth-abundant cobalt catalyst previously developed in our lab, [Cp*Co(C6H6)][B(C6F5)4]2 (Co(III)L)18,19. Notably, this robust catalyst is completely air stable and is prepared by a straightforward and scalable salt metathesis without the use of any precious metals. Only acetic acid in sub-stoichiometric amounts was required as an additive with heating to 50 °C. In determining reaction scope, 20 mol % of the robust earth-abundant catalyst (Co(III)L) was used. However, in preliminary studies on catalyst loading, product alcohol 4a was obtained with a modest reduction in yield to 70% when employing 5 mol % of (Co(III)L) with pivalic acid rather than acetic acid as an additive.
Figure 2: C–H Functionalization with benzamide 1a, butadiene and diverse aldehydes 3.
Reactions were performed on 0.2 mmol scale with 1a at [0.4 M] and proceeded with >98:2 dr unless otherwise noted. Isolated yields of product after purification by chromatography are reported. See Supplementary Methods for experimental details. a. Reaction performed with 5 mol % of (Co(III)L) and 20 mol % of pivalic acid in place of acetic acid. b. Reaction performed with 1a at [1.0 M] and at 70 °C.
Scope and limitations of the reaction.
Acetaldehyde also coupled in good yield to give alcohol 4b, whose structure was rigorously confirmed by X-ray crystallography (see Supplementary Table 1 and Supplementary Figure 3). Multiple linear aldehydes were evaluated to provide products 4c to 4h in good to excellent yields. These aldehydes illustrate that a variety of functionality is compatible with three-component coupling. A disubstituted alkene was incorporated without isomerization (4d), and a primary alkyl chloride was stable under the reaction conditions (4e). In addition, alcohol functionality could also be introduced when protected as either a benzyl (4f) or a silyl (4g) ether, although the tert-butyldiphenylsilyl (TBDPS) was necessary to minimize silyl deprotection under the reaction conditions. Finally, lactone 4h was obtained in near quantitative yield by coupling 4-oxo-butyric acid methyl ester with in situ cyclization of the initial alcohol product.
Several α-branched aldehydes were also evaluated providing alcohol products 4i, 4j and 4k in good to excellent yields. In particular, alcohol 4j demonstrates the incorporation of the privileged piperidine heterocycle motif20 with the nitrogen protected with the popular Boc protecting group21. However, the highly sterically hindered α,α-dibranched aldehyde, pivaldehyde, provided little product under the standard reaction conditions, and even at a 1 M concentration in benzamide 1a and a 70 °C reaction temperature, provided 4l in only a 14% isolated yield though as a single stereoisomer.
A broad range of aromatic aldehydes was also explored. Benzaldehyde (4m) along with a number of derivatives with electron deficient groups at the para-position (4n-4q) coupled in high yield. Moderately electron-rich p-tolualdehyde provided 4r though with a modest reduction in yield and diastereoselectivity. Aromatic aldehydes with substitution at the ortho site (4s) and at the meta site with electron-deficient (4t and 4u) and -rich (4v and 4w) substituents all coupled in high yields. The reactions performed with aromatic aldehydes further established the broad functional group compatibility of the transformation, with successful incorporation of electrophilic ester (4p) and ketone (4u) functionality, bromo (4o) and chloro (4t) substituents amenable to cross-coupling transformations, and acidic functionality exemplified by introduction of a phenol (4v) and an N-Boc aniline (4w).
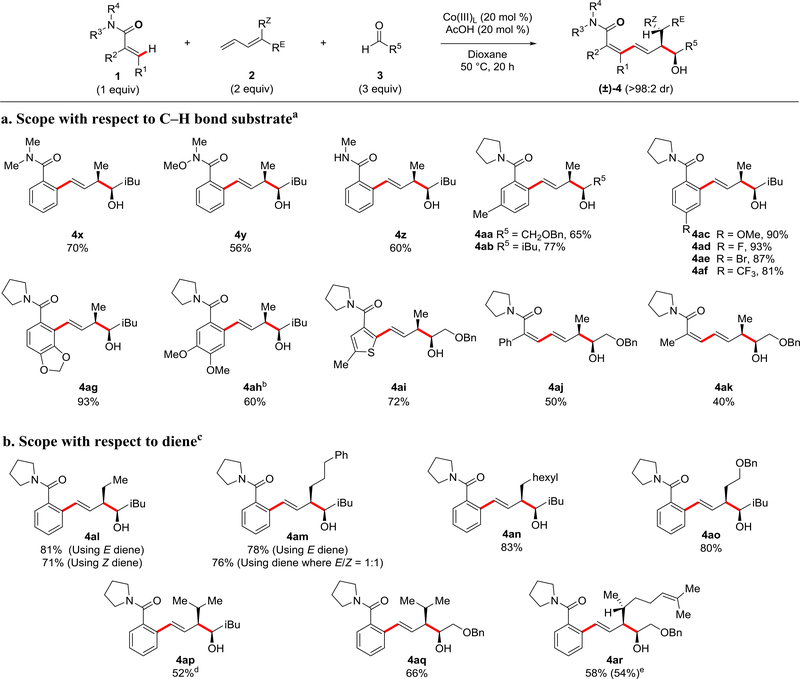
Different C–H bond substrates were also investigated (Figure 3a). In addition to the pyrrolidine amide directing group, a number of other amide derivatives were also shown to be effective. Both the N,N-dimethyl and the Weinreb22 tertiary benzamides provided products 4x and 4y in good yields, and the secondary amide N-methyl benzamide also coupled efficiently to give 4z. A number of different functionalities were examined on the aryl portion of the pyrrolidine benzamide. A range of electron-donating and electron-withdrawing groups were well-tolerated, resulting in products 4aa-af in good to excellent yield. With methylenedioxy substitution, alcohol 4ag was obtained in 93% yield as a single regioisomer, presumably through coordination of the oxygen to the cobalt in the C–H activation step. Conversely, attachment of two methoxy groups on meta- and para-positions resulted in product 4ah as the major regioisomer, isolated as a single compound in 60% yield. Here steric interactions between the two methoxy groups block the contiguous ortho-position. C–H functionalization was also successful for the heterocycle thiophene, giving alcohol 4ai in 72% yield. Alkene C(sp2)–H bonds also participated in the three-component reaction, generating products 4aj and 4ak in moderate yield and with high regio- and stereoselectivity for the introduction of six new stereocenters (four sp2 and two sp3) in a single transformation.
Figure 3: C–H Functionalization with dienes and aldehydes.
Reactions were performed on 0.2 mmol scale and proceeded with >98:2 dr unless otherwise noted, and isolated yields of product after purification by chromatography are reported. See Supplementary Methods for experimental details. a. 1a = [0.4 M]. b. 20% of the minor regioisomer was observed by NMR analysis. c. 1a = [1.0 M]. d. Isolated yield of major diastereomer, crude dr 92:8. e. Benzamide 1a (2.0 equiv), diene 2ar (1.0 equiv), and benzyloxyacetaldehyde (3.0 equiv).
Lastly, a number of mono and di-substituted butadienes were explored to expand the range of products that are accessible while also providing stereochemical information useful for deciphering the reaction mechanism (Figure 3b). Interestingly, both the (E)- and (Z)-isomers of 1,3-pentadiene furnished alcohol 4al with the (E)-isomer providing a slightly higher yield. Consistent with this stereochemical outcome, the pure (E)-isomer and a 1:1 mixture of (E)- and (Z)-isomers of hexa-3,5-dien-1-ylbenzene gave the same alcohol product 4am and in nearly identical yields. Additionally, dienes containing linear alkyl chains or benzyloxy substituents furnished products 4an and 4ao in 83% and 80% yield respectively. The monosubstituted butadiene with a phenyl group directly attached to the diene, 1-phenyl-1,3-butadiene, was also evaluated but did not provide the three-component coupling product (data not shown).
The symmetrical 1,1-disubstituted butadiene, 4-methyl-1,3-pentadiene, provided three-component products 4ap and 4aq in 52% and 66% yield, respectively. Moreover, a stereochemically pure unsymmetrical 1,1-disubstituted butadiene coupled to give 4ar with three asymmetric carbons and with very high stereoselectivity. The relative stereochemistry for 4ar was rigorously confirmed by X-ray structural analysis of an acylated derivative 4ar’ (see Supplementary Table 1 and Supplementary Figure 4). Because the unsymmetrical 1,1-disubstituted butadiene used in the three-component reaction to give 4ar required preparation, this diene was also evaluated as the limiting reagent for the three component reaction and resulted in a comparable 54% yield. The added stereochemical complexity introduced by unsymmetrical 1,1-disubstituted butadienes not only has potential synthetic utility but also provides useful mechanistic insight (vide infra).
Mechanistic studies.
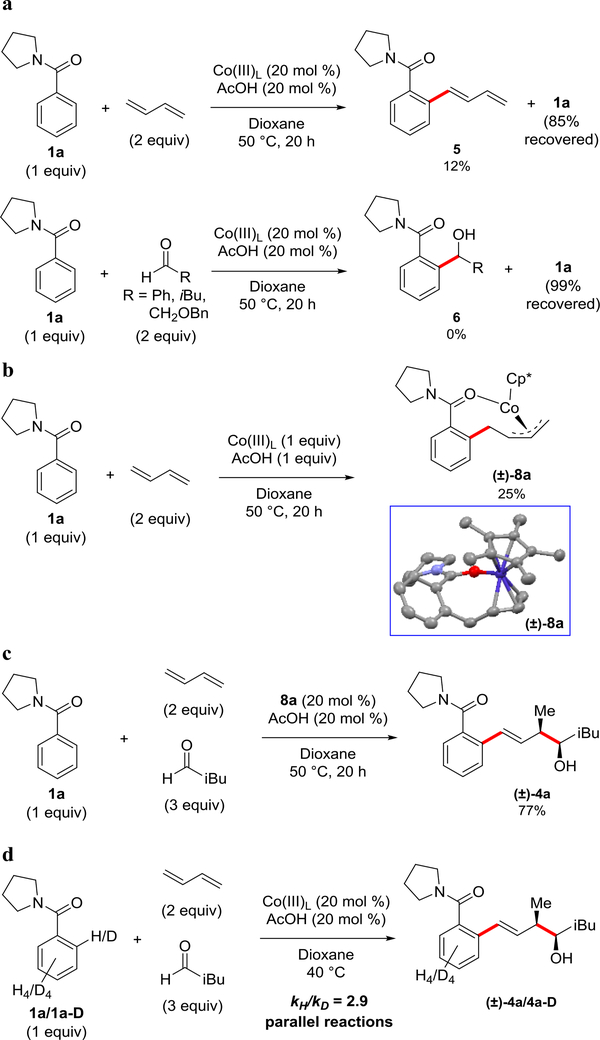
Experiments were conducted to help elucidate a mechanism for this three-component transformation (Figure 4). First, butadiene and multiple aldehyde coupling partners were separately submitted to two-component coupling under standard reaction conditions (Figure 4a). Butadiene resulted in only a 12% yield of the two-component coupling product, diene 5, with 85% recovery of 1a. Diene 5 is presumably released from the cobalt after β-hydride elimination to give an off-cycle cobalt hydride species (vide infra). More forcing conditions (100 °C) or addition of the common stoichiometric terminal oxidant, Cu(OAc)2, resulted in less than 5% of diene 5 or any other type of two-component product. Attempted coupling of 1a with alkyl and aryl aldehydes resulted in quantitative recovery of 1a without formation of any two-component product 6. This reaction outcome is expected because arene C(sp2)–H additions to standard aromatic and alkyl aldehydes are well-documented to be thermodynamically disfavored23,24.
Figure 4: Mechanistic Experiments.
a. Evaluation of two-component coupling reactions with either diene or aldehyde coupling partner. Benzamide 1a = [0.4 M]. b. Synthesis of Co-allyl species 8a. c. Co-allyl species 8a as the catalyst in the three-component reaction. 1a = [0.4 M]. d. Kinetic isotope effect determined from parallel reactions of 1a and 1a-D. Benzamides 1a and 1a-D = [0.4 M].
To trap possible intermediates along the catalytic cycle, substrate 1a was reacted with butadiene without any aldehyde but with stoichiometric amounts of the cobalt catalyst, resulting in isolation and rigorous X-ray structural characterization of the coordinatively saturated Co-allyl species 8a with η3-allyl binding (Figure 4b, Supplementary Table 1 and Supplementary Figure 5). Notably, the isolation and structural characterization of Cp*Co(III)-intermediates via direct C–H functionalization pathways has proven to be very challenging25. To ascertain whether the isolated Co-allyl species 8a is an intermediate along the reaction pathway, a reaction was conducted using 8a as the catalyst for the coupling of 1a with butadiene and isovaleraldehyde (Figure 4c). Using the standard reaction conditions, a 77% NMR yield of the desired alcohol 4a was obtained. This result establishes that 8a is a competent catalyst consistent with this allyl species serving an intermediate along the reaction pathway.
Experiments with deuterated substrate 1a-D also defined key features of the reaction (Figure 4d). When substrate 1a-D was subjected to the standard reaction conditions for only 2 h, no deuterium exchange was observed in either the product or the starting material (See Supplementary Methods). A primary kinetic isotope effect (KIE) of 2.90 ± 0.20 was observed (Figure 4d, Supplementary Figures 1 and 2), which is consistent with orthometallation as a rate-determining step26.
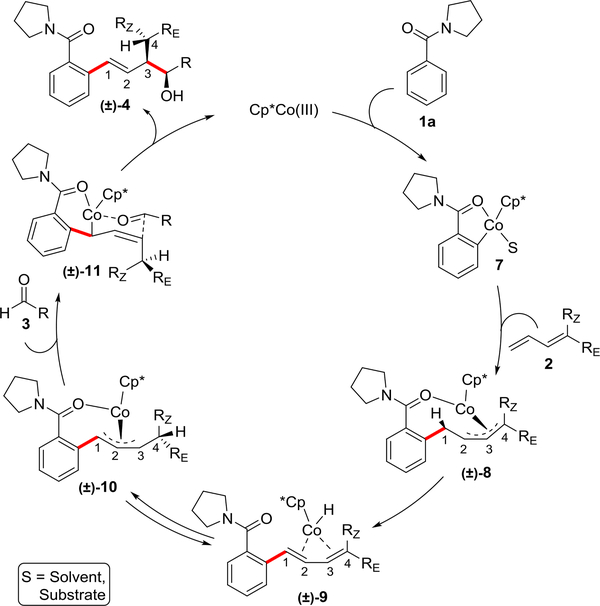
A mechanism consistent with all of the experimental data is shown in Figure 5. First, rate-determining C–H metallation of substrate 1a generates 5-membered metallocycle 7, which inserts into the terminal carbon of diene partner 2 to form Co-allyl species 8. This step is supported by the isolation and X-ray structural characterization of 8a (see Figure 4b). Formal migration of a hydrogen from carbon 1 to 4 in allyl intermediate 8 must occur to achieve the bond connectivity observed in the final product. Syn β-hydride elimination at carbon 1 in 8 would give the Co-diene complex 9, which is supported by the isolation of diene byproduct 5 when no aldehyde is present (Figure 4a). Reversible stereospecific syn-insertion of the Co-hydride from the same face but at carbon 4 would provide Co-allyl species 1027. Reaction of aldehyde 3 by the chair transition state depicted in 11 would provide the stereochemistry observed in product 4, which is obtained upon protonolysis with concomitant release of the cobalt catalyst.
Figure 5: Proposed mechanism for the three-component transformation.
The transformation proceeds through formation of Co-allyl species 8, which is supported by isolation of species 8a. Syn β-hydride elimination and hydride reinsertion leads to a new Co-allyl species 10, which can coordinate with the aldehyde to produce the chair transition state 11. Aldehyde addition and protonolysis yields the product 4. The depicted mechanism is consistent with the stereochemical outcomes observed for the substituted butadienes.
The stereochemistry observed for substituted dienes provides critical support for the proposed hydride migration from 8 to 10. (Z)- and (E)-monosubstituted dienes would be expected to provide the same intermediate 10 because a stereocenter is not introduced during hydride migration. Consequently, both (Z)- and (E)-monosubstituted dienes would be expected to provide the same product as was observed for both 4al and for 4am. Moreover, the high stereoselectivity and relative stereochemistry observed for 4ar is consistent with stereospecific hydride migration28 upon coupling with the unsymmetrical 1,1-disubstituted butadiene.
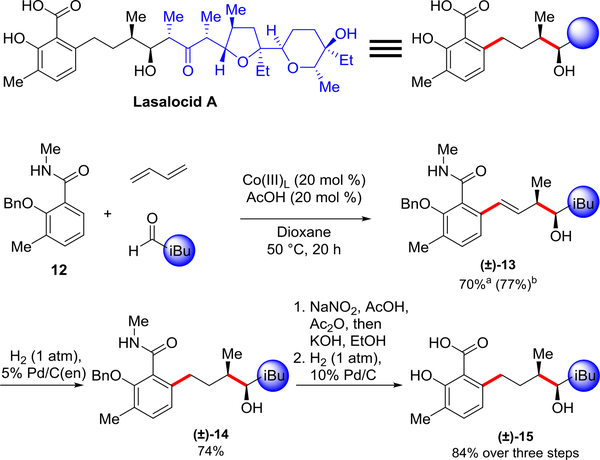
Synthesis of fragment of the ionophore antibiotic lasalocid A.
The carbon framework and stereochemistry obtained with this three-component reaction provides an opportunity to access structural motifs present in natural products as exemplified by the western fragment of the ionophore antibiotic lasalocid A29,30, which is used to treat coccidiosis in cattle (Figure 6). C–H bond functionalization of benzamide 12, butadiene, and isovaleraldehyde as a model aldehyde, provided alcohol 13 in good yield regardless of whether benzamide 12 (70% yield) or isovaleraldehyde (77%) was employed as the limiting reagent. Moreover, this reaction was also performed on the benchtop under an N2 atmosphere with benzamide 12 as the limiting reagent and gave a comparable 68% yield. In addition, when the reaction was performed on the benchtop with only 5 mol % of (Co(III)L) and with pivalic acid as the additive, the product was obtained with only a slight reduction in the yield to 60%. Alkene hydrogenation31 to provide 14 was followed by amide N-nitrosylation32, saponification and benzyl group cleavage to furnish alcohol 15 in excellent overall yield.
Figure 6: Synthesis of the core scaffold in lasalocid A.
Isolated yields are shown. aBenzamide 12 as the limiting reagent: benzamide 12 (1 equiv), butadiene (2 equiv) and isovaleraldehyde (3 equiv). bAldehyde as the limiting reagent: benzamide 12 (2 equiv), butadiene (2 equiv) and isovaleraldehyde (1 equiv).
Conclusion
These studies establish a general Co(III)-catalyzed three-component transformation for the synergistic, sequential C–H bond additions across dienes and aldehydes to form two new carbon-carbon σ-bonds and up to six stereocenters with high regio- and diastereoselectivity. A mechanism is proposed that involves stereospecific hydrogen migration and is supported by product connectivity and stereochemistry, X-ray structural characterization of a Co(III)-allyl intermediate, and isotope labeling studies. The utility of the three-component reaction is demonstrated by the rapid assembly of the western fragment of lasalocid A. Asymmetric variants of this three-component reaction might be possible using either enantiomerically pure coupling partners or chiral catalysts. Given the success of the disclosed three-component reaction, the discovery of additional synergistic three-component processes is anticipated.
Methods
General procedure for aldehyde and C–H bond substrate scope.
In a N2-filled glove box, a 0.5–2.0 mL microwave vial was charged with [Cp*Co(C6H6)[B(C6F5)4]2 (65.2 mg, 0.0400 mmol, 0.20 equiv), the indicated C–H bond partner (1) (0.200 mmol, 1.0 equiv) and corresponding aldehyde (3) (0.600 mmol, 3.0 equiv). Following this, 67 μL of a 0.6 M stock solution of acetic acid in 1,4-dioxane, followed by 333 μL of 1,4-dioxane. Finally, 100 μL of a 4 M stock solution of 1,3-butadiene in THF was added (0.400 mmol, 2.0 equiv) (use of commercial solution of butadiene in THF works equally well, for full details see Supplementary Table 2 and Supplementary Methods for the synthesis of the lasalocid A fragment 15). The reaction vial was then equipped with a magnetic stir bar, sealed with a microwave cap, and taken outside the glove box. The reaction mixture was stirred at 50 °C in a preset oil bath for 20 h. The reaction mixture was allowed to cool to room temperature and then was filtered through a small celite plug (1 cm long in a pipette) that was washed with ethyl acetate. The resulting mixture was then concentrated and purified by the corresponding chromatographic method to afford the desired product. Full experimental details and characterization of new compounds are provided in Supplementary Methods.
General procedure for diene substrate scope.
In a N2-filled glove box, a 0.5–2.0 mL microwave vial was charged with [Cp*Co(C6H6)[B(C6F5)4]2 (65.2 mg, 0.0400 mmol, 0.20 equiv), the indicated C–H bond partner (1) (0.200 mmol, 1.0 equiv) and corresponding aldehyde (3) (0.600 mmol, 3.0 equiv). Following this, 67 μL of a 0.6 M stock solution of acetic acid in 1,4-dioxane was added to the solid mixture, followed by 133 μL of 1,4-dioxane. Finally, diene (2) was added (0.400 mmol, 2.0 equiv). The reaction vial was then equipped with a magnetic stir bar, sealed with a microwave cap, and taken outside the glove box. The reaction mixture was stirred at 50 °C in a preset oil bath for 20 h. The reaction vial was allowed to cool to room temperature. The reaction mixture was filtered through a small celite plug (1 cm long in a pipette) that was washed with ethyl acetate. The resulting mixture was then concentrated and purified by the corresponding chromatographic method to afford the desired product. Full experimental details and characterization of new compounds are provided in Supplementary Methods.
Data availability.
The data that support the findings of this study are available within the paper and its Supplementary Information. X-ray crystal data for structures 4b, 4ar’, and 8a are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference numbers CCDC 1812525, CCDC 1826301, and CCDC 1812526, respectively.
Supplementary Material
Acknowledgements
This work was supported by the NIH (R35GM122473).
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary Information
Supporting Information
Supplementary Methods, Supplementary Figures 1–5, Supplementary Tables 1–2, Supplementary References
Compound 4b
Crystallographic data for compound 4b
Compound 4ar’
Crystallographic data for compound 4ar’
Compound 8a
Crystallographic data for compound 8a
References:
- 1.Dömling A & Ugi I Multicomponent reactions with isocyanides. Angew. Chem., Int. Ed. 39, 3168–3210 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Ramón DJ & Yus M Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew. Chem. Int. Ed 44, 1602–1634 (2005). [DOI] [PubMed] [Google Scholar]
- 3.D’Souza DM & Müller TJJ Multi-component syntheses of heterocycles by transition-metal catalysis. Chem. Soc. Rev 36, 1095–1108 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Ye J & Lautens M Palladium-catalysed norbornene-mediated C–H functionalization of arenes. Nat. Chem. 7, 863–870 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Davies HML, Manning JR Catalytic C–H bond functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamaguchi J, Yamaguchi AD & Itami K C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Wencel-Delord J & Glorius F C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 5, 369–375 (2013) [DOI] [PubMed] [Google Scholar]
- 8.Yoshino T, & Matsunaga S (Pentamethylcyclopentadienyl)cobalt(III)-catalyzed C–H bond functionalization: From discovery to unique reactivity and selectivity. Adv. Synth. Catal. 359, 1245–1262 (2017). [Google Scholar]
- 9.Crabtree RH & Lei A, Editors. Thematic issue: CH activation. Chem. Rev. 117, 8481–9520 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Blakemore DC, Castro L, Churcher I, Rees DC, Thomas AW, Wilson DW & Wood A Organic synthesis provides opportunities to transform drug discovery. Nat. Chem 10, Advanced online publication (2018) [doi: 10.1038/s41557-018-0021-z]. [DOI] [PubMed] [Google Scholar]
- 11.Boerth JA & Ellman JA A convergent synthesis of functionalized alkenyl halides through cobalt(III)-catalyzed three-component C–H bond addition. Angew. Chem. Int. Ed. 56, 9976–9980 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boerth JA, Hummel JR & Ellman JA Highly stereoselective cobalt(III)-catalyzed three-component C−H bond addition cascade. Angew. Chem., Int. Ed. 55, 12650–12654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen AE & MacMillan DWC Synergistic catalysis: a powerful synthetic strategy for new reaction development. Chem. Sci 3, 633–658 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery J Nickel-catalyzed reductive cyclizations and couplings. Angew. Chem. Int. Ed 43, 3890–3908 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Perry IB, Lu G, Liu P & Buchwald SL Copper-catalyzed asymmetric addition of olefin-derived nucleophiles to ketones. Science 353, 144–150 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zbieg JR, Yamaguchi E, McInturff EL & Krische MJ Enantioselective C-H crotylation of primary alcohols via hydrohydroxyalkylation of butadiene. Science 336, 324–327 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho HY & Morken, James P Diastereoselective construction of functionalized homoallylic alcohols by Ni-catalyzed diboron-promoted coupling of dienes and aldehydes. J. Am. Chem. Soc. 130, 16140–16141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hummel JR & Ellman JA Cobalt(III)-catalyzed synthesis of indazoles and furans by C–H bond functionalization/addition/cyclization cascades. J. Am. Chem. Soc. 137, 490–498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshino T, Ikemoto H, Matsunaga S, & Kanai M A cationic high-valent Cp*CoIII complex for the catalytic generation of nucleophilic organometallic species: directed C–H bond activation. Angew. Chem. Int. Ed. 52, 2207–2211 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Vitaku E, Smith DT & Njardson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Schneider N, Lowe DM, Sayle RA, Tarselli MA & Landrum GA Big Data from Pharmaceutical Patents: A Computational Analysis of Medicinal Chemists’ Bread and Butter. J. Med. Chem 59, 4385–4402 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Nahm S & Weinreb SM N-methoxy-N-methylamides as effective acylating agents. Tetrahedr. Lett 22, 3815–3818 (1981). [Google Scholar]
- 23.Li H, Li Y, Zhang XS, Chen K, Wang X & Shi Z-J J. Am. Chem. Soc Pyridinyl directed alkenylation with olefins via Rh(III)-catalyzed C–C bond cleavage of secondary arylmethanols. J. Am. Chem. Soc 133, 15244–15247 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Ozkal E, Cacherat B & Morandi B Cobalt(III)-catalyzed functionalization of unstrained carbon–carbon bonds through β-carbon cleavage of alcohols. ACS Catal. 5, 6458–6462 (2015). [Google Scholar]
- 25.Sen M, Rajesh N, Emayavaramban B, Premkumar JR & Sundararaju B Isolation of Cp*CoIII–alkenyl intermediate in efficient cobalt-catalyzed C−H alkenylation with alkynes. Chem. Eur. J. 24, 342–346 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Simmons EM & Hartwig JF On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 51, 3066–3072 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Korkis SE, Burns DJ & Lam HW Rhodium-catalyzed oxidative C−H allylation of benzamides with 1,3-dienes by allyl-to-allyl 1,4-Rh(III) migration. J. Am. Chem. Soc. 138, 12252–12257 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Hirano M, Shibasaki T, Komiya S & Bennett MA Synthesis of and stereospecific hydride migration in cationic (tricyclic arene)(cyclooctadiene)ruthenium(II) complexes. Organometallics 21, 5738–5745 (2002). [Google Scholar]
- 29.Nakata T, Schmid G, Vranesic B, Okigawa M, Smith-Palmer T, Kishi Y A total synthesis of lasalocid A. J. Am. Chem. Soc. 100, 2933–2935 (1978). [Google Scholar]
- 30.Ireland RE, Anderson RC, Badoud R, Fitzsimmons BJ, McGarvey GJ, Thaisrivongs S & Wilcox CS The total synthesis of ionophore antibiotics. A convergent synthesis of lasalocid A (X537A). J. Am. Chem. Soc. 105, 1988–2006 (1983). [Google Scholar]
- 31.Sajiki H, Hattori K & Hirota K The formation of a novel Pd/C-ethylenediamine complex catalyst: chemoselective hydrogenation without deprotection of the O-benzyl and N-Cbz groups. J. Org. Chem 63, 7990–7992 (1998). [Google Scholar]
- 32.White EH The chemistry of the N-alkyl-N-nitrosoamides. I. methods of preparation. J. Am. Chem. Soc 77, 6008–6010 (1955). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the paper and its Supplementary Information. X-ray crystal data for structures 4b, 4ar’, and 8a are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference numbers CCDC 1812525, CCDC 1826301, and CCDC 1812526, respectively.