Abstract
Thoracic aortic aneurysms, with an estimated prevalence in the general population of 1%, are potentially lethal, via rupture or dissection. Over the prior two decades, there has been an exponential increase in our understanding of the genetics of thoracic aortic aneurysm and/or dissection (TAAD). To date, 30 genes have been shown to be associated with the development of TAAD and ∼30% of individuals with nonsyndromic familial TAAD have a pathogenic mutation in one of these genes. This review represents the authors' yearly update summarizing the genes associated with TAAD, including implications for the surgical treatment of TAAD. Molecular genetics will continue to revolutionize the approach to patients afflicted with this devastating disease, permitting the application of genetically personalized aortic care.
Keywords: genetics, thoracic aortic aneurysm, thoracic aortic dissection
This review is the update to the 2017 paper “Genes Associated with Thoracic Aortic Aneurysm and Dissection” published in AORTA. 1 We have updated both Table 1 listing the genes known to predispose to thoracic aortic aneurysm or dissection (TAAD) and Fig. 1 , with the recommended sizes for surgical intervention for each specific mutation, based upon published findings in 2017.
Table 1. Genes associated with syndromic and nonsyndromic thoracic aortic aneurysm and/or dissection, associated vascular characteristics, and size criteria for elective surgical intervention (SMAD6 is the only gene that has been added to this table since publication of our 2017 AORTA review paper.).
| Gene | Protein | Animal model leading to vascular phenotype? | Syndromic TAAD | Nonsyndromic FTAAD | Associated disease/syndrome | Associated clinical characteristics of the vasculature | Ascending Aorta Size (cm) for Surgical Intervention | Mode of inheritance | OMIM |
|---|---|---|---|---|---|---|---|---|---|
| ACTA2 | Smooth muscle α-actin | Yes 10 | + | + | AAT6 + multisystemic smooth muscle dysfunction + MYMY5 | TAAD, early aortic dissection,* CAD, stroke (moyamoya disease), PDA, pulmonary artery dilation, BAV 11 12 | 4.5–5.0 a 13 14 15 | AD | 611788 613834 614042 |
| BGN | Biglycan | Yes 16 | + | − | Meester-Loeys syndrome | ARD, TAAD, pulmonary artery aneurysm, IA, arterial tortuosity 17 | Standard | X-linked | 300989 |
| COL1A2 | Collagen 1 α2 chain | No | + | − | EDS, arthrochalasia type (VIIb) + cardiac valvular type | Borderline aortic root enlargement 12 18 | Standard | AD + AR | 130060 225320 |
| COL3A1 | Collagen 3 α1 chain | Yes 19 | + | − | EDS, vascular type (IV) | TAAD, early aortic dissection,* visceral arterial dissection, vessel fragility, IA 20 21 22 | 5.0 b 22 | AD | 130050 |
| COL5A1 | Collagen 5 α1 chain | No e | + | − | EDS, classic type 1 | ARD, rupture/dissection of medium sized arteries 23 24 25 | Standard | AD | 130000 |
| COL5A2 | Collagen 5 α2 chain | Partially f | + | − | EDS, classic type 2 | ARD | Standard | AD | 130000 |
| EFEMP2 | Fibulin-4 | Yes 26 27 | + | − | Cutis laxa, AR type Ib | Ascending aortic aneurysms, other arterial aneurysms, arterial tortuosity and stenosis | Standard | AR | 614437 |
| ELN | Elastin | No | + | − | Cutis laxa, AD | ARD, ascending aortic aneurysm and dissection, BAV, IA possibly associated with SVAS 28 29 30 | Standard | AD | 123700 185500 |
| EMILIN1 | Elastin microfibril interfacer 1 | No | + | − | Unidentified CTD | Ascending and descending aortic aneurysm 31 | Standard | AD | Unassigned |
| FBN1 | Fibrillin-1 | Yes 32 33 34 35 36 | + | + | Marfan syndrome | ARD, TAAD, AAA, other arterial aneurysms, pulmonary artery dilatation, arterial tortuosity 37 | 5.0 15 38 | AD | 154700 |
| FBN2 | Fibrillin-2 | No | + | − | Contractual arachnodactyly | Rare ARD and aortic dissection, 39 BAV, PDA | Standard | AD | 121050 |
| FLNA | Filamin A | Yes 40 41 | + | − | Periventricular nodular heterotopia | Aortic dilatation/aneurysms, peripheral arterial dilatation, 42 PDA, IA, 43 BAV | Standard | XLD | 300049 |
| FOXE3 | Forkhead box 3 | Yes 44 | − | + | AAT11 | TAAD (primarily Type A dissection) 44 | Standard | AD | 617349 |
| LOX | Lysyl oxidase | Yes 45 46 47 48 | − | + | AAT10 | TAAD, AAA, hepatic artery aneurysm, BAV, CAD | Standard | AD | 617168 |
| MAT2A | Methionine adenosyltransferase II α | No g 49 | − | + | FTAA | Thoracic aortic aneurysms, BAV 49 | Standard | AD | Unassigned |
| MFAP5 | Microfibril-associated glycoprotein 2 | Partially h 50 | − | + | AAT9 | ARD, TAAD | Standard | AD | 616166 |
| MYH11 | Smooth muscle myosin heavy chain | Partially i 51 | − | + | AAT4 | TAAD, early aortic dissection,* PDA, CAD, peripheral vascular occlusive disease, carotid IA | 4.5–5.0 15 52 | AD | 132900 |
| MYLK | Myosin light chain kinase | No j 53 | − | + | AAT7 | TAAD, early aortic dissections* | 4.5–5.0 a 15 53 | AD | 613780 |
| NOTCH1 | NOTCH1 | Partially k | − | + | AOVD1 | BAV/TAAD 54 55 | Standard | AD | 109730 |
| PRKG1 | Type 1 cGMP-dependent protein kinase | No | − | + | AAT8 | TAAD, early aortic dissection,* AAA, coronary artery aneurysm/dissection, aortic tortuosity, small vessel CVD | 4.5–5.0 56 | AD | 615436 |
| SKI | Sloan Kettering proto-oncoprotein | No l | + | − | Shprintzen–Goldberg syndrome | ARD, arterial tortuosity, pulmonary artery dilation, other (splenic) arterial aneurysms 57 | Standard | AD | 182212 |
| SLC2A10 | Glucose transporter 10 | No m | + | − | Arterial tortuosity syndrome | ARD, 58 ascending aortic aneurysms, 58 other arterial aneurysms, arterial tortuosity, elongated arteries aortic/pulmonary artery stenosis | Standard | AR | 208050 |
| SMAD2 | SMAD2 | No | + | − | Unidentified CTD with arterial aneurysm/dissections | ARD, ascending aortic aneurysms, vertebral/carotid aneurysms and dissections, AAA 59 60 | Standard | AD | Unassigned |
| SMAD3 | SMAD3 | Partially n 61 | + | + | LDS type 3 | ARD, TAAD, early aortic dissection,* AAA, arterial tortuosity, other arterial aneurysms/dissections, IA, BAV 62 63 | 4.0–4.2 15 38 | AD | 613795 |
| SMAD4 | SMAD4 | Yes 64 | + | − | JP/HHT syndrome | ARD, TAAD, AVMs, IA 65 66 | Standard | AD | 175050 |
| SMAD6 | SMAD6 | No o | − | + | AOV2 | BAV/TAA 6 | Standard | AD | 602931 |
| TGFB2 | TGF-β2 | Yes 67 | + | + | LDS type 4 | ARD, TAAD, arterial tortuosity, other arterial aneurysms, BAV 67 68 | 4.5–5.0 c 69 | AD | 614816 |
| TGFB3 | TGF-β3 | No p | + | − | LDS type 5 | ARD, TAAD, AAA/dissection, other arterial aneurysms, IA/dissection 70 | Standard | AD | 615582 |
| TGFBR1 | TGF-β receptor type 1 | Yes 71 | + | + | LDS type 1 + AAT5 | TAAD, early aortic dissection,* AAA, arterial tortuosity, other arterial aneurysms/dissection, IA, PDA, BAV 72 | 4.0–4.5 d, 15 38 73 | AD | 609192 |
| TGFBR2 | TGF-β receptor type 2 | Yes 64 71 | + | + | LDS type 2 + AAT3 | TAAD, early aortic dissection,* AAA, arterial tortuosity, other arterial aneurysms/dissection, IA, PDA, BAV 72 | 4.0–4.5 d 15 38 73 | AD | 610168 |
Abbreviations: AAA, abdominal aortic aneurysm; AAT, aortic aneurysm, familial thoracic; AD, autosomal dominant; AOVD, aortic valve disease; AR, autosomal recessive; ARD, aortic root dilatation; AVM, arteriovenous malformation; BAV, bicuspid aortic valve; CAD, coronary artery disease; CTD, connective tissue disease; CVD, cerebrovascular disease; EDS, Ehlers–Danlos syndrome; FTAA, familial thoracic aortic aneurysm; FTAAD, familial thoracic aortic aneurysm and/or dissection; HHT, hereditary hemorrhagic telangiectasia; IA, intracranial aneurysm; JP, juvenile polyposis; LDS, Loeys-Dietz syndrome; MYMY, moyamoya disease; OMIM, Online Mendelian Inheritance in Man; PDA, patent ductus arteriosus; SVAS, supravalvular aortic stenosis; TGF, transforming growth factor; TAAD, thoracic aortic aneurysm and/or dissection; TGFBR, TGF-β receptor; XLD, X-linked dominant
It is important to note that since mutations in many of these genes are rare and have only recently been implicated in TAAD, there is a lack of adequate prospective clinical studies. Therefore, it is difficult to establish threshold diameters for intervention for TAAs, and each individual must be considered on a case by case basis, taking into account the rate of change in aneurysm size (> 0.5 cm per year is considered rapid), any family history of aortic dissection at diameters < 5.0 cm, and the presence of significant aortic regurgitation, which are all indications for early repair if present.
A “ + ” symbol in the syndromic TAAD column indicates that mutations in the gene have been found in patients with syndromic TAAD (same for the nonsyndromic TAAD column). A “-” symbol in the syndromic TAAD column indicates that mutations in the gene have not been found in patients with syndromic TAAD (same for the nonsyndromic TAAD column).
A reference is provided for each of the associated vascular characteristics not reported in the OMIM entry for that gene.
Standard = surgical intervention at 5.0 to 5.5 cm.
Early aortic dissection* = dissection at aortic diameters < 5.0 cm.
Individuals with MYLK and ACTA2 mutations have been shown to have aortic dissections at a diameter of 4.0 cm. 13 53
There are no data to set threshold diameters for the surgical intervention for EDS type IV. 38 The Canadian guidelines recommend surgery for aortic root sizes of 4.0 to 5.0 cm and ascending aorta sizes of 4.2 to 5.0 cm, though these patients are at high risk of surgical complications due to poor-quality vascular tissue. 74
There are limited data concerning the timing of surgical intervention for LDS type 4. However, there has been a case of a type A aortic dissection at an aortic diameter < 5.0 cm 69 hence, the recommended threshold range of 4.5 to 5.0 cm.
Current US guidelines recommend prophylactic surgery for LDS types 1 and 2 at ascending aortic diameters of 4.0 to 4.2 cm. 15 38 However, the European guidelines state that more clinical data are required. 22 Patients with TGFBR2 mutations have similar outcomes to patients with FBN1 mutations once their disease is diagnosed, 75 and the clinical course of LDS 1 and 2 does not appear to be as severe as originally reported. 73 76 77 Therefore, medically treated adult patients with LDS 1 or 2 may not require prophylactic surgery at ascending aortic diameters of 4.0 to 4.2 cm. 11 Individuals with TGFBR2 mutations are more likely to have aortic dissections at diameters < 5.0 cm than those with TGFBR1 mutations. 73 77 A more nuanced approach proposed by Jondeau et al utilizing the presence of TGFBR2 mutations (versus TGFBR1 mutations), the co-occurrence of severe systemic features (arterial tortuosity, hypertelorism, wide scarring), female gender, low body surface area, and a family history of dissection or rapid aortic root enlargement, which are all risk factors for aortic dissection, may be beneficial for LDS 1 and 2 patients to avoid unnecessary surgery at small aortic diameters. 73 Therefore, in LDS 1 or 2 individuals without the above features, Jondeau et al maintain that 4.5 cm may be an appropriate threshold, but females with TGFBR2 mutations and severe systemic features may benefit from surgery at 4.0 cm. 73
Wenstrup et al found that mice heterozygous for an inactivating mutation in Col5a1 exhibit decreased aortic compliance and tensile strength relative to wild-type mice. 78
Park et al recently demonstrated that Col5a2 haploinsufficiency increased the incidence and severity of AAA and led to aortic arch ruptures and dissections in an angiotensin II-induced aneurysm mouse model. 79 In an earlier paper, Park et al illustrated that mice heterozygous for a null allele in Col5a2 exhibited increased aortic compliance and reduced tensile strength compared with wild-type mice. 80
Guo et al found that knockdown of mat2aa in zebrafish led to defective aortic arch development. 49
Combs et al demonstrated that Mfap2 and Mfap5 double knockout (Mfap2 −/− ;Mfap5 −/− ) mice exhibit age-dependent aortic dilation, though this is not the case with Mfap5 single knockout mice.
While Kuang et al reported that a mouse knock-in model (Myh11 R247C/R247C ) does not lead to a severe vascular phenotype under normal conditions, 81 Bellini et al demonstrated that induced hypertension in this mouse model led to intramural delaminations (separation of aortic wall layers without dissection) or premature deaths (due to aortic dissection based on necroscopy according to unpublished data by Bellini et al) in over 20% of the R247C mice, accompanied by focal accumulation of glycosaminoglycans within the aortic wall (a typical histological feature of TAAD).
Wang et al demonstrated that SMC-specific knockdown of Mylk in mice led to histopathological changes (increased pools of proteoglycans) and altered gene expression consistent with medial degeneration of the aorta, though no aneurysm formation was observed.
Koenig et el recently found that Notch1 haploinsufficiency exacerbates the aneurysmal aortic root dilation in a mouse model of Marfan syndrome and that Notch1 heterozygous mice exhibited aortic root dilation, abnormal smooth muscle cell morphology, and reduced elastic laminae. 82
Doyle et al found that knockdown of paralogs of mammalian SKI in zebrafish led to craniofacial and cardiac anomalies, including failure of cardiac looping and malformations of the outflow tract. 57 Berk et al showed that mice lacking Ski exhibit craniofacial, skeletal muscle, and central nervous system abnormalities, which are all features of Shprintzen–Goldberg syndrome, but no evidence of aneurysm development was reported. 83
Mice with homozygous missense mutations in Slc2a10 have not been shown to have the vascular abnormalities seen with arterial tortuosity syndrome, 84 though Cheng et al did demonstrate that such mice do exhibit abnormal elastogenesis within the aortic wall. 85
Tan et al demonstrated that Smad3 knockout mice only developed aortic aneurysms with angiotensin II-induced vascular inflammation, though the knockout mice did have medial dissections evident on histological analysis of their aortas and exhibited aortic dilatation relative to wild-type mice prior to angiotensin II infusion. 61
Galvin et al demonstrated that Madh6, which encodes Smad6, mutant mice exhibited defects in cardiac valve formation, outflow tract septation, vascular tone, and ossification but no aneurysm development was observed. 86
Fig. 1.
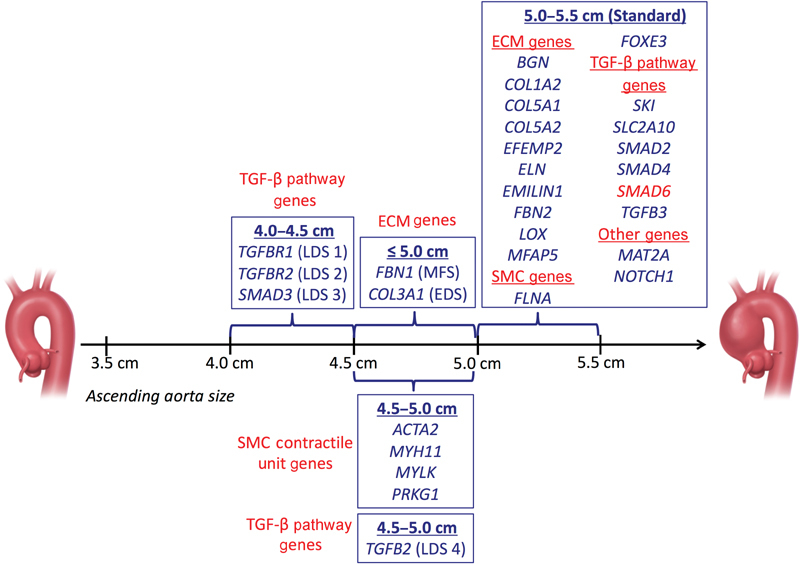
Ascending aorta dimensions for prophylactic surgical intervention. (Data derived from Table 1 and modified with permission from Brownstein et al. 1 ) Any gene newly reported during the past year to be associated with TAAD is highlighted in red. Abbreviations: ECM, extracellular matrix; SMC, smooth muscle cell; TAAD, thoracic aortic aneurysm and/or dissection; TGF, transforming growth factor.
Thoracic aortic aneurysms, with an estimated prevalence in the general population of 1%, 2 are potentially lethal, via rupture or dissection. Although significant progress has been made in decreasing the mortality of type A and type B aortic dissections, particularly among individuals who are diagnosed and undergo surgical repair, 3 almost 50% of patients with a type A aortic dissection still die before hospital admission. 4 Therefore, it is critical for clinicians to identify those individuals at risk of TAAD and to perform clinical and genetic risk stratification so that appropriate and personalized management can be provided.
To date, 30 genes have been found to be associated with TAAD ( Table 1 and Fig. 1 ) and ∼30% of individuals with familial nonsyndromic TAAD (clinical manifestations restricted to the aorta) have a pathogenic variant in one or more of these genes. 5 Mutations in these genes lead to a spectrum of risk and severity of type A and B aortic dissections, 5 as well as different extra-aortic manifestations. Specific mutations in ACTA2 are estimated to account for 12 to 21% of familial nonsyndromic TAAD, while mutations in syndromic genes ( FBN1, TGFBR1, TGFBR2, SMAD3, and TGFB2 ) are estimated to account for an additional 14% of cases of familial nonsyndromic TAAD. 5 Other genes listed in Table 1 are estimated to contribute to 1 to 2% each or less of familial nonsyndromic TAAD. 5 Given that the majority of familial nonsyndromic TAAD cannot be explained by a mutation in one of the known genes associated with TAAD, it is likely that additional genes remain to be identified.
Several important genetic findings have been reported during the past year. Using exome sequencing of 441 patients with bicuspid aortic valve and thoracic aortic aneurysm, Gillis et al identified pathogenic mutations in SMAD6 in 11 afflicted individuals, adding to the growing list of genes associated with TAAD. 6 Additionally, in an exome sequencing study of 27 patients with syndromic or familial TAAD (specifically focused on three pairs of first-degree relatives with the same pathogenic TAAD variant but differing phenotypic severity from three independent families), Landis et al found that variants within two genes, ADCK4 and COL15A1 , segregated with mild disease severity among thoracic aortic aneurysm patients, offering clues that may help explain the reduced penetrance and variable expression observed in those with TAAD. 7 Lastly, though not introducing a novel association, work by Franken et al on 290 Marfan syndrome (MFS) patients recently expanded our understanding of the genotype–phenotype relationships in TAAD—by demonstrating that among individuals with MFS, those with haploinsufficient mutations in FBN1 have larger aortic root diameters that exhibit a more rapid dilation rate than those with dominant negative mutations. 8 Similarly, De Cario et al found that the presence of certain common polymorphisms in TGFBR1 and TGFBR2 was associated with reduced cardiovascular disease severity among patients with MFS. 9
These studies completed in 2017 illustrate the dynamic nature of the field of TAAD genetics. Through continued investigation and expanded access to genetic testing for affected patients and their family members, whole genome sequencing will undoubtedly continue to add new genes to the roster of causes for familial TAAD. Molecular genetics will continue to revolutionize the approach to patients afflicted with this devastating disease, permitting the application of genetically personalized aortic care. A major challenge in the field remains the lack of functional studies to prove the pathogenicity of identified variants.
We will continue to provide a yearly update and a revised summary table and revised intervention criterion table in AORTA at the end of each calendar year.
Acknowledgements
None.
Funding Statement
Funding None.
Footnotes
Conflict of Interest The authors declare no conflict of interest related to this manuscript.
References
- 1.Brownstein A J, Ziganshin B A, Kuivaniemi H, Body S C, Bale A E, Elefteriades J A. Genes associated with thoracic aortic aneurysm and dissection: an update and clinical implications. Aorta (Stamford) 2017;5(01):11–20. doi: 10.12945/j.aorta.2017.17.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verstraeten A, Luyckx I, Loeys B. Aetiology and management of hereditary aortopathy. Nat Rev Cardiol. 2017;14(04):197–208. doi: 10.1038/nrcardio.2016.211. [DOI] [PubMed] [Google Scholar]
- 3.Mody P S, Wang Y, Geirsson A et al. Trends in aortic dissection hospitalizations, interventions, and outcomes among Medicare beneficiaries in the United States, 2000-2011. Circ Cardiovasc Qual Outcomes. 2014;7(06):920–928. doi: 10.1161/CIRCOUTCOMES.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard D P, Banerjee A, Fairhead J F, Perkins J, Silver L E, Rothwell P M; Oxford Vascular Study.Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study Circulation 2013127202031–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milewicz D M, Regalado E, Pagon R A, Adam M P, Ardinger H H, Wallace S E, Amemiya A, Bean L JH.Heritable Thoracic Aortic Disease OverviewIn:et al. Seattle, WA: GeneReviews(R)1993 [PubMed] [Google Scholar]
- 6.Gillis E, Kumar A A, Luyckx I et al. Candidate gene resequencing in a large bicuspid aortic valve-associated thoracic aortic aneurysm cohort: SMAD6 as an important contributor. Front Physiol. 2017;8:400. doi: 10.3389/fphys.2017.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landis B J, Schubert J A, Lai D et al. Exome sequencing identifies candidate genetic modifiers of syndromic and familial thoracic aortic aneurysm severity. J Cardiovasc Transl Res. 2017;10(04):423–432. doi: 10.1007/s12265-017-9753-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franken R, Teixido-Tura G, Brion M et al. Relationship between fibrillin-1 genotype and severity of cardiovascular involvement in Marfan syndrome. Heart. 2017;103(22):1795–1799. doi: 10.1136/heartjnl-2016-310631. [DOI] [PubMed] [Google Scholar]
- 9.De Cario R, Sticchi E, Lucarini Let al. Role of TGFBR1 and TGFBR2 genetic variants in Marfan syndrome J Vasc Surg 2017S0741-5214(17)31587-2. Article in Press [DOI] [PubMed] [Google Scholar]
- 10.Milewicz D M, Prakash S K, Ramirez F. Therapeutics targeting drivers of thoracic aortic aneurysms and acute aortic dissections: insights from predisposing genes and mouse models. Annu Rev Med. 2017;68:51–67. doi: 10.1146/annurev-med-100415-022956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milewicz D, Hostetler E, Wallace S et al. Precision medical and surgical management for thoracic aortic aneurysms and acute aortic dissections based on the causative mutant gene. J Cardiovasc Surg (Torino) 2016;57(02):172–177. [PubMed] [Google Scholar]
- 12.Bradley T J, Bowdin S C, Morel C F, Pyeritz R E. The expanding clinical spectrum of extracardiovascular and cardiovascular manifestations of heritable thoracic aortic aneurysm and dissection. Can J Cardiol. 2016;32(01):86–99. doi: 10.1016/j.cjca.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Disabella E, Grasso M, Gambarin F I et al. Risk of dissection in thoracic aneurysms associated with mutations of smooth muscle alpha-actin 2 (ACTA2) Heart. 2011;97(04):321–326. doi: 10.1136/hrt.2010.204388. [DOI] [PubMed] [Google Scholar]
- 14.Guo D C, Pannu H, Tran-Fadulu V et al. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet. 2007;39(12):1488–1493. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- 15.Andelfinger G, Loeys B, Dietz H. A decade of discovery in the genetic understanding of thoracic aortic disease. Can J Cardiol. 2016;32(01):13–25. doi: 10.1016/j.cjca.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Heegaard A M, Corsi A, Danielsen C C et al. Biglycan deficiency causes spontaneous aortic dissection and rupture in mice. Circulation. 2007;115(21):2731–2738. doi: 10.1161/CIRCULATIONAHA.106.653980. [DOI] [PubMed] [Google Scholar]
- 17.Meester J A, Vandeweyer G, Pintelon I et al. Loss-of-function mutations in the X-linked biglycan gene cause a severe syndromic form of thoracic aortic aneurysms and dissections. Genet Med. 2017;19(04):386–395. doi: 10.1038/gim.2016.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarze U, Hata R, McKusick V A et al. Rare autosomal recessive cardiac valvular form of Ehlers-Danlos syndrome results from mutations in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway. Am J Hum Genet. 2004;74(05):917–930. doi: 10.1086/420794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith L B, Hadoke P W, Dyer E et al. Haploinsufficiency of the murine Col3a1 locus causes aortic dissection: a novel model of the vascular type of Ehlers-Danlos syndrome. Cardiovasc Res. 2011;90(01):182–190. doi: 10.1093/cvr/cvq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Paepe A, Malfait F. The Ehlers-Danlos syndrome, a disorder with many faces. Clin Genet. 2012;82(01):1–11. doi: 10.1111/j.1399-0004.2012.01858.x. [DOI] [PubMed] [Google Scholar]
- 21.Germain D P. Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis. 2007;2:32. doi: 10.1186/1750-1172-2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erbel R, Aboyans V, Boileau C et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35(41):2873–2926. doi: 10.1093/eurheartj/ehu281. [DOI] [PubMed] [Google Scholar]
- 23.Monroe G R, Harakalova M, van der Crabben S N et al. Familial Ehlers-Danlos syndrome with lethal arterial events caused by a mutation in COL5A1. Am J Med Genet A. 2015;167(06):1196–1203. doi: 10.1002/ajmg.a.36997. [DOI] [PubMed] [Google Scholar]
- 24.Mehta S, Dhar S U, Birnbaum Y. Common iliac artery aneurysm and spontaneous dissection with contralateral iatrogenic common iliac artery dissection in classic Ehlers-Danlos syndrome. Int J Angiol. 2012;21(03):167–170. doi: 10.1055/s-0032-1325118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wenstrup R J, Meyer R A, Lyle J S et al. Prevalence of aortic root dilation in the Ehlers-Danlos syndrome. Genet Med. 2002;4(03):112–117. doi: 10.1097/00125817-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Davis E C, Chapman S L et al. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ Res. 2010;106(03):583–592. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igoucheva O, Alexeev V, Halabi C M et al. Fibulin-4 E57K knock-in mice recapitulate cutaneous, vascular and skeletal defects of recessive Cutis Laxa 1B with both elastic fiber and collagen fibril abnormalities. J Biol Chem. 2015;290(35):21443–21459. doi: 10.1074/jbc.M115.640425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jelsig A M, Urban Z, Hucthagowder V, Nissen H, Ousager L B. Novel ELN mutation in a family with supravalvular aortic stenosis and intracranial aneurysm. Eur J Med Genet. 2017;60(02):110–113. doi: 10.1016/j.ejmg.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Callewaert B, Renard M, Hucthagowder V et al. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Hum Mutat. 2011;32(04):445–455. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szabo Z, Crepeau M W, Mitchell A L et al. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. J Med Genet. 2006;43(03):255–258. doi: 10.1136/jmg.2005.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Capuano A, Bucciotti F, Farwell K D et al. Diagnostic exome sequencing identifies a novel gene, EMILIN1, associated with autosomal-dominant hereditary connective tissue disease. Hum Mutat. 2016;37(01):84–97. doi: 10.1002/humu.22920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira L, Andrikopoulos K, Tian J et al. Targeting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet. 1997;17(02):218–222. doi: 10.1038/ng1097-218. [DOI] [PubMed] [Google Scholar]
- 33.Pereira L, Lee S Y, Gayraud B et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci U S A. 1999;96(07):3819–3823. doi: 10.1073/pnas.96.7.3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Judge D P, Biery N J, Keene D R et al. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest. 2004;114(02):172–181. doi: 10.1172/JCI20641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habashi J P, Judge D P, Holm T Met al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome Science 2006312(5770):117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lima B L, Santos E J, Fernandes G R et al. A new mouse model for Marfan syndrome presents phenotypic variability associated with the genetic background and overall levels of Fbn1 expression. PLoS One. 2010;5(11):e14136. doi: 10.1371/journal.pone.0014136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris S A, Orbach D B, Geva T, Singh M N, Gauvreau K, Lacro R V. Increased vertebral artery tortuosity index is associated with adverse outcomes in children and young adults with connective tissue disorders. Circulation. 2011;124(04):388–396. doi: 10.1161/CIRCULATIONAHA.110.990549. [DOI] [PubMed] [Google Scholar]
- 38.Hiratzka L F, Bakris G L, Beckman J A et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55(14):e27–e129. doi: 10.1016/j.jacc.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Takeda N, Morita H, Fujita D et al. Congenital contractual arachnodactyly complicated with aortic dilatation and dissection: case report and review of literature. Am J Med Genet A. 2015;167A(10):2382–2387. doi: 10.1002/ajmg.a.37162. [DOI] [PubMed] [Google Scholar]
- 40.Retailleau K, Arhatte M, Demolombe S et al. Smooth muscle filamin A is a major determinant of conduit artery structure and function at the adult stage. Pflugers Arch. 2016;468(07):1151–1160. doi: 10.1007/s00424-016-1813-x. [DOI] [PubMed] [Google Scholar]
- 41.Feng Y, Chen M H, Moskowitz I P et al. Filamin A (FLNA) is required for cell-cell contact in vascular development and cardiac morphogenesis. Proc Natl Acad Sci U S A. 2006;103(52):19836–19841. doi: 10.1073/pnas.0609628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reinstein E, Frentz S, Morgan T et al. Vascular and connective tissue anomalies associated with X-linked periventricular heterotopia due to mutations in filamin A. Eur J Hum Genet. 2013;21(05):494–502. doi: 10.1038/ejhg.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lange M, Kasper B, Bohring A et al. 47 patients with FLNA associated periventricular nodular heterotopia. Orphanet J Rare Dis. 2015;10:134. doi: 10.1186/s13023-015-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuang S Q, Medina-Martinez O, Guo D C et al. FOXE3 mutations predispose to thoracic aortic aneurysms and dissections. J Clin Invest. 2016;126(03):948–961. doi: 10.1172/JCI83778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee V S, Halabi C M, Hoffman E P et al. Loss of function mutation in LOX causes thoracic aortic aneurysm and dissection in humans. Proc Natl Acad Sci U S A. 2016;113(31):8759–8764. doi: 10.1073/pnas.1601442113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hornstra I K, Birge S, Starcher B, Bailey A J, Mecham R P, Shapiro S D. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278(16):14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- 47.Mäki J M, Räsänen J, Tikkanen H et al. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106(19):2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- 48.Ren W, Liu Y, Wang X et al. β-Aminopropionitrile monofumarate induces thoracic aortic dissection in C57BL/6 mice. Sci Rep. 2016;6:28149. doi: 10.1038/srep28149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo D C, Gong L, Regalado E S et al. MAT2A mutations predispose individuals to thoracic aortic aneurysms. Am J Hum Genet. 2015;96(01):170–177. doi: 10.1016/j.ajhg.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Combs M D, Knutsen R H, Broekelmann T J et al. Microfibril-associated glycoprotein 2 (MAGP2) loss of function has pleiotropic effects in vivo. J Biol Chem. 2013;288(40):28869–28880. doi: 10.1074/jbc.M113.497727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellini C, Wang S, Milewicz D M, Humphrey J D. Myh11(R247C/R247C) mutations increase thoracic aorta vulnerability to intramural damage despite a general biomechanical adaptivity. J Biomech. 2015;48(01):113–121. doi: 10.1016/j.jbiomech.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pannu H, Tran-Fadulu V, Papke C L et al. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet. 2007;16(20):2453–2462. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang L, Guo D C, Cao J et al. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet. 2010;87(05):701–707. doi: 10.1016/j.ajhg.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKellar S H, Tester D J, Yagubyan M, Majumdar R, Ackerman M J, Sundt T M., III Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134(02):290–296. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 55.Proost D, Vandeweyer G, Meester J A et al. Performant mutation identification using targeted next-generation sequencing of 14 thoracic aortic aneurysm genes. Hum Mutat. 2015;36(08):808–814. doi: 10.1002/humu.22802. [DOI] [PubMed] [Google Scholar]
- 56.Guo D C, Regalado E, Casteel D E et al. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am J Hum Genet. 2013;93(02):398–404. doi: 10.1016/j.ajhg.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doyle A J, Doyle J J, Bessling S L et al. Mutations in the TGF-β repressor SKI cause Shprintzen-Goldberg syndrome with aortic aneurysm. Nat Genet. 2012;44(11):1249–1254. doi: 10.1038/ng.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Callewaert B L, Willaert A, Kerstjens-Frederikse W S et al. Arterial tortuosity syndrome: clinical and molecular findings in 12 newly identified families. Hum Mutat. 2008;29(01):150–158. doi: 10.1002/humu.20623. [DOI] [PubMed] [Google Scholar]
- 59.Micha D, Guo D C, Hilhorst-Hofstee Y et al. SMAD2 mutations are associated with arterial aneurysms and dissections. Hum Mutat. 2015;36(12):1145–1149. doi: 10.1002/humu.22854. [DOI] [PubMed] [Google Scholar]
- 60.Zhang W, Zeng Q, Xu Y et al. Exome sequencing identified a novel SMAD2 mutation in a Chinese family with early onset aortic aneurysms. Clin Chim Acta. 2017;468:211–214. doi: 10.1016/j.cca.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Tan C K, Tan E H, Luo B et al. SMAD3 deficiency promotes inflammatory aortic aneurysms in angiotensin II-infused mice via activation of iNOS. J Am Heart Assoc. 2013;2(03):e000269. doi: 10.1161/JAHA.113.000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Linde D, van de Laar I M, Bertoli-Avella A M et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol. 2012;60(05):397–403. doi: 10.1016/j.jacc.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 63.van de Laar I M, van der Linde D, Oei E H et al. Phenotypic spectrum of the SMAD3-related aneurysms-osteoarthritis syndrome. J Med Genet. 2012;49(01):47–57. doi: 10.1136/jmedgenet-2011-100382. [DOI] [PubMed] [Google Scholar]
- 64.Zhang P, Hou S, Chen J et al. Smad4 deficiency in smooth muscle cells initiates the formation of aortic aneurysm. Circ Res. 2016;118(03):388–399. doi: 10.1161/CIRCRESAHA.115.308040. [DOI] [PubMed] [Google Scholar]
- 65.Heald B, Rigelsky C, Moran R et al. Prevalence of thoracic aortopathy in patients with juvenile polyposis syndrome-hereditary hemorrhagic telangiectasia due to SMAD4. Am J Med Genet A. 2015;167A(08):1758–1762. doi: 10.1002/ajmg.a.37093. [DOI] [PubMed] [Google Scholar]
- 66.Wain K E, Ellingson M S, McDonald J et al. Appreciating the broad clinical features of SMAD4 mutation carriers: a multicenter chart review. Genet Med. 2014;16(08):588–593. doi: 10.1038/gim.2014.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindsay M E, Schepers D, Bolar N A et al. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet. 2012;44(08):922–927. doi: 10.1038/ng.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boileau C, Guo D C, Hanna N et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44(08):916–921. doi: 10.1038/ng.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Renard M, Callewaert B, Malfait F et al. Thoracic aortic-aneurysm and dissection in association with significant mitral valve disease caused by mutations in TGFB2. Int J Cardiol. 2013;165(03):584–587. doi: 10.1016/j.ijcard.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 70.Bertoli-Avella A M, Gillis E, Morisaki H et al. Mutations in a TGF-β ligand, TGFB3, cause syndromic aortic aneurysms and dissections. J Am Coll Cardiol. 2015;65(13):1324–1336. doi: 10.1016/j.jacc.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gallo E M, Loch D C, Habashi J P et al. Angiotensin II-dependent TGF-β signaling contributes to Loeys-Dietz syndrome vascular pathogenesis. J Clin Invest. 2014;124(01):448–460. doi: 10.1172/JCI69666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.MacCarrick G, Black J H, III, Bowdin S et al. Loeys-Dietz syndrome: a primer for diagnosis and management. Genet Med. 2014;16(08):576–587. doi: 10.1038/gim.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jondeau G, Ropers J, Regalado E et al. International Registry of Patients Carrying TGFBR1 or TGFBR2 mutations: results of the MAC (Montalcino Aortic Consortium) Circ Cardiovasc Genet. 2016;9(06):548–558. doi: 10.1161/CIRCGENETICS.116.001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boodhwani M, Andelfinger G, Leipsic J et al. Canadian Cardiovascular Society position statement on the management of thoracic aortic disease. Can J Cardiol. 2014;30(06):577–589. doi: 10.1016/j.cjca.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 75.Attias D, Stheneur C, Roy C et al. Comparison of clinical presentations and outcomes between patients with TGFBR2 and FBN1 mutations in Marfan syndrome and related disorders. Circulation. 2009;120(25):2541–2549. doi: 10.1161/CIRCULATIONAHA.109.887042. [DOI] [PubMed] [Google Scholar]
- 76.Teixidó-Tura G, Franken R, Galuppo V et al. Heterogeneity of aortic disease severity in patients with Loeys-Dietz syndrome. Heart. 2016;102(08):626–632. doi: 10.1136/heartjnl-2015-308535. [DOI] [PubMed] [Google Scholar]
- 77.Tran-Fadulu V, Pannu H, Kim D H et al. Analysis of multigenerational families with thoracic aortic aneurysms and dissections due to TGFBR1 or TGFBR2 mutations. J Med Genet. 2009;46(09):607–613. doi: 10.1136/jmg.2008.062844. [DOI] [PubMed] [Google Scholar]
- 78.Wenstrup R J, Florer J B, Davidson J M et al. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. J Biol Chem. 2006;281(18):12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 79.Park A C, Phan N, Massoudi D et al. Deficits in Col5a2 expression result in novel skin and adipose abnormalities and predisposition to aortic aneurysms and dissections. Am J Pathol. 2017;187(10):2300–2311. doi: 10.1016/j.ajpath.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park A C, Phillips C L, Pfeiffer F M et al. Homozygosity and heterozygosity for null col5a2 alleles produce embryonic lethality and a novel classic Ehlers-Danlos syndrome-related phenotype. Am J Pathol. 2015;185(07):2000–2011. doi: 10.1016/j.ajpath.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuang S Q, Kwartler C S, Byanova K L et al. Rare, nonsynonymous variant in the smooth muscle-specific isoform of myosin heavy chain, MYH11, R247C, alters force generation in the aorta and phenotype of smooth muscle cells. Circ Res. 2012;110(11):1411–1422. doi: 10.1161/CIRCRESAHA.111.261743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koenig S N, LaHaye S, Feller J D et al. Notch1 haploinsufficiency causes ascending aortic aneurysms in mice. JCI Insight. 2017;2(21):91353. doi: 10.1172/jci.insight.91353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berk M, Desai S Y, Heyman H C, Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial, patterning, and skeletal muscle development. Genes Dev. 1997;11(16):2029–2039. doi: 10.1101/gad.11.16.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zoppi N, Chiarelli N, Cinquina V, Ritelli M, Colombi M. GLUT10 deficiency leads to oxidative stress and non-canonical αvβ3 integrin-mediated TGFβ signalling associated with extracellular matrix disarray in arterial tortuosity syndrome skin fibroblasts. Hum Mol Genet. 2015;24(23):6769–6787. doi: 10.1093/hmg/ddv382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cheng C H, Kikuchi T, Chen Y H et al. Mutations in the SLC2A10 gene cause arterial abnormalities in mice. Cardiovasc Res. 2009;81(02):381–388. doi: 10.1093/cvr/cvn319. [DOI] [PubMed] [Google Scholar]
- 86.Galvin K M, Donovan M J, Lynch C A et al. A role for smad6 in development and homeostasis of the cardiovascular system. Nat Genet. 2000;24(02):171–174. doi: 10.1038/72835. [DOI] [PubMed] [Google Scholar]
- 87.Azhar M, Schultz J J, Grupp I et al. Transforming growth factor beta in cardiovascular development and function. Cytokine Growth Factor Rev. 2003;14(05):391–407. doi: 10.1016/s1359-6101(03)00044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


