Abstract
Naegleria fowleri is a free-living amoeba that can also act as an opportunistic pathogen causing severe brain infection, primary amebic meningoencephalitis (PAM), in humans. The high mortality rate of PAM (exceeding 97%) is attributed to (i) delayed diagnosis, (ii) lack of safe and effective anti-N. fowleri drugs, and (iii) difficulty of delivering drugs to the brain. Our work addresses identification of new molecular targets that may link anti-Naegleria drug discovery to the existing pharmacopeia of brain-penetrant drugs. Using inhibitors with known mechanism of action as molecular probes, we mapped the sterol biosynthesis pathway of N. fowleri by GC-MS analysis of metabolites. Based on this analysis, we chemically validated two enzymes downstream to CYP51, sterol C24-methyltransferase (SMT, ERG6) and sterol Δ8−Δ7 -isomerase (ERG2), as potential therapeutic drug targets in N. fowleri. The sterol biosynthetic cascade in N. fowleri displayed a mixture of canonical features peculiar to different domains of life: lower eukaryotes, plants and vertebrates. In addition to the cycloartenol→ergosterol biosynthetic route, a route leading to de novo cholesterol biosynthesis emerged. Isotopic labeling of the de novo-synthesized sterols by feeding N. gruberi trophozoites on the U13C-glucose-containing growth medium identified an exogenous origin of cholesterol, while 7-dehydrocholesterol (7DHC) had enriched 13C-content, suggesting a dual origin of this metabolite both from de novo biosynthesis and metabolism of scavenged cholesterol. Sterol homeostasis in Naegleria may be orchestrated over the course of its life-cycle by a “switch” between ergosterol and cholesterol biosynthesis. By demonstrating the growth inhibition and synergistic effects of the sterol biosynthesis inhibitors, we validated new, potentially druggable, molecular targets in N. fowleri. The similarity of the Naegleria sterol Δ8−Δ7 -isomerase to the human non-opioid σ1 receptor, implicated in human CNS conditions such as addiction, amnesia, pain and depression, provides an incentive to assess structurally diverse small-molecule brain-penetrant drugs targeting the human receptor for anti-Naegleria activity.
Author summary
Sterols are important constituents of cell membranes. In a unicellurar organism, such as the human pathogen N. fowleri, the cell membrane delineates the physical boundaries of the cell and mediates interactions of the organism with its environment. N. fowleri is a free-living amoeba that may infect the human brain causing a fulminant infection called primary amebic meningoencephalitis (PAM). PAM has resulted in death in >97% of reported cases. Understanding the molecular and cellular biology of N. fowleri will facilitate the rational development of new therapeutic interventions. Using inhibitors targeting different enzymatic steps in the sterol biosynthesis pathway, we mapped metabolic intermediates and delineated the biosynthetic routes contributing to N. fowleri sterol homeostasis. An array of sterol molecules suggests that two different sterol types, ergosterol-like and cholesterol-like sterols, co-exist and may be dynamically regulated in N. fowleri. Disruption of sterol biosynthesis by drug candidates was detrimental to N. fowleri. Drugs targeting three different enzymatic steps resulted in growth inhibition effect. This effect was amplified when drugs targeting different enzymes were combined. The observed synergistic effect of these drugs lowers the individual drug concentrations required for biological activity including delivering anti-PAM drugs across the blood-brain-barrier.
Introduction
Naegleria fowleri and its non-pathogenic relatives, Naegleria gruberi and Naegleria lovaniensies, belong to the genus Naegleria, class Heterolobosea, which is characterized by a capacity for quick differentiation from an amoeboid to a flagellate form. These amoebae feed mostly on bacteria, but can also act as opportunistic pathogens causing infections of the central nervous system (CNS) of humans and other animals. N. fowleri is the only species of the genus known to cause a severe primary amebic meningoencephalitis (PAM) in humans.[1] Naegleria occur in three forms–a cyst, a trophozoite (amoeboid), and a biflagellate. The trophozoite is the only feeding and reproductive stage of Naegleria spp., as well as the only one found in infected brain tissue[2], while the flagellate form was detected in the cerebrospinal fluid (CSF)[3]. PAM due to N. fowleri has a worldwide distribution although it occurs most frequently in tropical areas and during hot summer months.[4]
N. fowleri infection is problematic due to the rapid onset and destructive nature of the disease as well as to the lack of established success in treatment.[5] Until recently, no more than a dozen patients out of ~350 reported PAM cases worldwide have been treated successfully with Amphotericin B (AmpB), either alone or in combination with other drugs.[6–9]. The investigational anti-cancer and anti-leishmaniasis agent miltefosine[10] showed promise, but not all patients who received miltefosine as part of their treatment regimens survived. In 2013, two patients survived out of three treated with miltefosine, but one of the survivors had permanent brain damage.[11] In 2016–2017, two more patients receiving miltefosine survived out of 9 diagnosed with PAM. The lack of a single, proven, evidence-based treatment of PAM with a high probability of cure stimulates a need to further study Naegleria biology in order to understand molecular mechanisms maintaining N. fowleri homeostasis throughout different developmental stages and dietary conditions.
Sterols are an important class of lipids essential in all eukaryotes. It is assumed that the last eukaryotic common ancestor (LECA) already synthesized sterols.[12] Eukaryotes that lost the ability to synthesize sterols, e.g., insects, worms and most marine invertebrates, have to obtain them from food. Sterols are involved in both intra- and intercellular signaling and in the organization of membranes. They affect fluidity and permeability of membranes and are major players in the formation of lipid rafts, regions of reduced fluidity formed by the close association of sterols with sphingolipids. [13–15] In unicellular organisms, lipid rafts control endocytosis, vesicle trafficking, motility, and cell signaling and have been shown to regulate adhesion to host cells and control virulence (reviewed in [16]). Ergosterol, a fungal and kinetoplastid sterol, was shown to promote formation of the lipid rafts.[17]
By DNA sequence, Naegleria are close to kinetoplastids, however, in contrast to the lanosterol precursor in kinetoplastids,[18] de novo biosynthesis of ergosterol in amoebae occurs from cycloartenol, a precursor typical of photosynthetic organisms, ie., algae and plants.[19–21] Disruption of sterol biosynthesis by small-molecule inhibitors targeting CYP51 is detrimental for N. fowleri trophozoites, suggesting that ergosterol biosynthesis is essential for amoeboid survival.[22] Among the enzymes constituting the sterol biosynthetic pathway in eukaryotes, several targets have been studied for the development of therapeutic or agricultural agents. For instance, the HMG-CoA reductase inhibitors, known as statins, are drugs used for lowering serum cholesterol. Farnesyl diphosphate synthase (targeted by bisphosphonates), squalene synthase (aryloxyethyl thiocyanate and quinuclidine derivatives), squalene epoxidase (terbinafine), oxidosqualene cyclase (pyridinium-ion mimetics), sterol 14α-demethylase (CYP51) (azoles), sterol C24-methyltransferase (SMT) (arylguanidines, azasterols), and sterol Δ8−Δ7 isomerase (ERG2) (morpholines) have been validated as drug targets to treat fungal infections in humans and plants.
In this work, we have assessed the sterol biosynthesis pathway in N. fowleri downstream of CYP51 by GC-MS analysis of the metabolic intermediates accumulated in trophozoites in response to the inhibitors with known mechanisms of action (MOA). Using inhibitors as the molecular probes, we chemically validated SMT and ERG2 as essential enzymes in N. fowleri. In vitro growth inhibition effect were observed for inhibitors of each mechanistic group applied individually. When applied in combination, inhibitors with different MOA produced synergistic effects. The biosynthetic cascade reconstituted in N. fowleri, based on the flux of the metabolic intermediates, indicates that the steroidogenic pathway bifurcates into ergosterol and cholesterol arms. The observed sterol profile suggests a possibility of dynamic modulation of sterol homeostasis in N. fowleri in response to environmental factors or dietary conditions.
Results and discussion
Sterol biosynthesis enzymes in Naegleria
Biosynthetically, sterols are derived from isopentenyl diphosphate (IPP), the precursor of squalene and its downstream cyclized products. The first step leading to sterols adds an oxygen to squalene resulting in squalene epoxide, which is then cyclized in a second step either to lanosterol or to cycloartenol. Enzymes constituting the core sterol biosynthesis pathway in eukaryotes largely exhibit strong conservation in amino acid sequences; however, the pathway architecture and substrate specificity vary.[23] Steroidogenesis in the non-pathogenic environmental Naegleria species, N. gruberi and N. lovaniences, was assessed in the pre-genomic era.[20] Following up on results from earlier studies[22], we have now interrogated the sterol biosynthesis pathway in N. fowleri. Naegleria steroidogenic enzymes were identified by sequence homology to the yeast, human and plant orthologues. They are characterized by high intra-genus sequence similarities and lower similarity to the human counterparts, the latter ranging from 32 to 56% for homologous enzymes (S1 Table). The Δ24-reduction and Δ8−Δ7 -isomerization steps are performed by non-homologous enzyme pairs (<20% sequence identity), ERG4 and ERG2 in fungi, DHCR24/DWF1 and EBP/HYD1 in vertebrates/land plants respectively. The Naegleria enzymes are homologous to fungal orthologues, suggesting that both can be exploited therapeutically.
The 3-keto-steroid reductase and C22-desaturase could not be identified in Naegleria by homology with yeast, human or plant counterparts. The Naegleria 3-keto-steroid reductase that catalyzes the last of the three steps required to remove two C-4 methyl groups may be homologous to yet unknown C-3 ketoreductase in land plants.[12] A protein with similarity to CYP710A, equivalent to 22-desaturase in higher plants, is present in N. gruberi (XP_002678320.1), but its primary sequence is closer to that of CYP51. In animals, lack of 22-desaturase is a typical feature. The 24-alkylation performed by ERG6 is present in Naegleria but absent in animals.
The sterol metabolic network in N. fowleri
We used sterol biosynthesis inhibitors with different MOA to (i) interrogate the flux of sterol intermediates, (ii) access the physiological requirement for specific sterols and (iii) identify enzymatic chokepoints essential to trophozoite growth. The sterol biosynthetic cascade, reconstituted in N. fowleri based on the flux of the metabolic intermediates, is consistent with the earlier data [20, 22] that sterol biosynthesis in Naegleria proceeds from cycloartenol (22) via 31-norlanosterol (14) to ergosterol (7) (Fig 1). Cholesterol (1), 7-dehydrocholesterol (7DHC) (3), ergosterol (7) and its biosynthetic precursor ergosta-5,7-dienol (10) are the most abundant sterols dominating lipid extracts of the non-inhibited N. fowleri and N. gruberi trophozoites, whereas 4-monomethylsterols, 4,4-dimethyl intermediates and 14α-methylsterols are sparsely represented (Table 1).
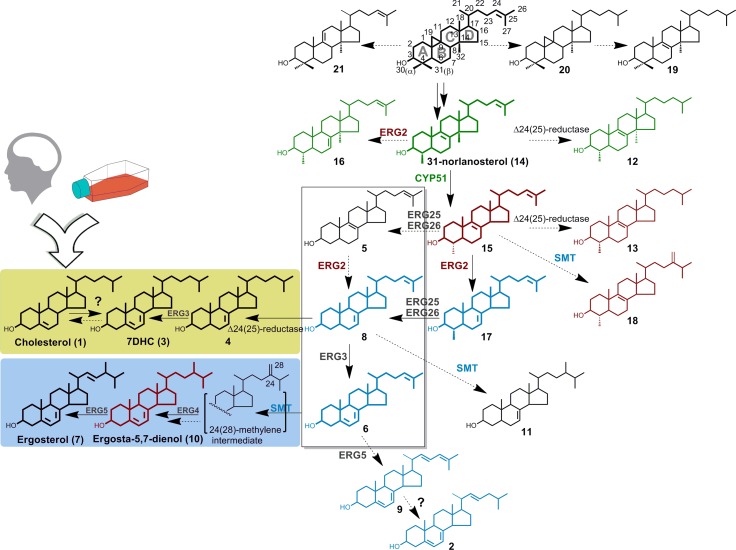
Fig 1. Sterol metabolic network in N. fowleri.
The pathway is reconstituted based on the metabolites observed in the untreated N. fowleri (shown in bold) and treated with the CYP51 (shown in green), SMT (in blue) and ERG2 (in dark red) inhibitors. Metabolites not observed in the wild-type N. fowleri are depicted in thin lines. The cholesterol arm of the pathway is highlighted by the yellow-green background, the ergosterol arm is highlighted by the blue background. Enclosed in frame are potential SMT substrates. Enzymes interrogated in these studies are labeled in colors corresponding to the accumulating metabolites. Numbering of atoms in sterol molecule is adapted from Nes and Venkatramesh, 1994.[41] Sterol labelling is as in Table 1. ERG enzyme nomenclature is according to the convention for S. cerevisiae.
Table 1. Sterol flux in N. fowleri.
| Non-inhibited | CYP51 | SMT | Sterol Δ8−Δ7−isomerase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ## | Metabolitesa | RRTb | NG | NFc | Posaconazolec | Epiminolanosterol | Abafungin | 25-Aza | Tamoxifen | AY9944 |
| 1 | Cholesterol | 1 | 7.6 | 25.6 | 26.0 | 15.6 | 2.0 | 12.7 | 3.0 | 9.5 |
| 4-Desmethylsterols (w/o cholesterol) | 77.6 | 62.9 | 23.8 | 61.9 | 88.8 | 74.5 | 71.9 | 54.6 | ||
| 2 | Cholesta-5,7,22-trienol | 1 | 3.3 | 4.3 | 2.9 | |||||
| 3 | 7-Dehydrocholesterol (7DHC) | 1.04 | 10.8 | 12.5 | 3.9 | 9.9 | 13.8 | 12.9 | 4.1 | 7.5 |
| 4 | Lathosterol | 1.06 | 0.8 | 0.2 | ||||||
| 5 | Zymosterol | 1.07 | 0.4 | 0.1 | 0.1 | |||||
| 6 | Cholesta-5,7,24-trienol | 1.11 | 30.8 | 30.0 | 38.8 | |||||
| 7 | Ergosterol | 1.11 | 35.5 | 26.7 | 14.2 | 7.7 | 20.7 | 8.8 | 24.3 | 22.8 |
| 8 | Cholesta-7,24-dienol | 1.12 | 1.6 | 5.5 | 2.8 | |||||
| 9 | Cholesta-5,7,22,24-Tetraenol | 1.20 | 6.9 | 8.6 | 5.3 | |||||
| 10 | Ergosta-5,7-dienol | 1.21 | 31.1 | 21.3 | 5.7 | 1.6 | 5.7 | 2.9 | 43.5 | 24.3 |
| 11 | Ergost-7-enol | 1.22 | 0.2 | 1.2 | ||||||
| 4-Monomethylsterols | 2.1 | 6.8 | 48.5 | 20.8 | 8.0 | 10.5 | 13.9 | 29.7 | ||
| 12 | 4α,14α-Dimethylcholest-8-enol | 1.08 | 0.1 | 1.1 | 15.5 | |||||
| 13 | 4α-Methylcholest-8-enol | 1.11 | 3.5 | 0.4 | 7.6 | 7.2 | 2.6 | 10.4 | 5.7 | |
| 14 | 31-Norlanosterol | 1.14 | 2.0 | 2.2 | 29.7 | 0.5 | 1.4 | |||
| 15 | 4α-Methylcholesta-8,24-dienol | 1.18 | 9.1 | 0.4 | 1.5 | 3.5 | 19.1 | |||
| 16 | Δ7-31-norlanosterol | 1.24 | 2.9 | |||||||
| 17 | 4α-Methylcholesta-7,24-dienol | 1.26 | 3.6 | 0.4 | 5.0 | 2.9 | ||||
| 18 | 4α-Methylergosta-8,24(28)dienol | 1.31 | 2.0 | |||||||
| 4,4-Dimethylsterols | 9.6 | 3.3 | 1.0 | 0.9 | 0.6 | 0.9 | 2.3 | 0.8 | ||
| 19 | Lanostanol | 1.30 | 0.5 | 0.2 | 0.2 | 0.4 | 0.5 | |||
| 20 | Cycloartanol | 1.32 | 6.9 | 1.1 | ||||||
| 21 | Parkeol | 1.40 | 0.3 | 0.1 | 0.1 | 0.3 | ||||
| 22 | Cycloartenol | 1.41 | 1.9 | 2.0 | 0.7 | 0.4 | 0.1 | 0.6 | 2.3 | 0.8 |
| Other sterols | 3.1 | 1.4 | 0.7 | 0.8 | 0.6 | 1.4 | 8.9 | 5.4 | ||
aAll listed sterol structures are shown in Fig 1. The metabolites were quantified based on the total ion current peak areas of each sterol. NF–N. fowleri, NG–N. gruberi
bRelative retention time compared to cholesterol
cPreviously published data[22] used here as a comparative companion in the context of the whole pathway.
Given structural similarity of sterol substrates, enzyme specificity is controlled kinetically rather than thermodynamically. Disruption of specific steps gives rise to the uncommon intermediates that are enzymatically converted to the end-products not operational in the wild-type organism. As reported previously,[22] upon treatment of N. fowleri with posaconazole, the cumulative content of the 14α-methylsterols, including 12, 14, 16, 19, 20, 21 and 22, increased from 6.5% to 49.1%, with the major intermediate being 31-norlanosterol (14) followed by its C24-C25 hydrogenated product, 4,14-dimethylcholest-8-enol (12). Posaconazole data reported elsewhere[22] are included in Table 1as a comparative companion in the context of the whole pathway. Given the unchanged content of 4,4-dimethylsterols in the CYP51 inhibitor-exposed samples, removal of the 4β-methyl group is unaffected by the blocking of CYP51 activity and likely occurs prior to the C-14 demethylation; removal of the 4α-methyl group occurs downstream to CYP51. The C-4 demethylation in two nonconsecutive steps is a feature of land plants.[12] In this work, by using molecular probes with the corresponding MOA, we mapped enzymatic steps downstream of CYP51, including sterol C8→C7-double bond isomerization, catalyzed by ERG2, and sterol C24-methylation, catalyzed by SMT (ERG6).
In an ERG2 inhibitor AY9944-treated N. fowleri, the 4α-methylcholesta-8,24-dienol (15) content increased from zero to 19.1% suggesting that the 4α-demethylation follows the Δ8−Δ7 -isomerization and preferably occurs on 4α-methylcholesta-7,24-dienol (17). The treatment of N. fowleri with another ERG2 inhibitor, tamoxifen, a non-steroidal estrogen receptor modulator, also resulted in an increase of the ergosta-5,7-dienol (10) content from 21.3% to 43.5% (Fig 1, Table 1), suggesting an inhibition of 22-desaturation performed by a yet unknown Naegleria enzyme.
Exposure of N. fowleri to the SMT inhibitors 24,25-epiminolanosterol (epiminolanosterol), abafungin and 25-azacycloartenol (25-Aza), led to high accumulation of cholesta-5,7,24-trienol (6) (≥30% of total sterols), an intermediate undetectable in the wild-type or posaconazole-inhibited N. fowleri (Table 1, Fig 2). This finding made three intermediates, 5, 8 and 6, potential candidates for endogenous SMT substrates in N. fowleri. All three contain a Δ24(25) olefin group that allows C-methylation with formation of the transient 24-methylene intermediate that ultimately is converted to the ergosterol-like physiological end-products (Fig 1).[24, 25] In the SMT-inhibited N. fowleri, the end-products 9 and 2, not operational in the wild-type N. fowleri, were also formed. Accumulation of cholesta-5,7,22,24-tetraenol (9) and cholesta-5,7,24-trienol (2), the end products representing a dysfunctional carbon flux, was previously reported in yeast carrying an ERG6 mutant defective in SMT activity.[26]
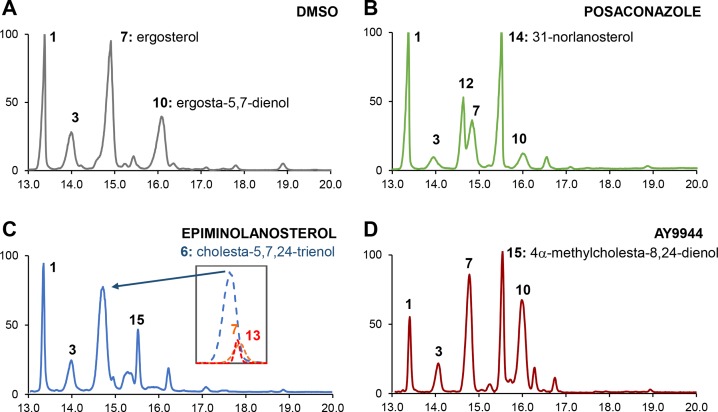
Fig 2. Lipid analysis by GC-MS.
Gas chromatography of the total sterol fractions derived from N. fowleri trophozoites treated with (A) 0.5% DMSO, (B) 0.2 μM posaconazole, (C) 5.4 μM epiminolanosterol, and (D) 5.6 μM AY9944. Peaks are labeled with numbers corresponding to metabolites listed in Table 1. Insert in (C) shows deconvolution of the major peak resulting from an overlap of three different sterols: 6, m/z = 349; 7, m/z = 363 and 13, m/z = 400. The color scheme is the same as in Fig 1: CYP51 inhibitor (shown in green), SMT inhibitor (in blue) and ERG2 inhibitor (in dark red).
Functional analysis of Naegleria sterol C24-methyltransferase (SMT)
To identify what configuration of the double-bond(s) in the sterol B-ring favors Naegleria SMT catalysis, the studies using recombinant enzyme and individually isolated sterol intermediates were conducted. In the N. gruberi genome, two candidate genes encoding SMT (77.6% sequence identity) were identified (S1 Table), which resemble those of higher plants in primary sequence (~50% identity). Higher plants have two SMT enzymes that use different substrates to give either C24-methylated or C24-ethylated phytosterols. N. fowleri has a single gene encoding SMT; no 24-ethylated sterols were detected in the lipid extracts of any Naegleria species. N. gruberi SMT (XP_002680047.1) sharing highest sequence identity (86%) with the N. fowleri counterpart, has been expressed in Escherichia coli and recombinant protein was characterized for substrate specificity. From the in vitro reconstitution of the SMT catalytic activity, among thirteen sterols tested, 5 (zymosterol) and 6 (cholesta-5,7,24-trienol) were equipotent as SMT substrates (set at 100%), while 8 (cholesta-7,24-dienol) showed 67.2% convertion (Table 2).
Table 2. Conversion of different sterols by N. gruberi SMT.
| Substrates | Relative Activities (%) |
|---|---|
| fecosterol | 0.0 |
| 24-methylenelophenol | 0.0 |
| zymosterol (5) | 100.0 |
| cholesta-5,7,24-trienol (6) | 100.0 |
| cholesta-7,24-dienol (8) | 67.2 |
| 14α-methylzymosterol | 29.0 |
| desmosterol | 17.10 |
| 4α-methylzymosterol | 9.4 |
| 31-norlanosterol (14) | 2.7 |
| cycloartenol (22) | 0.0 |
| 24-methylenecycloartenol | 0.0 |
| lanosterol (19) | 0.0 |
| obtusifoliol | 0.0 |
An origin of cholesterol in Naegleria
Remarkably, in addition to the ergosterol ‘arm’ (highlighted blue in Fig 1), a cholesterol arm, characterized by reactions 8→4→3→1 (highlighted orange in Fig 1), emerged in the metabolic flux studies. Together with the high cholesterol content, this observation raised a question about the origin of cholesterol: de novo biosynthesis or scavenging from the growth medium. To answer this question, we have conducted the 13C-labeling studies in N. gruberi grown in the ATCC1034 medium supplemented with U-13C glucose to the final concentration of 5% (w/v). Incorporation of the 13C-carbon was monitored by GC/MS; a percent of the 13C enrichment for each sterol was calculated from the isotopic distribution of the molecular mass using IsoCor software.[27] As expected, cycloartenol, ergosta-5,7-dienol and ergosterol made by amoeba biosynthetically, had the highest 13C-isotope content (Table 3). At the same time, cholesterol was unlabeled, proving its exogenous origin in cultured N. gruberi trophozoites. Remarkably, 13C-isotope content in 7-dehydrocholesterol (3) fall between the ergosterol and cholesterol values, suggesting dual origin of this intermediate in both the de novo biosynthesis and via a reverse reaction catalyzed in other species by cholesterol C-7 desaturase. Cholesterol 7-desaturase is a critical enzyme in insects and nematodes involved in the synthesis of steroid hormones (DAF-36).[28] These lineages lack sterol biosynthesis capacity and rely solely on cholesterol uptake from the diet. Both N. fowleri and N. gruberi genomes encode a putative DAF-36-like protein (S1 Table).
Table 3. Isotopic enrichment of N. gruberi sterols incubated with 50 mM U-13C-glucose.
| Sterols | Molecular Formula | 13C-content in normal medium (%) | 13C-content in U13C-glucose medium (%) |
|---|---|---|---|
| Cholesterol | C27H46O | 1.07 | 1.06 |
| Cholesta-5,7-dienol | C27H44O | 1.11 | 2.46 |
| Ergosterol | C28H44O | 1.06 | 4.02 |
| Erogsta-5,7-dienol | C28H46O | 1.11 | 4.03 |
| Cycloartenol | C30H50O | 1.15 | 4.15 |
The dual origin of 7DHC extends the cholesterol arm all the way from 8→4→3 (Fig 1). The last 3→1 step separating N. fowleri from making cholesterol de novo, the reduction of the C7-C8-double bond in 7-dehydrocholesterol usually performed by Δ7-dehydrocholesterol reductase, remains unobserved. Candidates for this enzymatic role are present in both N. fowleri and N. gruberi genomes (S1 Table). A possibility of the pathway bifurcation downstream of 8 suggests a “switch” between the C24-methylation and Δ24-reduction steps (Fig 1) committing Naegleria to either ergosterol or cholesterol biosynthesis, respectively. We hypothesize that the switch may be used to maintain dynamic sterol homeostasis over the course of the N. fowleri life-cycle.
Anti-proliferative and synergistic effects of sterol biosynthesis inhibitors
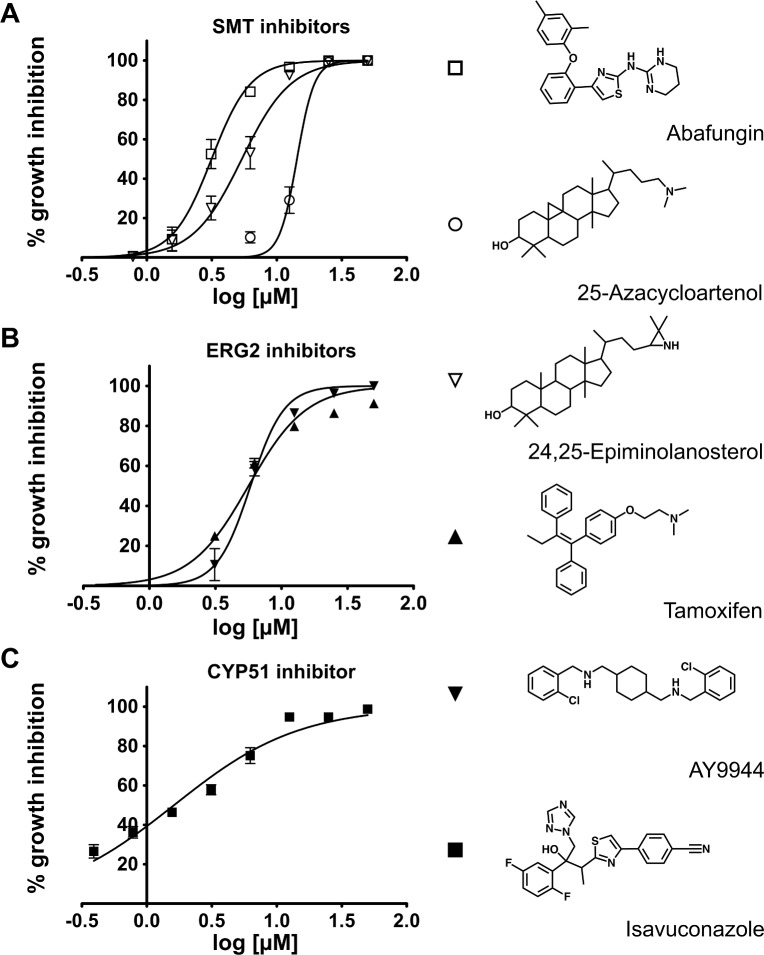
The growth inhibition of free-living amoebae by the sterol biosynthesis inhibitors has been reported previously. Thus, the effect of the agricultural systemic fungicides, tridemorph and fenpropimorph, which target both sterol Δ8−Δ7 -isomerase and Δ14-reductase,[29] has been reported in Acanthamoeba polyphaga, N. lovaniensis and N. gruberi in the mid 80’s.[20, 21] More recently, growth inhibition of Acanthamoeba castellanii and A. polyphaga was demonstrated by the pharmaceutical sterol biosynthesis inhibitors, itraconazole and voriconazole, targeting CYP51.[30] Finally, posaconazole and itraconazole were found superior to AmpB, a cornerstone of PAM therapy and a standard of care for CNS infections caused by molds, against N. fowleri trophozoites in vitro.[22] In this work, we demonstrated that blocking enzymatic steps downstream of CYP51, leads to a decline of ergosterol and/or accumulation of the end-products and metabolic intermediates incompatible with N. fowleri survival in vitro. The anti-proliferative effects of compounds were assessed using methods previously described.[7, 22] The EC50 values of the individual inhibitors deduced from the dose-response curves (Fig 3) are summarized in Table 4. For drug compatibility in combination experiments, all compound stocks were prepared in DMSO, which negatively affected the isavuconazole and posaconazole EC50 values compared to previously determined for the SBE-β-CD-solubilized drug.[22] The in vitro potency of all tested sterol biosynthesis inhibitors exceeded that of miltefosine (EC50 of 54.5 μM), an investigational drug currently recommended by the U.S. Centers for Disease Control and Prevention for the treatment of PAM.
Fig 3. Dose-response growth inhibition of N. fowleri trophosoites.
Dose-response curves are shown for (A) SMT inhibitors: abafungin, 25-azacycloartenol and 24,25-epiminolanosterol, (B) ERG2 inhibitors: tamoxifen and AY9944, (C) CYP51 inhibitor isavuconazole.
Table 4. Targeting N. fowleri with inhibitors of known MOA.
| Inhibitors | EC50, μM N. fowleri |
|---|---|
| CYP51 (ERG11) | |
| Posaconazolea | ≤0.01[22] |
| Posaconazole | 0.2±0.08 |
| Isavuconazolea | 0.1[22] |
| Isavuconazole | 1.6±0.03 |
| SMT (ERG6) | |
| Abafungin | 3.1±0.02 |
| Epiminolanosterol | 5.4±0.03 |
| 25-Azacycloartenol | 14.3±0.04 |
| Sterol Δ8−Δ7 isomerase (ERG2) | |
| Tamoxifen | 5.8±0.04 |
| AY9944 | 5.6±0.03 |
| Human σ1 receptor | |
| Fluoxetine (Prozac) | 31.8±0.01 |
| Fluvoxamine (Luvox) | 42% at 50 μM |
| Citalopram (Celexa) | 21% at 50 μM |
| Dextromethorphan (DXM) | 20% at 50μM |
| Standards of care | |
| AmpBa | 0.10±0.01[22] |
| Miltefosine | 54.4±0.01[22] |
asolubilised in SBE-β-CD
Blocking two essential enzymes in the ergosterol pathway is more detrimental for unicellular organisms than inhibiting one.[31] This synergistic effect is due to more efficient depletion of an essential end product(s) and/or redirection of substrate flow to produce intermediates and end-products harmful for key steroidogenic enzymes or membrane structures. In this work, we have demonstrated synergy between the CYP51 (isavuconazole), SMT (epiminolanosterol) and sterol ERG2 (tamoxifen) inhibitors paired in three different combinations as specified in Fig 4. Using classical isobolograms, combination indices (CI) and dose reduction indices (DRI) were calculated from the experimental data (Fig 4A, 4B and 4C) as described by Chou and Talalay (S2 Table). [32, 33] The isobolograms indicate synergy for all three drug pairs in achieving 95% of N. fowleri growth inhibition with 2- to 19-fold dose reduction for isavucanazole and 1- to 19-fold dose reduction for tamoxifen (Fig 4D); 3- to 109-fold dose reduction for epiminolanosterol and 2- to 16-fold dose reduction for tamoxifen (Fig 4E); and finally, the highest synergy with 4- to 265-fold dose reduction for isavucanazole and 13- to 132-fold dose reduction for epiminolanosterol is indicated for the isavuconazole-epiminolanosterol pair (Fig 4F).
Fig 4. Growth inhibition of N. fowleri as a function of drug combinations.
Heat maps show growth inhibition of N. fowleri by drug combinations: tamoxifen and isavuconazole (A), epiminlanosterol and tamoxifen (B), and epiminolanosterol and isavuconazole (C). Corresponding isobolograms (D, E, F) show the mean values of dose reduction index (DRI) ploted against calculated inhibitor doses required to achieve 95% growth inhibition. Standard deviations for each parameter mean value are shown in S2 Table.
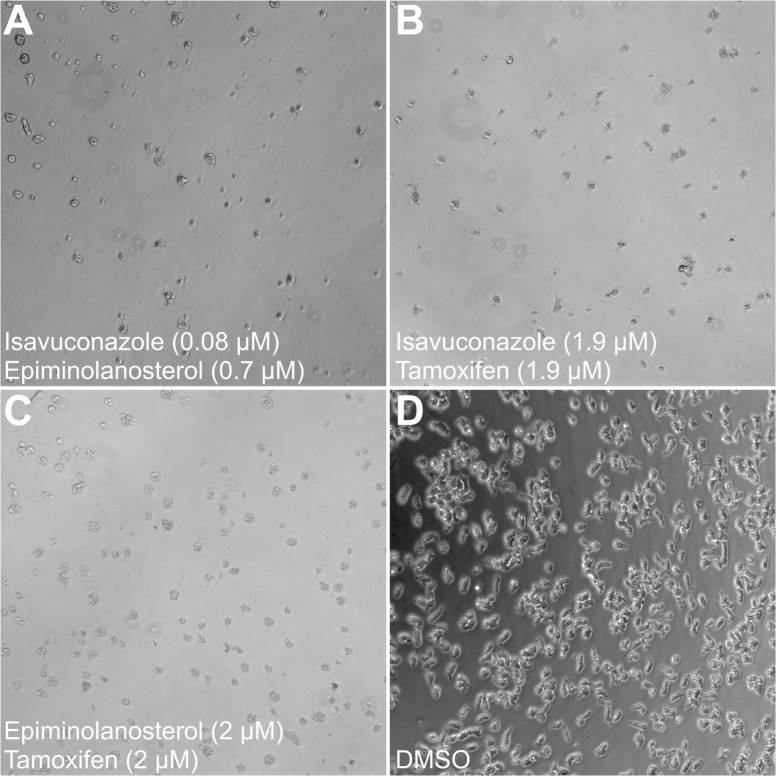
The drug combinations with the highest predicted synergy were analyzed microscopically for their effect on N. fowleri growth and morphology (Fig 5). The isavuconazole-epiminolanosterol pair, combined at concentrations of 0.08 μM and 0.7 μM, respectively, (CI = 0.07) (Fig 5A), the isavuconazole-tamoxifen pair, combined at 1.9 μM equimolar concentrations (CI = 0.36) (Fig 5B), and epiminolanosterol and tamoxifen pair, combined at 2 μM equimolar concentrations (CI = 0.38) (Fig 5C), completely inhibited the growth and led to the changed morphology and death of the majority of N. fowleri cells in 48 h of drug exposure, whereas DMSO-treated control cells grew and appeared normal (Fig 5D).
Fig 5. Synergistic effect of drugs at low concentrations.
The phase contrast microscope images show N. fowleri trophozoites treated for 48 hours with (A) 0.08 μM of isavuconazole and 0.7 μM of epiminolanosterol, (B) 1.9 μM equimolar concentrations of isavuconazole and tamoxifen, (C) 2 μM equimolar concentrations of epiminolanosterol and tamoxifen, and (D) 0.5% DMSO, which served as a negative control. The inhibitor-treated N. fowleri cells visible in the microscope field are rounded, much smaller in size and not viable, whereas DMSO-treated cells are irregularly shaped with visible cytoplasm. Magnification, ×20.
New therapeutic targets and blood-brain permeability of drugs
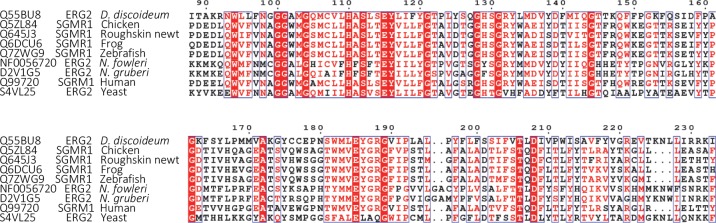
Drug ‘repurposing’ is a relevant and cost effective strategy for utilizing approved drugs for the treatment of rare diseases. The requirement of brain penetrance narrows down the pool of the FDA-approved candidates for the treatment of PAM. Only a few diseases of the brain like depression, affective disorders, chronic pain, and epilepsy respond to small-molecule therapy. The reason for the paucity of small-molecule drugs is that 98% of them do not cross the blood-brain barrier (BBB).[34, 35] Validation of sterol Δ8−Δ7 -isomerase (ERG2) as a new potential molecular drug target in N. fowleri provides a link to structurally diverse brain-penetrant small-molecule drugs targeting human non-opioid σ1 receptor sharing 30% sequence identity and 60% sequence homology to the catalytic domain of the N. fowleri sterol Δ8−Δ7 -isomerase (Fig 6). The non-opioid σ1 receptor is implicated in human CNS conditions such as addiction, amnesia, pain and depression.[36]
Fig 6. Sequence alignments between the catalytic domain of sterol Δ8−Δ7 -isomerase (ERG2) and a ligand binding site of non-opioid σ1 receptor (SGMR1) demonstrate high sequence similarity.
Accession numbers in UniProt or AmoebaDB are provided for each sequence. Numbering is according to the Dictyostelium discoideum ERG2 sequence.
We have tested four non-selective serotonin reuptake inhibitors (SSRIs) and σ1 receptor agonists with reported affinity to human σ1 receptors, fluvoxamine (Luvox), fluoxetine (Prozac), citalopram (Celexa) and dextromethorphan (DXM), [37, 38] for growth inhibition against N. fowleri. All four drugs demonstrated effect at the highest tested concentration (50 μM). Fluoxetine, the well-known antidepressant, was the most potent in this group (Table 4). Potency of fluoxetine (EC50 of 32 μM) exceeded that of miltefosine (54 μM). Other structurally diverse brain-penetrant small-molecule drugs targeting this human receptor will now be screened for anti-Naegleria activity to identify candidates for animal model studies.
Summary
The sterol biosynthetic pathway in N. fowleri displays a mixture of canonic features peculiar to different domains of life: lower eukaryotes, land plants, invertebrates and vertebrates. In addition to the cycloartenol→ergosterol biosynthetic route, our data suggest a route leading to de novo cholesterol biosynthesis with a single, yet to be experimentally observed, enzymatic step separating N. fowleri from making cholesterol de novo. N. fowleri also utilizes cholesterol obtained from diet by converting it into 7-dehydrocholesterol, a feature typical to the eukaryote lineages (e.g., insects, worms and marine invertebrates) that lost the ability to produce sterols from squalene. Hypothetically, a “switch” between ergosterol and cholesterol sterol types may occur in Naegleria, e.g., during encystation/excystation, to sustain environmental challenges by forming a viable cyst. By demonstrating the amoebicidal effect of the sterol biosynthesis inhibitors with different MOA, we validated multiple, potentially druggable, molecular targets in N. fowleri, linking the anti-Naegleria drug discovery to the existing small-molecule drugs targeting other human diseases. Synergy between alternative drug-targets is of interest for the treatment of PAM, as lower drug concentrations are required to achieve the biologic effect. Reducing therapeutic concentrations may, in part, alleviate the limitation imposed by the need of delivering anti-PAM drugs accross the blood-brain-barrier.
Materials and methods
Ethics statement
Research performed at UC San Diego was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals, National Research Council, 2011. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. Animal research was conducted under approved protocol S14187 from the Institutional Animal Care and Use Committee, University of California, San Diego. Euthanasia was accomplished by CO2 inhalation or by sodium pentobarbital overdose (60 mg/kg), followed by cervical dislocation. These methods of euthanasia have been selected because they cause minimal pain and distress to animals, are relatively quick, and do not adversely impact interpretation of the results of studies. All methods are in accord with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Compounds
The inhibitors tested were purchased from commercial vendors except epiminolasosterol and 25-azacycloartenol which were synthesized in Dr. Nes’ laboratory; the structure of the sterol was authenticated by both the GC-MS and NMR methods. AY9944 and abafungin were from Santa Cruz Biotechnology (SC-202965 and SC-474844); tamoxifen was from Sigma (T5648), isavuconazole was from StruChem (SC-98350), fluvoxamine (J90043), fluoxetine (C845), citalopram (K750) and dextromethorphan (X3585) were from AK Scientific.
N. fowleri strain maintenance
The N. fowleri KUL strain used in these studies was acquired from ATCC in 2015; aliquoted stock is maintained deep frozen in liquid nitrogen. N. fowleri amoebae maintained axenically (in culture) are weakly virulent. To maintain highly virulent status, N. fowleri strain is passaged every six months in mouse brain. For that, four week-old male Balb/c mice are inoculated by intranasal instillation of 10 μl of 3x104 N. fowleri trophozoites axenically cultured at 37°C in Nelson’s medium supplemented with 10% fetal bovine serum, resulting in 100% animal mortality at day 7. Female mice are less susceptible to N. fowleri infection, therefore only male mice are used in this model. At the time point when clinical signs of infection are apparent, the mice are sacrificed and the brains are harvested to propagate N. fowleri axenically for the next few months. The animals and tissues infected with N. fowleri are handled in the certified Biosafety Level-2 (BSL-2) environment according to the UCSD Institutional Animal Care and Use Committee guidelines and approved animal protocol. Safety glasses, gloves, mask, disposal gowns, shoe covers and head cover are worn. N. fowleri strain is handled in a biosafety cabinet mounted in the BSL-2 environment.
N. fowleri growth inhibition assay
To determine EC50 values, test compounds were tested for dose-response against N. fowleri trophozoites axenically cultured in Nelson’s medium supplemented with 10% fetal bovine serum at 37°C;[39] all the experiments were performed in triplicate using trophozoites harvested during the logarithmic phase of growth.[40] Drug concentration ranges of 0.4–50 μM and 0.008–25 μM were generated by transferring 0.5 μl of serially diluted compounds to a corresponding well of the 96-well plate followed by addition of 99.5 μl of N. fowleri trophozoites (10,000 amoebae). Assay plates were incubated for 48 h and cell viability was determined by the CellTiter-Glo Luminescent Cell Viability Assay.[7, 40] The experiments using trophozoites were conducted in a biosafety cabinet following the BSL-2 procedures as specified in the UCSD Biosafety Practices Guidelines.
GC-MS analysis of the Naegleria sterols
Twenty or fifty million N. gruberi or N. fowleri trophozoites per sample were exposed for 24 h to 0.5% DMSO or an inhibitor at EC50 concentration prior to lipid extraction. Sterols were analyzed by the use of GC-MS, wherein the lipids extracted from cells grown in the presence of non-lethal concentrations of inhibitors are separated by gas chromatography and subsequently analyzed by mass-spectrometry, as previously described.[22] The sterol identities were assigned based on relative chromatographic behavior, the characteristic molecular masses and electron ionization (EI) fragmentation patterns of free sterols (Fig 2). The sterols were quantified based on the total ion current peak areas of each sterol.
Catalytic activity of recombinant SMT
The cDNA coding sequence for XP_002680047 was retrieved from NCBI database and the gene was synthesized by Eurofin MWG (Huntsville, AL). The gene was cloned into the PQE30 plasmid (Qiagen) and expressed in E. coli M15 strain. Briefly, the M15 E.coli cells harboring expression vector were grown at 37°C in Luria broth (LB) containing ampicillin and kanamycin until OD600 reached 0.6. The gene expression was induced by 1.0 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 6 hours. The cells were collected by centrifugation and homogenized in 20 mM phosphate buffer in 5% (v/v) glycerol at pH 7.5. After removal of the cell debris by centrifugation at 100,000×g, the soluble fraction was analyzed by Bradford assay to determine the total protein concentration.
To assess the catalytic activity of SMT, thirteen different sterol substrates specified in Table 2 were used. Enzymatic assays were carried out using crude SMT preparation in 9-ml test tubes containing a total of 600 μl assay volume at 35°C for 4 hours. The assay mixture contained 100 μM sterol emulsified in 12 μl Tween 80 (1.2g/L) and 1.2 mg of total protein. The reaction was initiated by the addition of 200 μM of S-adenosyl methionine. The reactions were terminated with 1 ml of methanolic KOH. Sterols were extracted with hexane (3 x 1 ml) and dried under an N2 stream. The extracted sterols were separated by GC/MS and the substrate conversion rate (R) was calculated from the GC peak areas for the substrate (S) and the product (P) peaks according to the following equation: R = 1-P/(S+P). The conversion rates were normalized to the activity of the two best substrates, 5 and 6, which was considered as 100%.
Validation of the inhibitors for growth inhibition activity
Compounds were tested against N. fowleri KUL trophozoites axenically cultured in Nelson’s medium supplemented with 10% fetal bovine serum at 37°C.[22] All the experiments were performed using trophozoites harvested during the logarithmic phase of growth. Screening was performed in triplicate by using an ATP bioluminescence-based assay[40] as previously described.[22] The experiments using trophozoites were conducted in a biosafety cabinet following the BSL2 procedures as specified in the UCSD Biosafety Practices Guidelines.
Determination of FIC indices and isobologram construction
To determine if the combinations between the inhibitors are synergistic, additive, or antagonistic against N. fowleri trophozoites, classical isobolograms were used, and combination indices (CI) were calculated as described by Chou and Talalay.[32, 33] Briefly, compounds (epiminolanosterol/tamoxifen, isavuconazole/epiminolanosterol, isavuconazole/tamoxifen) were combined at different micromolar ratios (1:1, 1:2, 1:4, 1:8, 1:16, 2:1, 4:1, 8:1 and 16:1) at each drug concentrations ranging from 50 μM to 0.4 μM (Fig 4). Effect for drug pairs was assessed in triplicate in 96-well format with each drug being serially diluted either horizontally or vertically. Growth inhibition was assessed after 48 h using an ATP bioluminescence-based assay.[22]. The results were analyzed using CompuSyn software[32] that calculated combination indices serving as a quantitative measure for drug synergy (CI<1), additivity (CI = 1), or antagonism (CI>1). The dose reduction index (DRI), a fold of dose reduction that allows drug combination to achieve the same degree of inhibition as a dose of the drug used as a single agent, was also calculated by the CompuSyn software.
The predicted synergistic effect of drugs was experimentally tested in a 96-well clear bottom plate. N. fowleri trophozoites (1×104) were treated with pairs of compounds (isavuconazole/epiminolanosterol, isavuconazole/tamoxifen and epiminolanosterol/tamoxifen) mixed at concentrations that provided the highest CI at 48 h. Cells treated with 0.5% DMSO were used as control. After 48 hours, wells were visually inspected using an Axiovert 40 CFL phase contrast microscope (Carl Zeiss).
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Dean McKerrow for access to the lab instrumentation and facilities and for the critical reading of the manuscript, and Diane Thomas for excellent technical assistance and the proofreading of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the University of California San Diego start-up fund to (LMP), the National Institutes of Health grants 1KL2TR001444 (to AD) and R21AI119782 (to WDN). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Visvesvara GS. Infections with free-living amebae. Handb Clin Neurol. 2013;114:153–68. 10.1016/B978-0-444-53490-3.00010-8 [DOI] [PubMed] [Google Scholar]
- 2.Grace E, Asbill S, Virga K. Naegleria fowleri: pathogenesis, diagnosis, and treatment options. Antimicrob Agents Chemother. 2015;59(11):6677–81. 10.1128/AAC.01293-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomba M, Mucheleng'anga LA, Fwoloshi S, Ngulube J, Mutengo MM. A case report: primary amoebic meningoencephalitis in a young Zambian adult. BMC Infect Dis. 2017;17(1):532 10.1186/s12879-017-2638-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jonckheere JF. Origin and evolution of the worldwide distributed pathogenic amoeboflagellate Naegleria fowleri. Infect Genet Evol. 2011;11(7):1520–8. 10.1016/j.meegid.2011.07.023 [DOI] [PubMed] [Google Scholar]
- 5.Zysset-Burri DC, Muller N, Beuret C, Heller M, Schurch N, Gottstein B, et al. Genome-wide identification of pathogenicity factors of the free-living amoeba Naegleria fowleri. BMC Genomics. 2014;15:496 10.1186/1471-2164-15-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004;34(9):1001–27. 10.1016/j.ijpara.2004.06.004 [DOI] [PubMed] [Google Scholar]
- 7.Debnath A, Tunac JB, Galindo-Gomez S, Silva-Olivares A, Shibayama M, McKerrow JH. Corifungin, a new drug lead against Naegleria, identified from a high-throughput screen. Antimicrob Agents Chemother. 2012;56(11):5450–7. 10.1128/AAC.00643-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vargas-Zepeda J, Gomez-Alcala AV, Vasquez-Morales JA, Licea-Amaya L, De Jonckheere JF, Lares-Villa F. Successful treatment of Naegleria fowleri meningoencephalitis by using intravenous amphotericin B, fluconazole and rifampicin. Arch Med Res. 2005;36(1):83–6. [DOI] [PubMed] [Google Scholar]
- 9.Khanna V, Shastri B, Anusha G, Mukhopadhayay C, Khanna R. Acanthamoeba meningoencephalitis in immunocompetent: A case report and review of literature. Trop Parasitol. 2014;4(2):115–8. 10.4103/2229-5070.138540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaminsky R. Miltefosine Zentaris. Curr Opin Investig Drugs. 2002;3(4):550–4. [PubMed] [Google Scholar]
- 11.Cope JR, Conrad DA, Cohen N, Cotilla M, DaSilva A, Jackson J, et al. Use of the novel therapeutic agent miltefosine for the treatment of Primary Amebic Meningoencephalitis: report of 1 fatal and 1 surviving case. Clin Infect Dis. 2016;62(6):774–6. 10.1093/cid/civ1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmond E, Gribaldo S. Phylogenomics of sterol synthesis: insights into the origin, evolution, and diversity of a key eukaryotic feature. Genome Biol. Evol. 2009;1:364–81. 10.1093/gbe/evp036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma AI, Olson CL, Mamede JI, Gazos-Lopes F, Epting CL, Almeida IC, et al. Sterol targeting drugs reveal life cycle stage-specific differences in trypanosome lipid rafts. Sci Rep. 2017;7(1):9105 10.1038/s41598-017-08770-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387(6633):569–72. 10.1038/42408 [DOI] [PubMed] [Google Scholar]
- 15.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50. 10.1126/science.1174621 [DOI] [PubMed] [Google Scholar]
- 16.Goldston AM, Powell RR, Temesvari LA. Sink or swim: lipid rafts in parasite pathogenesis. Trends Parasitol. 2012;28(10):417–26. 10.1016/j.pt.2012.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276(36):33540–6. 10.1074/jbc.M104776200 [DOI] [PubMed] [Google Scholar]
- 18.Nes CR, Singha UK, Liu J, Ganapathy K, Villalta F, Waterman MR, et al. Novel sterol metabolic network of Trypanosoma brucei procyclic and bloodstream forms. Biochem J. 2012;443(1):267–77. 10.1042/BJ20111849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raederstorff D, Rohmer M. Sterol biosynthesis de nova via cycloartenol by the soil amoeba Acanthamoeba polyphaga. Biochem J. 1985;231(3):609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raederstorff D, Rohmer M. Sterol biosynthesis via cycloartenol and other biochemical features related to photosynthetic phyla in the amoebae Naegleria lovaniensis and Naegleria gruberi. Eur J Biochem. 1987;164:427–34. [DOI] [PubMed] [Google Scholar]
- 21.Raederstorff D, Rohmer M. The action of the systemic fungicides tridemorph and fenpropimorph on sterol biosynthesis by the soil amoeba Acanthamoeba polyphaga. Eur J Biochem. 1987;164(2):421–6. [DOI] [PubMed] [Google Scholar]
- 22.Debnath A, Calvet CM, Jennings G, Zhou W, Aksenov A, Luth MR, et al. CYP51 is an essential drug target for the treatment of primary amoebic meningoencephalitis (PAM). PLoS Negl Trop Dis. 2017;11(12):e0006104 10.1371/journal.pntd.0006104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nes WD. Biosynthesis of cholesterol and other sterols. Chem Rev. 2011;111(10):6423–51. 10.1021/cr200021m [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nes WD. Enzyme mechanisms for sterol C-methylations. Phytochemistry. 2003;64(1):75–95. [DOI] [PubMed] [Google Scholar]
- 25.Nes WD. Sterol methyl transferase: enzymology and inhibition. Biochim Biophys Acta. 2000;1529(1–3):63–88. [DOI] [PubMed] [Google Scholar]
- 26.Xu SH, Nes WD. Biosynthesis of cholesterol in the yeast mutant erg6. Biochem Biophys Res Commun. 1988;155(1):509–17. [DOI] [PubMed] [Google Scholar]
- 27.Millard P, Letisse F, Sokol S, Portais JC. IsoCor: correcting MS data in isotope labeling experiments. Bioinformatics. 2012;28(9):1294–6. 10.1093/bioinformatics/bts127 [DOI] [PubMed] [Google Scholar]
- 28.Wollam J, Magomedova L, Magner DB, Shen Y, Rottiers V, Motola DL, et al. The Rieske oxygenase DAF-36 functions as a cholesterol 7-desaturase in steroidogenic pathways governing longevity. Aging Cell. 2011;10(5):879–84. 10.1111/j.1474-9726.2011.00733.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercer EI. Morpholine antifungals and their mode of action. Biochem Soc Trans. 1991;19(3):788–93. [DOI] [PubMed] [Google Scholar]
- 30.Lamb DC, Warrilow AG, Rolley NJ, Parker JE, Nes WD, Smith SN, et al. Azole antifungal agents to treat the human pathogens Acanthamoeba castellanii and Acanthamoeba polyphaga through inhibition of sterol 14alpha-demethylase (CYP51). Antimicrob Agents Chemother. 2015;59(8):4707–13. 10.1128/AAC.00476-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Macedo-Silva ST, Visbal G, Urbina JA, de Souza W, Rodrigues JC. Potent in vitro antiproliferative synergism of combinations of ergosterol biosynthesis inhibitors against Leishmania amazonensis. Antimicrob Agents Chemother. 2015;59(10):6402–18. 10.1128/AAC.01150-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv. Enzyme Regul. 1984;22:27–55. [DOI] [PubMed] [Google Scholar]
- 33.Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–81. 10.1124/pr.58.3.10 [DOI] [PubMed] [Google Scholar]
- 34.Pardridge WM. Blood-brain barrier drug targeting: the future of brain drug development. Mol Interv. 2003;3(2):90–105, 51. 10.1124/mi.3.2.90 [DOI] [PubMed] [Google Scholar]
- 35.Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2(1):3–14. 10.1602/neurorx.2.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124(2):195–206. 10.1016/j.pharmthera.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albayrak Y, Hashimoto K. Sigma-1 receptor agonists and their clinical implications in neuropsychiatric disorders. Adv Exp Med Biol. 2017;964:153–61. 10.1007/978-3-319-50174-1_11 [DOI] [PubMed] [Google Scholar]
- 38.Niitsu T, Iyo M, Hashimoto K. Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases. Curr Pharm Des. 2012;18(7):875–83. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Kim JH, Sohn HJ, Yang HJ, Na BK, Chwae YJ, et al. Novel cathepsin B and cathepsin B-like cysteine protease of Naegleria fowleri excretory-secretory proteins and their biochemical properties. Parasitol Res. 2014;113(8):2765–76. 10.1007/s00436-014-3936-3 [DOI] [PubMed] [Google Scholar]
- 40.Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med. 2012;18(6):956–60. 10.1038/nm.2758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nes WD, Venkatramesh M. Molecular asymmetry and sterol evolution. Isopentenoids and other natural products. ACS Symposium Series. 562: American Chemical Society; 1994. p. 55–89.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.