ABSTRACT
This study explored the clinical implications of tumor mutational burden (TMB) in a well-defined HER2-positive metastatic breast cancer (MBC) patient population who had been previously treated but had subsequent disease progression. Whole exome sequencing was performed on formalin-fixed paraffin-embedded tumor samples and matched normal tissue. Among the 46 patients, 13 (28.3%) were estrogen receptor-positive and nine (19.6%) were progesterone receptor-positive by immunohistochemistry analysis. Twenty patients (43.5%) had recurrent MBC compared with de novo MBC (n = 26, 56.5%). Sixteen patients (34.6%) demonstrated more than 100 somatic non-synonymous SNV mutations, which was predefined as a high TMB. The median follow-up duration was 57.5 months. The median overall survival (mOS) differed significantly between low and high TMB status (44.9 months vs. 85.8 months, respectively, p = 0.016). In a multivariate Cox regression analysis, TMB was the only independent prognostic factor for good metastatic overall survival after adjusting for age and recurrence (Hazard ratio [HR] = 0.32, 95% confidence interval [CI], 0.103–0.998, p = 0.049). These data suggest that high TMB may be a prognostic marker for predicting good overall survival for patients undergoing conventional HER2-directed treatments and chemotherapy. Further, future clinical trials harnessing TMB may benefit by identifying an appropriate population who may have a favorable response to immunotherapy after recurrence following HER2-directed treatments.
KEYWORDS: Tumor mutational burden (TMB), Human epidermal growth factor receptor 2 (HER2), Metastatic breast cancer (MBC), Long survival outcomes, whole exome sequencing (WES)
Introduction
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer comprises 15–20% of total breast cancer diagnoses.1 HER2-targeted therapies, such as trastuzumab and/or pertuzumab plus cytotoxic chemotherapy, are an effective standard, first-line treatment option for HER2-positive metastatic breast cancer (MBC).2,3 Recent advances in HER2-targeting therapies, including dual HER2 blockade, have prolonged survival remarkably.3 In addition, several HER2-targeting therapeutics, including pertuzumab, ado-trastuzumab emtansine, and lapatinib, a reversible tyrosine kinase inhibitor (TKI), have been approved by the US Food and Drug Administration (FDA) for this treatment.3-5 However, HER2-positive MBC will eventually progress in most patients. Furthermore, primary and secondary resistance to anti-HER2-directed therapies, including trastuzumab, represent significant clinical problems.6 In patients with MBC treated with trastuzumab and chemotherapy, the median time to disease progression is 7.4 months and the objective response rate is 50%, suggesting that patients who initially respond to trastuzumab acquire resistance within a year.7
Analysis of tumor samples via whole exome sequencing (WES) has uncovered associations between clinical outcomes and tumor mutation burden (TMB).8,9 TMB has been defined as the sum of somatic non-synonymous single nucleotide variants (SNVs) that passed all the filters described. In non-breast cancer, TMB affected by DNA damage can predict overall survival.8 One emerging biomarker of outcome to immune-checkpoint blockade is the TMB. This finding is supported by the clinical activity of anti-PD-1 therapy in colorectal cancer with mismatch repair deficiency, a tumor subtype with mismatch repair proficiency, which has a significantly lower TMB and a poor response to these agents.10-12 Recent studies have suggested that the antigenicity of tumor cells is highly correlated with response to immune checkpoint inhibitors. It has also been hypothesized that highly mutated tumors are more likely to harbor neoantigens, which make them targets of activated immune cells. This metric has been shown, in several tumor types, to correlate with patient response to both CTLA-4 and PD-1 inhibition.9,13-15 Checkpoint blockade has also been found to be particularly effective in tumors with high TMB in certain indications such as melanoma8 and non-small cell lung cancer (NSCLC).9 However, cancers with lower TMB are less responsive to checkpoint inhibitors. One clinical trial showed that TMB was similarly associated with response rate compared with expression of PD-L1 as detected by immunohistochemistry in advanced metastatic urothelial carcinoma.16 Recently, several WES studies have demonstrated a significant correlation between median frequency of somatic mutations per megabase and median response rate to immunotherapy across solid tumors.17 Studies have also suggested that the antigenicity of tumor cells is highly correlated with response to immune checkpoint inhibitors.
We hypothesized that TMB is a potential prognostic or predictive marker candidate for conventional treatments, including HER2-targeting agents in HER2-positive MBC. Thus, this study explored the clinical implication of somatic TMB analyzed based on WES data in a well-defined HER2-positive MBC patient population who had been previously treated but eventually experienced disease progression.
The study was approved by the Institutional Review Board (IRB) of Samsung Medical Center. Our IRB approved the clinical sequencing program, Oncoseq 1, which enrolls patients with advanced breast cancer. Informed consent was obtained from all study participants.
Results
Patients
Among the 46 patients with HER2-positive MBC, the median age was 48 years (range: 29–68) and 36 patients were ≥40 years (78.3%). All (100%) patients were female and half were postmenopausal. The tumors of thirteen (28.3%) patients were determined to be estrogen receptor (ER)-positive and 9 (19.6%) were determined to be progesterone receptor (PR)-positive by immunohistochemical analysis. Twenty patients (43.5%) had recurrent MBC compared with initial de novo MBC (n = 26, 56.5%). In the first-line palliative chemotherapy regimen, 31 patients (67.4%) received docetaxel plus the HER2-targeting agent, trastuzumab, and seven patients (15.2%) received docetaxel and trastuzumab plus pertuzumab. A total of 16 (34.6%) tumors demonstrated more than 100 mutations (predefined as high TMB) based on WES. In second-line chemotherapy, adriamycin plus cyclophosphamide was the most common regimen (14 patients, 60.9%). There were no statistical differences in baseline characteristics between the high and low TMB groups (Table 1).
Table 1.
Baseline characteristics.
| Total | High TMB | Low TMB | ||
|---|---|---|---|---|
| (N = 46) | (n = 16) | (n = 30) | p value | |
| Median age (range), year | 48 (29–68) | 51 (32–68) | 43 (29–63) | 0.159 |
| Age ≥ 40 | 36 (78.3) | 15 (93.8) | 23 (76.7) | 0.145 |
| Sex | ||||
| Female | 46 (100.0) | 16 (100.0) | 30 (100.0) | |
| Menopausal status | 0.536 | |||
| Premenopausal | 23 (50.0) | 9 (56.3) | 14 (46.7) | |
| Postmenopausal | 23 (50.0) | 7 (43.8) | 16 (53.3) | |
| Histology | 0.644 | |||
| Invasive ductal carcinoma | 44 (95.7) | 15 (93.8) | 29 (96.7) | |
| Invasive lobular carcinoma | 2 (4.3) | 1 (6.3) | 1 (3.3) | |
| Tumor subtype at initial diagnosis | ||||
| ER positive | 13 (28.3) | 5 (31.3) | 7 (23.3) | 0.560 |
| PR positive | 9 (19.6) | 3 (18.8) | 6 (20.0) | 0.919 |
| Distant metastasis | 0.312 | |||
| Recurrence | 26 (56.5) | 11 (68.8) | 16 (53.3) | |
| Initial de novo stage IV | 20 (43.5) | 5 (31.3) | 14 (46.7) | |
| Regimen of chemotherapy (1st line) | 45 (97.8) | 16 (100.0) | 29 (96.7) | 0.137 |
| Docetaxel + Trastuzumab | 31 (67.4) | 14 (87.4) | 17 (56.7) | |
| Docetaxel + Trastuzumab + Pertuzumab | 7 (15.2) | 1 (6.3) | 6 (20.0) | |
| Capecitabine + Lapatinib | 5 (10.9) | 0 (0) | 5 (16.7) | |
| Other | 2 (4.3) | 1 (6.3) | 1 (3.3) | |
| Regimen of chemotherapy (2nd line) | 37 (80.4) | 13 (81.2) | 24 (80.0) | 0.165 |
| Adriamycin + Cyclophosphamide | 20 (54.1) | 6 (42.9) | 14 (60.9) | |
| Capecitabine + Lapatinib | 9 (24.3) | 5 (35.7) | 4 (17.4) | |
| Docetaxel + Trastuzumab | 4 (10.8) | 1 (7.1) | 3(13.0) | |
| Other | 4 (10.8) | 2 (14.3) | 2(8.7) |
TMB, tumor mutation burden; ER, estrogen receptor; PR, progesterone receptor.
Overall survival (OS) according to tumor mutation burden
The cutoff date for analyses was March 2017, resulting in a median follow-up duration of 82.4 months (95% confidence interval [CI]: 74.7–90.1 months). The median follow-up duration was 86.6 months in the low TMB group and 82.4 months in the high TMB group (p = 0.972). The median overall survival (mOS) value for those with low and high TMB status (44.9 months vs 85.8 months, respectively) differed significantly (p = 0.016) (Fig. 1). On univariate Cox regression, high TMB status provided a significant benefit for overall survival. Other clinical and pathological features were not statistically significant, even though age ≥ 40 years, recurrence status, ER positivity, and PR positivity were related to increased risk of death. For the final multivariate analysis using a stepwise approach, somatic mutation load ≥ 100, age ≥ 40 years, positive recurrence status, ER positivity, and PR positivity were selected (Supplementary Fig. 1). On the multivariate Cox regression analysis, high TMB was the independent prognostic factor associated with good metastatic OS after adjusting for age and recurrence status (hazard ratio [HR] = 0.213, 95% CI: 0.061–0.742, p = 0.015). Initial de novo metastatic disease was a poor prognostic factor for OS compared with recurrent breast cancer stage IV (Table 2).
Figure 1.
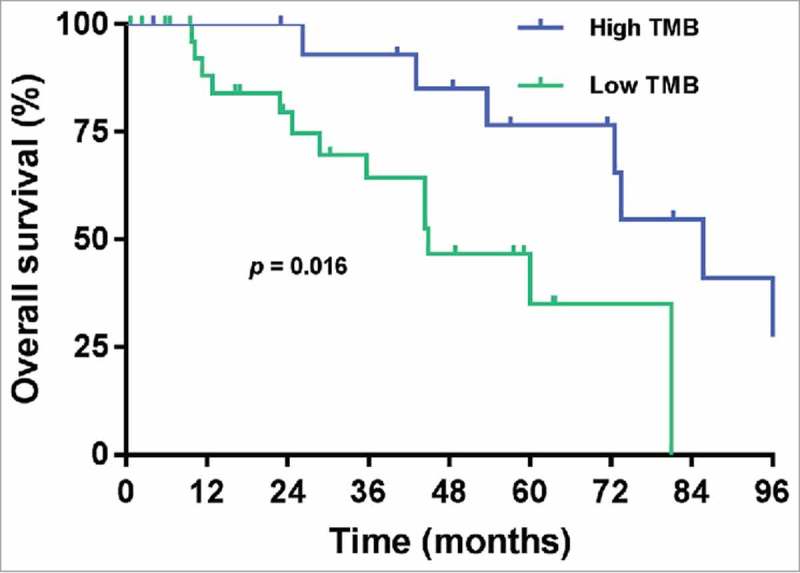
Overall survival (OS) according to tumor mutation burden (TMB) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer.
Table 2.
Univariate and multivariable Cox regression analyses for overall survival (OS).
| Univariate analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | p value | HR | 95% CI | p value |
| Somatic mutation load ≥ 100 | 0.276 | 0.091–0.833 | 0.022 | 0.213 | 0.061–0.742 | 0.015 |
| Age ≥ 40 years | 3.169 | 0.418–24.000 | 0.264 | 4.269 | 0.441–41.356 | 0.210 |
| Initial de novo | 2.028 | 0.835–4.924 | 0.118 | 3.348 | 1.106–10.136 | 0.032 |
| ER positive | 0.636 | 0.210–1.928 | 0.424 | 0.466 | 0.062–3.504 | 0.458 |
| PR positive | 1.275 | 0.421-3.860 | 0.667 | 6.989 | 0.859-56.859 | 0.069 |
HR, hazard ratio; ER, estrogen receptor; PR, progesterone receptor.
Statistically significant; C-index was calculated for the model from using a univariate Cox regression analysis.
Progression-free survival according to tumor mutation burden
Among the 46 patients, 45 (97.8%) received first-line palliative chemotherapy and one patient was deceased before treatment. All patients received HER2-targeting agents such as trastuzumab and/or pertuzumab or lapatinib. Disease progression or death in first-line palliative chemotherapy was observed in 37 patients (82.2%). The median progression-free survival (mPFS) based on low or high TMB status (9.6 months vs 17.1 months, respectively) was not significantly different in those who received first-line chemotherapy (p = 0.285) (Fig 2A). Thirty-seven patients received second-line chemotherapy. The mPFS based on low or high TMB status (4.4 months vs 5.6 months, respectively) was not significantly different in those who received second-line chemotherapy (p = 0.527) (Fig. 2B).
Figure 2.
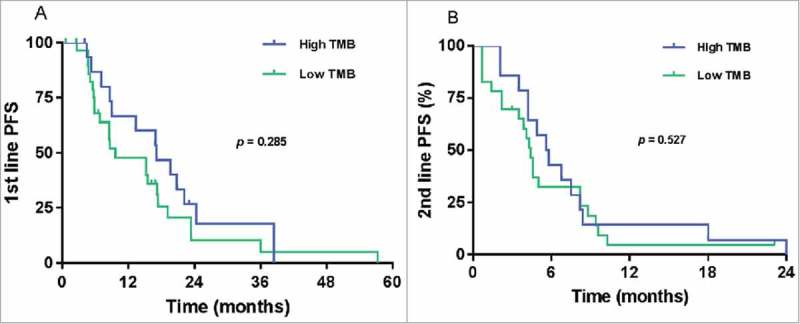
Progression-free survival (PFS) according to tumor mutation burden (TMB) in HER2-positive metastatic breast cancer in palliative first-line chemotherapy (A) and palliative second-line chemotherapy (B).
Pearson's linear correlation analysis according to TMB status
Patients with first-line palliative chemotherapy (n = 46) were stratified by tumor mutation burden, duration of OS, and duration of progression-free survival (PFS). All two-way comparisons were conducted by comparing patients who achieved clinical benefit (PFS > 12 months and OS > 2 years) with a HER2-targeting agent with patients who did not experience clinical benefits. Significantly greater clinical benefits were observed in the high TMB group compared with the low TMB group (p = 0.046) (Supplementary Fig. 2).
Correlation between TMB using by WES and number of molecular alteration using by FoundationOne®
We simulated a tumor mutation burden (TMB) after subsetting the FoundationOne® gene list in the WES results. Testing reported a range of 0 to 71 (mean, 11.67; standard deviation, 14.64; 95% CI, 7.85-15.96). There was significantly correlation between TMB using by WES and number of molecular alteration using by genes in FoundationOne® (Fig. 3A). When the FoundationOne® was classified as high molecular abnormality (≥ 20 mutations/Mb), the overall survival was significantly difference. The mOS at low and high number of molecular alteration using by genes in FoundationOne® (60.0 months vs. 104.3 months, respectively) was significantly different (p = 0.017) (Fig. 3B). And the mPFS by low and high number of molecular alteration using by genes in FoundationOne® (13.3 months vs. 22.1 months, respectively) was not significantly different in first line chemotherapy (p = 0.160) (Fig. 3C). However, there was no significant difference in overall survival when classified as intermediate molecular abnormality (≥ 6 mutations/Mb).
Figure 3.
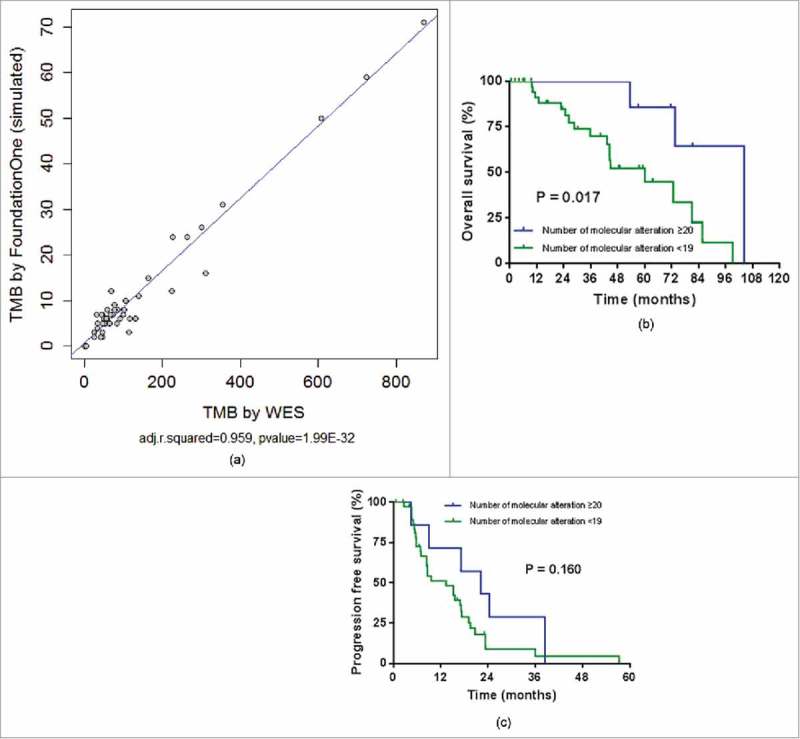
(A). Correlation between tumor mutation burden (TMB) using by whole exome sequencing (WES) and number of molecular alteration using by genes in FoundationOne®. (B). Overall survival by number of molecular alteration using by genes in FoundationOne®. (C). Progression free survival by number of molecular alteration using by genes in Foundation One®.
Discussion
A prognostic marker is needed to predict efficacy of the standard treatments, HER2-targeted agents and chemotherapy, because the prognosis is poor when HER2-positive MBC patients experience treatment failure. This study demonstrated that high TMB may be a good prognostic marker in HER2-positive MBC patients who have received standard treatments. The mOS was significantly longer in the high TMB status group based on WES.
Although immunotherapy has a good response rate in cancer patients with a high TMB, several immunotherapeutics that control immune check-points are still under exploration.14 There is some evidence that trastuzumab and immunotherapy act synergistically.18,19 There have also been reports that HER2-directed therapies, including trastuzumab and T-DM1, have some immunomodulatory therapeutic effects, providing a rationale for potential combination strategies with immunotherapy.11 Despite advances in HER2-directed therapies for patients with HER2-positive breast cancers, especially metastatic breast cancers, most HER2-positive MBC patients eventually progress after two or three lines of salvage therapy.20
We also hypothesized that a neoantigen might affect the clinical benefits of a HER2-targeting agent and cytotoxic chemotherapy. To test this, we performed WES on 46 patients with HER2-positive MBC. The neoantigen was a potential T cell target and predicted the response of specific treatment.21 The sum of somatic non-synonymous SNVs is a predictor of good response to chemotherapy due to increased neoantigen.16,17 The neoantigen causes non-synonymous SNVs that alter amino acid coding sequences.22 Some mutated peptides present on tumor cell surface and are recognized by neoantigen-specific T cells, isolated from tumor-infiltrating lymphocytes (TILs).23
Defining high TMB in each tumor type is still controversial. In this study, high TMB was predefined as >100 non-synonymous SNVs based on results showing that melanoma patients who receive clinical benefits from ipilimumab were classified as high neoantigen with mutational loads >100.24 In a different population using TCGA data, the authors defined high TML as median mutation load >65 mutations and reported no differences in prognosis in an ER-negative sub-group, while the HER2-positive sub-group was not analyzed. In addition, it has been reported that patients with high mutation load in ER-positive breast cancers demonstrate poor prognosis.25
In a two-way comparison based on mutation load, the average mOS has been reported at 20.7 months for first-line palliative chemotherapy, with or without a targeting agent, while the average median PFS of a placebo group was 12.4 months.3,26 Based on these studies, clinical benefit was defined as PFS > 12 months and OS > 24 months, and tumor burden mutation analysis and chi-square test were performed to demonstrate clinical benefits in the high TMB group.
One limitation of our study was a potential selection bias in relation to other studies due to including a small number of patients from a single center. Therefore, these results should be confirmed in larger number studies. However, it was meaningful that only HER2-positive breast cancer patients refractory to cytotoxic chemotherapy were enrolled. Given that the PD-1 and PD-L1 targeting immunotherapy in a high mutational burden population with other tumor types has shown favorable clinical benefits on preliminary analysis, we hypothesized that HER2-positive breast cancer patients with a high mutational burden and a poor response to cytotoxic chemotherapy would have good clinical outcomes with immunotherapy. In HER2 positive breast cancer, a HER2-targeting agent is the treatment of choice. If patients who progress after palliative chemotherapies have high-TMB on WES, we may consider a salvage immune-oncologic agent alone or in combination with other targeted agents. To our knowledge, this is the first report of TMB in HER2-positive breast cancer to show an association with response to standard chemotherapy with HER2-directed therapies. Further studies are needed to determine patient outcomes when immunotherapy is used.
In conclusion, high TMB was the only independent prognostic factor for good mOS. Based on these data and previous studies, we suggest that high TMB may not only be a prognostic marker for standard treatment but may also serve as a predictive marker to determine the next treatment option for immune checkpoint inhibitors when in progressed HER2-positive breast cancer patients who had previously received cytotoxic chemotherapy. Further, future clinical trials harnessing TMB could benefit from an ability to identify an appropriate population who may potentially demonstrate a favorable response to immunotherapy after recurrence following HER2-directed treatments. To our knowledge, this is the first report in which TMB has been shown to correlate with long-term survival in patients undergoing standard of care therapy for HER2-positive breast cancer.
Materials and methods
Sample collection
From 2007 to 2016, 46 patients with HER2-positive MBC who had progressed after more than two HER2-directed therapies were enrolled at Samsung Medical Center, Korea. TMB was defined as the sum of somatic non-synonymous somatic SNVs that passed all the filters described. WES and TMB analysis were performed using surgical specimens from patients or tissue biopsies of tumors performed at the time of breast cancer stage IV diagnosis. The data from all the patients who were enrolled were included in this analysis.
DNA extraction
Genomic DNA from tumor formalin-fixed paraffin-embedded (FFPE) samples was extracted with the QIAamp DNA FFPE Tissue kit (Qiagen, Valencia, CA, USA), according to the manufacturer's protocol. DNA from matched normal blood specimens was extracted with the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA).
Library preparation and next-generation sequencing
Genomic DNA was sheared in a Covaris S220 ultrasonicator (Covaris, Woburn, MA, USA). Genomic DNA from FFPE samples was used for the construction of a library using the Agilent SureSelect XT Human All Exon v5 kit (Agilent Technologies, Santa Clara, CA, USA) and the SureSelect XT Reagent Kit, HSQ (Agilent Technologies, Santa Clara, CA, USA). After hybridization of the library, the captured library was purified and amplified with an index barcode tag, and the library quality and quantity were assessed.
DNAseq analysis: Variant calling and annotation
WES was performed on FFPE tumor samples and matched normal tissue. Sequencing reads were aligned to a reference human genome using BWA-MEM, followed by standard preprocessing steps and the genome analysis tool kit (GATK) to generate analysis-ready BAM files. The MuTect software suite was used to generate somatic SNV calls using default parameters and by comparing BAM files from tumor and matched normal samples. SNVs present in the databases of common polymorphisms were filtered out. Mutation load (ML) for a subject was defined as the sum of somatic non-synonymous SNVs that passed all the filters described. We also divided the 100 mutation predefined cutoff into the top third (top 33%) mutational load. To investigate the correlation between the tumor mutational burden (TMB) from WES and those from the targeted sequencing, we generated simulation data by subsetting genes in the FoundationOne® panel from the WES results. FoundationOne® is the targeted panel sequencing that involved 315 cancer-related genes plus select introns from 28 genes, providing an accurate assessment of TMB.27 We applied 20 molecular/Mb abnormality as the cutoff of the high TMB level in this analysis, which was the top 20 percent number of molecular alterations.
Statistical analyses
Prognostic associations were assessed with Cox proportional hazard regression models and Kaplan–Meier survival curves. Variables included patient age, recurrence status, ER status, PR status, and somatic mutation load. Patients were divided into two groups for each variable: age < 40 years or ≥ 40 years, positive or negative recurrence status, positive or negative ER status, positive or negative PR status, and somatic mutation load < 100 or ≥ 100. Survival curves for the two groups were estimated using the Kaplan–Meier method, and a log-rank test was used to compare mOS curves between the two groups. Univariate and multivariable Cox proportional hazard regression models with a backward stepwise procedure were used to assess the impact of potential prognostic variables on mOS. Results were expressed as HR and 95% CIs, and graphically displayed in GraphPad Prism 7. All statistical analyses and plots were conducted using SPSS v.24 (IBM Corp., Armonk, NY) software package and p-values < 0.05 were considered statistically significant.
Supplementary Material
Funding Statement
This work was supported by and a grant from the National Research Foundation of Korea (NRF-2018R1A2B6004690) and a grant from the Ministry of Health & Welfare, Republic of Korea (HI13C2096).
Author's contribution
Conception and design: Hun Jung, Kyunghee Park, Yeon Hee Park
Development of methodology: Razvan Cristescue, Yeon Hee Park
Acquisition of data: Song Ee Park, Kyunghee Park, Eunjin Lee, Woong-Yang Park, Soo Youn Cho
Analysis and interpretation of data: Ji-Yeon Kim, Jin Seok Ahn, Young-Hyuck Im, Soo Youn Cho
Writing, review, revision of manuscript: Hun Jung, Song Ee Park, Kyunghee Park, Choonghoon Lee, Hun Jung, Razvan Cristescue, Yeon Hee Park
Study supervision: Yeon Hee Park
Disclosure of potential conflicts of interest
RC is a contracted employee of eMerck & Co.
CL is a contracted employee of MSD Korea Ltd.
References
- 1.Chen X, Yuan Y, Gu Z, Shen K. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;134:957–67. doi: 10.1007/s10549-012-1990-z. PMID:22370627. [DOI] [PubMed] [Google Scholar]
- 2.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, et al.. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. PMID:15911866. [DOI] [PubMed] [Google Scholar]
- 3.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A, et al.. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–71. doi: 10.1016/S1470-2045(13)70130-X. PMID:23602601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, et al.. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. PMID:17192538. [DOI] [PubMed] [Google Scholar]
- 5.Muller P, Kreuzaler M, Khan T, Thommen DS, Martin K, Glatz K, Savic S, Harbeck N, Nitz U, Gluz O, et al.. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med. 2015;7:315ra188. doi: 10.1126/scitranslmed.aac4925. PMID:26606967. [DOI] [PubMed] [Google Scholar]
- 6.Chung A, Cui X, Audeh W, Giuliano A. Current status of anti-human epidermal growth factor receptor 2 therapies: predicting and overcoming herceptin resistance. Clin Breast Cancer. 2013;13:223–32. doi: 10.1016/j.clbc.2013.04.001. PMID:23829888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, et al.. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. PMID:11248153. [DOI] [PubMed] [Google Scholar]
- 8.Mar VJ, Wong SQ, Li J, Scolyer RA, McLean C, Papenfuss AT, Tothill RW, Kakavand H, Mann GJ, Thompson JF, et al.. BRAF/NRAS wild-type melanomas have a high mutation load correlating with histologic and molecular signatures of UV damage. Clin Cancer Res. 2013;19:4589–98. doi: 10.1158/1078-0432.CCR-13-0398. PMID:23833303. [DOI] [PubMed] [Google Scholar]
- 9.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al.. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–8. doi: 10.1126/science.aaa1348. PMID:25765070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 Inhibition. N Engl J Med. 2017;377:2500–1. doi: 10.1056/NEJMc1713444. PMID:29262275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al.. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357:409–13. doi: 10.1126/science.aan6733. PMID:28596308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al.. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–20. doi: 10.1056/NEJMoa1500596. PMID:26028255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al.. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. PMID:21498393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, Foppen MHG, Goldinger SM, et al.. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–11. doi: 10.1126/science.aad0095. PMID:26359337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al.. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–9. doi: 10.1056/NEJMoa1406498. PMID:25409260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, et al.. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. PMID:26952546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Champiat S, Ferte C, Lebel-Binay S, Eggermont A, Soria JC. Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy. Oncoimmunology. 2014;3:e27817. doi: 10.4161/onci.27817. PMID:24605269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Varadan V, Gilmore H, Miskimen KL, Tuck D, Parsai S, Awadallah A, Krop IE, Winer EP, Bossuyt V, Somlo G, et al.. Immune signatures following single dose trastuzumab predict pathologic response to preoperative trastuzumab and chemotherapy in HER2-positive early breast cancer. Clin Cancer Res. 2016;22:3249–59. doi: 10.1158/1078-0432.CCR-15-2021. PMID:26842237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loi S, Andre F, Maibach R, Hui R, Bartsch R, Jerusalem G, Gombos A, Campone M, Bonnefoi H, Bachelot T, et al.. Abstract OT3-01-05: PANACEA (IBCSG 45-13/BIG 4–13): A phase Ib/II trial evaluating the efficacy of pembrolizumab and trastuzumab in patients with trastuzumab-resistant, HER2-positive, metastatic breast cancer. Cancer Res. 2016;76:OT3–01–5–OT3–5. doi: 10.1158/1538-7445.sabcs15-ot3-01-05. [DOI] [Google Scholar]
- 20.Roche H, Vahdat LT. Treatment of metastatic breast cancer: second line and beyond. Ann Oncol. 2011;22:1000–10. doi: 10.1093/annonc/mdq429. PMID:20966181. [DOI] [PubMed] [Google Scholar]
- 21.Overwijk WW, Wang E, Marincola FM, Rammensee HG, Restifo NP. Mining the mutanome: developing highly personalized immunotherapies based on mutational analysis of tumors. J Immunother Cancer. 2013;1:11. doi: 10.1186/2051-1426-1-11. PMID:24829748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu YC, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28:22–7. doi: 10.1016/j.smim.2015.11.002. PMID:26653770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, et al.. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–37. doi: 10.1158/1078-0432.CCR-09-0737. PMID:19723653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roszik J, Haydu LE, Hess KR, Oba J, Joon AY, Siroy AE, Karpinets TV, Stingo FC, Baladandayuthapani V, Tetzlaff MT, et al.. Novel algorithmic approach predicts tumor mutation load and correlates with immunotherapy clinical outcomes using a defined gene mutation set. BMC Med. 2016;14:168. doi: 10.1186/s12916-016-0705-4. PMID:27776519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haricharan S, Bainbridge MN, Scheet P, Brown PH. Somatic mutation load of estrogen receptor-positive breast tumors predicts overall survival: an analysis of genome sequence data. Breast Cancer Res Treat. 2014;146:211–20. doi: 10.1007/s10549-014-2991-x. PMID:24839032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saad ED, Katz A, Buyse M. Overall survival and post-progression survival in advanced breast cancer: a review of recent randomized clinical trials. J Clin Oncol. 2010;28:1958–62. doi: 10.1200/JCO.2009.25.5414. PMID:20194852. [DOI] [PubMed] [Google Scholar]
- 27.Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J, et al.. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9:34. doi: 10.1186/s13073-017-0424-2. PMID:28420421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


