ABSTRACT
This study evaluated the effects of combining an OX40 agonistic antibody (aOX40) with a cell vaccine targeting HER2/neu, called “Triplex”. Such HER2/neu cell vaccine included two biological adjuvants (interleukin 12 (IL12) and allogeneic histocompatibility antigens) and was previously found able to prevent autochthonous HER2/neu-driven mammary carcinogenesis. Timing of aOX40 administration, concomitantly or after cell vaccination, gave opposite results. Unexpectedly, vaccine efficacy was hampered by concomitant OX40 triggering. Such decreased immunoprevention was likely due to a reduced induction of anti-HER2/neu antibodies and to a higher level of Treg activation. On the contrary, aOX40 administration after the completion of vaccination slightly but significantly increased immunopreventive vaccine efficacy, and led to increased production of GM-CSF and IL10. In conclusion, OX40 triggering can either impair or ameliorate immunoprevention of HER2/neu-driven mammary carcinogenesis depending on the schedule of aOX40 administration.
KEYWORDS: Cancer vaccine, HER2/neu, immunoprevention, mammary cancer, OX40
Introduction
Immunoprevention in individuals prone to develop cancers is an appealing approach.1 Cancer vaccines applied to advanced tumors often fail, likely because of multiple immune escape mechanisms. On the contrary, vaccination of cancer prone mice succeeded in significantly delaying cancer onset. A well-studied model for cancer immunoprevention is that of HER2/neu transgenic mice, in which autochthonous mammary cancer can be almost totally prevented by a cell vaccine combining different stimuli (HER2/neu antigen and, as adjuvants, allogeneic major histocompatibility antigens and IL12).2-4 Cancer immunoprevention of autochthonous tumors by cell vaccine relied on the induction of anti-HER2/neu antibodies and IFNγ.5 However, vaccine optimal effects were dependent on two conditions (start of vaccination at a preneoplastic stage and lifelong administration of the vaccine)6 that could be hardly implemented in a translational perspective.
In tumor-bearing mice, hyporesponsivity to tumor antigens can be driven by high proportion of specific regulatory T cells (Treg) and a Treg immune-suppressive microenvironment.7 Treg were found to play a role in tolerance to HER2/neu and depletion of Treg combined to immunization elicited a stronger immune response.8,9 Chronic IL12 administration to HER2/neu tolerant mice can effectively induce IFNγ, but in the long term this was countered by the induction of Treg cells.10 In search of more effective immunopreventive approaches, we investigated the combination of the cell vaccine with a Treg targeting approach consisting in engagement of OX40.
OX40 is a co-stimulatory molecule belonging to the TNFR immune checkpoints family.7,11,12 In mice OX40 is constitutively expressed on Treg, as well as on activated CD4 and CD8 immune cells. OX40 triggering with agonistic antibodies determines the functional inactivation of Treg, prolongs the survival of antigen-activated immune cells, and leads to tumor rejection, tolerance prevention and reversion of the immune anergy.13-17 Such activities prompted the inclusion of OX40 engagement in murine models and in human trials of cancer immunotherapy, often in combination with other immunological approaches.7,11,12
A combined immunotherapy based on OX40 triggering plus vaccination showed an increased ability to eradicate or prevent the growth of HER2/neu cells.18-22 Most studies, however, used cancer cell injection to induce tumors or to evaluate vaccine power, often in non-tolerant or partially HER2/neu-tolerant mice. Under these conditions the main response evoked was a T cell response. HER2/neu transgenic mice showing onset of autochthonous mammary tumors could more faithfully model the situation of spontaneously arising tumors and relationships with their immunologically tolerant host.
In the present study we investigated the effect of OX40 triggering in combination with a cancer vaccine for the prevention of autochthonous HER2/neu-driven mammary tumors.
Results and discussion
HER2/neu transgenic BALB-neuT mice show spontaneous onset of mammary carcinoma, with the first tumor being observed at a median time of 16 weeks of age.2 In these mice mammary carcinoma can be almost completely prevented by the life-long vaccination with an engineered IL12-producing allogeneic HER2/neu-positive cell vaccine through mechanisms that include host production of anti-neu antibodies and IFNγ.3,5,6 In search of more efficient vaccine schedules, we investigated the efficacy of combining vaccination with a monoclonal antibody triggering OX40 (aOX40). A suboptimal vaccine schedule was chosen to observe either increased or decreased preventive efficacy: mice were therefore vaccinated starting at 10 weeks of age for three monthly cycles. Two schedules of aOX40 administration were used: concomitant to cell vaccine (aOX40+vax) or after the completion of 3 cycles of vaccination (aOX40postvax) (Fig. 1A). Cell vaccine alone, even at the suboptimal conditions here employed, was able to delay the spontaneous onset of mammary carcinoma, with the first tumor being observed at a median time of 35 weeks (Fig. 1B and C, black circles). OX40 triggering with the agonistic OX86 antibody concomitant to vaccine (aOX40+vax) partially impaired prevention efficacy, causing a significantly earlier tumor onset (at a median time of 28.5 weeks) and higher numbers of tumors per mouse with respect to vaccine alone (Fig. 1B and C, red squares). The aOX40postvax schedule yielded a slight but significant increase in the immunopreventive activity of the vaccine, with tumor onset at a median age of 39 weeks and decreased number of tumors per mouse (Fig. 1B and 1C, blue triangles).
Figure 1.
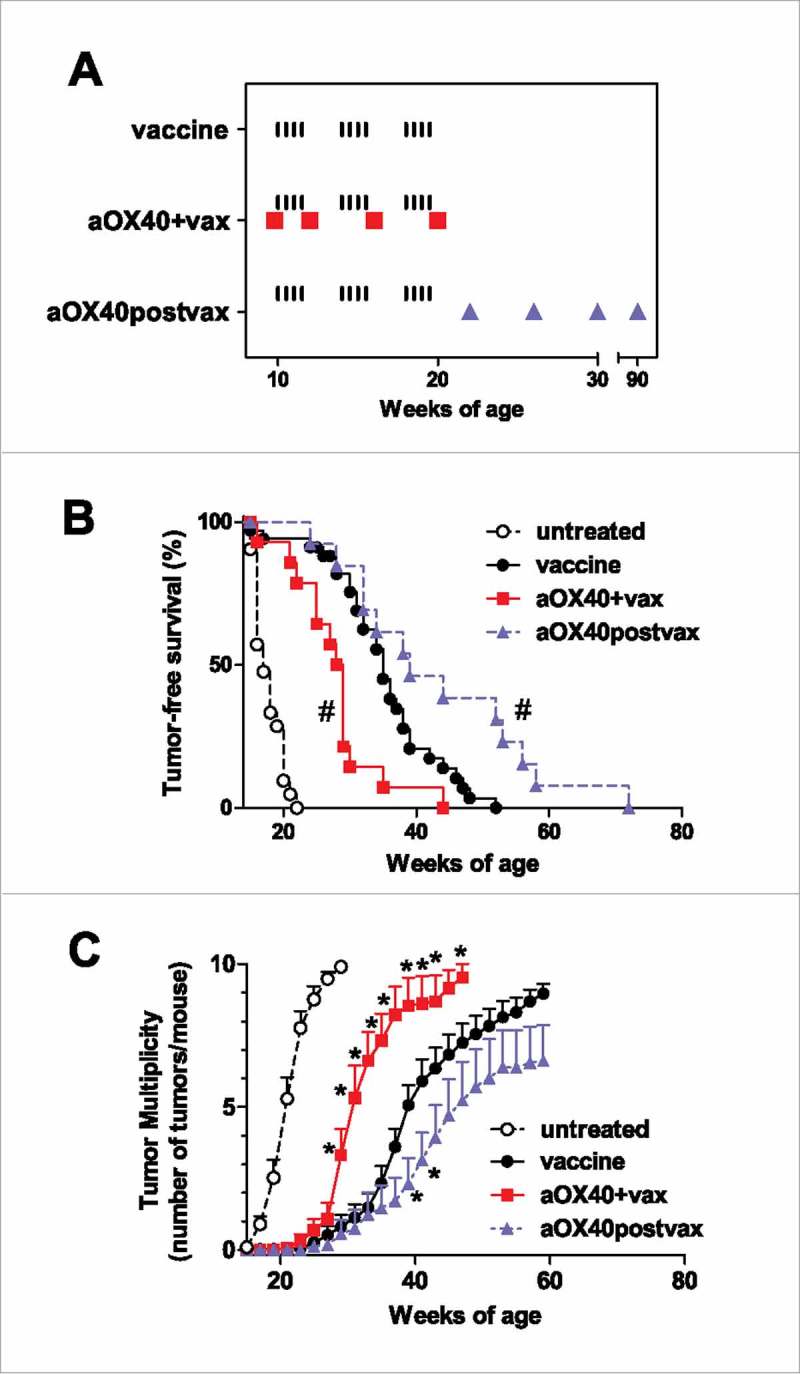
Effect of different timings of aOX40 administration combined to vaccination on mammary carcinogenesis in BALB-neuT mice. (A) Schedule of mice treatment. Black ticks: i.p. injection of a cell vaccine dose. Red squares: i.p. injection of aOX40 concomitant to vaccine (aOX40+vax). Blue triangles: i.p. injection of aOX40 after completion of vaccine cycles (aOX40postvax). (B) Tumor-free survival curves. Groups: untreated, n = 21; vaccine: n = 34; aOX40+vax, n = 13; aOX40postvax, n = 18. #p < 0.01 vs vaccine only group (Mantel-Haenszel test). All treated groups were significantly different from untreated (p < 0.01 at least). (C) Tumor multiplicity. Mean ± SEM. *p < 0.05 at least vs vaccine only (Student's t test). Untreated mice received vehicle (PBS) alone.
Immunopreventive efficacy in BALB-neuT mice was dependent on humoral mechanisms.5,6 We therefore evaluated the induction of anti-HER2/neu antibodies in the different groups of treatment (Fig. 2). Mice treated with cell vaccine alone showed an increase of anti-HER2/neu antibodies during vaccination that peaked at 20 weeks. Thereafter vaccination was discontinued and antibody level progressively declined. Such decrease preceeded the onset of mammary tumors, confirming the role played by anti-neu antibodies in the immunopreventive effect.5,6 Mice receiving aOX40 concomitant with cell vaccine (aOX40+vax) had a significantly decreased production of anti-neu antibodies, which further decreased after vaccine discontinuation, reaching very low levels at about 37 weeks. aOX40+vax-treated mice produced significantly less total IgG against HER2/neu (Fig. 2A) and significantly less IgG2a and IgG3 than mice subjected to vaccine only, with unaltered isotype ratios (Fig. 2B-C). Antibody response included anti-H-2q IgG antibodies (Fig. 2A inset). No preferential isotype induction was observed in anti-H-2q response (Fig. 2D). The decreased antibody levels correlated well with the decreased immunoprevention by cell vaccine. Mice treated with aOX40 after the completion of the three vaccine cycles (aOX40postvax) showed kinetics and isotypes of anti-neu antibodies superimposable to that of vaccine alone (Fig. 2A, B, C). Since the administration of aOX40postvax did not maintain higher anti-neu antibody levels, the increased preventive efficacy of the OX40 triggering after the completion of cell vaccination should rely upon antibody-independent mechanisms.
Figure 2.
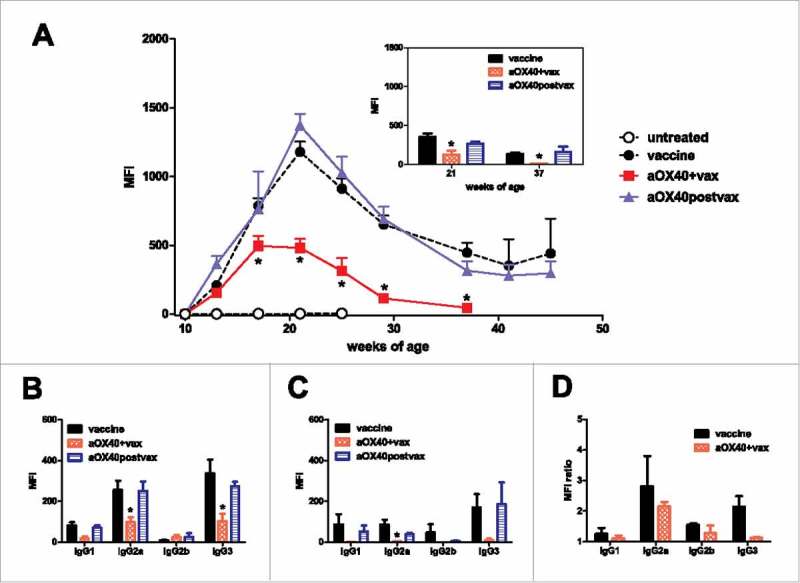
Effect of different timings of aOX40 administration combined to vaccination on the induction of anti-vaccine antibodies. (A) Kinetics of anti-vaccine antibodies in mice groups of Fig. 1. MFI = Median fluorescence intensity. Mean ± SEM is shown for each point. *p < 0.01 at least vs vaccine only group (Student's t test). Inset: anti-H-2q antibodies at two time points (21 and 37 weeks of age). *p < 0.05 at least vs vaccine only group (Student's t test). (B) Anti-vaccine antibody isotypes at 21 weeks of age. MFI as in A. Mean ± SEM is shown for each point. *p < 0.05 at least vs vaccine only group (Student's t test). (C) Anti-vaccine antibody isotypes at 37 weeks of age. MFI as in A. Mean ± SEM is shown for each point. *p < 0.05 at least vs vaccine only group (Student's t test). (D) Ratio between anti-vaccine and anti-H-2q isotype antibodies at 21 weeks of age. MFI ratio = MFI on TT12.E2 cells/MFI on N202.1E cells.
HER2/neu transgenic mice showed about 15% of Treg both in blood and in lymphoid organs (axillary and mesentheric lymph nodes and spleen) (Fig. 3 and data not shown). aOX40+vax did not affect Treg number, but significantly increased the frequency of Treg expressing the activation marker CD103. aOX40+vax treatment did not modify Treg expression of GITR (see Fig. 3) or CD44 (data not shown) in comparison to vaccine only. Therefore, in aOX40+vax-treated mice, Treg frequency was unaffected but Treg appeared more activated.
Figure 3.
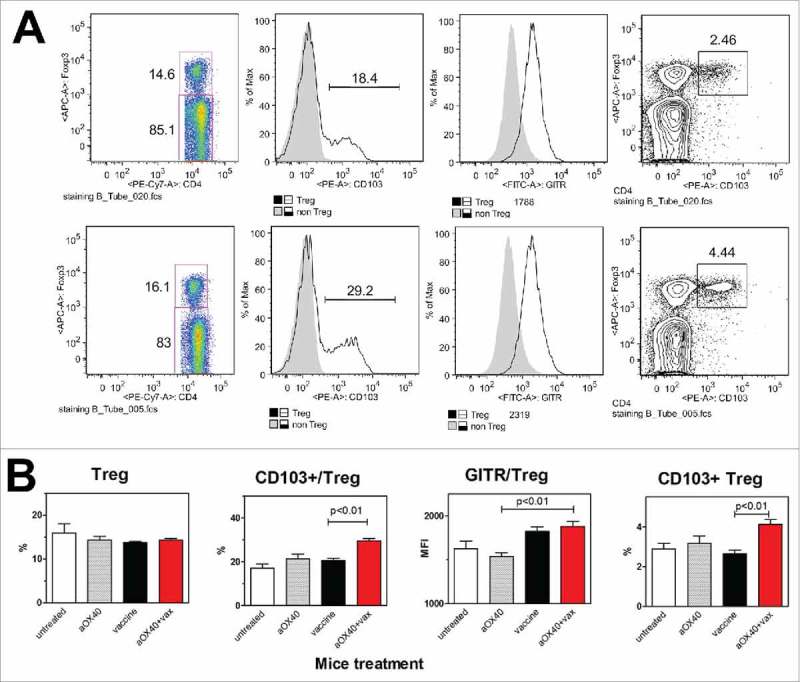
Effect of aOX40+vax combined treatment on Treg number and phenotype. (A) Cytofluorometric plots of a representative mouse per group (at 17 weeks of age): vaccine alone (upper row), aOX40+vax (lower row). Panels from left to right show: Treg frequency over total splenocytes, CD103+ cells over Treg, GITR expression level (Mean fluorescence intensity, MFI) over Treg, CD103+ Treg frequency in gated CD4+ splenocytes. (B) Each bar represents the mean and SEM from mice of the different groups studied (at 17 weeks of age). For comparison, data from untreated mice and from mice treated with aOX40 alone are shown. Groups: untreated, n = 3; aOX40, n = 10; vaccine, n = 20; aOX40+vax, n = 25. Significance at the Student's t test is reported.
In the aOX40postvax schedule, the frequency of Treg and the frequency of effector memory T cells (Tem) were unaltered, with respect to vaccine only group (Fig. 4A). Splenocytes of aOX40postvax mice, restimulated for 6 days with HER2/neu cells, showed a significantly higher production of GM-CSF and IL10 than splenocytes of mice treated with vaccine only, whereas IFNγ showed no difference (Fig. 4B).
Figure 4.
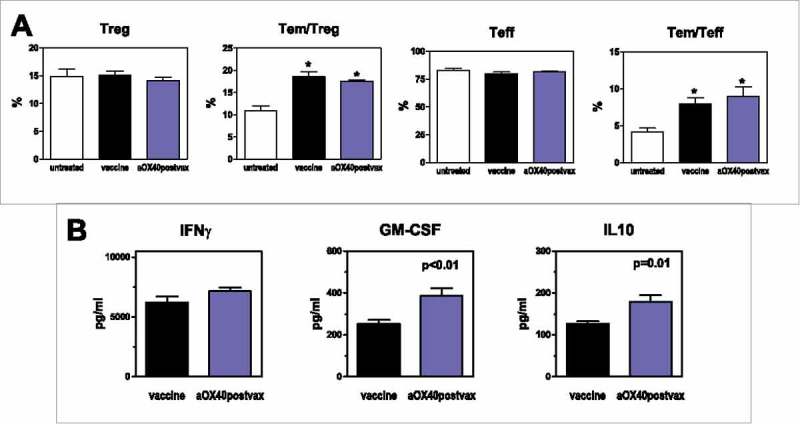
Effects of aOX40postvax combined treatment. (A) Frequency of Treg, effector memory (Tem)/Treg, T effector (Teff) and effector memory/Teff. Each bar represents the mean and SEM from mice of the different groups studied (at 32 weeks of age). Groups: untreated, n = 4; vaccine, n = 8; aOX40postvax, n = 8. *p≤0.01 vs untreated group (Student's t test). (B) Cytokine production by restimulated spleen cells (collected from mice at 32 weeks of age). Each bar represents the mean and SEM from mice of the different groups studied. Groups: vaccine, n = 6; aOX40postvax, n = 6. Significance at the Student's t test is reported.
Our study shows that the administration of an agonistic antibody triggering OX40 combined to a powerful cell vaccine can tune the immunopreventive ability depending on aOX40 timing: vaccine efficacy was hampered by concomitant OX40 triggering, but was increased by aOX40 administration after the completion of vaccination. Contrasting results have been reported regarding the effect of OX40 triggering on Treg perhaps in dependency of tumor microenvironment.11,12 Here we showed for the first time that OX40 triggering can have opposite results when combined with a cell vaccine even within the same tumor model depending on the timing of aOX40 administration.
Treatment with aOX40 after the completion of vaccinations induced a weak but significant increase in vaccine efficacy, in accordance with published results showing that OX40 activation boosted a previous cell vaccination.23 However, increased efficacy was not related to variations in antibody response or in Treg and effector memory T cell frequencies in the spleen of treated mice. The only observed variations were the increased production of GM-CSF and IL10 but not IFNγ in splenocytes. Increased expression of IL10 and GM-CSF was reported by OX40 triggering in some studies.21,24 It is worth noting that IL10, which is generally considered as a suppressor cytokine, also has antitumor activities.25-27
The decreased preventive efficacy induced by concomitant OX40 triggering was unexpected but correlated well with the decreased antibody response (the main effector of immunoprevention) and with the induction of a more activated Treg phenotype. Both reduction and increase of immune suppression upon OX40 engagement in different model systems are possible.11,12
In addition, some studies report OX40 triggering combined with anti-tumor immunization in mice injected with cancer cell lines. Using a tumor cell line derived from HER2/neu transgenic mice, an immunotherapeutic approach combining OX40 agonist and CTLA-4 blockade together with HER2 vaccination reversed T-cell anergy and extended survival of tumor-bearing mice.21
In our study, combined immunotherapy was investigated to prevent the progression of a HER2/neu-driven spontaneous carcinogenic program. It was started when only preneoplastic lesions were present and lasted for several months during which tumor growth was delayed but progressed anyway. This implies that mice had already been exposed to tumor antigen, i.e. even the pretreatment with aOX40 did not find truly naïve mice. Several models studied so far administered aOX40 to truly naïve mice and then vaccinated them or challenged them with tumor cells. Patients are much more similar to our model (not truly naïve) than to vaccination-challenge model systems. Furthermore prevention studies span several months, during which the immune response is edited by tumor growth, and the microenvironment is continuously changing, because studies last up to one year and a half. Treg frequency increases with age28 and strong age-related differences in responses induced by OX40 triggering have been reported.29 In a comprehensive immunological perspective, it should be kept in mind that our vaccine was adjuvanted by transduced expression of IL12 and by allogeneic histocompatibility antigens, therefore the observed effects are due to the combined activity of all the immune stimuli. Finally, HER2/neu driven mammary carcinogenesis ultimately affect all the mammary glands and, within each gland, multifocal neoplasms can arise. This is the reason why we choose a systemic administration route. Better results were obtained with the intratumoral administration of aOX40, however it is a route that could be hardly applied to a disseminated neoplasm.12
Cancer immunotherapy has entered a new renaissance since checkpoint inhibitors have shown the ability to elicit powerful long-lasting immune responses leading to clinical benefit in 20–30% of patients.12 However, most patients are resistant, and about 10% patients undergo a rapid progression under checkpoint inhibitor treatment.30 Combining immunomodulating strategies could increase the proportion of responders, provided that the optimal timing of combination is choosen.31,32 Our study shows that when combining OX40 engagement with an adjuvanted cancer vaccine opposite results can be obtained depending on timing of OX40 triggering. OX40 is therefore emerging as a relevant tuning molecule that might make the difference in combination therapies in either a positive or negative way.32,33 The negative interference observed with OX40 triggering concomitant with cancer vaccine suggests that preclinical models should be thoroughly interrogated before planning clinical trials of combined approaches.
Materials and methods
Mice and tumor growth
BALB-neuT female mice (H-2d haplotype), transgenic for a mutant rat HER2/neu oncogene driven by the mouse mammary tumor virus promoter, were bred and genetically screened as reported.2 Experiments were approved by the institutional review board of the University of Bologna, authorized by the Italian Ministry of Health and done according to Italian and European laws and guidelines. Individually tagged virgin females used in the experiments were treated and inspected twice weekly for mammary tumor onset. Progressively growing masses of >3 mm in mean diameter were regarded as tumors. Mice with tumors in multiple mammary glands, or one tumor exceeding a mean diameter of 1.5 cm, were killed for ethical reasons. Tumor multiplicity is the number of tumors per mouse at each time point and is expressed as mean ± SEM for each experimental group.6
Vaccine
Vaccine consisted of allogeneic (H-2q) murine mammary carcinoma cells expressing high levels of HER2/neu and releasing transduced IL12.3 Each vaccine dose consisted of 2 × 106 cells, proliferation-blocked by treatment with mitomycin C (40 μg/ml Sigma-Aldrich, Milan, Italy), administered intraperitoneally in 0.4 ml of phosphate-buffered saline (PBS) (Invitrogen, Milan, Italy). Control mice received PBS alone. Mice were vaccinated at 10, 11, 14, 15, 18, 19 weeks of age (two doses per week, 12 vaccinations in total).
aOX40 treatment
The rat IgG1 monoclonal antibody OX86 (European Collection of Cell Cultures), which binds OX40 with agonist activity (here referred to as aOX40) was administered i.p. (100 μg in 200 μl),14 according the following schedules (as shown in Fig. 1A):
aOX40+vax: treatment with aOX40 the day before the first vaccination (at 10 weeks of age) and in weeks 12, 16, 20.
aOX40postvax: treatment with aOX40 every 4 weeks starting at 22 weeks of age.
In Vitro restimulation, cytotoxicity assay and cytokine release
Mixed lymphocyte-tumor cell cultures (MLTC) were performed with spleen mononuclear cells cocultured at a 50:1 ratio with proliferation-blocked Neu/H-2q cells for 6 days in RPMI 1640 supplemented with 10% fetal bovine serum and with 20 units/ml of recombinant IL-2. Supernatants from MLTC were assayed for IFNγ, GM-CSF and IL10 by ELISA assays (R&D Systems Inc.).
Antibody Response
Sera were collected from individual mice at different time points and stored at −80°C. To determine the level of anti-vaccine antibodies, sera diluted 1:65 were used in indirect immunofluorescence assay to stain viable TT12.E2 cells (HER2/neu overexpressing and H-2q positive) followed by cytofluorometric analysis as previously described.3 The intensity of fluorescence of each serum sample was normalized to the expression of rat HER2/neu by the target cells (determined using the monoclonal antibody Ab4, clone 7.16.4, 5 μg/ml, Oncogene Research Products, Cambridge, MA, USA). Anti-H-2q antibodies were determined by indirect immunofluorescence assay against N202.1E cells (HER2/neu-negative and H-2q positive). Isotype subclasses analyses were carried out by indirect immunofluorescence assays with sera diluted 1:20. Secondary fluorescein-conjugated monoclonal antibodies directed against Ig subclasses were as follows: anti–mouse IgG1 clone A85–1; anti–mouse IgG2a clone R19–15; anti–mouse IgG2b clone R12–3; anti–mouse IgG3 clone R40–82. All of them were purchased from BD PharMingen.
Lymphocyte subpopulations
Cell suspensions were obtained from individual lymphoid organs (spleen, bone marrow, lymph nodes) as described.16 PE-Cy7 anti-CD4 (RM4–5), APC anti-Foxp3 (FJK-16s), PE anti-CD103 (2E7) and FITC anti-GITR were purchased from eBioscience. Surface staining was performed on cells obtained from each organ by incubating antibodies at 5 μg/mL on ice for 30 min in PBS containing 2% FBS. Flow cytometry data were acquired on a FACSCalibur (Becton Dickinson) and analysed with FlowJo software (version 8.8.4; Treestar). Data from different organs of each mouse, if statistically not different, were pooled in the final elaboration.
Statistical analysis
Differences in tumor-free survival curves were analyzed by the Mantel-Haenszel test. Tumor multiplicity, T cell frequency, antibody or cytokine levels were compared by the Student's t test or the nonparametric Wilcoxon test.
Funding Statement
This work was supported by grants from the Italian Association for Cancer Research (AIRC) (IG15324 to P-L. Lollini), the Department of Experimental, Diagnostic and Specialty Medicine of the University of Bologna (DIMES) (“Pallotti” Fund) and the University of Bologna.
Disclosure of potential conflicts of interests
No potential conflicts of interest were disclosed.
Acknowledgments
This paper is dedicated to the memory of Giorgio Prodi, thirty years after his untimely death.
References
- 1.Finn OJ, Beatty PL. Cancer immunoprevention. Curr Opin Immunol. 2016;39:52–58. doi: 10.1016/j.coi.2016.01.002. PMID:26799207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nanni P, Nicoletti G, De Giovanni C, Landuzzi L, Di Carlo E, Cavallo F, Pupa SM, Rossi I, Colombo MP, Ricci C, et al.. Combined allogeneic tumor cell vaccination and systemic interleukin 12 prevents mammary carcinogenesis in HER-2/neu transgenic mice. J Exp Med. 2001;194(9):1195–205. doi: 10.1084/jem.194.9.1195. PMID:11696586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Giovanni C, Nicoletti G, Landuzzi L, Astolfi A, Croci S, Comes A, Ferrini S, Meazza R, Iezzi M, Di Carlo E, et al.. Immunoprevention of HER-2/neu transgenic mammary carcinoma through an interleukin 12-engineered allogeneic cell vaccine. Cancer Res. 2004;64(11):4001–9. doi: 10.1158/0008-5472.CAN-03-2984. PMID:15173014. [DOI] [PubMed] [Google Scholar]
- 4.Lollini PL, Cavallo F, Nanni P, Forni G. Vaccines for tumour prevention. Nat Rev Cancer. 2006;6(3):204–16. doi: 10.1038/nrc1815. PMID:16498443. [DOI] [PubMed] [Google Scholar]
- 5.Nanni P, Landuzzi L, Nicoletti G, De Giovanni C, Rossi I, Croci S, Astolfi A, Iezzi M, Di Carlo E, Musiani P, et al.. Immunoprevention of mammary carcinoma in HER-2/neu transgenic mice is IFN-gamma and B cell dependent. J Immunol. 2004;173(4):2288–96. doi: 10.4049/jimmunol.173.4.2288. PMID:15294941. [DOI] [PubMed] [Google Scholar]
- 6.Palladini A, Nicoletti G, Pappalardo F, Murgo A, Grosso V, Stivani V, Ianzano ML, Antognoli A, Croci S, Landuzzi L, et al.. In silico modeling and in vivo efficacy of cancer-preventive vaccinations. Cancer Res. 2010;70(20):7755–63. doi: 10.1158/0008-5472.CAN-10-0701. PMID:20924100. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa H, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Curr Opin Immunol. 2014;27:1–7. doi: 10.1016/j.coi.2013.12.005. PMID:24413387. [DOI] [PubMed] [Google Scholar]
- 8.Jacob JB, Kong YC, Meroueh C, Snower DP, David CS, Ho YS, Wei WZ. Control of Her-2 tumor immunity and thyroid autoimmunity by MHC and regulatory T cells. Cancer Res. 2007;67(14):7020–7. doi: 10.1158/0008-5472.CAN-06-4755. PMID:17638915. [DOI] [PubMed] [Google Scholar]
- 9.Weiss VL, Lee TH, Jaffee EM, Armstrong TD. Targeting the right regulatory T-cell population for tumor immunotherapy. Oncoimmunology. 2012;1(7):1191–3. doi: 10.4161/onci.20664. PMID:23170276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair RE, Kilinc MO, Jones SA, Egilmez NK. Chronic immune therapy induces a progressive increase in intratumoral T suppressor activity and a concurrent loss of tumor-specific CD8+ T effectors in her-2/neu transgenic mice bearing advanced spontaneous tumors. J Immunol. 2006;176(12):7325–34. doi: 10.4049/jimmunol.176.12.7325. PMID:16751376. [DOI] [PubMed] [Google Scholar]
- 11.Linch SN, McNamara MJ, Redmond WL. OX40 Agonists and Combination Immunotherapy: Putting the Pedal to the Metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034. PMID:25763356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria JC, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. PMID:26645943. [DOI] [PubMed] [Google Scholar]
- 13.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7(11):880–7. doi: 10.1038/nrc2250. PMID:17957190. [DOI] [PubMed] [Google Scholar]
- 14.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205(4):825–39. doi: 10.1084/jem.20071341. PMID:18362171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68(13):5206–15. doi: 10.1158/0008-5472.CAN-07-6484. PMID:18593921. [DOI] [PubMed] [Google Scholar]
- 16.Burocchi A, Pittoni P, Gorzanelli A, Colombo MP, Piconese S. Intratumor OX40 stimulation inhibits IRF1 expression and IL-10 production by Treg cells while enhancing CD40L expression by effector memory T cells. Eur J Immunol. 2011;41(12):3615–26. doi: 10.1002/eji.201141700. PMID:22229156. [DOI] [PubMed] [Google Scholar]
- 17.Bulliard Y, Jolicoeur R, Zhang J, Dranoff G, Wilson NS, Brogdon JL. OX40 engagement depletes intratumoral Tregs via activating FcgammaRs, leading to antitumor efficacy. Immunol Cell Biol. 2014;92(6):475–80. doi: 10.1038/icb.2014.26. PMID:24732076. [DOI] [PubMed] [Google Scholar]
- 18.Cuadros C, Dominguez AL, Lollini PL, Croft M, Mittler RS, Borgstrom P, Lustgarten J. Vaccination with dendritic cells pulsed with apoptotic tumors in combination with anti-OX40 and anti-4-1BB monoclonal antibodies induces T cell-mediated protective immunity in Her-2/neu transgenic mice. Int J Cancer. 2005;116(6):934–43. doi: 10.1002/ijc.21098. PMID:15856473. [DOI] [PubMed] [Google Scholar]
- 19.Murata S, Ladle BH, Kim PS, Lutz ER, Wolpoe ME, Ivie SE, Smith HM, Armstrong TD, Emens LA, Jaffee EM, et al.. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176(2):974–83. doi: 10.4049/jimmunol.176.2.974. PMID:16393983. [DOI] [PubMed] [Google Scholar]
- 20.Kitamura N, Murata S, Ueki T, Mekata E, Reilly RT, Jaffee EM, Tani T. OX40 costimulation can abrogate Foxp3+ regulatory T cell-mediated suppression of antitumor immunity. Int J Cancer. 2009;125(3):630–8. doi: 10.1002/ijc.24435. PMID:19455675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linch SN, Kasiewicz MJ, McNamara MJ, Hilgart-Martiszus IF, Farhad M, Redmond WL. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. Proc Natl Acad Sci U S A. 2016;113(3):E319–27. doi: 10.1073/pnas.1510518113. PMID:26729864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foote JB, Kok M, Leatherman JM, Armstrong TD, Marcinkowski BC, Ojalvo LS, Kanne DB, Jaffee EM, Dubensky TW Jr., Emens LA. A STING Agonist Given with OX40 Receptor and PD-L1 Modulators Primes Immunity and Reduces Tumor Growth in Tolerized Mice. Cancer Immunol Res. 2017;5(6):468–79. doi: 10.1158/2326-6066.CIR-16-0284. PMID:28483787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Curti A, Parenza M, Colombo MP. Autologous and MHC class I-negative allogeneic tumor cells secreting IL-12 together cure disseminated A20 lymphoma. Blood. 2003;101(2):568–75. doi: 10.1182/blood-2002-03-0991. PMID:12393660. [DOI] [PubMed] [Google Scholar]
- 24.Shibahara I, Saito R, Zhang R, Chonan M, Shoji T, Kanamori M, Sonoda Y, Kumabe T, Kanehira M, Kikuchi T, et al.. OX40 ligand expressed in glioblastoma modulates adaptive immunity depending on the microenvironment: a clue for successful immunotherapy. Mol Cancer. 2015;14:41. doi: 10.1186/s12943-015-0307-3. PMID:25744203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2(3):194–9. doi: 10.1158/2326-6066.CIR-13-0214. PMID:24778315. [DOI] [PubMed] [Google Scholar]
- 26.Giovarelli M, Musiani P, Modesti A, Dellabona P, Casorati G, Allione A, Consalvo M, Cavallo F, Di Pierro F, De Giovanni C, et al.. Local release of IL-10 by transfected mouse mammary adenocarcinoma cells does not suppress but enhances antitumor reaction and elicits a strong cytotoxic lymphocyte and antibody-dependent immune memory. J Immunol. 1995;155(6):3112–23. PMID:7673726. [PubMed] [Google Scholar]
- 27.Wang L, Liu JQ, Talebian F, Liu Z, Yu L, Bai XF. IL-10 enhances CTL-mediated tumor rejection by inhibiting highly suppressive CD4+ T cells and promoting CTL persistence in a murine model of plasmacytoma. Oncoimmunology. 2015;4(7):e1014232. doi: 10.1080/2162402X.2015.1014232. PMID:26140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez AL, Lustgarten J. Implications of aging and self-tolerance on the generation of immune and antitumor immune responses. Cancer Res. 2008;68(13):5423–31. doi: 10.1158/0008-5472.CAN-07-6436. PMID:18593945. [DOI] [PubMed] [Google Scholar]
- 29.Ruby CE, Weinberg AD. OX40-enhanced tumor rejection and effector T cell differentiation decreases with age. J Immunol. 2009;182(3):1481–9. doi: 10.4049/jimmunol.182.3.1481. PMID:19155495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Champiat S, Dercle L, Ammari S, Massard C, Hollebecque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, et al.. Hyperprogressive Disease Is a New Pattern of Progression in Cancer Patients Treated by Anti-PD-1/PD-L1. Clin Cancer Res. 2017;23(8):1920–8. doi: 10.1158/1078-0432.CCR-16-1741. PMID:27827313. [DOI] [PubMed] [Google Scholar]
- 31.Karaki S, Anson M, Tran T, Giusti D, Blanc C, Oudard S, Tartour E. Is There Still Room for Cancer Vaccines at the Era of Checkpoint Inhibitors. Vaccines (Basel). 2016;4(4):37. PMID:27827885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messenheimer DJ, Jensen SM, Afentoulis ME, Wegmann KW, Feng Z, Friedman DJ, Gough MJ, Urba WJ, Fox BA. Timing of PD-1 Blockade Is Critical to Effective Combination Immunotherapy with Anti-OX40. Clin Cancer Res. 2017;23(20):6165–77. doi: 10.1158/1078-0432.CCR-16-2677. PMID:28855348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy R. Eradication of spontaneous malignancy by local immunotherapy. Sci Transl Med. 2018;10(426) doi: 10.1126/scitranslmed.aan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]


