ABSTRACT
Multiple myeloma (MM) derives from malignant transformation of plasma cells (PC), which accumulate in the bone marrow (BM), where microenvironment supports tumor growth and inhibits anti-tumor immune responses. Adenosine (ADO), an immunosuppressive molecule, is produced within MM patients' BM by adenosinergic ectoenzymes, starting from ATP (CD39/CD73) or NAD+ [CD38/CD203a(PC-1)/CD73]. These ectoenzymes form a discontinuous network expressed by different BM cells. We investigated the expression and function of ectoenzymes on microvesicles (MVs) isolated from BM plasma samples of patients with MM, using asymptomatic forms of monoclonal gammopathy of undetermined significance (MGUS) and smoldering MM (SMM) as controls.
The percentage of MVs expressing ectoenzymes at high levels was higher when derived from MM patients than controls. BM CD138+ PC from MM patients expressed high levels of all ectoenzymes. Paired MVs samples confirmed a higher percentage of MVs with high ectoenzymes expression in MM patients than controls. Pooled MVs from MM patients or controls were tested for ADO production. The catabolism of ATP, NAD+, ADPR and AMP to ADO was higher in MVs from MM patients than in those from controls.
In conclusion, our results confirmed the hypothesis that MVs in MM niche are main contributor of ADO production. The ability of MVs to reach biological fluids strongly support the view that MVs may assume diagnostic and pathogenetic roles.
KEYWORDS: Multiple myeloma, extracellular vesicles, ectoenzymes, adenosine, immunosuppression
Introduction
Multiple myeloma (MM) is the result of an expansion of malignant plasma cells (PC) in the bone marrow (BM).1 Active MM must be differentiated from asymptomatic monoclonal gammopathies in the absence of organ damage, that are classified as i) monoclonal gammopathy of undetermined significance (MGUS), with a percentage of PC in the BM less than 10%, M-spike less than 3 mg/dL, and progression rate to active MM of approximately 1%. Further, ii) smoldering MM (SMM) presents a percentage of PC in the BM ranging from 10% to 60% and/or M-spike above 3 mg/dL, with a rate of progression to MM of approximately 10% in the first 5 years, without myeloma-defining events.1
The mechanisms behind growth and diffusion of MM cells into the BM have been defined by exploiting different approaches.2-8 The critical role of the alterations of the immune-microenviroment in the support of MM cell growth and survival has been amply reported.5 Cell populations present in the BM niche of MM patients [e.g. PC, osteoblasts (OB), osteoclasts (OC), myeloid derived suppressor cells (MDSC) and regulatory T cells (Treg)] are characterized by the expression of different adenosinergic ectoenzymes belonging to the classical and alternative pathway for adenosine (ADO) production9 (Figure 1). The classical pathway is initiated by CD39, an ecto-nucleoside-triphosphate-diphosphohydrolase (NTPDase), that converts ATP to ADP. CD39 converts the latter molecule into AMP, that is fully converted to ADO by the 5’-nucleotidase (5’-NT) CD73.10 CD39 and CD73 in the BM niche are expressed by MDSC and Treg, leading to the formation of ADO from ATP, produced by PC in normoxic conditions.11 ADO may be alternatively generated from NAD+.12 The reaction is run by CD38, a molecule with ADP-ribosyl-cyclase/cyclic ADP ribose-hydrolase enzymatic activities. The ectoenzyme converts NAD+ (extruded from MM cells under hypoxic conditions) to ADPR, that is subsequently converted to AMP by CD203a(PC-1) (an ectonucleotide-pyrophosphatase-phosphodiesterase-1). The same enzyme can also convert ATP to AMP. The last step is shared with the conventional pathway, and CD73 metabolizes AMP to ADO.13,14 Ectoenzymes belonging to the alternative pathway in the BM niche are expressed by different cell populations, since CD38 is expressed at high levels by PC [which are CD73−/CD203a(PC-1)−], CD203a(PC-1) is expressed by MSC and OB, while CD73 is expressed by OB and OC.9 The CD38/CD203a(PC-1)/CD73 pathway is operative to generate ADO in a discontinuous fashion, whereby all the enzymatic molecules are expressed on different cells present in a closed BM environment.9,11,12 This conclusion was confirmed by co-cultures between MM cells and other BM cell populations (such as OC, OB and stromal cells), that were able to lead the generation of ADO.9,12 ADO production in the BM niche may be followed by an abrogation of anti-tumor immune response, through the inhibition of effector functions of T lymphocytes and through the activity of MDSC.15 Moreover, it has been demonstrated that ADO stimulates the release of anti-inflammatory cytokines (i.e. IL-10) and inhibits the release of pro-inflammatory cytokines (i.e. IL-1, IL-12 and IL-6) by BM-resident cells following tissue damage, thus contributing to the abrogation of inflammatory response.16
Figure 1.
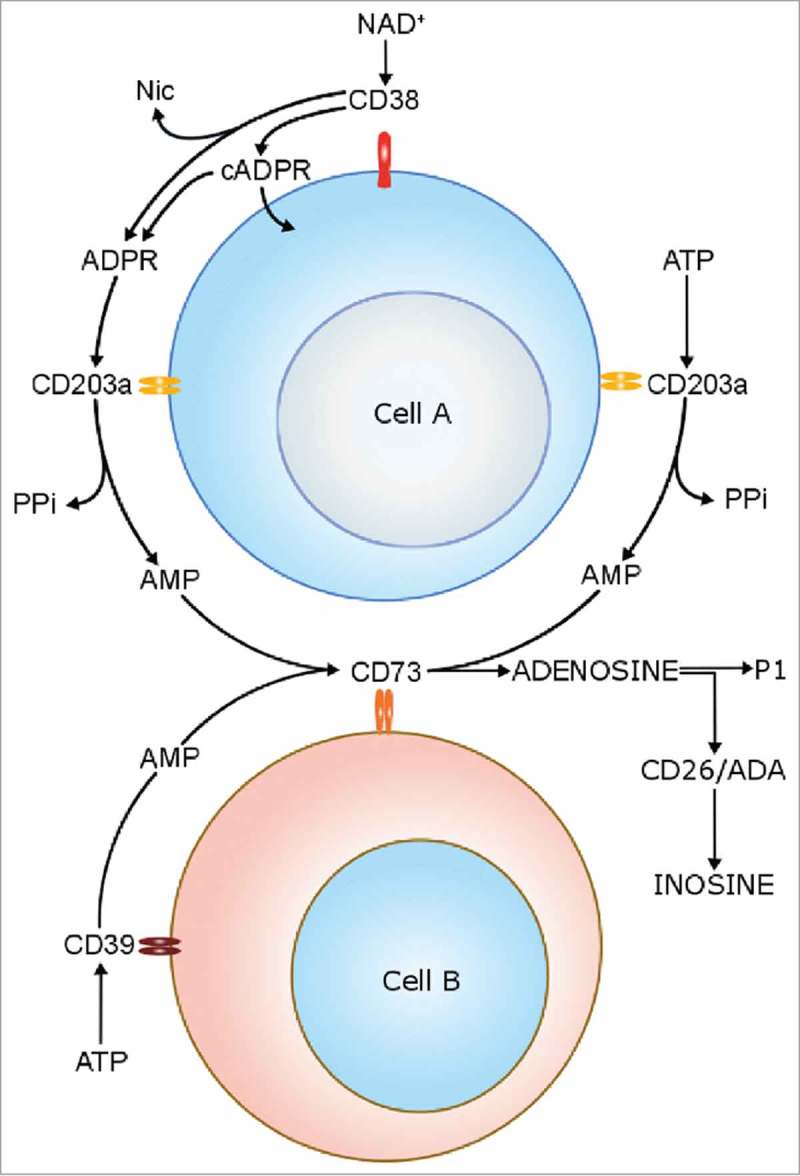
Schematic representation of enzymatic activities of canonical and non-canonical adenosinergic pathways.
We have previously demonstrated that ADO levels were significantly higher in BM plasma samples collected at diagnosis from MM patients than in those from MGUS/SMM patients. Moreover, ADO levels in BM plasma samples at diagnosis were higher in patients at advanced stage (ISS stage III) than in those from early stages (pooled stage I-II MM patients). These findings confirmed that ADO production in the BM niche correlated with disease progression and may represent a prognostic marker for ISS staging in complement with other markers.9
The working hypothesis of this study is that extracellular vesicles (EV) may contribute to ADO production within and outside the BM niche in MM. EV include microvesicles (MVs), exosomes and apoptotic bodies.17 MVs differ from exosomes in diameter (range 50–1000 nm versus 40–120 nm) and nature. Indeed, exosomes originate through an endocytic pathway, while MVs are released from the plasma membrane of activated or neoplastic cells. Among other surface molecules, MVs express integrins, selectins, CD40 or CD81, and are involved in inter-cellular communications, being captured by target cells through endocytosis, receptor-mediated endocytosis or fusion with the plasma membrane.18,19 Tumor-derived EV are reported as involved in disease progression20-22 and as dampening anti-tumor immune response.17,18 Involvement of MVs in tissue damage23 and disease progression.24 was recently reported in MM patients.
To define a mechanistic background of the above observations, and to answer whether MVs contribute to ADO production and hence to tumor-driven immune suppression within the BM niche, we analyzed the expression and function of adenosinergic ectoenzymes in MVs isolated from BM plasma samples of MM patients and controls. The power of our approach was in adopting as controls BM samples from MGUS and SMM patients. This strategic choice guarantees the highest sensitivity and specificity of our observations.
Results
Characterization of MVs
MVs size, polydispersity and mean count rate were defined by performing a dynamic light scattering analysis. Plasma-derived MVs formed a bell-shaped size distribution profile with a peak ranging from 219.8 nm to 303.7 nm, a polydispersity factor ranging from 0.226 to 0.515 and a mean count rate ranging from 235.2 kcps to 302.6 kcps. These results confirm the presence of homogeneous MVs preparations (Figure 2A).
Figure 2.
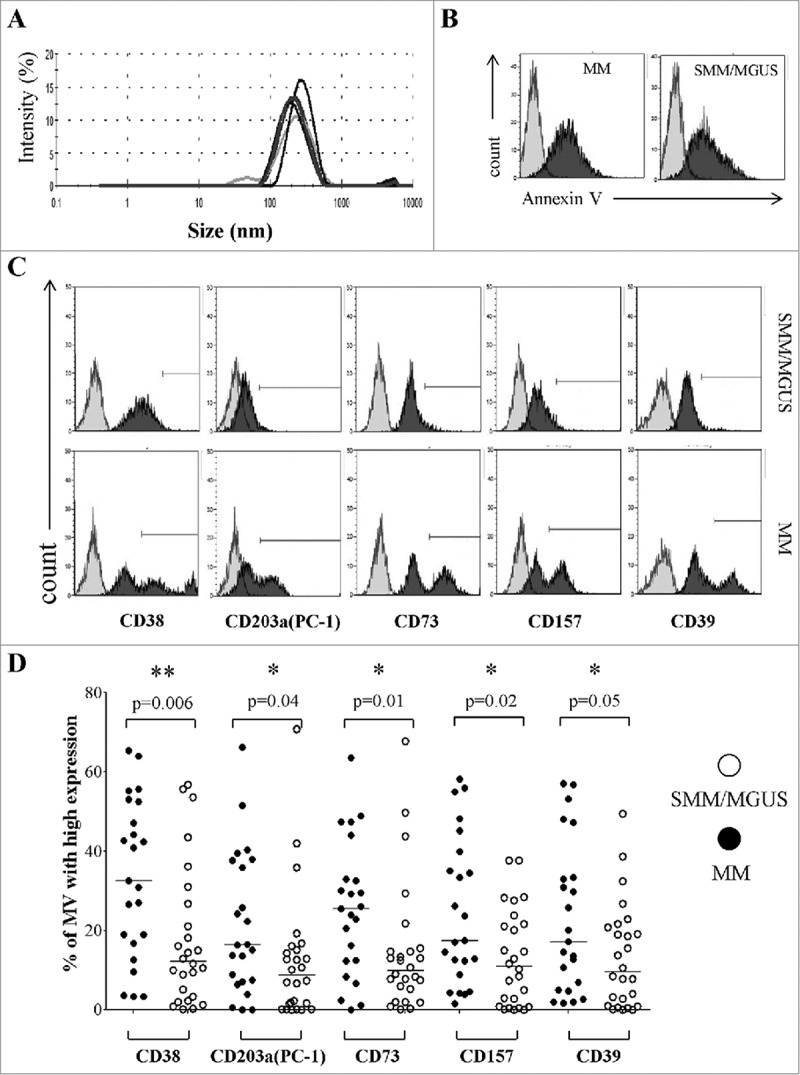
Expression of CD38, CD203a(PC-1), CD73, CD157 and CD39 in MVs from the BM of patients with MM or MGUS/SMM. Panel A represents the characterization of the size and distribution of MVs by Particle Sizer. Panel B shows a representative experiment performed in pooled MV from 5 MM patients and 5 controls (MGUS/SMM). Grey profiles indicate unstained MVs and black profiles indicate MVs stained with Annexin V. Panel C shows a representative experiment performed in MM patients and controls (MGUS/SMM). Grey profiles indicate MVs stained with irrelevant isotype-matched mAbs and black profiles indicate MVs stained with specific mAbs. Panel D shows percentages of MVs expressing high levels of ectoenzymes. Mv were isolated from 27 MM patients (black dots) or 25 MGUS/SMM patients (controls, white dots). Horizontal lines indicated medians. p values are indicated when differences are statistically significant.
It has been previously demonstrated that MV formation is an energy-dependent phenomenon and results from cell membrane remodeling and loss of lipid asymmetry, leading to phosphatidylserine (PS) exposure. Thus, PS represents a specific marker for MV and discriminate between MV and other EV.25-27 To further characterize our MV preparation, we have performed a flow cytometric analysis on pooled MV from 5 MM patients and 5 SMM/MGUS patients, using Annexin V (a specific ligand of PS). As shown in Figure 2B, both pool of MV display a high staining with Annexin V, thus confirming that our preparations contain prevalently MV.
Ectoenzymes are expressed on MVs from BM plasma samples
The profile of the ectoenzyme expression was determined by a preliminary detailed flow cytometric analysis. The presence of CD38, CD203a(PC-1), CD73, CD157 and CD39 was assessed on MVs purified from BM plasma samples obtained from MM patients and controls. A representative experiment is shown in Figure 2C.
Ectoenzymes tested were detected in all MVs preparations, irrespectively of their source. Control samples were characterized by a homogenous expression, as witnessed by single, sharp peaks. In contrast, MVs from MM patients displayed at least two discrete peaks. This finding might indicate that the MVs originated from different cell populations present in the MM niche, each releasing a peculiar set of MVs.
The immunophenotype of MVs was characterized according to a quantitative criterion, as described in Materials & Methods. Figure 2D shows that MVs displaying high amounts of CD38 molecules (CD38high) were significantly more abundant in BM samples from MM patients than in those MVs from the BM of MGUS/SMM controls. Similar results were observed for CD203a(PC-1)high, CD73high, CD157high and CD39high MVs. Data are summarized in Table 2A. These findings indicate that MVs from MM patient BM are characterized by the presence of high amounts of CD38 molecules, and apparently of functionally related ectoenzymes.
Table 2.
Numerical results of ectoenzyme expression.
| A) MV with high expression of ectoenzymes in MM patients and controls (% of MV with high expression; mean ± SD) |
MM patients |
MGUS/SMM patients |
Statistics |
| CD38 | 33.3 ± 4.1 | 17.9 ± 3.4 | p = 0.006 |
| CD39 | 22.9 ± 3.9 | 13.2 ± 2.5 | p = 0.05 |
| CD157 | 24.7 ± 3.8 | 13 ± 2.3 | p = 0.04 |
| CD203a/PC-1 | 21.3 ± 3.7 | 12.2 ± 3.1 | p = 0.04 |
| CD73 | 25.3 ± 3.5 | 14.5 ± 3.2 | p = 0.01 |
| B) MV with high expression of ectoenzymes in BM cells from MM patients (% of MV with high expression; mean ± SD) |
CD138+ cells |
CD138- cells |
Statistics |
| CD38 | 28 ± 1.1 | 4.6 ± 0.32 | p = 0.05 |
| CD39 | 16.3 ± 0.3 | 10.5 ± 0.3 | p = 0.05 |
| CD73 | 4.4 ± 0.3 | 6.6 ± 0.3 | p = 0.05 |
| CD203a/PC-1 | 16.9 ± 0.5 | 16.4 ± 0.3 | ns |
| C) MV with high expression of ectoenzymes in fresh BM samples from MM patients and controls (% of MV with high expression; mean ± SD) |
MM patients |
MGUS/SMM patients |
Statistics |
| CD38 | 37 ± 16.9 | 7.3 ± 0.69 | p = 0.05 |
| CD39 | 31.4 ± 12.1 | 2.4 ± 0.17 | p = 0.05 |
| CD203a/PC-1 | 43.1 ± 16.2 | 2.5 ± 0.2 | p = 0.05 |
| CD73 | 48.7 ± 11.3 | 4.5 ± 1.3 | p = 0.05 |
Characterization of the source of CD38high MVs expressing other potential adenosinergic ectoenzymes
Directly originating from the plasma membrane, MVs are expected to mirror the surface antigenic composition of the parental cells. This issue was firstly elucidated by discriminating the neoplastic PC from the other potential sources of MVs in the BM niche. To this aim, cells obtained from BM samples of 4 MM patients and 4 MGUS/SMM patients were double stained for CD138 (a dependable marker of neoplastic PC) and for the ectoenzymes here investigated. A representative experiment is shown in Figure 3A and 3B. CD138+ PC from MM patients expressed higher levels of CD38 and CD39 than the CD138− counterpart. In contrast, the expression of CD73 was higher in the CD138− than in CD138+ population. Finally, the expression of CD203a(PC-1) was similar between these two subsets (Figure 3C). Data are summarized in Table 2B.
Figure 3.
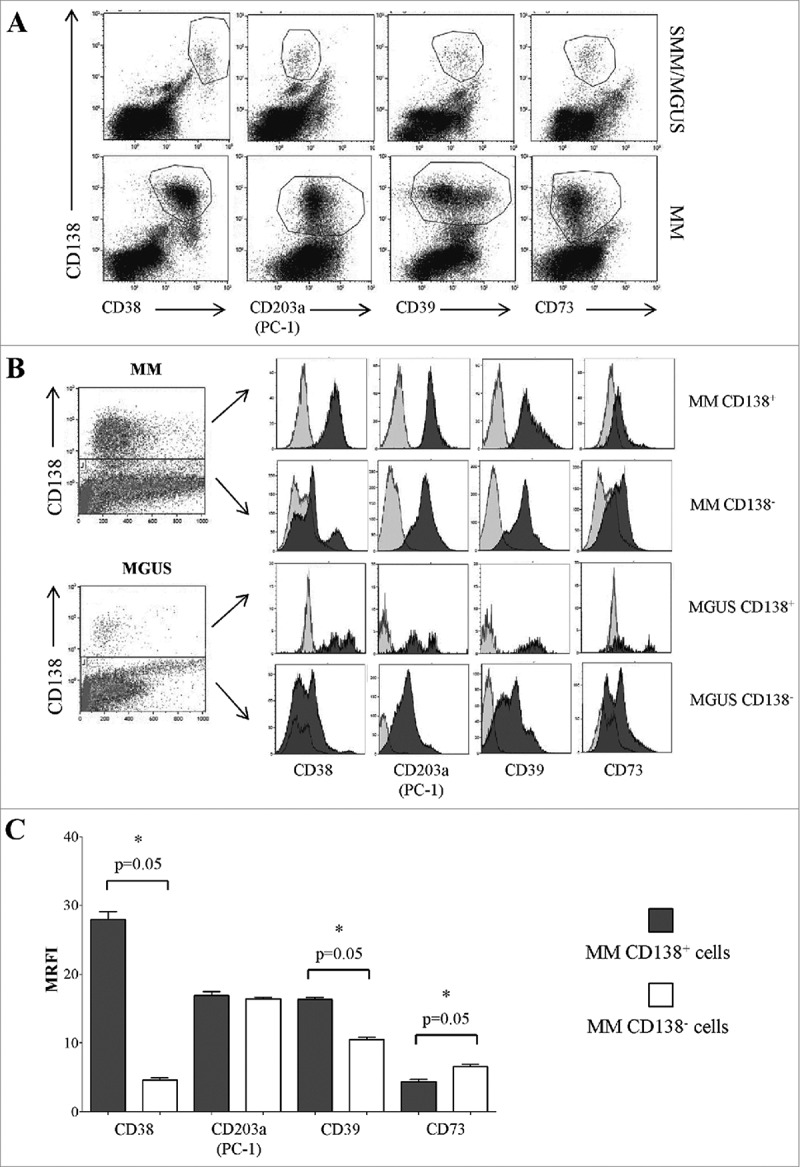
Expression of ectoenzymes in CD138+ cells from BM samples obtained from MM patients and controls (MGUS/SMM). Flow cytometric dot plots in Panel A show a representative experiment, where BM cells from MM and MGUS patients were stained with anti-CD138 mAb (y axes) and anti-CD38, anti-CD203a(PC-1), anti-CD39 and anti-CD73 mAbs (x axes). Flow cytometric profiles in Panel B show a representative experiment, where BM cells from MM and MGUS patients were stained with anti-CD138 mAb (y axes) and anti-CD38, anti-CD203a(PC-1), anti-CD39 and anti CD73 mAbs (x axes). The expression of each ectoenzyme was evaluated in CD138+ and CD138− cells. Grey profiles indicate MVs stained with irrelevant isotype-matched mAbs and black profiles indicate MVs stained with specific mAbs. Histograms in Panel C summarize the MRFI of each ectoenzyme calculated gating on BM CD138+ cells in samples from MM patients (n = 4).
Next, we analyzed the expression of other ectoenzymes on plasma-derived MVs obtained from the same BM samples used for the above experiments. Figure 4A shows a representative experiment. Once more, MVs derived from MM patients' BM plasma samples displayed significantly higher percentages of CD38high, CD203a(PC-1)high, CD73high and CD39high MVs than those from controls (Figure 4B). Data are summarized in Table 2C. Since the expression of these molecules is different between CD138+ and CD138− cells in the corresponding BM samples, we speculate that the portion of MVs expressing high levels of ectoenzymes, that has been predominantly detected in MV originated from MM patients, might be derived from both tumor cells and resident cells in the BM niche.
Figure 4.
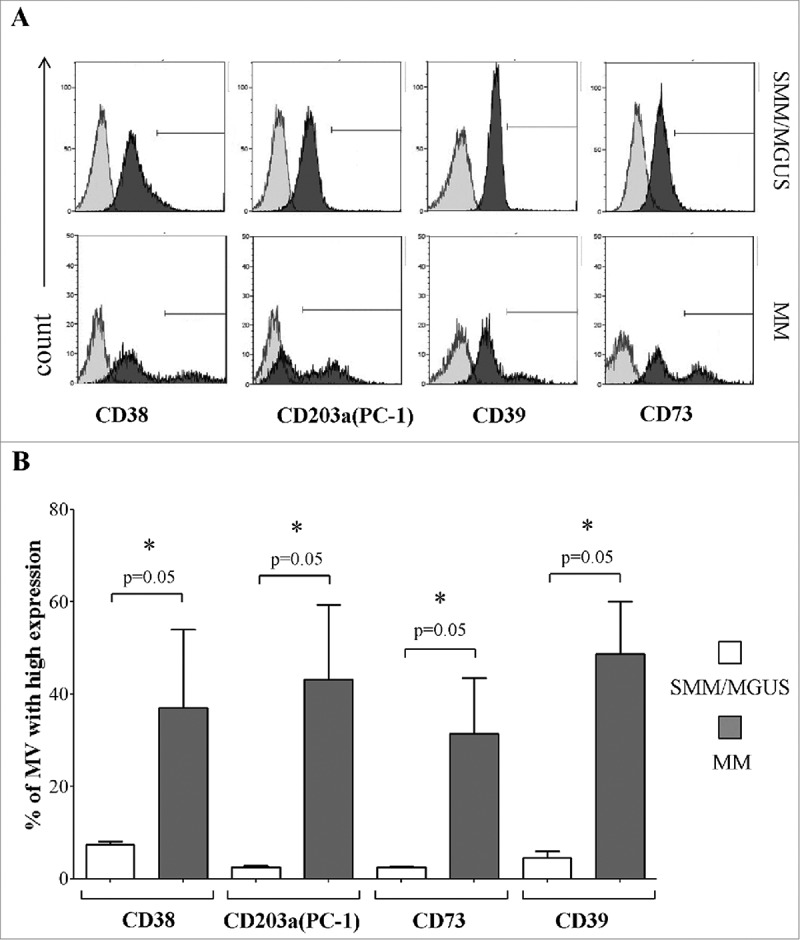
Expression of different ectoenzymes in MVs obtained from fresh BM samples. Panel A shows a representative experiment performed in fresh BM samples obtained from MM patients and controls (MGUS/SMM), where grey profiles indicated MVs stained with irrelevant isotype-matched mAbs and black profiles indicated MVs stained with specific mAbs. Panel B shows results obtained from MM patients (n = 4) and controls (MGUS/SMM, n = 4). p values are indicated when differences are statistically significant.
MVs from MM patients produce ADO from different substrates and with a higher efficiency than MVs obtained from controls
The immunophenotype of MVs from MM patients closely resembles that of the cells from which they likely originated as witnessed by the shared high expression level of CD38, CD39, CD73 and CD203a ectoenzymes. The next question was whether the ectoenzymes expressed by MVs isolated from BM samples were functional. To address this issue, we evaluated ADO produced by MVs following exposure to different substrates. Thus, MVs obtained from 6 different MM patients were pooled and challenged with ATP, NAD+, ADPR and AMP. The same procedure was conducted with samples pooled from 6 different MGUS/SMM controls.
The consumption of ATP was lower in MVs obtained from controls than in those obtained from MM patients. Accordingly, the production of AMP from ATP [either via CD39 or CD203a(PC-1)] was higher in MVs from MM patients. Consequently, the level of ADO detected in MVs from MM patients was higher than in those from controls (Figure 5A, Table 3A). These observations demonstrated the enzymatic functionality of CD39 and of CD203a(PC-1) in both MVs pools, showing that the ability to metabolize ATP is higher in MVs obtained from MM patients than in those derived from controls. Moreover, CD73 molecules on the surface of MVs are endowed with ecto-5'-nucleotidase activity. Indeed, the consumption of AMP (CD73 substrate) is higher in MVs from MM patients than in controls. Consequently, the production of ADO (the CD73 product from AMP metabolization) was higher in MVs from MM patients than in controls (Figure 5A and Table 3B). A possible interpretation is that ectoenzyme clustering/arrangement on the surface of MVs leads to an improvement of their efficiency.
Figure 5.
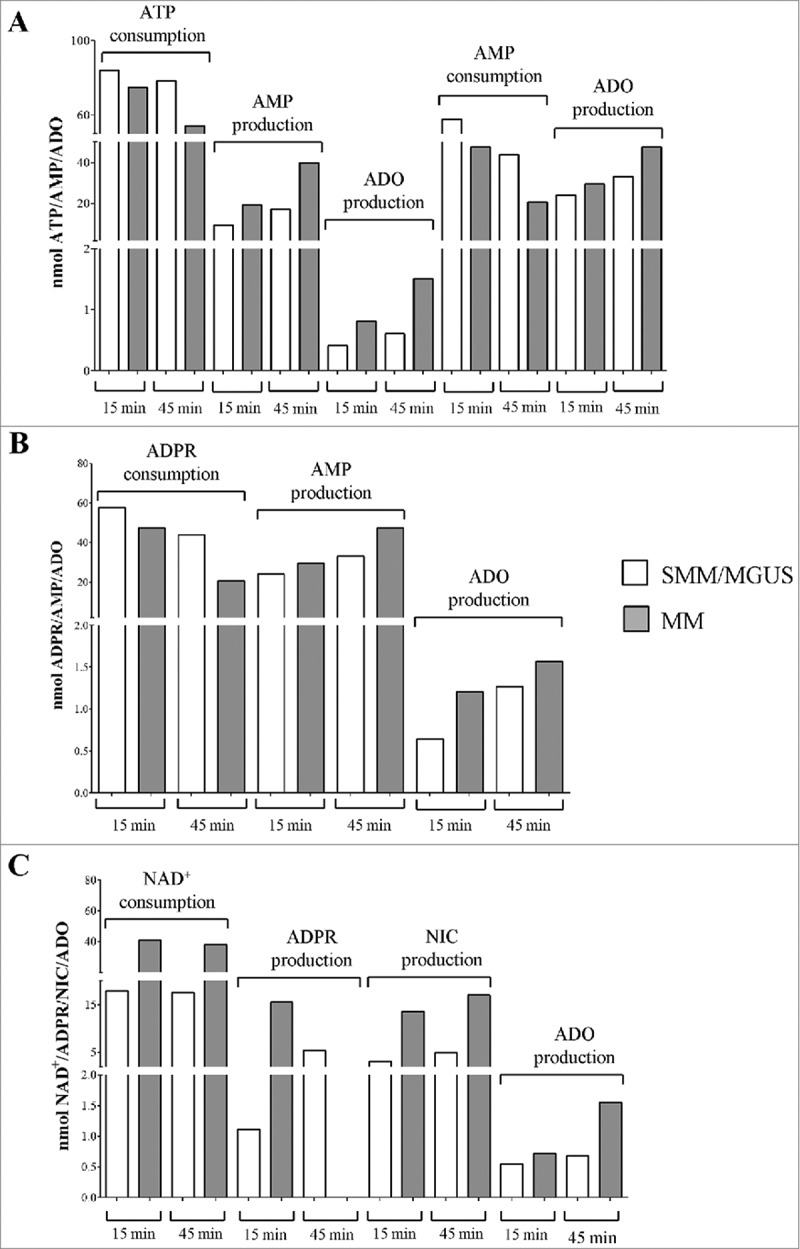
Functional and time-dependent analysis of ectoenzymes expressed on MVs pooled from MM patients and controls (MGUS/SMM). Different metabolites were evaluated by HPLC on supernatants of MVs pooled from MM patients (grey columns) and controls (MGUS/SMM, white columns). Panel A shows i) the consumption of ATP and the corresponding production of AMP and ADO and ii) the consumption of AMP the corresponding production of ADO. Panel B shows the consumption of ADPR and the corresponding production of AMP and ADO. Panel C shows the consumption of NAD+ and the corresponding production of ADPR, AMP, NIC and ADO.
Table 3.
Numerical results of ADO production from pooled MV.
| MM patients |
MGUS/SMM patients |
|||
|---|---|---|---|---|
| 15 min | 45 min | 15 min | 45 min | |
| (A) Substrate: ATP (nmol) | ||||
| ATP | 74.8 | 54.4 | 84.3 | 78.3 |
| AMP | 19.5 | 40 | 9.5 | 17.3 |
| ADO | 0.8 | 1.5 | 0.4 | 0.6 |
| (B) Substrate: AMP (nmol) | ||||
| AMP | 43.9 | 20.6 | 57.8 | 47.4 |
| ADO | 33.2 | 47.4 | 24.1 | 29.6 |
| (C) Substrate: ADPR (nmol) | ||||
| ADPR | 83.8 | 79.1 | 83.4 | 79.5 |
| AMP | 11.1 | 10.6 | 10 | 8.5 |
| ADO | 1.2 | 1.5 | 0.6 | 1.2 |
| (D) Substrate: NAD+ (nmol) | ||||
| NAD+ | 18 | 17.6 | 40.9 | 38.2 |
| ADPR | 15.5 | 0 | 1.1 | 5.3 |
| Nic | 13.6 | 17.01 | 3.1 | 4.9 |
| ADO | 0.7 | 1.5 | 0.5 | 0.6 |
The consumption of ADPR (main CD38 product obtained from NAD+ enzymatic metabolization) was higher but not significantly different in MVs from MM patients than in controls. As apparent, the production of AMP from ADPR [due to CD203a(PC-1) enzymatic activity] was similar in MVs from MM patients and in those from controls. On the contrary, the final product of mono- and di-nucleotides (ATP and NAD+, respectively) metabolization resulted in production of the immunosuppressive nucleoside ADO, that was detected at higher amount in MVs from MM patients than in those from controls (Figure 5B and Table 3C). These observations suggest that the enzymatic efficiency of CD39 and of CD203a(PC-1) is generally low in both MVs pools. However, the ability to metabolize ATP, NAD+, ADPR and AMP, as well as the production of the final product ADO, is higher in MVs obtained from MM patients than in those derived from controls. Indeed, residual NAD+ was higher in MVs from controls than in those from MM patients. Accordingly, the production of ADPR from NAD+ (catalyzed by CD38) was higher in MVs from MM patients than in those from controls at 15 min, but opposite results were obtained at 45 min, probably due to exhaustion of the substrate highly metabolized by MVs from MM patients. Consequently, following the same pattern, the production of NIC from NAD+ (byproduct of metabolization of NAD+ by CD38) was higher in MVs from MM patients than in those from controls. The amount of ADO produced was higher in those from MM patients than in those from controls (Figure 5C and Table 3D). Taken together, these observations suggest that the enzymatic activity/efficiency of the adenosinergic pathway driven by CD38 [CD38/CD203a(PC-1)/CD73] in MVs was functionally different between MM patients and controls, thus suggesting an heterogeneity of the ectoenzyme expression. A general interpretation of our findings is that CD38 is expressed in a plasma cell membrane domain with active or passive array with the other ectonucleotidase enzymes. The observed outcome is that MVs isolated from the BM niche from MM are equipped with enzymatic machinery capable of metabolizing nucleotides to produce immunosuppressive ADO.
CD38high and CD157high MVs are correlated with the presence of neoplastic plasma cells in the BM
In the attempt to identify a qualitative/quantitative correlation between the presence of MVs with high expression of adenosinergic ectoenzymes and clinical parameters of MM patients, MM patients were split in two groups on the basis of i) International Staging System (ISS) stage (stage I-II vs stage III), ii) newly diagnosed (D) or relapsed (R) disease and iii) percentage of PC in the BM (% of PC, < or > 10).
As shown in Figure 6A, the percentage of MVs expressing high levels of CD203a(PC-1) and CD73 was higher in ISS stage I-II than in ISS stage III MM patients (data are summarized in Table 4A). Moreover, MVs expressing high levels of CD38 and its paralogue CD157 (BST-1), contiguous gene duplicates on human chromosome 4 (4p15), represent a fraction which is higher in patients with a significant higher number of PC as compared with patients with a reduced presence of PC. The bulk of these findings is summarized in Figure 6B (data are summarized in Table 4B). In contrast, no correlation was found between the percentage of MVs highly expressing ectoenzymes and other clinical parameters of MM patients.
Figure 6.
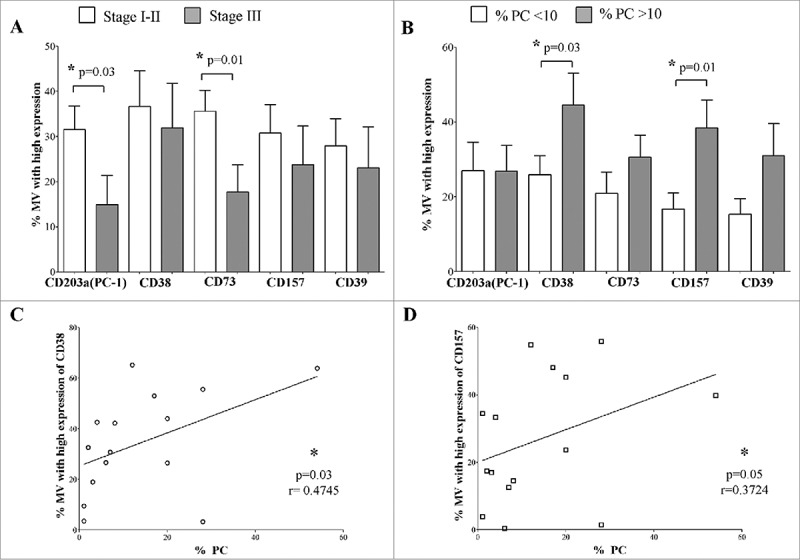
Correlations between MVs highly expressing ectoenzymes and clinical parameters of MM patients. MM patients were split in two groups on the basis of clinical parameters, and the percentage of MVs highly expressing CD203a(PC-1), CD38, CD73, CD157 and CD39 was compared between these two groups. Panel A shows the analysis of the percentage of MVs highly expressing ectoenzymes in MM patients with ISS Stage I-II (white columns) or Stage III (grey columns). Panel B shows the analysis of the percentage of MVs highly expressing ectoenzymes in MM patients with a percentage of plasma cells below (white columns) or above (grey columns) 10% of total BM cells. The analysis of the correlation between percentage of MVs highly expressing CD38 or CD157 and the percentage of plasma cells in the BM is shown in Panel C and Panel D, respectively.
Table 4.
Numerical results of clinical correlations.
| A) Correlation between MV and disease stage (% of MV with high expression; mean ± SD) |
Stage I/II MM patients |
Stage III MM patients |
Statistics |
| CD203a/PC-1 | 31.5 ± 13.8 | 14.9 ± 15.7 | p = 0.01 |
| CD73 | 35.6 ± 12.1 | 17.7 ± 14.6 | p = 0.01 |
| B) Correlation between MV and % of PC in the BM (% of MV with high expression; mean ± SD) |
<10 % of PC in the BM |
>10 % of PC in the BM |
Statistics |
| CD38 | 25.9 ± 14.3 | 44.5 ± 22.4 | p = 0.03 |
| CD157 | 16.7 ± 12.2 | 38.4 ± 19.5 | p = 0.01 |
| C) Correlation between MV and % of PC in the BM (% of MV with high expression; mean ± SD) |
Correlation (r) |
Significance (p) |
|
| CD38 | 0.47 | 0.03 | |
| CD157 | 0.37 | 0.05 | |
On the basis of the latter results, we analyzed the correlation between the percentage of MVs with high expression of CD38 and CD157 and the percentage of PC in the BM of MM patients. The percentage of PC positively correlated with the percentage of MVs displaying high expression of either CD38 or CD157 (Figure 6C and 6D, respectively, and Table 4C).
Collectively, these data suggested that MVs expressing high levels of adenosinergic ectoenzymes are released by malignant PC in the BM microenvironment.
Discussion
Several studies have shown the involvement of MVs in the progression of different solid and hematological human tumors. The underlying mechanism(s) are various, and sometimes contradictory. In fact, MVs are capable of stimulating tumor cell proliferation,24 inducing angiogenesis,20,28 suppressing normal cell development,21,29,30 modulating tumor/microenvironment interactions31 and inhibiting anti-tumor immune response.17,22,32 The role of MVs in the context of human MM is now being investigated. Zhao et al reported that MVs contribute to inducing renal function impairment in MM patients, by inducing apoptosis of proximal tubular cells.23 Furthermore, MVs derived from MM cells induce neoplastic cell proliferation24 and stimulate angiogenesis by inducing proliferation, tube formation, IL-6 secretion and VEGF release by endothelial cells.33
The expression and function of adenosinergic ectoenzymes have been demonstrated on exosomes derived from cancer patients.34-37 Less information is currently available regarding the expression and function of adenosinergic ectoenzymes on tumor-derived MVs, and their contribution to ADO production within the tumor microenvironment. In particular, no information is present in literature regarding ectoenzymes belonging to the non-canonical adenosinergic pathway. One of such ectoenzyme, CD73, is observed on MVs derived from mesenchymal stromal cells (MSC). This is likely due to the high density of the molecule on the cells of origin.38
Our starting point is that CD73 and the chain of other ectoenzymes involved in the generation of ADO are also operative in the context of the BM niche in MM. It is already known that adenosinergic ectoenzymes are present on different cell subsets in the BM microenvironment of MM patients, and that both the canonical and alternative pathways for ADO production are functional within BM niche.11,12 This work was designed to determine whether MVs participate in the cross-talk taking place between different districts of MM patients.
Our original findings stem from a comparison of MVs derived from BM plasma samples from MM patients with those derived from MGUS/SMM patients, taken as controls. The choice of these controls was dictated by the desire to obtain the highest sensitivity and specificity from our observations. The first observation is that MM samples contain MVs expressing higher levels of ectoenzymes belonging to both canonical (CD39, CD73) and non-canonical [CD38, CD203a(PC-1), CD73] pathways than controls. In addition, the expression of these molecules is not only higher, but their profile is more heterogeneous than in controls. This could be explained if the MVs obtained from the MM niche derive from different cell populations, the most likely of which is represented by malignant PC. Indeed, the percentage of MVs expressing high levels of CD38 and CD157 correlates with the percentage of PC in the BM. However, the fraction of MVs expressing high levels of CD203a(PC-1) and CD73 is also higher in MM than in controls. While it is true that these molecules are also present on OB, OC, Tregs and MDSC (and not only on PC), the main populations of the BM niche are not significantly different between MM patients and controls. Therefore, it is possible that environmental factors (differentially present in BM niche from MM patients as compared to controls) may account for variations in MVs release from these cell subsets. In line with this, it has been demonstrated that MVs release can be modulated by ARF6, a GTP-binding protein that regulates ERK signaling pathway.39 Among the different cytokines and growth factors released in the BM microenvironment, some of them activate the ERK signaling pathway. One may speculate that the activation state of BM-resident cells of MM patients is paralleled by the release of MVs from the niche.
In our view, another relevant finding is that MVs express functional adenosinergic ectoenzymes, capable of producing ADO from ATP and NAD+. This function is enhanced in MVs derived from MM patients, likely due to the increased numbers of MVs expressing high levels of each ectoenzyme, as compared to controls. This is in line with our previous studies, which highlighted a correlation between the production of ADO in the BM and progression of the disease.9 Therefore, a bona fide conclusion is that MVs may contribute to ADO production in the context of the BM niche. In this scenario, it is also important to consider that cells within the BM niche are equipped with high affinity (A1 and A2A receptors) and low affinity (A2B and A3) purinergic receptors for ADO. Accordingly, activation of A2A receptors occurs at 1–20 nm ADO, A1 receptors at 0.3–3 nM, while A2B or A3 receptor activation requires an ADO concentration >1,000 nM.40-42 Consequently, the micromolar concentration of ADO produced in the BM niche fluid (with the contribution of MVs) is greater than the affinity constant (Km) of adenosinergic receptors expressed by BM cells either in situ or other cells farther away. Therefore, accumulated extracellular ADO can indiscriminately activate A1, A2A and A3, as well as the A2B subtype, which requires considerably higher concentrations of ADO, such as those generated under pathophysiological conditions (e.g., MM progression).9 Since ADO is able to modulate the anti-tumor immune response15 and the release of pro- and anti-inflammatory cytokines within the BM microenvironment,16 we can speculate that MV can participate to this process through their ability to produce ADO from different substrates.
In conclusion, the results presented here support the view that MVs are endowed with a functional immunosuppressive role in the context of BM microenvironment in MM patients. This function is mediated by the production of ADO. Since we have previously demonstrated that anti-CD38 monoclonal antibodies (i.e., daratumumab), used in the treatment of MM patients, is caused by the release of MVs from the plasma membrane of malignant plasma cells,12,43 the generation of adenosinergic MVs must be taken into account to prevent immunosuppression and abrogation of local anti-tumor immune responses. The results derived from the present work may complement those derived from the in vivo experience on the use of therapeutic anti-CD38 mAbs.44,45 The more ambitious objective is to transfer in therapy the observations derived from in vitro findings. At the same time, disease models become confirmatory of experimental hypothesis designed in basic science.
Materials and methods
Isolation of microvesicles
All patients involved in the study gave their written informed consent, according to Helsinki Declaration. Patient diagnosis was made according to the International Myeloma Working Group46 criteria. Patients' demographic features are summarized in Table 1. Plasma samples were obtained from BM biopsies at diagnosis and stored at −80°C until use.
Table 1.
Characteristics of patients enrolled in the study.
| sex |
age |
status |
stage |
type |
% PC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M | F | range | median | D | R | I | II | III | kappa | lambda | unknown | range | median | ||
| patients | MM | 27 | 8 | 19 | 41–90 | 68 | 18 | 9 | 6 | 8 | 13 | 19 | 8 | 0 | 1–60 | 20 |
| controls | SMM | 14 | 7 | 7 | 41–83 | 63 | — | — | — | — | — | 9 | 5 | 0 | 0–6 | 2 |
| MGUS | 11 | 3 | 8 | 44–83 | 64 | — | — | — | — | — | 6 | 2 | 3 | 0.5–4 | 1 | |
MVs were isolated from BM plasma samples of 27 MM patients and 25 control subjects (11 MGUS and 14 SMM patients). In some experiments, paired fresh BM plasma and whole blood samples from MM patients (n = 4) and controls (n = 4) were analyzed.
MVs were isolated from BM plasma samples by differential centrifugation, as reported.47 Briefly, 500 μl of each BM plasma sample was diluted (1:3) in PBS and centrifuged (3,000 g for 15 min at 4°C) to pellet large cell debris and remove remaining platelets. The supernatant was collected in a suitable centrifugation tube and centrifuged (20,000 g for 1 h at 4°C) in a fixed-angle rotor, washed once in PBS and resuspended in 50 μl of binding buffer [PBS containing 0.5% BSA and 2 mM EDTA (both purchased from Sigma Aldrich)].
MVs were resuspended in 50 μl of binding buffer (PBS containing 0.5% BSA, 2 mM EDTA, both purchased from Sigma Aldrich). MVs size and polydispersity were analyzed using the Zetasizer Nano ZS90 particle sizer at a 90° fixed angle (Malvern Instruments, Worcestershire, UK), as previously described.48
Flow cytometric analysis
The expression of phosphatidylserine (PS) has been evaluated on pooled MVs obtained from BM plasma samples from 5 MM patients and 5 SMM/MGUS patients, using PE Annexin V Apoptosis Detection Kit I (BD Pharmingen), following manufacturer's protocol.
The expression of ectoenzymes was evaluated on MVs and BM cells using the following monoclonal (m)Abs generated in our Lab and FITC- or APC-conjugated by Aczon (Bologna, Italy): anti-CD38 (#IB4), anti-CD73 (#CB73), anti-CD157 (#SY/11B5). Anti-CD203a(PC-1) (#3E8) was kindly provided by J. Goding, and anti-CD39 PE-Cy7 mAb was purchased from eBiosciences. FITC- or APC-conjugated irrelevant isotype-matched mAbs were purchased from Beckman Coulter. MVs were resuspended in binding buffer, incubated with specific mAbs (20 min in the dark, at 4°C), and then washed with 500 µl of binding buffer. Samples were then centrifuged (14,000 g for 1 h at 4°C). MVs resuspended in staining buffer (400 µl) were then subjected to flow cytometric analysis.
In some experiments, BM whole blood samples (50 µl) were incubated with specific mAbs (20 min in the dark at 4°C). Erythrocytes were then lysed using BD FACS lysis buffer (BD Biosciences, 15 min at RT in the dark). Cells were then washed, resuspended in binding buffer and then analyzed by flow cytometry.
Flow cytometric analysis was performed using a Gallios cytometer and Kaluza software (Beckman Coulter). Data related to MVs were expressed as percentage of MVs presenting high expression of specific molecules. The first peak of mean fluorescence intensity (MFI) for each molecule was used as marker to define the percentage of MV with high expression of the selected marker. Thus, MV with high expression are defined as MV with a MFI higher than the first peak.
Data related to BM cells were expressed either as percentage of positive cells or mean relative of fluorescence intensity (MRFI), obtained as follows: mean fluorescence obtained with specific mAb / mean fluorescence obtained with irrelevant isotype-matched mAb.
ADO production by MVs
MVs pooled from 6 different MM patients and 6 different MGUS/SMM controls were resuspended in PBS + 0.1% glucose and incubated at 37°C and 5% CO2 in 96-well plates (Costar Corning), in the presence (or absence) of ATP, NAD+, ADPR or AMP (all at a concentration of 50 µM). Supernatants were collected after 15 or 45 min incubation and acetonitrile (ACN, Sigma Aldrich) immediately added (1:2 supernatnat:ACN ratio) to stabilize ADO. Samples were then centrifuged at 12,000 g and supernatants were collected and stored at -80°C until use. The presence of ATP, NAD+, ADPR, Nicotinamide (NIC) and ADO was analyzed by HPLC assay.
HPLC analysis
MVs supernatants (see above) were evaporated by Speed-vac (Eppendorf), reconstituted in mobile-phase buffer, and assayed by HPLC, as previously described.49 Chromatographic analysis was performed with an HPLC System (Beckman Gold 126/166NM, Beckman Coulter) equipped with a reverse-phase column (Synergi Fusion C18, 5 µm; 150 × 4.5 mm, Phenomenex). Substrates and products of the enzymatic reactions were separated using a pH 5.1 mobile-phase buffer (0.125 M citric acid and 0.025 M KH2PO4) containing 8% ACN over 10 min, at a flow rate of 0.8 mL/min. UV absorption spectra were measured at 254 nm. HPLC-grade standards used to calibrate the signals were dissolved in AIM V serum-free medium (Invitrogen, Paisley, UK), pH 7.4, 0.2 μm sterile-filtered and injected in a buffer volume of 20 μL. The retention times (Rt, in min) of standards were: ATP, 1.9; AMP, 2.15; NAD+, 2.8; ADPR, 3.2; NIC, 4.5; and ADO; 5.56. Peak integration was performed using a Karat software (Beckman Coulter).
The qualitative identity of HPLC peaks was confirmed by co-migration of known reference standards. The presence of ADO was also confirmed by spiking standard (50 μM ADO), followed by chromatography. Quantitative measurements were inferred by comparing the peak area of samples with calibration curves for peak areas of each standard compound. Concentrations were expressed as nmol of substrates and products.
Statistical analysis
Statistical analysis was performed using Prism 5.03 software (GraphPad Software). Gaussian distribution of data was analyzed using Kolmogorov-Smirnov test. The Student t test or the Mann-Whitney test were used to compare data, depending on data distribution. The significance range as follows: *p < 0.05 (significant), **p < 0.005, and ***p <0.0005.
Funding Statement
This work has been supported by A.I.R.C. IG 17273 to V. Pistoia and IG 16985 to M. Massaia, and grant from Compagnia San Paolo to V. Pistoia, F. Malavasi and N. Giuliani. F. Morandi was the recipient of a Fellowship from Fondazione Umberto Veronesi. B. Castella is supported by an AACR – Takeda Fellowship in multiple myeloma. M. Bolzoni and D. Toscani were supported by Fellowships from the Fondazione Italiana per la Ricerca sul Cancro (id. 18152 and 16462, respectively). A.C. Faini is a student of the MD/PhD Program of the University of Torino, Italy.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work has been supported by A.I.R.C. IG 17273 to V. Pistoia and IG 16985 to M. Massaia, and grant from Compagnia San Paolo to V. Pistoia, F. Malavasi and N. Giuliani. F. Morandi was the recipient of a Fellowship from Fondazione Umberto Veronesi. B. Castella is supported by an AACR – Takeda Fellowship in multiple myeloma. M. Bolzoni and D. Toscani were supported by Fellowships from the Fondazione Italiana per la Ricerca sul Cancro (id. 18152 and 16462, respectively). A.C. Faini is a student of the MD/PhD Program of the University of Torino, Italy.
References
- 1.Affer M, Chesi M, Chen WG, Keats JJ, Demchenko YN, Roschke AV, Van Wier S Fonseca R, Bergsagel PL, Kuehl WM. Promiscuous MYC locus rearrangements hijack enhancers but mostly super-enhancers to dysregulate MYC expression in multiple myeloma. Leukemia. 2014;28:1725–35. doi: 10.1038/leu.2014.70. PMID:24518206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd KD, Ross FM, Walker BA, Wardell CP, Tapper WJ, Chiecchio L, Dagrada G, Konn ZJ, Gregory WM, Jackson GH, et al.. Mapping of chromosome 1p deletions in myeloma identifies FAM46C at 1p12 and CDKN2C at 1p32.3 as being genes in regions associated with adverse survival. Clin Cancer Res. 2011;17:7776–84. doi: 10.1158/1078-0432.CCR-11-1791. PMID:21994415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chesi M, Matthews GM, Garbitt VM, Palmer SE, Shortt J, Lefebure M, Stewart AK, Johnstone RW, Bergsagel PL. Drug response in a genetically engineered mouse model of multiple myeloma is predictive of clinical efficacy. Blood. 2012;120:376–85. doi: 10.1182/blood-2012-02-412783. PMID:22451422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dispenzieri A, Katzmann JA, Kyle RA, Larson DR, Melton LJ, Colby CL 3rd, Therneau TM, Clark R, Kumar SK, Bradwell A, et al.. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8. doi: 10.1016/S0140-6736(10)60482-5. PMID:20472173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein B, Zhang XG, Jourdan M, Content J, Houssiau F, Aarden L, Piechaczyk M, Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989;73:517–26. PMID:2783861. [PubMed] [Google Scholar]
- 6.Martinez-Garcia E, Popovic R, Min DJ, Sweet SM, Thomas PM, Zamdborg L, Heffner A, Will C, Lamy L, Staudt LM, et al.. The MMSET histone methyl transferase switches global histone methylation and alters gene expression in t(4;14) multiple myeloma cells. Blood. 2011;117:211–20. doi: 10.1182/blood-2010-07-298349. PMID:20974671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikhael JR, Dingli D, Roy V, Reeder CB, Buadi FK, Hayman SR, Dispenzieri A, Fonseca R, Sher T, Kyle RA, et al.. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo. Clin Proc. 2013;88:360–76. doi: 10.1016/j.mayocp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Rajkumar SV, Leong T, Roche PC, Fonseca R, Dispenzieri A, Lacy MQ, Lust JA, Witzig TE, Kyle RA, Gertz MA, et al.. Prognostic value of bone marrow angiogenesis in multiple myeloma. Clin Cancer Res. 2000;6:3111–6. PMID:10955791. [PubMed] [Google Scholar]
- 9.Horenstein AL, Quarona V, Toscani D, Costa F, Chillemi A, Pistoia V, Giuliani N, Malavasi F. Adenosine generated in the bone marrow niche through a CD38-mediated pathway correlates with progression of human myeloma. Mol Med. 2016;22. doi: 10.2119/molmed.2016.00198. PMID:27761584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–67. doi: 10.1016/j.molmed.2013.03.005. PMID:23601906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quarona V, Ferri V, Chillemi A, Bolzoni M, Mancini C, Zaccarello G, Roato I, Morandi F, Marimpietri D, Faccani G, et al.. Unraveling the contribution of ectoenzymes to myeloma life and survival in the bone marrow niche. Ann N Y Acad Sci. 2015;1335:10–22. doi: 10.1111/nyas.12485. PMID:25048519. [DOI] [PubMed] [Google Scholar]
- 12.Horenstein AL, Chillemi A, Quarona V, Zito A, Roato I, Morandi F, Marimpietri D, Bolzoni M, Toscani D, Oldham RJ, et al.. NAD(+)-Metabolizing Ectoenzymes in Remodeling Tumor-Host Interactions: The Human Myeloma Model. Cells. 2015;4:520–37. doi: 10.3390/cells4030520. PMID:26393653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fedele G, Sanseverino I, D'Agostino K, Schiavoni I, Locht C, Horenstein AL, Malavasi F, Ausiello CM. Unconventional, adenosine-producing suppressor T cells induced by dendritic cells exposed to BPZE1 pertussis vaccine. J Leukoc Biol. 2015;98:631–9. doi: 10.1189/jlb.3A0315-101R. PMID:26089537. [DOI] [PubMed] [Google Scholar]
- 14.Horenstein AL, Chillemi A, Zaccarello G, Bruzzone S, Quarona V, Zito A, Serra S, Malavasi F. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology. 2013;2:e26246. doi: 10.4161/onci.26246. PMID:24319640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karakasheva TA, Waldron TJ, Eruslanov E, Kim SB, Lee JS, O'Brien S, Hicks PD, Basu D, Singhal S, Malavasi F, et al.. CD38-Expressing myeloid-derived suppressor cells promote tumor growth in a murine model of esophageal cancer. Cancer Res. 2015;75:4074–85. doi: 10.1158/0008-5472.CAN-14-3639. PMID:26294209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10:1081–7. doi: 10.1038/nm1103. PMID:15448683. [DOI] [PubMed] [Google Scholar]
- 17.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, László V, Pállinger E, Pap E, Kittel A, et al.. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. PMID:21560073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawamoto T, Ohga N, Akiyama K, Hirata N, Kitahara S, Maishi N, Osawa T, Yamamoto K, Kondoh M, Shindoh M, et al.. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS One. 2012;7:e34045. doi: 10.1371/journal.pone.0034045. PMID:22479517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyiadzis M, Whiteside TL. The emerging roles of tumor-derived exosomes in hematological malignancies. Leukemia. 2017;31:1259–68. doi: 10.1038/leu.2017.91. PMID:28321122. [DOI] [PubMed] [Google Scholar]
- 20.Feng Q, Zhang C, Lum D, Druso JE, Blank B, Wilson KF, Welm A, Antonyak MA, Cerione RA, et al.. A class of extracellular vesicles from breast cancer cells activates VEGF receptors and tumour angiogenesis. Nat Commun. 2017;8:14450. doi: 10.1038/ncomms14450. PMID:28205552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh AK, Secreto CR, Knox TR, Ding W, Mukhopadhyay D, Kay NE. Circulating microvesicles in B-cell chronic lymphocytic leukemia can stimulate marrow stromal cells: implications for disease progression. Blood. 2010;115:1755–64. doi: 10.1182/blood-2009-09-242719. PMID:20018914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Luca L, D'Arena G, Simeon V, Trino S, Laurenzana I, Caivano A, La Rocca F, Villani O, Mansueto G, Deaglio S, et al.. Characterization and prognostic relevance of circulating microvesicles in chronic lymphocytic leukemia. Leuk Lymphoma. 2017;58:1424–32. doi: 10.1080/10428194.2016.1243790. PMID:27739922. [DOI] [PubMed] [Google Scholar]
- 23.Zhao A, Kong F, Liu CJ, Yan G, Gao F, Guo H, Guo AY, Chen Z, Li Q. Tumor cell-derived microvesicles induced not epithelial-mesenchymal transition but apoptosis in human proximal tubular (HK-2) cells: implications for renal impairment in multiple myeloma. Int J Mol Sci. 2017;18:513. doi: 10.3390/ijms18030513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arendt BK, Walters DK, Wu X, Tschumper RC, Jelinek DF. Multiple myeloma dell-derived microvesicles are enriched in CD147 expression and enhance tumor cell proliferation. Oncotarget. 2014;5:5686–99. doi: 10.18632/oncotarget.2159. PMID:25015330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima LG, Chammas R, Monteiro RQ, Moreira ME, Barcinski MA. Tumor-derived microvesicles modulate the establishment of metastatic melanoma in a phosphatidylserine-dependent manner. Cancer Lett. 2009;283:168–75. doi: 10.1016/j.canlet.2009.03.041. PMID:19401262. [DOI] [PubMed] [Google Scholar]
- 26.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood. 1997;89:1121–32. PMID:9028933. [PubMed] [Google Scholar]
- 27.Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, et al.. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci U S A. 2015;112:E1433–42. PMID:25713383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Treps L, Perret R, Edmond S, Ricard D, Gavard J. Glioblastoma stem-like cells secrete the pro-angiogenic VEGF-A factor in extracellular vesicles. J Extracell Vesicles. 2017;6:1359479. doi: 10.1080/20013078.2017.1359479. PMID:28815003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Razmkhah F, Soleimani M, Mehrabani D, Karimi MH, Amini Kafi-Abad S, Ramzi M, Iravani Saadi M, Kakoui J. Leukemia microvesicles affect healthy hematopoietic stem cells. Tumour Biol. 2017;39:1010428317692234. doi: 10.1177/1010428317692234. PMID:28218044. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson T, Lundholm M, Widmark A, Persson E. Tumor cell-derived exosomes from the prostate cancer cell line TRAMP-C1 impair osteoclast formation and differentiation. PLoS One. 2016;11:e0166284. doi: 10.1371/journal.pone.0166284. PMID:27832183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang L, Valencia CA, Dong B, Chen M, Guan PJ, Pan L. Transfer of microRNAs by extracellular membrane microvesicles: a nascent crosstalk model in tumor pathogenesis, especially tumor cell-microenvironment interactions. J Hematol Oncol. 2015;8:14. doi: 10.1186/s13045-015-0111-y. PMID:25885907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berchem G, Noman MZ, Bosseler M, Paggetti J, Baconnais S, Le Cam E, Nanbakhsh A, Moussay E, Mami-Chouaib F, Janji B, et al.. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-beta and miR23a transfer. Oncoimmunology. 2016;5:e1062968. doi: 10.1080/2162402X.2015.1062968. PMID:27141372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Zhu XJ, Zeng C, Wu PH, Wang HX, Chen ZC, Li QB. Microvesicles secreted from human multiple myeloma cells promote angiogenesis. Acta Pharmacol Sin. 2014;35:230–8. doi: 10.1038/aps.2013.141. PMID:24374814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–83. doi: 10.4049/jimmunol.1003884. PMID:21677139. [DOI] [PubMed] [Google Scholar]
- 35.Schuler PJ, Saze Z, Hong CS, Muller L, Gillespie DG, Cheng D, Harasymczuk M, Mandapathil M, Lang S, Jackson EK, et al.. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol. 2014;177:531–43. doi: 10.1111/cei.12354. PMID:24749746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. doi: 10.1038/srep20254. PMID:26842680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zumaquero E, Munoz P, Cobo M, Lucena G, Pavon EJ, Martin A, Navarro P, García-Pérez A, Ariza-Veguillas A, Malavasi F, et al.. Exosomes from human lymphoblastoid B cells express enzymatically active CD38 that is associated with signaling complexes containing CD81, Hsc-70 and Lyn. Exp Cell Res. 2010;316:2692–706. doi: 10.1016/j.yexcr.2010.05.032. PMID:20570673. [DOI] [PubMed] [Google Scholar]
- 38.Henao Agudelo JS, Braga TT, Amano MT, Cenedeze MA, Cavinato RA, Peixoto-Santos AR, Muscará MN, Teixeira SA, Cruz MC, Castoldi A, et al.. Mesenchymal stromal cell-derived microvesicles regulate an internal pro-inflammatory program in activated macrophages. Front Immunol. 2017;8:881. doi: 10.3389/fimmu.2017.00881. PMID:28824619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D'Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19:1875–85. doi: 10.1016/j.cub.2009.09.059. PMID:19896381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cieślak M, Komoszyński M, Wojtczak A. Adenosine A(2A) receptors in Parkinson's disease treatment. Purinergic Signalling. 2008;4:305–12. doi: 10.1007/s11302-008-9100-8. PMID:18438720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohta A, Sitkovsky M. The adenosinergic immuno-modulatory drugs. Current opinion in pharmacology. 2009;9:501–6. doi: 10.1016/j.coph.2009.05.005. PMID:19539527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–87. doi: 10.1146/annurev.pharmtox.41.1.775. PMID:11264476. [DOI] [PubMed] [Google Scholar]
- 43.Chillemi A, Quarona V, Antonioli L, Ferrari D, Horenstein AL, Malavasi F. Roles and modalities of ectonucleotidases in remodeling the multiple myeloma niche. Front Immunol. 2017;8:305. doi: 10.3389/fimmu.2017.00305. PMID:28373875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood. 2018;4:13–29. doi: 10.1182/blood2017-06-740944. Epub 2017 Nov 8. [DOI] [PubMed] [Google Scholar]
- 45.Malavasi F, Chillemi A, Castella B, Schiavoni I, Incarnato D, Oliva S, et al.. CD38 and Antibody Therapy: What Can Basic Science Add? Blood. 2016;128:SCI-36–SCI-. [Google Scholar]
- 46.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos M-V, Kumar S, Hillengass J, Kastritis E, Richardson P, et al.. International myeloma working group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 15:e538–e48. doi: 10.1016/S1470-2045(14)70442-5. PMID:25439696. [DOI] [PubMed] [Google Scholar]
- 47.Ferretti E, Tripodo C, Pagnan G, Guarnotta C, Marimpietri D, Corrias MV, Ribatti D, Zupo S, Fraternali-Orcioni G, Ravetti JL, et al.. The interleukin (IL)-31/IL-31R axis contributes to tumor growth in human follicular lymphoma. Leukemia. 2015;29:958–67. doi: 10.1038/leu.2014.291. PMID:25283844. [DOI] [PubMed] [Google Scholar]
- 48.Marimpietri D, Petretto A, Raffaghello L, Pezzolo A, Gagliani C, Tacchetti C, Mauri P, Melioli G, Pistoia V. Proteome profiling of neuroblastoma-derived exosomes reveal the expression of proteins potentially involved in tumor progression. PLoS One. 2013;8:e75054. doi: 10.1371/journal.pone.0075054. PMID:24069378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morandi F, Morandi B, Horenstein AL, Chillemi A, Quarona V, Zaccarello G, Carrega P, Ferlazzo G, Mingari MC, Moretta L, et al.. A non-canonical adenosinergic pathway led by CD38 in human melanoma cells induces suppression of T cell proliferation. Oncotarget. 2015;6:25602–18. doi: 10.18632/oncotarget.4693. PMID:26329660. [DOI] [PMC free article] [PubMed] [Google Scholar]


