ABSTRACT
In this study, we extensively dissected the phenotypic complexity of the splenic tumor microenvironment (TME) in chronic lymphocytic leukemia (CLL) by high-dimensional mass cytometry (CyTOF). As a result, we identified potential new targets and tested a dual immune checkpoint blockade targeting the TME in pre-clinical mouse models of CLL.
KEYWORDS: chronic lymphocytic leukemia, tumor microenvironment, immunotherapy, immune-checkpoint blockade, mass cytometry
The progression of chronic lymphocytic leukemia (CLL), the most frequent leukemia in adults, is highly dependent on complex interactions with cells populating the CLL tumor microenvironment (TME), which is associated with dysfunctional anti-tumor immunity.1 To escape surveillance by the immune system, tumor cells are able to co-opt multiple immunosuppressive pathways2,3 such as the engagement of immune checkpoints that prevents an effective anti-tumor immunity. Immunotherapy aims to stimulate anti-tumor activity by reactivating the immune system. Recently, immune checkpoints blockade showed promising results in a mouse preclinical model of CLL.4 Despite recent advances in treatments, CLL remains an incurable disease. Therefore, finding specific targets and combining immunotherapy approaches represent new challenges for efficient CLL treatment.
As first CyTOF (Cytometry by Time Of Flight) study in CLL, we deeply characterized the splenic CLL microenvironment by high-resolution mass cytometry using a custom 35-marker panel (Fig. 1A). Over the last years, mass cytometry emerged as a new technology for high-dimensional multi-parameter single cell analysis that overcomes the limitations of conventional flow cytometry. By using antibodies labeled with heavy-metal isotopes instead of fluorescent tags, mass cytometry enables the simultaneous quantification of multiple surface and intracellular proteins and thus provides the most comprehensive understanding of the cellular phenotypic signature within the TME. For example, mass cytometry enabled the identification of specific cancer-associated fibroblast (CAF) subsets in human breast cancer5 as well as the analysis of immune responses in several tissues after immunotherapy in genetically-engineered cancer models.6 However, interpretation of the complex cytometry data remains challenging and requires tools that capture the multi-parametric relationship of cells.
Figure 1.
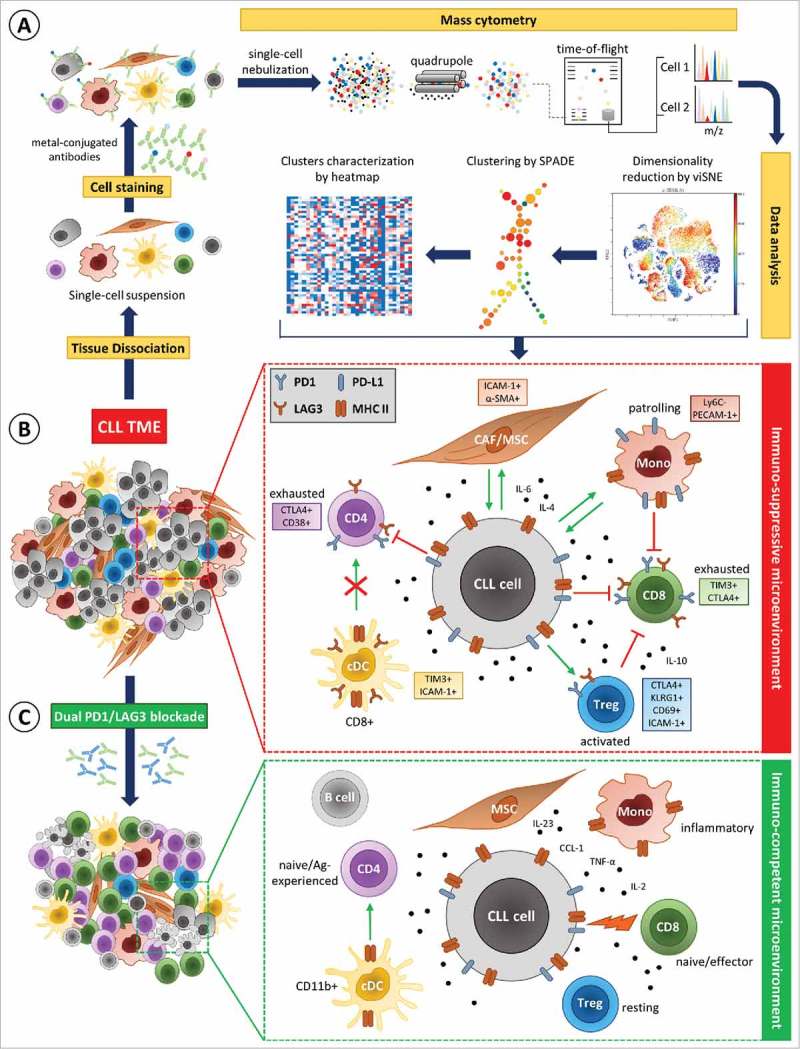
Mass cytometry analysis of the CLL microenvironment before and after dual PD1/LAG3 blockade. (A) High-dimensional single-cell mass cytometry of the splenic immune infiltrate in CLL was performed to dissect the complexity of the tumor microenvironment (TME). (B) Mass cytometry revealed relevant alterations in the composition of the splenic CLL TME, the upregulation of immune checkpoints and other specific surface molecules (colored boxes), as well as cytokines. Enhanced PD1/PD-L1 and LAG3/MHC II signaling in the CLL TME leads to cellular activation (green arrows) or inhibition (red arrows) of target cells depending on cell types resulting in an immuno-suppressive microenvironment. (C) Dual immune checkpoint blockade (anti-PD1/anti-LAG3) resolved alterations induced by CLL and restored an immuno-competent microenvironment with an effective anti-tumor immune response.
To establish an extensive cartography of immune cell subsets populating the leukemic spleen, we combined multiple tools as viSNE (visualization for high-dimensional single-cell data based on the t-Distributed Stochastic Neighbor Embedding (t-SNE) algorithm) and SPADE (SPAnning tree progression of Density normalized Events) to provide a comprehensive view of the data, and more especially to facilitate statistical comparison of cell populations (SPADE) while maintaining single-cell resolution (viSNE) (Fig. 1A).
We observed that malignant CLL cells, when compared to normal B cells, show a higher expression of Programmed cell death ligand 1 (PD-L1) and Lymphocyte-activation gene 3 (LAG3) immune checkpoints as well as several maturation and adhesion molecules such as CD44 and ICAM-1. Furthermore, PD-L1 expression was heterogeneous on CLL cells, a high expression being concomitant with a higher expression of maturation and adhesion molecules. Excluding B cells from our analysis pipeline, we identified a significant alteration in the immune cell composition in CLL that is associated with strong immune suppression (Fig. 1B). More particularly, we revealed a significant enrichment of CD8+ T cell subpopulations displaying features of exhaustion such as the expression of the immune checkpoints PD1, LAG3, T-cell immunoglobulin and mucin-domain containing-3 (TIM3) and cytotoxic T-lymphocyte-associated Protein 4 (CTLA4). Our analysis revealed as well an expansion of two subsets of activated regulatory T cells (Treg) expressing PD1, LAG3, CTLA4 and killer-cell lectin-like receptor G1 (KLRG1), both described to have enhanced suppressive capacities.7 Concerning the myeloid compartment, tolerogenic CD8+ dendritic cells also expressing immune checkpoints such as LAG3 or TIM3 as well as PECAM-1+ PD-L1+ patrolling (PAT) monocytes8 were enriched in CLL. Overall, we recognized an upregulation of PD1 and LAG3 on the majority of immune cell populations in CLL TME. This is particularly relevant as both PD-L1 and MHC II, the ligands for PD1 and LAG3, respectively, are expressed by CLL cells and monocytes thus indicating enhanced PD1/PD-L1 and LAG3/MHC II signaling in the CLL microenvironment. Therefore, we evaluated a dual anti-PD1/anti-LAG3 immunotherapy in preclinical mouse models of CLL. Indeed, dual PD1/LAG3 blockade efficiently limited CLL development and restored an immuno-competent microenvironment as identified by mass cytometry (Fig. 1C). Additionally, the cytokine profile in serum after dual checkpoint blockade was comparable to healthy controls and showed the restoration of an effective immune response illustrated by increased levels of IL-2, IL-23, TNF-α, and CCL1.
Some checkpoint blockades such as anti-CTLA4 and anti-PD1 are already approved and show beneficial effects in the treatment of melanoma9 and non-small cell lung cancer (NSCLC).10 The number of open clinical trials investigating checkpoint blockade is increasing in hematological malignancies. In particular, PD1 blockade is currently tested as monotherapy or combination therapy with anti-LAG3 in CLL and other B-cell malignancies (ClinicalTrials.gov ID NCT02061761). Indeed, the majority of patients do not respond to single-agent therapies, thus highlighting a clinical need to design combination therapeutic approaches for multiple targets. In this study, we demonstrated that a dual anti-PD1/LAG3 therapy is a potential combination that could be beneficial for CLL patients.
In conclusion, this study underlines that high-dimensional mass cytometry represents a powerful tool to profile the immune contexture and characterize complex phenotypic populations within a heterogeneous microenvironment to identify potential new therapeutic targets for leukemia treatment. Thus, integration of immune phenotyping appears as a novel branch of precision/targeted medicine in which therapeutic decisions are based on the composition of the TME. Clinically, mass cytometry arises as a new technology allowing rapid implementation and adoption of novel advances in clinical immunology.
Importantly and in contrast to a wide array of solid tumors, the TME of hematological malignancies is per se composed of a high number of immune cells and particularly T lymphocytes, and thus can be recognized as a “hot tumor”. Combination immunotherapies appear especially suitable to restore functional anti-tumor immunity and therefore more clinical trials are required to identify and test promising therapies.
Funding Statement
This work was supported by funding from Fonds National de la Recherche Luxembourg (FNR, INTER/DFG/11509946), Fonds de la Recherche Scientifique (FNRS, Télévie/7.4508.16) and from Luxembourg Institute of Health (HEMATEXO).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood. 2009;114:3367–75. doi: 10.1182/blood-2009-06-225326. PMID:19636060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nat Biotechnol. 2009;27:129–39. doi: 10.1038/nbt0209-129. PMID:19204689. [DOI] [PubMed] [Google Scholar]
- 3.Cutucache CE. Tumor-induced host immunosuppression: special focus on CLL. Int Immunopharmacol. 2013;17:35–41. doi: 10.1016/j.intimp.2013.05.021. PMID:23751895. [DOI] [PubMed] [Google Scholar]
- 4.McClanahan F, Hanna B, Miller S, Clear AJ, Lichter P, Gribben JG, Seiffert M. PD-L1 checkpoint blockade prevents immune dysfunction and leukemia development in a mouse model of chronic lymphocytic leukemia. Blood. 2015;126:203–11. doi: 10.1182/blood-2015-01-622936. PMID:25800048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa A, Kieffer Y, Scholer-Dahirel A, Pelon F, Bourachot B, Cardon M, Sirven P, Magagna I, Fuhrmann L, Bernard C, et al.. Fibroblast heterogeneity and immunosuppressive environment in human breast cancer. Cancer Cell. 2018;33:463–79e10. doi: 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Spitzer MH, Carmi Y, Reticker-Flynn NE, Kwek SS, Madhireddy D, Martins MM, Gherardini PF, Prestwood TR, Chabon J, Bendall SC, et al.. Systemic immunity is required for effective cancer immunotherapy. Cell. 2017;168:487–502e15. doi: 10.1016/j.cell.2016.12.022. PMID:28111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–5. doi: 10.1126/science.1160062. PMID:18845758. [DOI] [PubMed] [Google Scholar]
- 8.Hanna BS, McClanahan F, Yazdanparast H, Zaborsky N, Kalter V, Rossner PM, Benner A, Dürr C, Egle A, Gribben JG, et al.. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2016;30:570–9. doi: 10.1038/leu.2015.305. PMID:26522085. [DOI] [PubMed] [Google Scholar]
- 9.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al.. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. PMID:26027431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. PMID:25891174. [DOI] [PubMed] [Google Scholar]


