Abstract
Background
Although people living with HIV or AIDS (PLWHA) are at higher risk for many cancers, breast, prostate, and colorectal cancer rates are lower in this patient population. Because these tumors are often screen-detected, these inverse associations could be driven by HIV-related differences in utilization of cancer screening.
Methods
We ascertained incident breast, prostate, and colorectal cancer in PLWHA using data from the HIV/AIDS Cancer Match Study (1996–2012). Comparisons with general population cancer rates were made using standardized incidence ratios (SIRs), overall and stratified by tumor stage/size, breast cancer estrogen receptor status, and colorectal site. We also examined the potential effect of study design and unmeasured confounding on inverse standardized incidence ratios.
Results
Compared with the general population, PLWHA had lower rates of invasive breast (SIR = 0.63, 95% confidence interval [CI] = 0.58 to 0.68), prostate (SIR = 0.48, 95% CI = 0.46 to 0.51), proximal colon (SIR = 0.67, 95% CI = 0.59 to 0.75), distal colon (SIR = 0.51, 95% CI = 0.43 to 0.59), and rectal cancers (SIR = 0.69, 95% CI = 0.61 to 0.77). Reduced risk persisted across tumor stage/size for prostate and colorectal cancers. Although distant-stage breast cancer rates were not reduced (SIR = 0.94, 95% CI = 0.73 to 1.20), HIV-infected women had lower rates of large (>5 cm) breast tumors (SIR = 0.65, 95% CI = 0.50 to 0.83). The magnitude of these inverse standardized incidence ratios could not plausibly be attributed to case underascertainment, out-migration, or unmeasured confounding.
Conclusions
Breast, prostate, and colorectal cancer rates are markedly lower among PLWHA, including rates of distant-stage/large tumors that are not generally screen-detected. This set of inverse HIV-cancer associations is therefore unlikely to be due primarily to differential screening and may instead represent biological relationships requiring future investigation.
Human immunodeficiency virus (HIV) leads to progressive immunosuppression and has been linked to increased cancer risk since the beginning of the HIV epidemic in the United States (1–5). Despite decreases in the incidence of certain virally associated cancers such as Kaposi sarcoma (KS) and non-Hodgkin lymphoma (NHL) after the widespread introduction of highly active antiretroviral therapy (HAART) in 1996 (6–8), more recent data (post-2010) indicate that HIV-related immunosuppression remains a risk factor for numerous cancers in people living with HIV or AIDS (PLWHA) (9–11). A small number of intriguing exceptions have been identified, suggesting that there may be a subset of tumors that occur less frequently in PLWHA. This unique set of cancers includes three common, solid organ tumors that are often targets of screening in the United States: breast, prostate, and colorectal cancers (12–19). Rates of these three cancers in PLWHA are approximately half those observed in the general US population (20).
Because these tumors are often screen-detected, observed cancer deficits have been hypothesized to result from lower uptake of screening tests such as mammography or prostate-specific antigen (PSA) in PLWHA compared with the general population (13). Lower screening rates could result in less frequent early tumor detection, leading to decreased rates of local-stage tumors relative to a population receiving screening (ie, screening effect). However, this scenario would not lead to lower cancer rates for larger tumors, which are generally clinically detected. In fact, in the absence of frequent screening, a higher proportion of cancers are likely to be diagnosed at advanced stages, which could result in an elevation in risk for distant-stage disease.
To test whether such a screening effect, rather than underlying biology, could be the primary explanation for the observed HIV-related deficits in these three common tumors, we examined cancer rates in PLWHA stratified by tumor stage and size at diagnosis. We also assessed whether inverse associations could be driven by artifacts induced by our data linkage study design, including underascertainment of HIV-positive cancer cases, out-migration of PLWHA from registry areas, and unmeasured confounding.
Methods
Data Source
We utilized data from the HIV/AIDS Cancer Match (HACM) Study, a linkage of nine US population-based cancer and HIV registries (https://hivmatch.cancer.gov/) (8). Among PLWHA, we used cancer registry data to ascertain incident cases of breast, prostate, and colorectal cancers beginning at four months following the earlier of HIV report or AIDS diagnosis date and continuing until death or end of cancer registry follow-up. Cases were captured during years when HIV infection and an AIDS diagnosis were both reportable conditions and cancer registries had complete case ascertainment. Included registries (by year) were: Colorado (1996–2007), Connecticut (2005–2010), Georgia (2004–2012), Maryland (2008–2012), Michigan (1996–2010), New Jersey (1996–2012), New York (2001–2012), Puerto Rico (2003–2012), and Texas (1999–2009). This study was approved by institutional review boards at the National Cancer Institute and participating registries as required.
Case Ascertainment
Prostate cancers were identified using the International Classification of Diseases for Oncology, version 3 (ICD-O3), topography code C619. The Surveillance, Epidemiology, and End Result (SEER) cancer site–specific factor-1 variable was available between 2004 and 2012 to classify female breast cancers (ICD-O3 C500-509) as estrogen receptor (ER) positive or negative. Colorectal cancers were evaluated by site: proximal colon (ICD-O3 C180-84), distal colon (ICD-O3 C185-87), and rectum (ICD-O3 C199, C209). Rectal tumors with squamous histology (ICD-O-3 histology codes 8050-8084, 8094, 8123, 8124, 8215) were excluded, as these may be misclassified anal cancers (21). We considered only the first primary, nonrecurrent cancer of each type (breast, prostate, colorectal). Each registry provided tumor stage data, and all but Colorado also provided tumor size data for breast and colorectal tumors from 2004 onward. Data on tumor size was missing for a proportion of these breast (14%) and colorectal cancers (proximal colon: 21%, distal colon: 26%, rectum: 35%).
Statistical Analysis
To determine whether cancer rates in PLWHA differed from the general population, we calculated standardized incidence ratios (SIRs), defined as the ratio of observed cases in PLWHA to the expected cancer counts. The expected counts were determined using cancer rates from the general population of the registry regions, standardized to the characteristics of the HIV population by age, sex, race/ethnicity, calendar year, and registry. We calculated the overall standardized incidence ratio for each cancer type, as well as standardized incidence ratios stratified by attained age (0–39, 40–49, 50–59, 60–69, 70+ years), race/ethnicity (non-Hispanic whites, non-Hispanic blacks, Hispanics), HIV risk group (men who have sex with men [MSM], injection drug users [IDUs], and male and female heterosexuals), history of an AIDS diagnosis, and calendar year (1996–2000, 2001–2005, 2006+). Ninety-five percent confidence intervals around each standardized incidence ratio estimate and P values testing whether the standardized incidence ratio was different than the null value of 1.0 were calculated using the exact method in SAS version 9.3 (SAS Institute, Inc.). P values of less than .05 (alpha error rate = 5%) were considered statistically significant. All statistical tests were two-sided.
To evaluate whether observed associations were consistent with a screening effect, we calculated standardized incidence ratios stratified by tumor stage for all cancers (SEER summary stage: local, regional, and distant), as well as standardized incidence ratios stratified by tumor size (small: <2 cm, medium: 2–4.9 cm, large: >5 cm) for breast and colorectal cancer. Because smaller, local-stage tumors are often detected through screening, inverse standardized incidence ratios observed only in that stratum, and not among larger, distant-stage tumors that are more likely to be clinically detected, would be consistent with a screening effect. We further compared standardized incidence ratios for invasive breast cancer, which can be detected through screening or after symptomatic presentation, with standardized incidence ratios for noninvasive ductal carcinoma in situ (DCIS), a diagnosis that is exclusively screen-detected.
Implications of Study Design
We next examined whether imperfect follow-up ascertainment or failure to capture case HIV status within the HACM registry linkages (22) could serve as alternative explanations for inverse associations. A proportion of PLWHA with longer-term follow-up (>10 years) may have moved out of the registry catchment area, potentially resulting in an excess number of person-years being part of the expected cancer count calculation, which could artificially lower standardized incidence ratios. We recalculated overall standardized incidence ratios by cancer type after decreasing the person-years for PLWHA by 10% starting 10 years after HIV report or AIDS diagnosis date. In addition, prior reports have noted imperfect sensitivity of the HACM registry linkages, with approximately 82% of HIV-infected cancer patients successfully linking to their respective HIV registry (ie, 18% of HIV-infected cases misclassified at HIV-uninfected) (23). This imperfect sensitivity could artificially decrease the estimated standardized incidence ratios by decreasing observed HIV-infected case counts. We recalculated standardized incidence ratios after increasing the observed case counts in PLWHA by 22% (ie, dividing by 82% sensitivity [1/0.82 = 1.22]).
Potential Confounding
Finally, we considered the possibility that lower cancer risk could be due to unmeasured confounding. We estimated potential combinations of risk factor prevalence in PLWHA, risk factor prevalence in the general population, and the relative risk (RR) between a given risk factor and cancer that would be required to induce inverse standardized incidence ratios of the magnitude we observed, given the baseline assumption that the “true” association was null (SIR = 1.0). To do this, we applied the following formula, where “A” denotes the prevalence of the hypothetical risk factor in the general population; “B” denotes the prevalence of the risk factor in HIV-infected individuals; and “RR” represents the relative risk between the risk factor and cancer (4).
Results
During approximately 3.1 million person-years of follow-up in PLWHA, we observed: 688 invasive breast cancers in women (incidence rate [IR] = 77.0/100 000), 1522 prostate cancers in men (IR = 49.34/100 000), and 713 colorectal cancers (269 proximal colon IR = 8.7/100 000; 173 distal colon IR = 5.6/100 000, 271 rectum IR = 8.8/100 000). These observed counts represented statistically significantly lower cancer rates than would be expected for persons in the general population from these nine HACM registry regions (Table 1). HIV-infected women were nearly 40% less likely to be diagnosed with invasive breast cancer (SIR = 0.63, 95% CI = 0.58 to 0.68, P < .001), an inverse association that persisted for both ER-positive (SIR = 0.55, 95% CI = 0.49 to 0.61, P < .001) and -negative (SIR = 0.68, 95% CI = 0.58 to 0.79, P < .001) tumors. HIV-infected men were more than 50% less likely to be diagnosed with prostate cancer (SIR = 0.48, 95% CI = 0.46 to 0.51, P < .001). The risk of colorectal cancer was also reduced in PLWHA compared with the general population (proximal colon SIR = 0.67, 95% CI = 0.59 to 0.75, P < .001; distal colon SIR = 0.51, 95% CI = 0.43 to 0.59, P < .001; rectum SIR = 0.69, 95% CI = 0.61 to 0.77, P < .001).
Table 1.
SIRs and 95% CIs comparing observed cancers in PLWHA to expected counts from the US general population, according to cancer diagnosis and stage
| Overall |
Local stage |
Regional stage |
Distant stage |
Unknown stage |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cancer diagnosis | No. | SIR (95% CI) | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) |
| Invasive breast cancer | 688 | 0.63 (0.58 to 0.68) | 336 (48.8)* | 0.58 (0.52 to 0.64) | 254 (36.9) | 0.61 (0.54 to 0.69) | 68 (9.9) | 0.94 (0.73 to 1.20) | 30 (4.4) | 1.01 (0.68 to 1.45) |
| Estrogen receptor positive | 305 | 0.55 (0.49 to 0.61) | 166 (54.4) | 0.54 (0.46 to 0.63) | 114 (37.4) | 0.55 (0.45 to 0.65) | 24 (7.9) | 0.74 (0.48 to 1.05) | 1 (<1) | 0.23 (0.01 to 1.28) |
| Estrogen receptor negative | 164 | 0.68 (0.58 to 0.79) | 73 (44.5) | 0.59 (0.47 to 0.75) | 69 (42.1) | 0.71 (0.55 to 0.89) | 21 (12.8) | 1.10 (0.68 to 1.68) | 1 (<1) | 0.42 (0.01 to 2.36) |
| Prostate cancer | 1522 | 0.48 (0.46 to 0.51) | 1242 (81.6) | 0.49 (0.46 to 0.52) | 132 (8.7) | 0.40 (0.33 to 0.47) | 61 (4.0) | 0.55 (0.42 to 0.71) | 87 (5.7) | 0.40 (0.33 to 0.47) |
| Proximal colon cancer | 269 | 0.67 (0.59 to 0.75) | 95 (35.3) | 0.69 (0.56 to 0.85) | 97 (36.1) | 0.62 (0.50 to 0.75) | 67 (24.9) | 0.69 (0.54 to 0.88) | 10 (3.7) | 0.81 (0.39 to 1.50) |
| Distal colon cancer | 173 | 0.51 (0.43 to 0.59) | 62 (35.8) | 0.49 (0.37 to 0.62) | 61 (35.3) | 0.50 (0.38 to 0.64) | 40 (23.1) | 0.51 (0.37 to 0.70) | 10 (5.8) | 0.71 (0.34 to 1.31) |
| Rectal cancer | 271 | 0.69 (0.61 to 0.77) | 106 (39.1) | 0.66 (0.54 to 0.80) | 75 (27.7) | 0.60 (0.47 to 0.75) | 55 (20.3) | 0.74 (0.56 to 0.97) | 35 (12.9) | 1.02 (0.71 to 1.42) |
Percentage of overall cancer diagnoses by stage. CI = confidence interval; PLWHA = people living with HIV or AIDS; SIR = standardized incidence ratio.
Cancer rates remained lower in PLWHA when considering stratification by stage for prostate (SIR range = 0.40–0.55), proximal colon (SIR range = 0.62–0.69), distal colon (SIR range = 0.49–0.51), and rectal tumors (SIR range = 0.60–0.74). Lower risk also persisted for small (<2 cm), medium (2–4.9 cm), and large (>5 cm) tumors of the distal colon (SIR range = 0.46–0.67) and rectum (SIR range = 0.62–0.75), as well as medium and large tumors of the proximal colon (SIR range = 0.63–0.73) (Table 2). Data on tumor size were not available for prostate cancer.
Table 2.
SIRs and 95% CIs comparing observed cancers in PLWHA to expected counts from the general US population, according to cancer diagnosis and tumor size
| Overall |
Small tumors <2 cm |
Medium tumors 2–4.9 cm |
Large tumors >5 cm |
|||||
|---|---|---|---|---|---|---|---|---|
| Cancer diagnosis | No. | SIR (95% CI) | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) |
| Invasive breast cancer | 688 | 0.63 (0.58 to 0.68) | 256 (37.2)* | 0.56 (0.49 to 0.63) | 176 (25.6) | 0.61 (0.52 to 0.70) | 64 (9.3) | 0.65 (0.50 to 0.83) |
| Proximal colon cancer | 269 | 0.67 (0.59 to 0.75) | 26 (9.7) | 0.72 (0.47 to 1.06) | 79 (29.4) | 0.73 (0.58 to 0.91) | 79 (29.4) | 0.63 (0.50 to 0.78) |
| Distal colon cancer | 173 | 0.51 (0.43 to 0.59) | 25 (14.5) | 0.67 (0.43 to 0.99) | 42 (24.3) | 0.46 (0.33 to 0.62) | 41 (23.7) | 0.51 (0.36 to 0.69) |
| Rectal cancer | 271 | 0.69 (0.61 to 0.77) | 41 (15.1) | 0.67 (0.48 to 0.91) | 60 (22.1) | 0.75 (0.57 to 0.97) | 43 (15.9) | 0.62 (0.45 to 0.83) |
Percentages of overall cancer diagnoses by tumor size calculated among cases from 2004 onward, when tumor size data were available from all registries except Colorado (breast: 574, proximal colon: 232, distal colon: 145, rectum: 223). Missing tumor size data by diagnosis: breast (14%), prostate (100%), proximal colon (21%), distal colon (26%), rectum (35%). CI = confidence interval; PLWHA = people living with HIV or AIDS; SIR = standardized incidence ratio.
Lower breast cancer risk in HIV-infected women was observed for in situ breast tumors (SIR = 0.59, 95% CI = 0.50 to 0.68) and invasive local- (SIR = 0.58, 95% CI = 0.52 to 0.64) and regional-stage tumors (SIR = 0.61, 95% CI = 0.54 to 0.69). Lower risk was not observed for distant-stage disease (SIR = 0.94, 95% CI = 0.73 to 1.20). However, large breast tumors (>5 cm) did occur less frequently in HIV-infected women (SIR = 0.65, 95% CI = 0.50 to 0.83). In addition, we observed a consistent pattern of inverse standardized incidence ratios for breast cancer across demographic strata, including lower breast cancer rates among HIV-infected women younger than age 40 years (SIR = 0.73, 95% CI = 0.59 to 0.92), a group unlikely to be screened (Table 3).
Table 3.
SIRs and 95% CIs comparing observed cancers in PLWHA to expected count from the general US population, according to cancer diagnosis and demographics
| Invasive breast cancer |
Prostate cancer |
Proximal colon cancer |
Distal colon cancer |
Rectal cancer |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) | No. (%) | SIR (95% CI) |
| Total cases | 688 | 0.63 (0.58 to 0.68) | 1522 | 0.48 (0.46 to 0.51) | 269 | 0.67 (0.59 to 0.75) | 173 | 0.51 (0.43 to 0.59) | 271 | 0.69 (0.61 to 0.77) |
| Age, y | ||||||||||
| 0–39 | 82 (11.9) | 0.73 (0.59 to 0.92) | 8 (<1%) | 1.57 (0.68 to 3.08) | 10 (3.7) | 0.56 (0.27 to 1.03) | 8 (4.6) | 0.59 (0.25 to 1.16) | 31 (11.4) | 1.69 (1.15 to 2.40) |
| 40–49 | 277 (40.3) | 0.63 (0.55 to 0.70) | 173 (11.4) | 0.52 (0.45 to 0.61) | 50 (18.6) | 0.56 (0.42 to 0.74) | 38 (22.0) | 0.48 (0.34 to 0.66) | 80 (29.5) | 0.78 (0.62 to 0.97) |
| 50–59 | 232 (33.7) | 0.63 (0.55 to 0.71) | 627 (41.2) | 0.44 (0.41 to 0.48) | 95 (35.3) | 0.61 (0.49 to 0.74) | 68 (39.3) | 0.46 (0.36 to 0.59) | 100 (36.9) | 0.59 (0.48 to 0.71) |
| 60–69 | 77 (11.2) | 0.57 (0.45 to 0.71) | 550 (36.1) | 0.49 (0.45 to 0.54) | 76 (28.3) | 0.80 (0.63 to 1.00) | 39 (22.5) | 0.51 (0.37 to 0.70) | 43 (15.9) | 0.55 (0.40 to 0.74) |
| 70+ | 20 (2.9) | 0.54 (0.33 to 0.84) | 164 (10.8) | 0.56 (0.48 to 0.65) | 38 (14.1) | 0.86 (0.61 to 1.19) | 20 (11.6) | 0.73 (0.44 to 1.12) | 17 (6.3) | 0.68 (0.40 to 1.09) |
| Race† | ||||||||||
| White | 105 (15.3) | 0.60 (0.49 to 0.73) | 317 (20.8) | 0.43 (0.39 to 0.48 | 61 (22.7) | 0.68 (0.52 to 0.87) | 32 (18.5) | 0.39 (0.27 to 0.56) | 77 (28.4) | 0.72 (0.57 to 0.90) |
| Black | 442 (64.2) | 0.65 (0.59 to 0.71) | 954 (62.7) | 0.51 (0.48 to 0.54) | 145 (53.9) | 0.62 (0.53 to 0.73) | 95 (54.9) | 0.54 (0.43 to 0.66) | 142 (52.4) | 0.74 (0.63 to 0.88) |
| Hispanic | 141 (20.5) | 0.59 (0.50 to 0.70) | 251 (16.5) | 0.45 (0.39 to 0.51) | 63 (23.4) | 0.79 (0.61 to 1.01) | 46 (26.6) | 0.55 (0.40 to 0.73) | 52 (19.2) | 0.54 (0.40 to 0.71) |
| Risk group‡ | ||||||||||
| MSM | − * | – | 644 (42.3) | 0.54 (0.50 to 0.59) | 67 (24.9) | 0.53 (0.41 to 0.67) | 44 (25.4) | 0.39 (0.29 to 0.53) | 75 (27.7) | 0.53 (0.42 to 0.66) |
| Male IDU | – | – | 314 (20.6) | 0.34 (0.30 to 0.38) | 35 (13.0) | 0.44 (0.31 to 0.62) | 30 (17.3) | 0.42 (0.28 to 0.60) | 49 (18.1) | 0.59 (0.44 to 0.79) |
| Male het. | – | – | 181 (11.9) | 0.55 (0.47 to 0.63) | 27 (10.0) | 0.88 (0.58 to 1.28) | 23 (13.2) | 0.87 (0.55 to 1.31) | 29 (10.7) | 0.97 (0.65 to 1.40) |
| Female IDU | 156 (22.7) | 0.50 (0.42 to 0.58) | – | – | 21 (7.8) | 0.80 (0.49 to 1.2) | 19 (11.0) | 0.87 (0.52 to 1.35) | 17 (6.3) | 0.78 (0.45 to 1.25) |
| Female het. | 334 (48.5) | 0.71 (0.63 to 0.79) | – | – | 45 (16.7) | 1.04 (0.76 to 1.39) | 16 (9.2) | 0.47 (0.27 to 0.77) | 39 (14.4) | 1.17 (0.83 to 1.60) |
| Other, %§ | 198 (28.8) | 383 (25.2) | 74 (27.5) | 41 (23.7) | 62 (22.9) | |||||
| Clinical diagnosis | ||||||||||
| HIV only | 251 (36.5) | 0.66 (0.58 to 0.75) | 454 (29.8) | 0.54 (0.49 to 0.59) | 87 (32.3) | 0.74 (0.60 to 0.92) | 55 (31.8) | 0.56 (0.42 to 0.73) | 68 (25.1) | 0.59 (0.46 to 0.75) |
| AIDS | 437 (63.5) | 0.61 (0.55 to 0.67) | 1068 (70.2) | 0.46 (0.43 to 0.49) | 182 (67.7) | 0.64 (0.55 to 0.74) | 118 (68.2) | 0.48 (0.40 to 0.58) | 203 (74.9) | 0.73 (0.63 to 0.83) |
| Year | ||||||||||
| 1996–2000 | 37 (5.4) | 0.75 (0.53 to 1.03) | 33 (2.2) | 0.34 (0.23 to 0.48) | 7 (2.6) | 0.43 (0.17 to 0.89) | 5 (2.9) | 0.34 (0.11 to 0.80) | 17 (6.3) | 1.03 (0.60 to 1.65) |
| 2001–2005 | 188 (27.3) | 0.69 (0.60 to 0.80) | 350 (23.0) | 0.48 (0.43 to 0.53) | 67 (24.9) | 0.65 (0.50 to 0.82) | 49 (28.3) | 0.53 (0.39 to 0.70) | 58 (21.4) | 0.57 (0.43 to 0.73) |
| 2006+ | 463 (67.3) | 0.60 (0.54 to 0.65) | 1139 (74.8) | 0.49 (0.46 to 0.52) | 195 (72.5) | 0.69 (0.60 to 0.79) | 119 (68.8) | 0.51 (0.42 to 0.61) | 196 (72.3) | 0.71 (0.61 to 0.82) |
Breast cancer calculations were restricted to females; prostate cancer calculations were restricted to males. CI = confidence interval; het. = heterosexual; IUD = injection drug user; MSM = men who have sex with men; PLWHA = people living with HIV or AIDS; SIR = standardized incidence ratio.
Cases were restricted to adults reporting white, black, or Hispanic race/ethnicity.
HIV risk group defined as follows: het. = heterosexual; IUD = injection drug user; MSM = men who have sex with men.
The proportion of cases not classified into one of the HIV risk groups noted above is listed as other.
Both HIV-infected patients with a prior AIDS diagnosis and HIV-only patients had lower rates of breast, prostate, and colorectal cancers compared with the general population. Those with a prior AIDS diagnosis had statistically significantly lower risks for cancers of the prostate and rectum (P ≤ .01) compared with HIV-only patients. Breast, proximal colon, and distal colon cancer rates did not statistically significantly differ between HIV-only patients and those with a prior AIDS diagnosis (P = .74, P = .39, and P = .56, respectively).
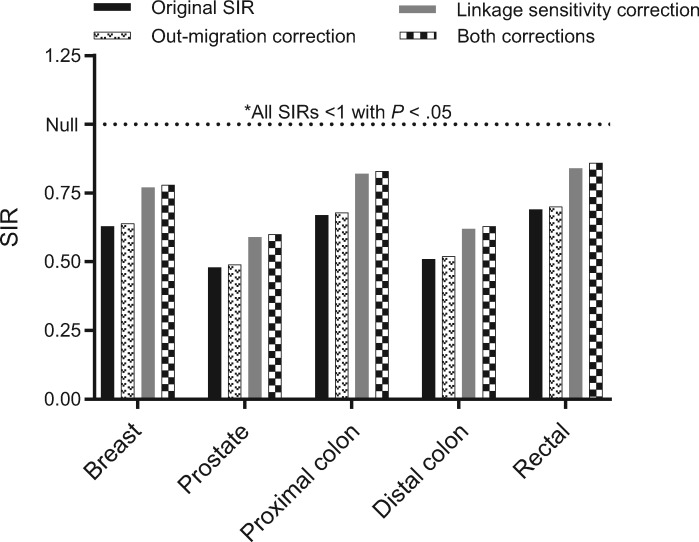
Figure 1 illustrates the effect of accounting for potential data artifacts inherent to the registry linkage design. Neither decreasing follow-up time in the denominator to account for potential out-migration of long-term HIV survivors nor increasing the proportion of cancer cases that are HIV-infected to allow for imperfect sensitivity of the linkage between HIV and cancer registries altered our conclusions. Even standardized incidence ratios corresponding to the most conservative scenario (applying both corrections) represented lower cancer rates in PLWHA: invasive breast (SIR = 0.77, 95% CI = 0.71 to 0.82), prostate (SIR = 0.60, 95% CI = 0.58 to 0.63), proximal colon (SIR = 0.83, 95% CI = 0.75 to 0.93), distal colon (SIR = 0.63, 95% CI = 0.55 to 0.72), and rectum (SIR = 0.86, 95% CI = 0.77 to 0.95).
Figure 1.
Effect of potential data artifacts inherent to registry linkage study design on observed standardized incidence ratios. SIR = standardized incidence ratio.
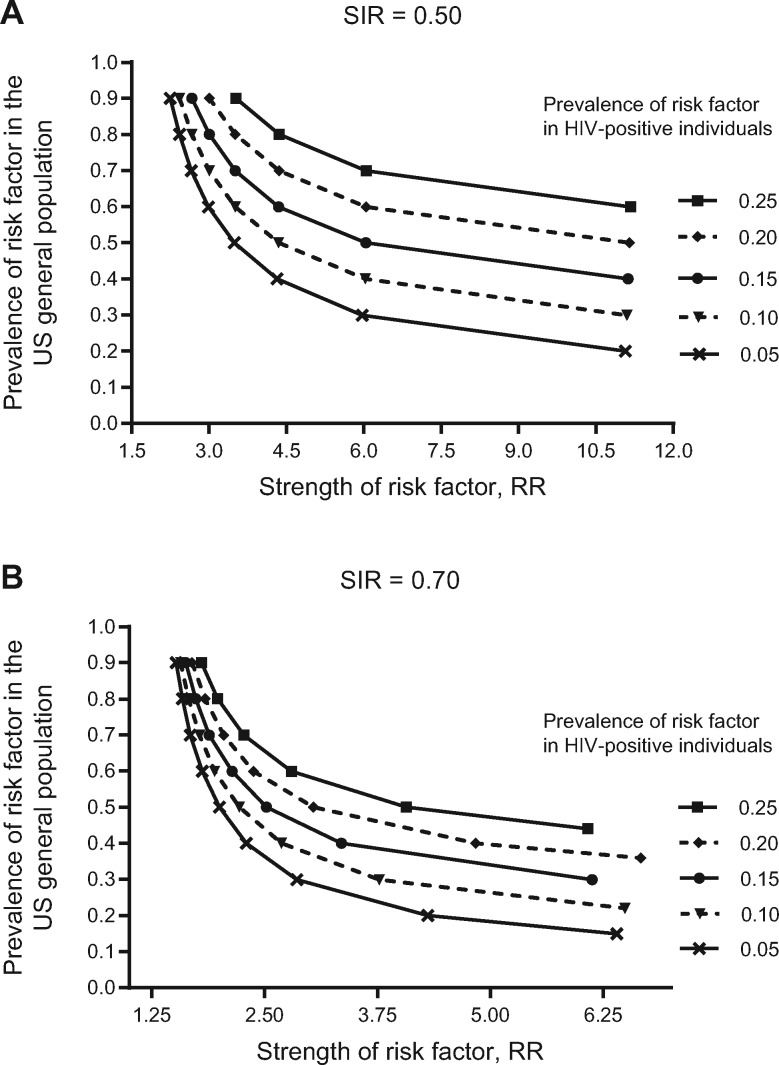
Figure 2 illustrates the characteristics of potentially unmeasured confounders that would be required to artificially induce inverse standardized incidence ratios of the magnitude we report if the underlying association were truly null (SIR = 1.0). For example, to bias a null association down to a standardized incidence ratio of 0.50, a risk factor would have to double prostate cancer risk (risk ratio [RR] along x-axis = 2.0) (Figure 2A), be nearly absent in HIV-infected men (5% prevalence line), and be present in nearly all men in the general population (90% prevalence along y-axis). The characteristics of an unmeasured confounder explaining standardized incidence ratios of 0.70 are also presented (Figure 2B). Across most of the risk factor prevalence spectrum illustrated, a harmful confounder could only induce a false association if it more than doubled cancer rates (eg, RR = 2.5, PLWHA prevalence = 15%, general population prevalence = 50%).
Figure 2.
Risk factor characteristics needed for an unmeasured confounder to induce standardized incidence ratios of the magnitude reported: (A) SIR = 0.50; (B) SIR = 0.70. SIR = standardized incidence ratio.
Discussion
PLWHA in the United States have markedly lower breast, prostate, and colorectal cancer rates compared with the general population. This lower risk was present for both small, local-stage tumors that are primarily screen-detected and larger tumors that are likely clinically detected. This argues against screening behavior as the sole explanation for this unique set of inverse HIV-cancer relationships. Correcting for both uncaptured out-migration and imperfect sensitivity of our data linkages did not account for these inverse associations. Finally, the magnitude of the effect estimates we observed could not plausibly be explained by unmeasured confounding. In combination, these observations lead us to conclude that lower risk for these specific cancers in PLWHA is not a data artifact but may instead represent an underlying biological relationship requiring further study.
This set of cancers is unique in that each is a target of cancer screening—the 2016 US Preventive Services Task Force (USPSTF) guidelines recommend mammography for early detection of invasive breast cancers and colon-/sigmoidoscopy for the detection and removal of pre-invasive lesions in the colon and rectum. In contrast, as of 2012, USPSTF guidelines discourage widespread PSA testing due to concerns of overdiagnosis of prostate cancer (ie, diagnosing indolent tumors that would never progress to invasive, life-threatening disease) (24–26). Although there is evidence that mammography may overdiagnose a proportion of breast cancers (27,28), mammography is still recommended because early detection of invasive breast tumors has resulted in declines in breast cancer mortality (29,30). Given the close link between screening and diagnosis for these common cancers, cancer deficits among PLWHA are often hypothesized to be attributable to lower screening rates in the HIV population (ie, lack of screening resulting in lower detection rates). The largely consistent inverse standardized incidence ratios that we report across both cancers likely to be screen-detected (local-stage), as well as those not likely to be identified through screening (distant-stage), do not support this hypothesis.
The lower rates observed in HIV-infected women for local-stage but not distant-stage breast cancer did raise the possibility of a screening effect, namely that lower screening uptake in HIV-infected women could be a potential explanation. However, HIV-infected women exhibited substantial breast cancer deficits for regional tumors, which are not generally screen-detected. Additionally, if breast cancer incidence were truly similar by HIV status (ie, SIR = 1), but simply diagnosed later in women living with HIV due to a lack of screening, at least a fraction of local-stage tumors that were purportedly missed early should have appeared later as an increased risk in HIV-infected women of distant-stage disease—this was not observed in our data.
In addition to the early detection of invasive disease (secondary prevention), colorectal cancer screening is also a means of primary prevention—colon-/sigmoidoscopy identifies and removes precancerous lesions, decreasing cancer incidence rather than simply shifting stage at diagnosis. Accordingly, rates of colorectal cancer in US adults age 50 years and older, the target group for screening, have declined over the past two decades across disease stage (31). The question of whether rates of cancer screening differ in PLWHA compared with the general US population is not completely understood. Survey data suggest that HIV-infected women are less likely to be current with breast cancer screening (32). However, data from colon-/sigmoidoscopy surveys are conflicting—certain evidence points to lower screening rates in PLWHA, whereas other data suggest that frequent gastrointestinal symptoms in PLWHA may have the opposite effect, increasing the likelihood of clinical contact and subsequent receipt of screening (33,34). If the documented declines in colorectal cancer in the US general population are due to higher uptake of screening (31), the hypothesis that PLWHA receive less screening would result in comparatively higher colorectal cancer rates, which is inconsistent with our findings. It is likely that both the frequency of clinical contact and factors related to health care access play important, and possibly opposing, roles in determining screening uptake in PLWHA. Cancer screening recommendations should be evidence-based and carefully balance the benefits and harms of screening—the data presented here are not adequate to make recommendations specific to PLWHA. Current screening guidelines for PLWHA recommend clinical attention and regular testing similar to the general US population (35).
HIV and cancer registries do not collect data on important cancer risk factors, which is a limitation, but we demonstrated that theoretical HIV-related differences in the distribution of unmeasured confounders were unlikely to induce biased standardized incidence ratios of the magnitude we observed. For example, well-known risk factors for breast cancer (eg, parity and age at first birth) are associated with changes in cancer risk of no greater than 50% (RR ∼ 1.5) (36,37). Our calculations (Figure 2B) indicated that a truly null association between HIV and cancer risk could only be biased to a standardized incidence ratio of 0.70 if such a risk factor were nearly absent (<5% prevalence) in PLWHA but ubiquitous (≥80% prevalence) in the general population—a highly unlikely scenario. A risk factor distributed more evenly across the population (eg, PLWHA = 20%, general population = 40%) would have to increase cancer risk approximately fivefold to induce a false association. Even established risk factor–cancer associations (eg, family history and colorectal cancer) do not fit this profile; a fourfold increase in colorectal cancer incidence has only been observed for those with multiple affected relatives (38), a trait unlikely to be either common enough in the population or related to HIV in a way that could explain our observations.
HIV-infected patients with a prior AIDS diagnosis (ie, history of severe immunosuppression) had marginally lower risks for cancers of the prostate, proximal colon, and rectum compared with individuals with HIV only, but we did not have detailed CD4 T-cell counts to explore this association further. In a previous HACM analysis, the lowest prostate cancer risk was observed for HIV-infected men with CD4 T-cell counts of less than 50 cells/mm3 (13). These data, along with deficits of breast and prostate cancer observed in immunosuppressed transplant recipients (39), raise the possibility that the severity of immunosuppression may impact cancer risk.
A question that deserves further attention, given the sex-specific nature of prostate and breast cancers, is whether HIV affects cancer risk through altering hormone levels. Data from Kaiser Permanente of Northern California (14) indicate that the prostate cancer deficit in HIV-infected men persists after adjustment for testosterone deficiency to account for hypogonadism as a potential link between HIV and lower prostate cancer risk (40–42). Interestingly, additional female sex organ tumors (ovarian and endometrial cancer) occur at lower rates in HIV-infected women (SIR = 0.69 and 0.43, respectively) (20). HIV is also associated with premature menopause (43,44), an outcome affected by endogenous hormones. However, the uniformity of inverse standardized incidence ratios across female cancer sites suggests that a link between HIV and reproductive hormones is not adequate to explain all of our data. For example, whereas oral contraceptive use is associated with increased postmenopausal breast cancer risk (45), it markedly decreases the risk of endometrial cancer (46). HIV altering estrogen levels would therefore not be expected to halve cancer risk uniformly across both cancer types.
A broader mechanism could be postulated, such as HIV perturbing estrogen/androgen metabolism and overall levels of circulating hormones through direct interactions with hormone receptors. The idea that HIV–cell surface interactions can alter cancer risk has been hypothesized in the context of breast cancer. Apoptosis resulting from HIV binding to the CXCR4 chemokine receptor on breast epithelium has been suggested as a possible mechanism for lower breast cancer incidence in HIV-infected women (16). Supporting this hypothesis, an examination of 20 breast cancers in women with HIV viral loads greater than 500 copies/mL reported lower odds of infection with CXCR4-tropic HIV virus compared with controls (16), although this association may be less relevant in the HAART era as CXCR4 tropism develops largely in advanced HIV disease (47).
Our study addresses a currently unanswered question in the field—is an etiologic link between HIV and lower risks of breast, prostate, and colorectal cancers plausible, or is it an artifact due to differential screening, biases in registry data, or unmeasured confounding? The large, population-based nature of the HACM Study allowed us to evaluate the effect of HIV on the risk of clinically relevant tumor categories (eg, ER-positive breast cancer) that have not been reported previously. We evaluated and subsequently eliminated alternative explanations for our findings, strengthening our confidence in the conclusion that PLWHA are at lower risk for this set of common, solid organ tumors.
However, this study was not without limitations. The lack of clinical information describing patient immunosuppression and receipt of antiretroviral therapy precluded us from further examining the link between immunity and cancer risk. Future work should extend our findings by including CD4 T-cell counts and HIV therapy dosage to more completely understand the biological underpinning of these inverse HIV-cancer associations.
In the United States during the HAART era, PLWHA have lower risks of breast, prostate, and colorectal cancer compared with the general population. This lower risk is present for both early-stage tumors that are primarily screen-detected and larger tumors that are likely clinically detected, arguing against a screening effect as the primary explanation for these HIV-related cancer deficits. Further attempts to uncover artifactual explanations for the lower rates of these tumors in PLWHA did not alter our conclusions, suggesting that the lower risk potentially represents an underlying biological relationship.
Funding
This research was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
Notes
Affiliations of authors: Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Rockville, MD (AEC, EAE, PM, MSS); New York State Cancer Registry (MJS).
The funder had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
This research was supported by the Intramural Research Program of the National Cancer Institute at the National Institutes of Health. No authors report any conflicts of interest.
The authors gratefully acknowledge the support and assistance provided by individuals at the following state HIV/AIDS and cancer registries: Colorado, Connecticut, Georgia, Maryland, Michigan, New Jersey, New York, Puerto Rico, and Texas. We also thank Timothy McNeel at Information Management Services for programming support.
The views expressed in this paper are those of the authors and should not be interpreted as reflecting the views or policies of the National Cancer Institute, HIV/AIDS or cancer registries, or their contractors. This research was supported in part by the Intramural Research Program of the National Cancer Institute.
The following cancer registries were supported by the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute: Connecticut (HHSN261201300019I) and New Jersey (HHSN261201300021I, N01-PC-2013-00021). The following cancer registries were supported by the National Program of Cancer Registries of the Centers for Disease Control and Prevention: Colorado (NU58DP006347-01), Georgia (5U58DP003875-01), Maryland (5NU58DP003919-05-00), Michigan (17NU58DP006334), New Jersey (NU58/DP003931-05-00), New York (U58/DP003879), and Texas (5U58DP000824-04). The New Jersey State Cancer Registry was also supported by the state of New Jersey, the Maryland Cancer Registry was supported by the State of Maryland and the Maryland Cigarette Restitution Fund, and the New York State Cancer Registry was also supported by the state of New York. The following HIV registries were supported by HIV Incidence and Case Surveillance Branch of the Centers for Disease Control and Prevention, National HIV Surveillance Systems: Colorado (NU62PS003960), Connecticut (5U62PS001005-05), Michigan (U62PS004011-02), and New Jersey (U62PS004001-2).
References
- 1. Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: Impact of immunosuppression. J Acquir Immune Defic Syndr. 2009;522:203–208. 10.1097/QAI.0b013e3181b033ab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engels EA, Biggar RJ, Hall HI, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer. 2008;1231:187–194. 10.1002/ijc.23487 [DOI] [PubMed] [Google Scholar]
- 3. Patel P, Hanson DL, Sullivan PS, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008;14810:728–736. 10.7326/0003-4819-148-10-200805200-00005 [DOI] [PubMed] [Google Scholar]
- 4. Engels EA, Brock MV, Chen J, et al. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol. 2006;249:1383–1388. 10.1200/JCO.2005.03.4413 [DOI] [PubMed] [Google Scholar]
- 5. Frisch M, Biggar RJ, Engels EA, et al. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;28513:1736–1745. 10.1001/jama.285.13.1736 [DOI] [PubMed] [Google Scholar]
- 6. Eltom MA, Jemal A, Mbulaiteye SM, et al. Trends in Kaposi's sarcoma and non-Hodgkin's lymphoma incidence in the United States from 1973 through 1998. J Natl Cancer Inst. 2002;9416:1204–1210. 10.1093/jnci/94.16.1204 [DOI] [PubMed] [Google Scholar]
- 7. Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi's sarcoma and non-Hodgkin's lymphomas among HIV-1-infected individuals. Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1999;21(suppl 1):S34–S41. [PubMed] [Google Scholar]
- 8. Engels EA, Pfeiffer RM, Goedert JJ, et al. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;2012:1645–1654. 10.1097/01.aids.0000238411.75324.59 [DOI] [PubMed] [Google Scholar]
- 9. Simard EP, Pfeiffer RM, Engels EA.. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med. 2010;17015:1337–1345. 10.1001/archinternmed.2010.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robbins HA, Pfeiffer RM, Shiels MS, et al. Excess cancers among HIV-infected people in the United States. J Natl Cancer Inst. 2015;1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Silverberg MJ, Lau B, Achenbach CJ, et al. Cumulative incidence of cancer among persons with HIV in North America: A cohort study. Ann Intern Med. 2015;1637:507–518. 10.7326/M14-2768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shiels MS, Cole SR, Kirk GD, et al. A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr. 2009;525:611–622. 10.1097/QAI.0b013e3181b327ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shiels MS, Goedert JJ, Moore RD, et al. Reduced risk of prostate cancer in U.S. men with AIDS. Cancer Epidemiol Biomarkers Prev. 2010;1911:2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marcus JL, Chao CR, Leyden WA, et al. Prostate cancer incidence and prostate-specific antigen testing among HIV-positive and HIV-negative men. J Acquir Immune Defic Syndr. 2014;665:495–502. 10.1097/QAI.0000000000000202 [DOI] [PubMed] [Google Scholar]
- 15. Goedert JJ, Schairer C, McNeel TS, et al. Risk of breast, ovary, and uterine corpus cancers among 85,268 women with AIDS. Br J Cancer. 2006;955:642–648. 10.1038/sj.bjc.6603282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hessol NA, Napolitano LA, Smith D, et al. HIV tropism and decreased risk of breast cancer. PLoS One. 2010;512:e14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: A meta-analysis. Lancet. 2007;3709581:59–67. 10.1016/S0140-6736(07)61050-2 [DOI] [PubMed] [Google Scholar]
- 18. O'Neill TJ, Nguemo JD, Tynan AM, et al. Risk of colorectal cancer and associated mortality in HIV: A systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2017;754:439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hessol NA, Seaberg EC, Preston-Martin S, et al. Cancer risk among participants in the women's interagency HIV study. J Acquir Immune Defic Syndr. 2004;364:978–985. 10.1097/00126334-200408010-00013 [DOI] [PubMed] [Google Scholar]
- 20. Hernandez-Ramirez RU, Shiels MS, Dubrow R, et al. Cancer risk in HIV-infected people in the USA from 1996 to 2012: A population-based, registry-linkage study. Lancet HIV. 2017;411:e495–e504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coghill AE, Shiels MS, Rycroft RK, et al. Rectal squamous cell carcinoma in immunosuppressed populations: Is this a distinct entity from anal cancer? AIDS. 2016;301:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robbins HA, Shiels MS, Pfeiffer RM, et al. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS. 2014;286:881–890. 10.1097/QAD.0000000000000163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980-2007. JAMA. 2011;30514:1450–1459. 10.1001/jama.2011.396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Catalona WJ, Smith DS, Ratliff TL, et al. Detection of organ-confined prostate cancer is increased through prostate-specific antigen-based screening. JAMA. 1993;2708:948–954. 10.1001/jama.1993.03510080052031 [DOI] [PubMed] [Google Scholar]
- 25. Drazer MW, Huo D, Eggener SE.. National prostate cancer screening rates after the 2012 US Preventive Services Task Force recommendation discouraging prostate-specific antigen-based screening. J Clin Oncol. 2015;3322:2416–2423. 10.1200/JCO.2015.61.6532 [DOI] [PubMed] [Google Scholar]
- 26. Vickers AJ, Sjoberg DD, Ulmert D, et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med. 2014;12:26. 10.1186/1741-7015-12-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harding C, Pompei F, Burmistrov D, et al. Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med. 2015;1759:1483–1489. 10.1001/jamainternmed.2015.3043 [DOI] [PubMed] [Google Scholar]
- 28. Welch HG, Prorok PC, O'Malley AJ, et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;37515:1438–1447. 10.1056/NEJMoa1600249 [DOI] [PubMed] [Google Scholar]
- 29. Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2016;1644:244–255. [DOI] [PubMed] [Google Scholar]
- 30. Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: An update for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;15110:727–737, W237–W242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Austin H, Henley SJ, King J, et al. Changes in colorectal cancer incidence rates in young and older adults in the United States: What does it tell us about screening. Cancer Causes Control. 2014;252:191–201. 10.1007/s10552-013-0321-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Momplaisir F, Mounzer K, Long JA.. Preventive cancer screening practices in HIV-positive patients. AIDS Care. 2014;261:87–94. 10.1080/09540121.2013.802276 [DOI] [PubMed] [Google Scholar]
- 33. Antoniou T, Jembere N, Saskin R, et al. A population-based study of the extent of colorectal cancer screening in men with HIV. BMC Health Serv Res. 2015;15:51. 10.1186/s12913-015-0711-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reinhold JP, Moon M, Tenner CT, et al. Colorectal cancer screening in HIV-infected patients 50 years of age and older: Missed opportunities for prevention. Am J Gastroenterol. 2005;1008:1805–1812. 10.1111/j.1572-0241.2005.50038.x [DOI] [PubMed] [Google Scholar]
- 35. Mani D, Aboulafia DM.. Screening guidelines for non-AIDS defining cancers in HIV-infected individuals. Curr Opin Oncol. 2013;255:518–525. 10.1097/CCO.0b013e328363e04a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schottenfeld DaF, Joseph F.. Cancer Epidemiology and Prevention, 3rd ed.New York: Oxford University Press; 2006 [Google Scholar]
- 37. Rosner B, Colditz GA, Willett WC.. Reproductive risk factors in a prospective study of breast cancer: The Nurses' Health Study. Am J Epidemiol. 1994;1398:819–835. 10.1093/oxfordjournals.aje.a117079 [DOI] [PubMed] [Google Scholar]
- 38. Johns LE, Houlston RS.. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;9610:2992–3003. 10.1111/j.1572-0241.2001.04677.x [DOI] [PubMed] [Google Scholar]
- 39. Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;30617:1891–1901. 10.1001/jama.2011.1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wunder DM, Bersinger NA, Fux CA, et al. Hypogonadism in HIV-1-infected men is common and does not resolve during antiretroviral therapy. Antivir Ther. 2007;122:261–265. [PubMed] [Google Scholar]
- 41. Gomes AR, Souteiro P, Silva CG, et al. Prevalence of testosterone deficiency in HIV-infected men under antiretroviral therapy. BMC Infect Dis. 2016;161:628. 10.1186/s12879-016-1892-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rochira V, Zirilli L, Orlando G, et al. Premature decline of serum total testosterone in HIV-infected men in the HAART-era. PLoS One. 2011;612:e28512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Calvet GA, Grinsztejn BG, Quintana Mde S, et al. Predictors of early menopause in HIV-infected women: A prospective cohort study. Am J Obstet Gynecol. 2015;2126:765 e1–e13. [DOI] [PubMed] [Google Scholar]
- 44. Schoenbaum EE, Hartel D, Lo Y, et al. HIV infection, drug use, and onset of natural menopause. Clin Infect Dis. 2005;4110:1517–1524. 10.1086/497270 [DOI] [PubMed] [Google Scholar]
- 45. Sampson JN, Falk RT, Schairer C, et al. Association of estrogen metabolism with breast cancer risk in different cohorts of postmenopausal women. Cancer Res. 2017;774:918–925. 10.1158/0008-5472.CAN-16-1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iversen L, Sivasubramaniam S, Lee AJ, et al. Lifetime cancer risk and combined oral contraceptives: The Royal College of General Practitioners' Oral Contraception Study. Am J Obstet Gynecol. 2017;2166:580 e1–e9. [DOI] [PubMed] [Google Scholar]
- 47. Weber J, Piontkivska H, Quinones-Mateu ME.. HIV type 1 tropism and inhibitors of viral entry: Clinical implications. AIDS Rev. 2006;82:60–77. [PubMed] [Google Scholar]