Abstract
Background
Germline genetic testing is standard practice in oncology. Outcomes of telephone disclosure of a wide range of cancer genetic test results, including multigene panel testing (MGPT) are unknown.
Methods
Patients undergoing cancer genetic testing were recruited to a multicenter, randomized, noninferiority trial (NCT01736345) comparing telephone disclosure (TD) of genetic test results with usual care, in-person disclosure (IPD) after tiered-binned in-person pretest counseling. Primary noninferiority outcomes included change in knowledge, state anxiety, and general anxiety. Secondary outcomes included cancer-specific distress, depression, uncertainty, satisfaction, and screening and risk-reducing surgery intentions. To declare noninferiority, we calculated the 98.3% one-sided confidence interval of the standardized effect; t tests were used for secondary subgroup analyses. Only noninferiority tests were one-sided, others were two-sided.
Results
A total of 1178 patients enrolled in the study. Two hundred eight (17.7%) participants declined random assignment due to a preference for in-person disclosure; 473 participants were randomly assigned to TD and 497 to IPD; 291 (30.0%) had MGPT. TD was noninferior to IPD for general and state anxiety and all secondary outcomes immediately postdisclosure. TD did not meet the noninferiority threshold for knowledge in the primary analysis, but it did meet the threshold in the multiple imputation analysis. In secondary analyses, there were no statistically significant differences between arms in screening and risk-reducing surgery intentions, and no statistically significant differences in outcomes by arm among those who had MGPT. In subgroup analyses, patients with a positive result had statistically significantly greater decreases in general anxiety with telephone disclosure (TD –0.37 vs IPD +0.87, P = .02).
Conclusions
Even in the era of multigene panel testing, these data suggest that telephone disclosure of cancer genetic test results is as an alternative to in-person disclosure for interested patients after in-person pretest counseling with a genetic counselor.
Germline genetic testing is standard practice in oncology and is increasingly utilized to identify high-risk patients across a range of medical disciplines (1–6). Professional societies recommend that germline genetic testing be paired with pre- and post-test counseling to optimize informed consent, understanding, psychosocial responses, and preventive behaviors (2,7–9). Given the complexity of genetic information, potential for false reassurance and psychosocial distress, genetic counseling has traditionally been delivered in person (10–13).
While in-person genetic counseling has been the standard of care, alternative delivery models have been considered to reduce patient time and travel burdens, improve access, and address the shortage of genetic providers (14,15). Despite limited data establishing comparable outcomes, telephone delivery has been increasingly utilized by some genetic providers (11,15–19). Yet, there have been concerns that telephone communication may be associated with poorer patient understanding and increased distress, particularly in the setting of complex genetic information (20–22). Two randomized studies have reported that genetic counseling by phone is no worse than in-person counseling for BRCA1/2 testing (23–25). However, broader sequencing platforms, such as multigene panel testing (MGPT) are increasingly utilized in oncology and other specialties (1,2,26–29). Broader sequencing is associated with a greater risk for uncertainty, including more variants of uncertain significance, uncertainties regarding risk estimates for some genes, and uncertainties regarding medical management (1,2,30). There are limited data on patient outcomes with MGPT, and additional concerns about misunderstanding of results, anxiety, uncertainty, and potentially the adoption of inappropriate screening or risk-reducing surgeries (1,2,30–36). Thus, it remains unknown if telephone counseling is an adequate alternative in the era of MGPT (24,25,37). Further, there are no data on outcomes by test result, which are important given the potential for greater distress, uncertainty, or misunderstanding for subgroups with positive, variant of uncertain significance (VUS), or negative results (19,25).
To better understand the risks and benefits of telephone disclosure of genetic test results in real-world clinical practice and in the era of MGPT, we conducted the first randomized multicenter noninferiority trial comparing telephone disclosure of a range of cancer genetic testing, including MGPT. All pretest counseling was conducted in person given stakeholder data from patients and providers supporting preferences for face-to-face pretest counseling prior to telephone disclosure (21,22). We hypothesized that knowledge and anxiety with telephone disclosure (TD) would not be inferior to the current usual care, in-person disclosure (IPD). Further, we hypothesized that there would not be statistically significant differences in outcomes among patients undergoing MGPT and those receiving positive results.
METHODS
Study Design, Setting, and Participants
From December 2012 to October 2015, participants were recruited to the Communication Of GENetic Test results by telephone (COGENT) Study, a multicenter, randomized, noninferiority trial (NCT01736345) comparing disclosure of genetic test results by telephone with a genetic counselor with usual care (in-person disclosure with a genetic counselor). At the time the study was conducted, the two randomized studies suggesting noninferiority for telephone counseling for BRCA1/2 testing had not been published. Thus, all sites still predominantly utilized in-person counseling (eg, usual care). Participants were recruited from the clinical cancer genetic programs at the University of Pennsylvania, Fox Chase Cancer Center, MD Anderson Cancer Center at Cooper, University of Chicago, and John H. Stroger Jr. Hospital at Cook County. Participants were English-speaking adults who had completed in-person pretest counseling with a genetic counselor and were proceeding with clinical cancer genetic testing. Initially, this was limited to BRCA1/2 testing. In 2013, as multigene panels became clinically available, eligibility was expanded to include any clinical genetic testing for hereditary breast, gynecological, and/or gastrointestinal cancer syndromes (May 2014). The cost of genetic testing and services was covered by insurance or self-pay. Individuals with uncorrected or uncompensated hearing, vision, or speech impairments were not eligible.
The institutional review boards at all sites approved this study. Participants were recruited at the end of their in-person pretesting counseling session. After written informed consent, participants completed a baseline survey (T0).
Random Assignment
Participants were equally assigned to telephone or in-person (usual care) disclosure, stratified by study site and sex, and informed of their random assignment arm after completion of their baseline survey (T0). Participants who expressed a preference for in-person communication were enrolled into a third, nonrandomized arm, where outcomes were collected but they received results in-person.
Procedures
Genetic test disclosures were delivered by 22 board-certified genetic or nurse counselors. Standardized theoretical and stakeholder-informed communication protocols and visual aids were utilized (38–40). Genetic counselors (GCs) underwent protocol training and completed counseling checklists (40). All sessions were audiotaped.
Telephone disclosuresessions were scheduled, and all participants in the TD arm were recommended to return for a clinic visit with a site medical provider (physician or nurse practitioner) with expertise in cancer genetics to further discuss medical management.
In-person disclosure sessions with a genetic counselor and medical provider were scheduled as per usual care. Medical management discussions with medical providers occurred during the same visit, after patients received their results with the genetic counselor, consistent with usual care at all sites.
Primary Outcomes
Participants completed a postdisclosure survey (T1) within seven days after their disclosure. Outcomes were informed by our theoretical model informed by the Self-regulation Theory of Health Behavior (38,40–42), including potential risks of telephone communication (eg, poorer understanding of results, greater short-term distress, and poorer behavioral outcomes), as previously described (40). Genetic knowledge was evaluated (T0 and T1) using an adapted six-item Cancer Genetics Knowledge scale (43), which is applicable to a broad range of cancer genes and focused on understanding of test results and cancer risk (Cronbach’s α = .58–.60). This scale included items evaluating understanding of dominant inheritance (one item) and risk of genetic disease with positive (two items) and negative results (three items). MGPT knowledge was evaluated in individuals who were offered and elected for MGPT. This scale includes 11 items evaluating the benefits (three items), limitations (six items), and interpretation (two items) of MGPT, adapted from the ClinSeq scale and utilized in related research (Cronbach’s α = .57–.61) (44).
State anxiety, a sensitive indicator of transient or situational changes in anxiety (past 24 hours) was measured (T0 and T1) with the 20-item State Inventory of the State-Trait Anxiety Inventory (Cronbach’s α = .96) (45).
General anxiety was assessed (T0 and T1) with the seven-item Hospital Anxiety and Depression Scale (HADS) anxiety subscale (46), which has been used to assess more persistent anxiety (past week) in a wide range of medical patients (Cronbach’s α = .86) .
Secondary Outcomes
Cancer-specific distress was evaluated (T0 and T1) with 14 items of Impact of Events Scale (IES) (47). We excluded one item lacking face validity in our population (Cronbach’s α = .89–.90).
Depression was assessed (T0 and T1) with the seven-item HADS depression subscale (Cronbach’s α = .82–.84) (46).
Uncertainty was assessed (T0 and T1) using a three-item scale adapted from the Multi-dimensional Impact of Cancer Risk Assessment Questionnaire (MICRA) (48). This scale was added with the advent of MGPT in 2014 given increased risks for uncertainty with MGPT (Cronbach’s α = .81–.84).
Satisfaction with genetic services was measured (T0 and T1) with a nine-item scale evaluating participants’ cognitive and affective perceptions of their genetic counseling and testing experience (Cronbach’s α = .74–.81) (49,38).
Behavioral intention (T0, T1) was included as an early surrogate of performance of screening and risk reduction behaviors. These included behavioral intent to perform mammography, breast magnetic resonance imaging (MRI), colonoscopy, and prophylactic surgeries (mastectomy and oophorectomy). Patients responded on a seven-point Likert scale and could mark “not applicable” if the screening behavior had not been recommended for them.
Statistical Analysis
The study was designed as a noninferiority trial, to conclude that TD is as good as, or better than IPD at T1 (postdisclosure) relative to predisclosure (ie, change scores). Our primary outcomes included genetic knowledge, state anxiety, and general anxiety to show that telephone disclosure is not associated with unacceptably worse understanding or increased distress than in-person disclosure. We used a Bonferroni correction to account for multiple hypothesis testing. To declare noninferiority, we calculated the 1-0.0167 = 98.33% one-sided confidence interval of the standardized effect (TD minus IPD), and if the bound of the 98.33% confidence interval is within 0.247 units of zero in the direction indicating that TD is worse, then TD is determined to be noninferior to IPD. We chose .247 as a relevant bound since 0.5 standard deviations has been considered a clinically relevant and medium-sized effect in the behavioral literature, and .247 was slightly less than half this clinically relevant metric (50). The decision rule was chosen to have greater than 90% power to declare TD noninferior to IPD with 412 participants with complete data for the primary analyses, and 80% power with 290 participants with complete data for the longitudinal analyses. We assumed 1.67% type I error (one-sided) and a 0.5 within-subject correlation of the change scores. We used an intention-to-treat approach, comparing outcomes between random assignment arms rather than the actual intervention received. For primary analyses, we compared random assignment arms among those with complete data. In sensitivity analyses, we compared random assignment arms among the full sample after using multiple imputation (51). We used complete case analyses for the primary analyses, rather than the imputation analyses as the primary analyses, because we felt that complete case analyses were more conservative with respect to a noninferiority trial. For secondary outcomes, we also performed noninferiority analyses, but set the type I error rate to 5% (one-sided).
In secondary analyses, we also compared outcomes using t tests in subgroups of clinical interest, those who received an MGPT result and outcomes by test result. In these secondary subgroup analyses, the criteria for statistical significance was a nominal P value of 5% or smaller. We assessed balance between randomization arms using t tests and chi-square tests as appropriate for continuous and categorical variables. We similarly compared characteristics of those who declined participation with those who did not, and those who did and did not complete baseline surveys, and those lost and not lost to follow-up. Noninferiority tests were one-sided, with a 1.67% type I error rate, but all other statistical tests were two-sided; a P value of less than .05 was considered statistically significant.
Results
Study Participants
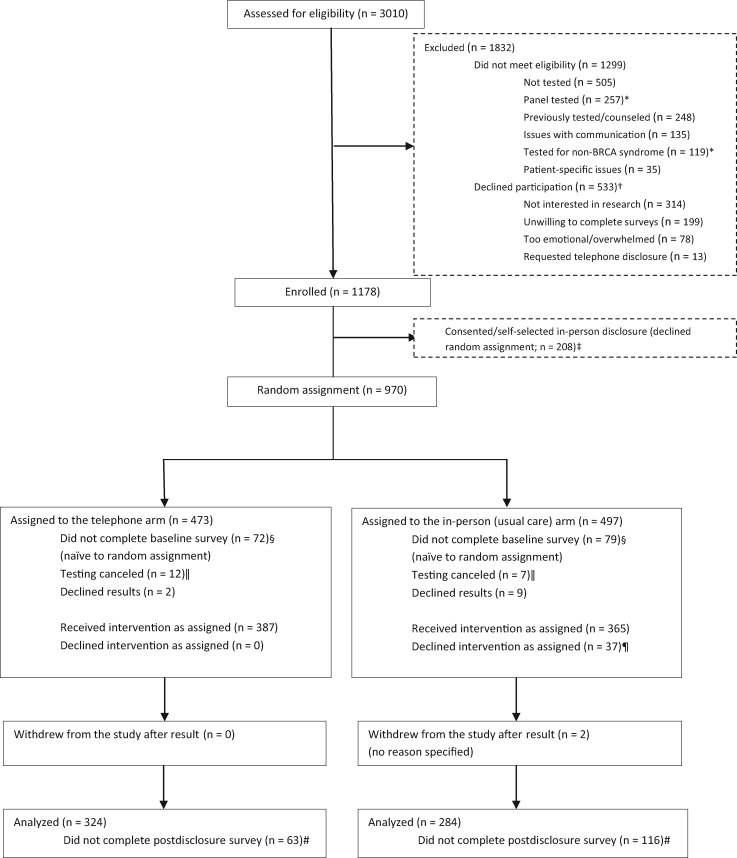
Enrollment, random assignment, and survey completion data are shown in Figure 1; 1178 (68.8%) patients consented to the study, and 970 (82.3%) agreed to be randomly assigned. Two hundred eight (17.7%) declined random assignment due to a strong preference for IPD. Those who declined participation were more likely to be older (mean = 52.31 vs 49.34 years, P = .001), have a history of cancer (63.4% vs 55.3%, P = .002), be of Ashkenazi heritage (21.4% vs 14.3%, P = .001), not have a known genetic mutation in the family (83.3% vs 79.0%, P = .01), and live closer to the study site (18.9 miles vs 31 miles, P = .03) compared with consented participants (data not shown).
Figure 1.
Consort diagram.
*Individuals approached prior to non-BRCA1/2 and multigene testing adaptation. †Multiple reasons for decline were reported per participant. ‡Participants who expressed a preference for in-person communication were enrolled into a third, nonrandomized arm, where outcomes were collected, but they received results in-person. §Survey not completed within seven days. ‖Canceled because of lack of insurance coverage. ¶Individuals who were disclosed by telephone at the participant’s behest (n = 31), disclosed by telephone due to illness/financial burden (n = 3), and received results in-person with a non-COGENT provider (n = 3). #This includes 37 who declined intervention as assigned and did not complete the postdisclosure survey within seven days.
Four hundred seventy-three participants were randomly assigned to TD, 497 to IPD (usual care); 291 (30.0%) had MGPT. There were no statistically significant differences between randomly assigned groups (Table 1). The mean age of randomly assigned participants (SD) was 48.25 (12.86) years, 83.2% were non-Latino white, 59.2% reported a college education, and 28.2% had MGPT (Table 1). Those who declined random assignment were more likely to be older (P = .008), to have had MGPT (P = .001), or to be at Fox Chase Cancer Center, and were least likely to be at the University of Chicago (P < .001). Genetic counselor fidelity to communication topics was high (88.4% for IPD, 87.9% for TD).
Table 1.
Characteristics of participants who completed a baseline survey and agreed to random assignment to phone or in-person (usual care) disclosure of genetic test results
| Characteristic | Telephone disclosure (n = 401) | In-person disclosure (n = 418) |
|---|---|---|
| Mean age (SD), y | 48.20 (12.54) | 48.96 (12.88) |
| Sex, No. (%) | ||
| Female | 374 (93.3) | 385 (92.1) |
| Male | 27 (6.7) | 33 (7.9) |
| Race, No. (%) | ||
| White | 340 (84.8) | 341 (81.6) |
| Nonwhite | 61 (15.2) | 77 (18.4) |
| Ashkenazi Jewish, No. (%) | ||
| Yes | 56 (14.0) | 71 (17.0) |
| No | 274 (68.3) | 284 (67.9) |
| Not captured | 71 (17.7) | 63 (15.1) |
| Education, No. (%) | ||
| Some college or less | 166 (41.4) | 164 (39.2) |
| College degree or more | 234 (58.4) | 251 (60.0) |
| Missing | 1 (<1) | 3 (<1) |
| Household income, yearly, No. (%) | ||
| $49 999 or less | 86 (21.4) | 97 (23.2) |
| $50 000–$99 999 | 101 (25.2) | 112 (26.8) |
| $100 000–$149 999 | 75 (18.7) | 69 (16.5) |
| $150 000 or more | 85 (21.2) | 81 (19.4) |
| Missing | 54 (13.5) | 59 (14.1) |
| Marital status, No. (%) | ||
| Married/domestic partner | 284 (70.8) | 285 (68.2) |
| Not currently married/in a domestic partnership | 117 (29.2) | 133 (31.8) |
| Personal history of cancer, No. (%) | ||
| No | 193 (48.1) | 188 (45.0) |
| Yes | 208 (51.9) | 230 (55.0) |
| Breast, No. (%) | 154 (74.0) | 160 (70.0) |
| Ovary, No. (%) | 21 (10.1) | 30 (13.0) |
| Single primary, nonbreast/ovary, No. (%) | 22 (10.6) | 27 (11.7) |
| Multiple primaries, No. (%) | 11 (5.3) | 13 (5.7) |
| Testing for surgical treatment decision, No. (%) | ||
| Yes | 32 (8.0) | 25 (6.0) |
| No | 369 (92.0) | 393 (94.0) |
| Known genetic mutation in family, No. (%) | ||
| Yes | 91 (22.7) | 95 (22.7) |
| No | 310 (77.3) | 323 (77.3) |
| Number of FDR/SDR with cancer, mean (SD) | 3.63 (2.27) | 3.79 (2.39) |
| Had multigene testing, No. (%) | ||
| Yes | 110 (27.4) | 121 (28.9) |
| No | 87 (21.7) | 93 (22.2) |
| NA (recruited prior to inclusion of multigene testing) | 204 (50.9) | 204 (48.8) |
| Result, No. (%) | ||
| Positive | 51 (12.7) | 57 (13.6) |
| Variant of uncertain significance | 36 (9.0) | 40 (9.6) |
| Negative | 249 (62.1) | 261 (62.4) |
| True negative | 51 (12.7) | 44 (10.5) |
| Testing canceled | 12 (3.0) | 7 (1.7) |
| Participant declined/unable to receive results | 2 (<1) | 9 (2.2) |
| Recruitment site, No. (%) | ||
| University of Pennsylvania | 103 (275.7) | 108 (25.8) |
| Fox Chase Cancer Center | 150 (37.4) | 150 (35.9) |
| University of Chicago | 76 (19.0) | 85 (20.3) |
| John H. Stroger Jr. Hospital at Cook County | 26 (6.5) | 24 (5.7) |
| MD Anderson at Cooper University Hospital | 46 (11.5) | 51 (12.2) |
| Distance to site*, mean (SD) | 37.47 (161.77) | 34.24 (127.23) |
Straight-line distance was calculated using participant ZIP code and the corresponding site ZIP code to represent travel distance for participants. FDR=First Degree Relative; SDR=Second Degree Relative.
There were no statistically significant differences between randomized group when including only the subset with paired data.
There were no demographic differences between the random assignment arms among those who completed baseline and T1 surveys (Supplementary Table 1, available online). There were no other baseline psychosocial differences between the arms. Those lost to follow-up at T1 had lower baseline knowledge in the TD arm compared with the IPD arm (P = .05), while those not lost to follow-up had higher baseline knowledge in the TD arm compared with the IPD arm (P = .005). Differences between those with and without follow-up are included in Supplementary Table 2 (available online).
Noninferiority Analyses and Differences in Cognitive, Affective, and Behavioral Outcomes
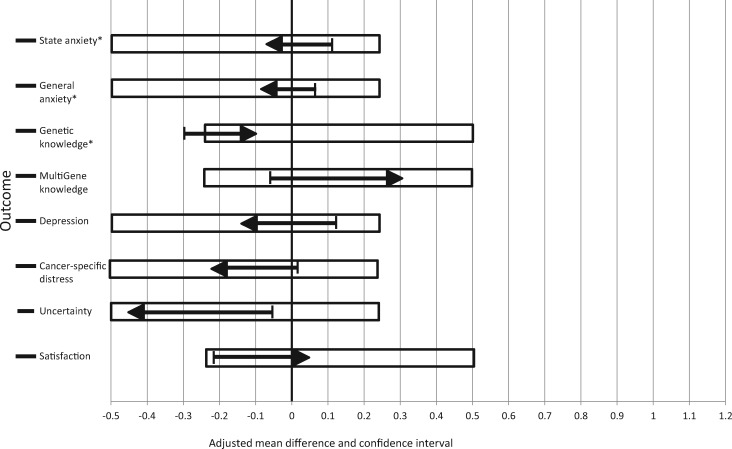
Of our primary outcomes, TD was noninferior to IPD for general and state anxiety, but it did not meet noninferiority thresholds for knowledge (Figure 2). The mean reductions in state anxiety and general anxiety were greater for TD participants than IPD participants, and the bounds of the one-sided 98.33% confidence intervals that would indicate that TD was inferior did not cross the noninferiority threshold (a CI bound of 0.247 standardized units suggesting that TD was inferior). The confidence interval bound for increase in knowledge did cross the noninferiority threshold in the complete case analysis, but not in the multiple imputation analysis (Figure 2), suggesting that TD may not be noninferior to IPD for knowledge gain. TD was noninferior to IPD for all secondary outcomes, including cancer-specific distress, depression and uncertainty, and multigene knowledge.
Figure 2.
Noninferiority analyses comparing telephone to usual care in-person disclosure of genetic test results. *Primary postdisclosure outcomes. Black arrows depict the 98.3% confidence interval (one-sided) for primary outcomes/95% confidence interval (one-sided) for all other outcomes. The blocked area indicates the noninferiority range. If the confidence intervals completely fall within the noninferiority range, then noninferiority can be concluded. The arrows indicate the direction in which the effect would show that telephone disclosure is more favorable than in-person disclosure. For example, more negative average change scores (ie, less than 0) in state anxiety between arms would indicate that telephone disclosure decreased state anxiety more than in-person disclosure. Similarly, more positive average change scores (ie, greater than 0) in knowledge would indicate that telephone disclosure increased knowledge more than in-person disclosure. With imputed data, the estimated effects (one-sided CI) were state anxiety –0.04 (0.09), general anxiety –0.08 (0.05), and genetic knowledge –0.07 (–0.24), which were all within the noninferiority range.
There were no statistically significant differences between randomly assigned arms in change in or postdisclosure intention to perform cancer screening or risk-reducing surgeries (Table 2). With the exception of knowledge, results with imputed data were consistent with results from complete case analyses (Supplementary Table 3, available online). Per-protocol analyses did not differ from the intention-to-treat analyses.
Table 2.
Patient-reported outcomes at baseline and postdisclosure by randomized arm
| Scale | Score range | Telephone disclosure (n = 324) |
In-person (usual care) disclosure (n = 284) |
TD minus IPD difference in change (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | Post disclosure Mean (SD) | Change score (SD) Mean (SD) | Baseline Mean (SD) | Post disclosure Mean (SD) | Change score (SD) | |||
| Genetic knowledge | 6–28 | 23.10 (2.58) | 23.29 (2.53) | +0.20 (2.24) | 22.53 (2.59) | 23.07 (2.72) | +0.52 (2.58) | –0.32 (–0.72 to +0.06) |
| State anxiety | 20–80 | 34.60 (12.50) | 34.20 (11.96) | –0.39 (8.92) | 34.83 (12.91) | 34.82 (12.59) | –0.06 (10.18) | –0.33 (–1.86 to +1.19) |
| General anxiety | 0–21 | 6.75 (3.77) | 6.27 (3.53) | –0.48 (2.54) | 6.83 (3.67) | 6.60 (3.91) | –0.26 (2.70) | –0.22 (–0.64 to +0.19) |
| Depression | 0–21 | 2.74 (2.86) | 2.81 (2.99) | +0.08 (2.29) | 2.70 (2.85) | 2.75 (2.88) | +0.06 (2.30) | +0.02 (–0.34 to +0.39) |
| Cancer-specific distress | 0–70 | 17.21 (13.70) | 17.43 (14.23) | +0.21 (11.14) | 16.91 (13.89) | 18.52 (14.57) | +1.48 (10.24) | –1.27 (–2.99 to +0.45) |
| Satisfaction | 9–45 | 38.60 (3.95) | 38.47 (5.86) | –0.14 (5.45) | 38.88 (4.11) | 38.65 (5.49) | –0.23 (5.70) | +0.09 (–0.81 to +0.99) |
| Behavioral intention items* | ||||||||
| Planning screening mammogram | 1–7 | 6.24 (1.65) | 6.40 (1.60) | +0.10 (1.63) | 6.44 (1.34) | 6.41 (1.60) | –0.03 (1.61) | +0.13 (–0.19 to +0.44) |
| Planning screening breast MRI | 1–7 | 4.37 (2.10) | 4.64 (2.31) | +0.21 (2.18) | 3.97 (2.03) | 4.25 (2.41) | +0.18 (2.07) | +0.03 (–0.45 to +0.52) |
| Planning prophylactic mastectomy | 1–7 | 2.99 (1.95) | 2.44 (2.03) | –0.64 (2.01) | 2.96 (1.87) | 2.35 (1.80) | –0.70 (1.78) | +0.06 (–0.40 to +0.52) |
| Planning prophylactic oophorectomy | 1–7 | 3.65 (1.81) | 3.03 (2.19) | –0.74 (1.87) | 3.53 (1.81) | 3.09 (2.25) | –0.70 (2.20) | –0.04 (–0.54 to +0.45) |
| Planning colonoscopy | 1–7 | 3.13 (2.43) | 3.22 (2.41) | +0.19 (1.89) | 3.36 (2.48) | 3.63 (2.52) | +0.16 (1.88) | +0.03 (–0.51 to +0.56) |
Patients could respond “not applicable” if screening behavior had not been recommended for them; mammogram (n = 222 TD, n = 185 IPD); breast MRI (n = 167 TD, n = 136 IPD); prophylactic mastectomy (n = 146 TD, n = 121 IPD); prophylactic oophorectomy (n = 154 TD, n = 110 IPD); colonoscopy (n = 96 TD, n = 98 IPD). CI = confidence interval; IPD = in-person disclosure; MRI = magnetic resonance imaging; TD = telephone disclosure.
Differences in Outcomes Among Participants Who Had Multigene Testing and by Test Result
In secondary subgroup analyses, there were no statistically significant differences in any outcomes among the subset of patients who had MGPT (Table 3). Among participants who received a positive result, those in the TD arm had statistically significantly greater decreases in general anxiety (change in anxiety = –0.37 in TD arm as compared with +0.87 in the IPD arm, P = .02) (Supplementary Table 4, available online). Those with uninformative negative results had greater declines in uncertainty in the TD arm (change in uncertainty = –0.22 in TD arm as compared with +0.89 in the IPD arm, P = .08), among the subset who completed this measure. Those with a true-negative result had greater declines in general anxiety in the TD arm.
Table 3.
Patient-reported outcomes at baseline and postdisclosure among participants who had multigene testing
| Scale | Score range | Telephone disclosure (n = 87) |
In-person disclosure (n = 82) |
TD-IPD difference in change (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline Mean (SD) | Post disclosure Mean (SD) | Change score (SD) | Baseline Mean (SD) | Post disclosure Mean (SD) | Change score (SD) | |||
| Genetic knowledge | 6–28 | 22.92 (2.28) | 23.08 (2.34) | +0.14 (1.93) | 22.28 (2.29) | 22.75 (2.63) | +0.43 (2.32) | –0.29 (–0.95 to 0.36) |
| Multigene knowledge | 11–54 | 36.22 (5.11) | 37.21 (4.03) | +0.75 (6.07) | 37.46 (4.07) | 36.92 (4.30) | –0.45 (4.93) | 1.20 (–0.52 to 2.91) |
| State anxiety | 20–80 | 32.60 (12.50) | 32.66 (12.33) | +0.06 (9.98) | 33.78 (12.90) | 33.0 (10.59) | –0.78 (8.4) | 0.84 (–1.96 to 3.65) |
| General anxiety | 0–21 | 6.30 (3.71) | 5.87 (3.65) | –0.43 (2.31) | 6.67 (3.27) | 5.74 (3.50) | –0.92 (2.19) | 0.49 (–0.20 to 1.17) |
| Depression | 0–21 | 2.55 (3.03) | 2.54 (2.96) | –0.01 (2.19) | 2.83 (2.93) | 2.75 (2.78) | –0.07 (1.87) | 0.06 (–0.56 to 0.68) |
| Cancer-specific distress | 0–70 | 15.89 (13.07) | 15.98 (13.44) | +0.09 (11.17) | 17.64 (13.64) | 17.54 (13.24) | –0.36 (9.38) | 0.44 (–2.71 to 3.60) |
| Satisfaction | 9–45 | 39.26 (4.07) | 40.11 (4.62) | +0.85 (4.27) | 39.57 (4.02) | 40.50 (4.24) | +1.02 (4.91) | –0.17 (–1.57 to 1.24) |
| Behavioral intention items* | ||||||||
| Planning screening mammogram | 1–7 | 6.41 (1.58) | 6.64 (1.24) | +0.19 (1.66) | 6.50 (1.19) | 6.36 (1.61) | –0.20 (1.47) | 0.39 (–0.20 to 0.97) |
| Planning screening breast MRI | 1–7 | 4.57 (2.28) | 4.82 (2.29) | 0 (1.99) | 4.06 (2.33) | 4.21 (2.44) | –0.15 (1.98) | 0.15 (–0.73 to 1.02) |
| Planning prophylactic mastectomy | 1–7 | 2.71 (1.92) | 2.50 (2.14) | –0.27 (2.48) | 2.60 (1.69) | 2.08 (1.75) | –0.66 (1.53) | 0.39 (–0.61 to 1.38) |
| Planning prophylactic oophorectomy | 1–7 | 3.34 (1.95) | 2.96 (2.14) | –0.72 (1.95) | 2.98 (1.61) | 2.63 (1.96) | –0.73 (1.89) | 0.01 (–0.90 to 0.90) |
| Planning colonoscopy | 1–7 | 3.47 (2.51) | 3.57 (2.49) | +0.08 (2.15) | 3.44 (2.42) | 3.61 (2.51) | 0 (1.94) | 0.08 (–0.70 to 0.86) |
Patients could respond “not applicable” if screening behavior had not been recommended for them; mammogram (n = 58 TD, n = 56 IPD); breast MRI (n = 40 TD, n = 41 IPD); prophylactic mastectomy (n = 33 TD, n = 35 IPD); prophylactic oophorectomy (n = 40 TD, n = 33 IPD); colonoscopy (n = 49 TD, n = 60 IPD). There were no statistically significant differences in baseline characteristics between arms in the subset of patients who had multigene panel testing. CI = confidence interval; IPD = in-person disclosure; MRI = magnetic resonance imaging; TD = telephone disclosure.
Discussion
This study provides evidence that telephone disclosure is, in general, not inferior to usual care, in-person disclosure of genetic test results for affective outcomes (eg, no greater increase in anxiety or distress) immediately postdisclosure, even in the era of more complex multigene panel testing. This finding is similar to previously reported outcomes with BRCA1/2 testing, but importantly was identified in this multicenter study including participants undergoing cancer genetic testing for a wider range of cancer types and including multigene panel testing. There have been concerns that counseling patients for testing of multiple genes of varied risk and cancer type could increase risks for misunderstanding and distress (23,24). Yet, in this study, telephone disclosure was still found to be noninferior to in-person disclosure of results for all outcomes except for knowledge. Although knowledge did not meet the noninferiority threshold in the complete case analysis, it did in the multiple imputation analysis, suggesting that telephone may not be noninferior to in-person disclosure for knowledge gain.
It is important to note that in contrast to the prior studies, our study included in-person pretest counseling, based on patient and provider stakeholder data, suggesting this is the preferred model for delivery when in-person counseling is available. Additionally, the 22 participating genetic counselors utilized the tiered-binned model for pretest counseling (39), and our telephone communication protocols include specific provider probes to assess patient understanding and emotional responses in the absence of visual cues (40). Thus, outcomes could differ with different approaches as telephone disclosure is more widely adopted into cancer care. Given our findings, we recommend careful attention to patient understanding as telephone disclosure becomes increasingly utilized in a wider range of clinical practice settings.
These findings have several clinical implications. First, telephone disclosure of results after in-person pretest counseling may be a reasonable alternative to requiring patients to return for in-person disclosure, minimizing burden for many patients without introducing clinically significant risks. Second, almost 20% of patients in our study and 30% in Schwartz et al. declined participation given a preference for in-person counseling (23,52). Other studies have shown that uptake of testing is lower with telephone pretest counseling (23–25). Collectively, these data provide rationale for maintaining in-person pretest counseling when available and for providing the option for in-person disclosure for patients who prefer in-person communication, particularly in the era of more complex MGPT (52).
To our knowledge, this is the first study to report outcomes comparing telephone and in-person disclosure by test result. Patients and providers have raised concerns about the potential for greater distress with telephone disclosure among patients receiving a positive result, or the potential for misunderstanding for those receiving a VUS or uninformative negative result (11,21,22). In secondary subgroup analyses by test result received, we found no statistically significant differences between groups for most outcomes, and there was less increase in anxiety with telephone disclosure for those receiving a positive result. It is possible that the process of traveling to and waiting in the medical setting has the potential to cause greater distress and negative anticipation (52). Alternatively, it may be that receiving results from a genetic counselor by phone (without a discussion of medical management with a medical provider in the same visit) is associated with less anxiety.
We acknowledge several limitations to these data. We utilized multiple imputation to account for missing follow-up data. While this did not change our findings for most outcomes, this was not the case for knowledge. As using imputed data can be associated with potential biases, careful attention to patient understanding with telephone communication is recommended. These data represent immediate postdisclosure outcomes, and longitudinal data will inform any impact on risk-reducing and screening behaviors. We selected a knowledge measure that is applicable to a range of cancer genetic results (eg, many knowledge scales utilized to assess genetic testing outcomes are BRCA1/2 specific), but we had modest internal consistency for this outcome. This scale may represent several discrete knowledge constructs evaluated (eg, lower correlation between test items), and further analyses evaluating which construct patients don’t understand could inform improvements in genetic counseling. These data don’t address the use of telephone for pretest counseling. Subgroup analyses were limited by sample size, although they represent the largest available samples to date. Genetic counselors utilized counseling checklists to limit variability in the content of disclosures and therefore were not blinded to research participation status. Lack of reimbursement remains a key barrier to widespread implementation of telephone disclosure, providing additional support for changes in billing and reimbursement for telephone services (11,23,52).
These data suggest that telephone disclosure is a reasonable alternative to in-person disclosure of genetic test results among interested patients after in-person pretest counseling, even with multigene panel testing and with careful attention to patient understanding as telephone disclosure becomes increasingly utilized in a wider range of clinical practice settings.
Funding
This work was supported by the National Cancer Institute (R01 CA160847:
Trial Registration NCT01736345). This work was also supported by the National Institutes of Health (P30 CA006927).
Notes
Affiliations of authors: Division of Hematology-Oncology, Department of Medicine (ARB, SMD, DF, AB, JML, DM, JP, JES), Department of Medical Ethics and Health Policy (ARB), and Abramson Cancer Center (ARB, SMD), University of Pennsylvania, Philadelphia, PA; Division of Hematology-Oncology (RC, CG, SM, SN) and Section of Gastroenterology, Hepatology, and Nutrition (JS), Department of Medicine, and Center for Clinical Cancer Genetics and Global Health (LJPM, OIO), The University of Chicago, Chicago, IL; Biostatistics and Bioinformatics Facility (BLE) and Department of Medical Genetics (MJH, MBD, AF, SM, KR, CR, MS), Fox Chase Cancer Center, Temple University Health System, Philadelphia, PA; Department of Internal Medicine, The John H. Stroger Jr. Hospital of Cook County, Chicago, IL (PG, RG, TL, CS); Division of Hematology-Oncology, MD Anderson Cancer Center at Cooper, Camden, NJ (GG, DFC, JH, KM, XY).
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Dr. Angela Bradbury had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Bradbury, Patrick-Miller, Egleston. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: Bradbury. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Egleston.
Supplementary Material
References
- 1. Bradbury AR, Patrick-Miller L, Domchek S.. Multiplex genetic testing: Reconsidering utility and informed consent in the era of next-generation sequencing. Genet Med .2015;172:97–98. 10.1038/gim.2014.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robson ME, Bradbury AR, Arun B, et al. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2015;3331:3660–3667. 10.1200/JCO.2015.63.0996 [DOI] [PubMed] [Google Scholar]
- 3. Goldman JS, Hahn SE, Catania JW, et al. Genetic counseling and testing for Alzheimer disease: Joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;136:597–605. 10.1097/GIM.0b013e31821d69b8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;12424:2761–2796. 10.1161/CIR.0b013e318223e230 [DOI] [PubMed] [Google Scholar]
- 5. Benatar M, Stanislaw C, Reyes E, et al. Presymptomatic ALS genetic counseling and testing: Experience and recommendations. Neurology. 2016;8624:2295–2302. 10.1212/WNL.0000000000002773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tchan M, Savige J, Patel C, et al. KHA-CARI autosomal dominant polycystic kidney disease guideline: Genetic testing for diagnosis. Semin Nephrol .2015;356:545–549, e542. [DOI] [PubMed] [Google Scholar]
- 7. Robson ME, Storm CD, Weitzel J, Wollins DS, Offit K.. American Society of Clinical Oncology policy statement update: Genetic and genomic testing for cancer susceptibility. J Clin Oncol . 2010;285:893–901. [DOI] [PubMed] [Google Scholar]
- 8. Schwartz GF, Hughes KS, Lynch HT, et al. Proceedings of the international consensus conference on breast cancer risk, genetics, & risk management, April, 2007. Cancer. 2008;11310:2627–2637. 10.1002/cncr.23903 [DOI] [PubMed] [Google Scholar]
- 9. ACMG Board of Directors. Policy statement: Points to consider in the clinical application of genomic sequencing. 2012. https://www.acmg.net/StaticContent/PPG/Clinical_Application_of_Genomic_Sequencing.pdf. Accessed July 1, 2017.
- 10. Biesecker BB, Boehnke M, Calzone K, et al. Genetic counseling for families with inherited susceptibility to breast and ovarian cancer. JAMA. 1993;26915:1970–1974. 10.1001/jama.1993.03500150082032 [DOI] [PubMed] [Google Scholar]
- 11. Trepanier AM, Allain DC.. Models of service delivery for cancer genetic risk assessment and counseling. J Genet Couns. 2014;232:239–253. 10.1007/s10897-013-9655-6 [DOI] [PubMed] [Google Scholar]
- 12. Berliner JL, Fay AM.. Risk assessment and genetic counseling for hereditary breast and ovarian cancer: Recommendations of the National Society of Genetic Counselors. J Genet Couns. 2007;163:241–260. 10.1007/s10897-007-9090-7 [DOI] [PubMed] [Google Scholar]
- 13. Trepanier A, Ahrens M, McKinnon W, et al. Genetic cancer risk assessment and counseling: Recommendations of the national society of genetic counselors. J Genet Couns. 2004;132:83–114. 10.1023/B:JOGC.0000018821.48330.77 [DOI] [PubMed] [Google Scholar]
- 14. Jenkins J, Calzone KA, Dimond E, et al. Randomized comparison of phone versus in-person BRCA1/2 predisposition genetic test result disclosure counseling. Genet Med. 2007;98:487–495. 10.1097/GIM.0b013e31812e6220 [DOI] [PubMed] [Google Scholar]
- 15. Scheuner MT, Sieverding P, Shekelle PG.. Delivery of genomic medicine for common chronic adult diseases: A systematic review. JAMA. 2008;29911:1320–1334. 10.1001/jama.299.11.1320 [DOI] [PubMed] [Google Scholar]
- 16. Baumanis L, Evans JP, Callanan N, Susswein LR.. Telephoned BRCA1/2 genetic test results: Prevalence, practice, and patient satisfaction. J Genet Couns. 2009;185:447–463. 10.1007/s10897-009-9238-8 [DOI] [PubMed] [Google Scholar]
- 17. Chen WY, Garber JE, Higham S, et al. BRCA1/2 genetic testing in the community setting. J Clin Oncol. 2002;2022:4485–4492. 10.1200/JCO.2002.08.147 [DOI] [PubMed] [Google Scholar]
- 18. Shanley S, Myhill K, Doherty R, et al. Delivery of cancer genetics services: The Royal Marsden telephone clinic model. Fam Cancer. 2007;62:213–219. 10.1007/s10689-007-9131-2 [DOI] [PubMed] [Google Scholar]
- 19. Burgess KR, Carmany EP, Trepanier AM.. A comparison of telephone genetic counseling and in-person genetic counseling from the genetic counselor's perspective. J Genet Couns. 2016;251:112–126. 10.1007/s10897-015-9848-2 [DOI] [PubMed] [Google Scholar]
- 20. Peshkin BN, Demarco TA, Graves KD, et al. Telephone genetic counseling for high-risk women undergoing BRCA1 and BRCA2 testing: Rationale and development of a randomized controlled trial. Genet Test. 2008;121:37–52. [DOI] [PubMed] [Google Scholar]
- 21. Bradbury AR, Patrick-Miller L, Fetzer D, et al. Genetic counselor opinions of, and experiences with telephone communication of BRCA1/2 test results. Clin Genet. 2011;792:125–131. 10.1111/j.1399-0004.2010.01540.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patrick-Miller L, Bradbury AR, Terry MB.. Controversies in communication of genetic screening results for cancer: A report from the American Society of Preventive Oncology's Screening Special Interest Group (ASPO's 33rd Annual Meeting, March 8 to 10, 2009, Tampa, Florida). Cancer Epidemiol Biomarkers Prev. 2010;192:624–627. 10.1158/1055-9965.EPI-19-2-ASPO01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schwartz MD, Valdimarsdottir HB, Peshkin BN, et al. Randomized noninferiority trial of telephone versus in-person genetic counseling for hereditary breast and ovarian cancer. J Clin Oncol. 2014;327:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kinney AY, Butler KM, Schwartz MD, et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: A cluster randomized trial. J Natl Cancer Inst. 2014;10612:dju328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kinney AY, Steffen LE, Brumbach BH, et al. Randomized noninferiority trial of telephone delivery of BRCA1/2 genetic counseling compared with in-person counseling: 1-year follow-up. J Clin Oncol. 2016;3424:2914–2924. 10.1200/JCO.2015.65.9557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xue Y, Ankala A, Wilcox WR, Hegde MR.. Solving the molecular diagnostic testing conundrum for Mendelian disorders in the era of next-generation sequencing: Single-gene, gene panel, or exome/genome sequencing. Genet Med. 2015;176:444–451. 10.1038/gim.2014.122 [DOI] [PubMed] [Google Scholar]
- 27. Kurian AW, Hare EE, Mills MA, et al. Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol. 2014;3219:2001–2009. 10.1200/JCO.2013.53.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. LaDuca H, Stuenkel AJ, Dolinsky JS, et al. Utilization of multigene panels in hereditary cancer predisposition testing: Analysis of more than 2,000 patients. Genet Med. 2014;1611:830–837. 10.1038/gim.2014.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Desmond A, Kurian AW, Gabree M, et al. Clinical actionability of multigene panel testing for hereditary breast and ovarian cancer risk assessment. JAMA Oncol. 2015;17:943–951. 10.1001/jamaoncol.2015.2690 [DOI] [PubMed] [Google Scholar]
- 30. Domchek SM, Bradbury A, Garber JE, Offit K, Robson ME.. Multiplex genetic testing for cancer susceptibility: Out on the high wire without a net? J Clin Oncol. 2013;3110:1267–1270. [DOI] [PubMed] [Google Scholar]
- 31. Bradbury AR, Patrick-Miller LJ, Egleston BL, et al. Patient feedback and early outcome data with a novel tiered-binned model for multiplex breast cancer susceptibility testing. Genet Med. 2016;181:25–33. [DOI] [PubMed] [Google Scholar]
- 32. Kurian AW, Ford JM.. Multigene panel testing in oncology practice: How should we respond? JAMA Oncol. 2015;13:277–278. 10.1001/jamaoncol.2015.28 [DOI] [PubMed] [Google Scholar]
- 33. Robson M. Multigene panel testing: Planning the next generation of research studies in clinical cancer genetics. J Clin Oncol. 2014;3219:1987–1989. 10.1200/JCO.2014.56.0474 [DOI] [PubMed] [Google Scholar]
- 34. Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med. 2015;37324:2336–2346. 10.1056/NEJMoa1508054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN.. Gene panel testing for inherited cancer risk. J Natl Compr Canc Netw. 2014;129:1339–1346. [DOI] [PubMed] [Google Scholar]
- 36. Lumish HS, Steinfeld H, Koval C, et al. Impact of panel gene testing for hereditary breast and ovarian cancer on patients. J Genet Couns. 2017;265:1116–1129. 10.1007/s10897-017-0090-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peshkin BN, Kelly S, Nusbaum RH, et al. Patient perceptions of telephone vs. in-person BRCA1/BRCA2 genetic counseling. J Genet couns. 2016;253:472–482. 10.1007/s10897-015-9897-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patrick-Miller L, Egleston BL, Daly M, et al. Implementation and outcomes of telephone disclosure of clinical BRCA1/2 test results. Patient Educ Couns. 2013;933:413–419. 10.1016/j.pec.2013.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bradbury AR, Patrick-Miller L, Long J, et al. Development of a tiered and binned genetic counseling model for informed consent in the era of multiplex testing for cancer susceptibility. Genet Med. 2015;176:485–492. 10.1038/gim.2014.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Patrick-Miller LJ, Egleston BL, Fetzer D, et al. Development of a communication protocol for telephone disclosure of genetic test results for cancer predisposition. JMIR Res Protoc. 2014;34:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Leventhal H, Benyamini Y, Brownlee S, Diefenbach M, Leventhal EA, Patrick-Miller L.. Perceptions of health and illness: Current research and applications In: Petrie KJ, Weinman JA, eds. Illness Representations: Theoretical Foundations. Amsterdam: Harwood; 1997:19–46. [Google Scholar]
- 42. Shiloh S. Illness representations, self-regulation, and genetic counseling: A theoretical review. J Genet Couns. 2006;155:325–337. 10.1007/s10897-006-9044-5 [DOI] [PubMed] [Google Scholar]
- 43. Kelly K, Leventhal H, Marvin M, Toppmeyer D, Baran J, Schwalb M.. Cancer genetics knowledge and beliefs and receipt of results in Ashkenazi Jewish individuals receiving counseling for BRCA1/2 mutations. Cancer Control. 2004;114:236–244. [DOI] [PubMed] [Google Scholar]
- 44. Kaphingst KA, McBride CM, Wade C, et al. Patients' understanding of and responses to multiplex genetic susceptibility test results. Genet Med. 2012;147:681–687. 10.1038/gim.2012.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Speilberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA.. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 46. Zigmond AS, Snaith RP.. The Hospital Anxiety and Depression Scale. Acta Psych Scand. 1983;676:361–370. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 47. Horowitz M, Wilner N, Alvarez W.. Impact of event scale: A measure of subjective stress. Psychosomat Med. 1979;413:209–218. 10.1097/00006842-197905000-00004 [DOI] [PubMed] [Google Scholar]
- 48. Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: The Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psych. 2002;216:564–572. 10.1037/0278-6133.21.6.564 [DOI] [PubMed] [Google Scholar]
- 49. Pieterse AH, van Dulmen AM, Beemer FA, Bensing JM, Ausems MG.. Cancer genetic counseling: Communication and counselees' post-visit satisfaction, cognitions, anxiety, and needs fulfillment. J Genet Couns. 2007;161:85–96. 10.1007/s10897-006-9048-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cohen J. A power primer. Psychol Bull. 1992;1121:155–159. 10.1037/0033-2909.112.1.155 [DOI] [PubMed] [Google Scholar]
- 51. Raghunathan TE LJ, Van Hoewyz J, Solenberger P.. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodol. 2001;271:85–95. [Google Scholar]
- 52. Madlensky L. Is it time to embrace telephone genetic counseling in the oncology setting? J Clin Oncol. 2014;327:611–612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.