Summary
Nodal signaling controls asymmetric organ placement during vertebrate embryogenesis. Nodal is induced by a leftward fluid flow at the ciliated left-right organizer (LRO). The mechanism of flow sensing, however, has remained elusive. pkd2 encodes the calcium channel Polycystin-2, which is required for kidney development and laterality, and may act in flow perception. Here, we have studied the role of Polycystin-2 in Xenopus and show that pkd2 is indispensable for left-right (LR) asymmetry. Knockdown of pkd2 prevented left-asymmetric nodal cascade induction in the lateral plate mesoderm. Defects were due to failure of LRO specification, morphogenesis, and, consequently, absence of leftward flow. Polycystin-2 synergizes with the unconventional nodal-type signaling molecule Xnr3 to induce the LRO precursor tissue before gastrulation, upstream of symmetry breakage. Our data uncover an unknown function of pkd2 in LR axis formation, which we propose represents an ancient role of Polycystin-2 during LRO induction in lower vertebrates.
Subject Areas: Zoology, Evolutionary Developmental Biology, Developmental Biology
Graphical Abstract
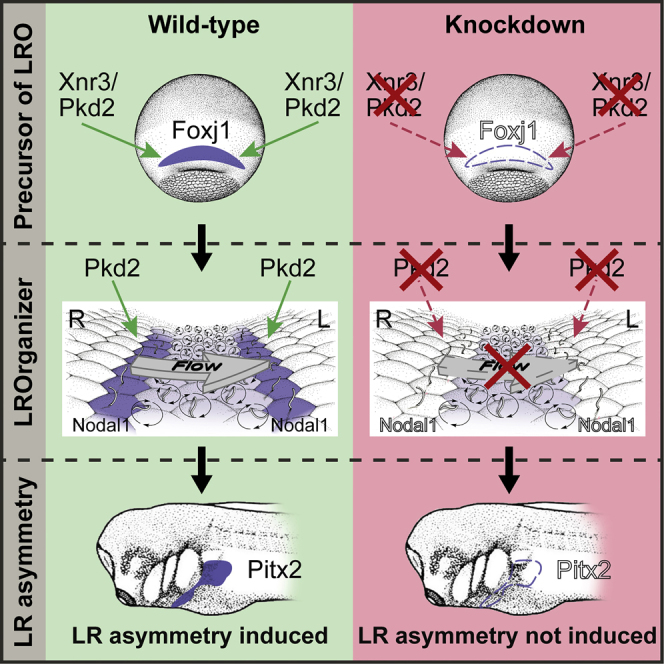
Highlights
-
•
Loss of Polycystin-2 in Xenopus results in LR asymmetry defects upstream of leftward flow
-
•
LR defects are caused by lack of LR organizer induction
-
•
Polycystin-2 is required upstream of foxj1 for specification of superficial mesoderm
-
•
Polycystin-2 and Xnr3 synergistically induce foxj1 in the superficial mesoderm
Zoology; Evolutionary Developmental Biology; Developmental Biology
Introduction
Asymmetries along the left-right (LR) body axis are a common feature of most animals. Vertebrates, for example, show asymmetric arrangements of most visceral organs (Grimes and Burdine, 2017). In all deuterostomes examined, as well as in several protostomes, asymmetries are induced by unilateral activation of the Nodal signaling cascade, consisting of nodal, lefty, and pitx2, before onset of asymmetric organogenesis (Namigai et al., 2014, Shiratori and Hamada, 2014). How the Nodal cascade is activated unilaterally is a matter of intense research. The most common symmetry-breaking mechanism in vertebrates is a transient extracellular leftward fluid flow at the archenteron of gastrula/neurula embryos (Blum et al., 2009). Although this flow lost in birds, it is absolutely required for symmetry breakage in rabbit, mouse, frog, and fish (Essner et al., 2005, Nonaka et al., 1998, Schweickert et al., 2007). Flow is generated at the left-right organizer (LRO), a transient ciliated epithelium. Owing to the clockwise rotation of monocilia and their posterior tilting, they generate a leftward flow of extracellular fluids (Blum et al., 2014b, Yoshiba and Hamada, 2014). Putative LROs have been pinpointed in further vertebrates (axolotl, additional frog species, sturgeon), the ancient chordate amphioxus, and the sea urchin Paracentrotus (Blum et al., 2009, Sáenz-Ponce et al., 2011, Tisler et al., 2016).
How flow is sensed on the left side of the LRO and how it activates the Nodal cascade in the lateral plate mesoderm (LPM) is still largely unknown. The Nodal inhibitor Dand5 is one decisive factor in chordates. dand5 and nodal are expressed bilaterally on both sides of the LRO, just before flow becomes established. As a result of flow, dand5 becomes down-regulated on the left (Schweickert et al., 2010, Shinohara et al., 2012). In Xenopus, knockdown of dand5 rescues flow-deficient embryos, establishing dand5 mRNA asymmetry as the first detectable molecular feature of LR asymmetry. How this inhibition is realized at the cellular level is not known. One mechanism that has been proposed is a calcium-dependent down-regulation of dand5 during flow (Yoshiba et al., 2012). Left-sided calcium fluxes during flow stages have been described and implicated in flow sensing, but the molecular consequences have remained elusive (Sarmah et al., 2005, Yuan et al., 2015). According to the two-cilia model of symmetry breakage, motile cilia at the center of the LRO create a leftward flow that bends immotile mechanosensory cilia at the left margin. This bending results in left-sided calcium fluxes, which supposedly activate downstream calcium-dependent events that break the bilateral symmetry (McGrath et al., 2003, Tabin and Vogan, 2003). Genetic experiments in the mouse have unequivocally demonstrated that the calcium channel Polycystin-2 is specifically required in lateral cells of the mouse LRO to break symmetry (Yoshiba et al., 2012).
We and others have previously shown that pkd2, the gene encoding Polycystin-2, is necessary for kidney and LR development in mouse and for kidney development in Xenopus, as it is in other vertebrates (Futel et al., 2015, Pennekamp et al., 2002, Sullivan-Brown et al., 2008, Tran et al., 2010). Here, we revisited the role of Polycystin-2 in LR development in Xenopus, in which the sequential steps of laterality development are known in great detail and can be experimentally manipulated in a sided manner. pkd2 knockdown resulted in failure of Nodal cascade activation, as previously shown for fish and mouse. LRO morphogenesis and function were lost in morphants, as revealed by altered marker gene expression and a lack of ciliation and leftward flow. This phenotype was due to a lack of pkd2-dependent induction of the LRO precursor tissue, the superficial mesoderm (SM), during early gastrulation. Polycystin-2 and the FGFR-binding ligand Xnr3 synergize to induce foxj1 in the SM, establishing an unknown function of Polycystin-2.
Results and Discussion
pkd2 Is Required for LR Axis Development in Xenopus
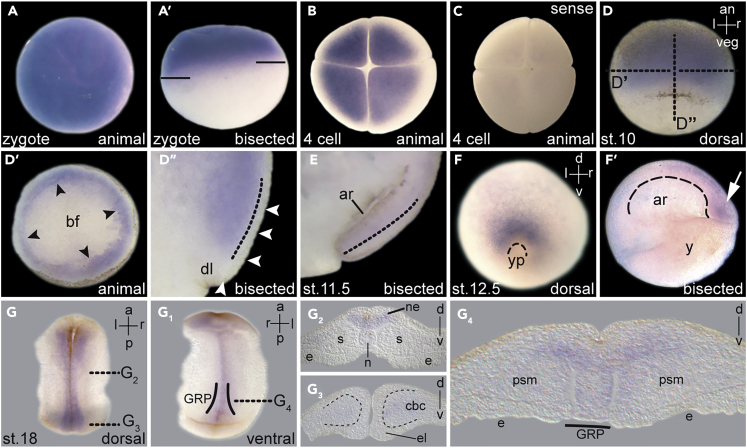
As a reference point for the functional assessment of pkd2 during symmetry breakage, we analyzed mRNA expression during embryogenesis. Signals at the animal pole of the zygote and during early cleavage stages represented maternally deposited transcripts (Figures 1A and 1B). Sense probe control specimens lacked staining (Figure 1C; data not shown). During early gastrulation, pkd2 was restricted to the deep mesoderm, excluding the dorsal lip and the superficial epithelial layer, i.e., the SM, from which the frog LRO, the gastrocoel roof plate (GRP), develops during gastrulation (Figure 1D; Blum et al., 2014a). Later expression sites included the notochord, trunk organizer, deep neuroectoderm, intermediate mesoderm, and developing pronephric kidney (Figures 1E–1G and S1A–S1E; cf. Tran et al., 2010). Remarkably, zygotic pkd2 mRNA was not detected in the SM or in the GRP (Figures 1D and 1G), i.e., the early LR-relevant tissues.
Figure 1.
Expression of pkd2 mRNA during Xenopus Embryogenesis
(A–C) Maternally deposited mRNA localized to the animal hemisphere of the zygote (A, A′) and four-cell stage embryos (B). Note animal-vegetal shift of expression as indicated in the zygote. (C) Lack of a specific signal in specimen hybridized with a sense probe.
(D–F) pkd2 transcripts in deep mesodermal layers of the early gastrula (D, D′, D″; black arrowheads; plane of section indicated in D by dotted line), in dorsal notochordal mesoderm at mid gastrula (E), and in tail organizer (white arrow) and posterior notochord at late gastrula stages (F, F′). Please note the lack of transcripts in the dorsal lip and the SM (white arrowheads). Border of inner and outer layer of marginal zone indicated by dotted line. Yolk plug and archenteron roof indicated by dashed lines.
(G) Deep neuroectodermal, tail organizer, and posterior notochordal expression at neurula stages; no expression of GRP detected (G4).
a, anterior; an, animal; ar, archenteron; bf, blastocoel floor; cbc, circumblastoporal collar; dl, dorsal lip; e, endoderm; el, epithelial layer (of cbc); l, left; n, notochord; ne, neuroectoderm; psm, presomitic mesoderm; r, right; s, somites; v, ventral; veg, vegetal; yp, yolk plug.
See also Figure S1.
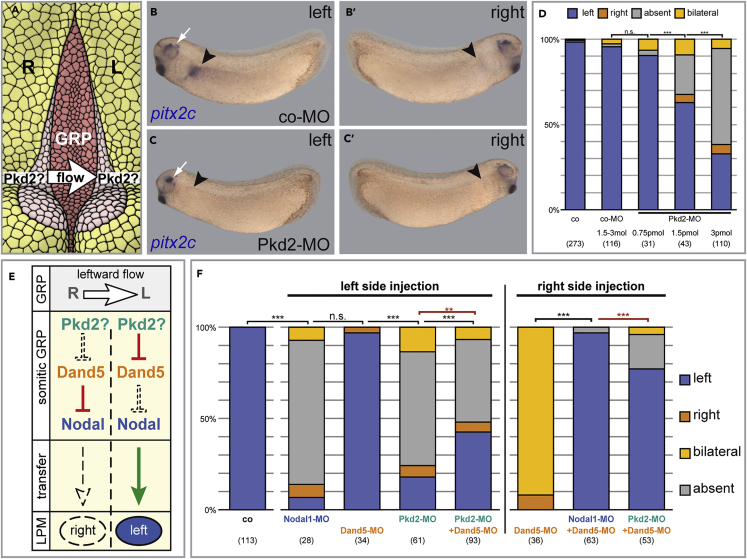
In the mouse, Polycystin-2 acts downstream of flow but upstream of flow-dependent dand5 repression and LPM Nodal induction (Yoshiba et al., 2012). To test whether this function was conserved in Xenopus, we targeted a previously characterized antisense morpholino oligomer (MO) that blocks translation of pkd2 (Pkd2-MO; Tran et al., 2010) to the future GRP (cf. Figure 2A). Morphant embryos lacked induction of the Nodal cascade in the left LPM in a dose-dependent manner, in agreement with this notion (Figures 2B–2D). As expected for a flow-sensing function, pkd2 was required on the left side of the GRP, as revealed by unilateral injections (Figures S2A and S2B; cf. Vick et al., 2009 for left-sided flow knockdown). Knockdown in the LPM itself did not cause LR defects, suggesting that pkd2 was not relevant for Nodal propagation (Figure S2B). In summary, these results showed that pkd2 was required for the activation of the Nodal cascade in the left LPM, suggesting a conserved role during flow-dependent symmetry breakage.
Figure 2.
pkd2 Is Required for LR Asymmetry in Xenopus, Independently of dand5
(A) Putative sensory function of Polycystin-2 during symmetry breakage downstream of GRP-flow.
(B–D) Dose-dependent loss of left-sided pitx2c expression following Pkd2-MO targeting to the GRP. Black arrowhead indicates approximate location of present or absent pitx2c expression on both sides of the embryo. White arrow highlights expression of pitx2c around the eye as proof of working ISH.
(E) Model of hierarchy of the flow sensing module and possible role of pkd2 on the lateral side of the LRO downstream of flow and upstream of Dand5.
(F) Epistatic single and double knockdown experiments, performed on the left or right side, as indicated. Differences in expected and experimental outcome highlighted by red significance. See text for details.
See also Figure S2.
**p < 0.01, ***p < 0.001 in all Figures.
Numbers in parentheses indicate the number of embryos analyzed for each condition.
Pkd2 Functions Independently of dand5
Dand5 plays a key role at the interface between flow and Nodal induction (Figure 2E). To test whether pkd2 acted upstream of dand5 in the process of flow sensing, we performed combinatory knockdown experiments. When Pkd2-MO was injected on the left side of the GRP, embryos failed to induce pitx2c in the left LPM, similar to a GRP-specific nodal1 knockdown. Inhibition of dand5 alone had no effect, as described (Figure 2F; cf. Schweickert et al., 2010), because flow down-regulates dand5 as a physiological target. When Pkd2-MO and Dand5-MO were co-injected, most specimens still did not induce pitx2c (Figure 2F). Dand5 knockdown thus failed to rescue loss of Polycystin-2, as was expected from the sensor function described in mouse (Figure 2E). Pkd2 therefore could act upstream of nodal induction in the LPM. Knockdown of pkd2 in the LPM, however, did not affect pitx2 expression (Figure S2B). Alternatively, pkd2 could be required for nodal function at the lateral cells (sensory part) of the GRP. The possibility of sided injections in Xenopus afforded the opportunity of investigating this notion on the right side of the GRP, independently of flow. Here, dand5 knockdown induces right-sided Nodal cascade induction (Figure 2F; cf. Schweickert et al., 2010). Parallel knockdown of dand5 and nodal1 at the right GRP margin counteracted this effect, as loss of the Nodal inhibitor Dand5 can only be effective in the presence of Nodal. Simultaneous targeting of Dand5-MO and Pkd2-MO to the right GRP margin still prevented right-sided Nodal cascade induction (Figure 2F), suggesting that pkd2 was required for Nodal function at the GRP or GRP function in general.
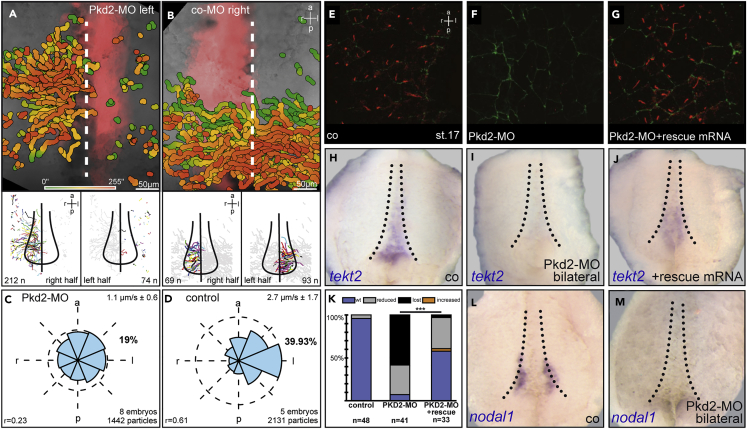
Leftward Flow and GRP Morphogenesis Are Compromised in pkd2 Morphants
The high efficiency of Pkd2-MO, comparable with a nodal1 knockdown in the left GRP, asked for an in-depth analysis of GRP function itself. Leftward flow was analyzed in dorsal explants prepared from flow stage embryos that were injected with control (co-)MO or Pkd2-MO as described (Vick et al., 2009). Time-lapse videography of fluorescent beads added to the explants demonstrated that flow was absent at the targeted area in morphants (Figures 3A, 3C, and Video S1), whereas co-MO injected specimens displayed the previously reported directionality and velocity of bead transport (Figures 3B and 3D; cf. Schweickert et al., 2007). This result suggested that GRP function was lost in pkd2 morphants. A morphological analysis of GRP tissue using immunofluorescence (IF) to highlight cilia and cell boundaries confirmed this notion, as cilia were literally absent (Figures 3E–3G). To gain further insights into the nature of GRP defects, scanning electron microscopy (SEM) was employed. co-MO-injected specimens (Figure S3A; n = 6) displayed on average 88% ciliated GRP cells, whereas this rate dropped to 34% in pkd2 morphants, and remaining ciliated cells lacked polarization (Figure S3B; n = 5). Individual specimens lacked cilia altogether. Remarkably, non-ciliated morphant cells revealed an increase in cell surface area and thus resembled endodermal cells rather than the small GRP cells (Figure S3B), indicating a potential change of fate. GRP marker genes, such as the tektin isoform tekt2 as well as the axonemal dynein motor protein dnah9, were absent from morphant explants, whereas control samples exhibited wild-type expression patterns (Figures 3H–3K, S3C, and S3D). Importantly, GRP ciliation and gene expression were rescued by co-injection of a full-length pkd2 mRNA construct that was not targeted by the Pkd2-MO (Figures 3G, 3J, and 3K).
Figure 3.
Leftward Flow and GRP Morphogenesis Are Compromised in pkd2 Morphants
(A–D) Unilateral injection of Pkd2-MO (A) but not co-MO (B) caused disruption of leftward flow at targeted area (red fluorescence: lineage tracer), as revealed by gradient time trail (GTT); see Transparent Methods section and Schweickert et al., (2007) analysis. Quantification of directionality and velocity of bead transport on morphant (C) and control (D) sides of dorsal explants. Rho (r) represents a value for robustness of directionality of leftward flow. Scale bars represent 50 micrometers.
(E–G) Stage 17 GRP ciliation (E) was lost in pkd2 morphants (F) and regained in specimens co-injected with pkd2-MO and a full-length pkd2 mRNA. IF with acetylated α-tubulin antibodies (red) and Alexa 488-phalloidin (green) to highlight GRP cilia and cell borders.
(H–K) tekt2 expression at the GRP of stage 17 dorsal explants (H) was lost in pkd2 morphants (I) and rescued upon co-injection of a full-length pkd2 mRNA (J). (K) Quantification of results.
(L and M) nodal1 expression in lateral GRP cells (L) was lost in morphants (M).
To investigate the GRP fate in depth, we analyzed marker genes that highlight the lateral sensory cells, namely, nodal1 and dand5. Both were absent from morphant specimens, in which Pkd2-MO was targeted to these cells (Figures 3L, 3M and S3E–S3H). A further SEM analysis revealed that lateral GRP cells were missing completely and that remaining ciliated central GRP cells directly bordered non-ciliated endodermal cells (Figure S3I). In the absence of nodal1, the Dand5-MO thus remained without effect, providing a stringent explanation for the nearly complete lack of pitx2c induction upon dand5 knockdown in pkd2 morphants (cf. Figure 2F).
Polycystin-2 is mainly part of a cilia- or endoplasmic reticulum (ER)-located calcium channel complex, which can modulate cellular calcium levels, and itself can be regulated by intracellular calcium (Busch et al., 2017). To test if manipulations of intracellular calcium levels also affected nodal1 expression, we treated embryos with Thapsigargin (Tg), a well-known antagonist of ER-located SERCA pumps (Thastrup et al., 1990). Embryos treated at early gastrulation showed blastopore closure defects, caused by lack of necessary intracellular calcium waves, as previously reported (Wallingford et al., 2001; data not shown). Treatment from late gastrula stages onward, however, did not affect gastrulation and resulted in reduced or absent nodal1 expression at neurula stages, i.e., when nodal1 is initiated in lateral GRP cells before flow occurs (Figures S3J–S3L). This outcome was supported by another set of experiments. When embryos were treated in the same way with BAPTA-AM, an intracellular calcium chelator, they also showed reduced expression of nodal1 at neurula stages (Figures S3M–S3P). These results support the notion that Polycystin-2 is necessary to induce the lateral cell fate of the LRO in Xenopus, and this required intracellular calcium changes. Yet, these findings were unexpected, as altered LRO nodal expression was not reported from pkd2-knockout mice or zebrafish morphants (Bisgrove et al., 2005, Pennekamp et al., 2002). Mouse embryos treated with Tg, however, showed loss of nodal1 in the LRO and LPM as well, arguing for conservation of a calcium-dependent induction of lateral LRO fates (Takao et al., 2013). Taken together, these analyses demonstrated that LR defects in Pkd2 morphants were caused by impaired GRP morphogenesis and function and, specifically, the absence of lateral LRO cells expressing nodal1 and dand5.
pkd2 Is Required for SM Specification
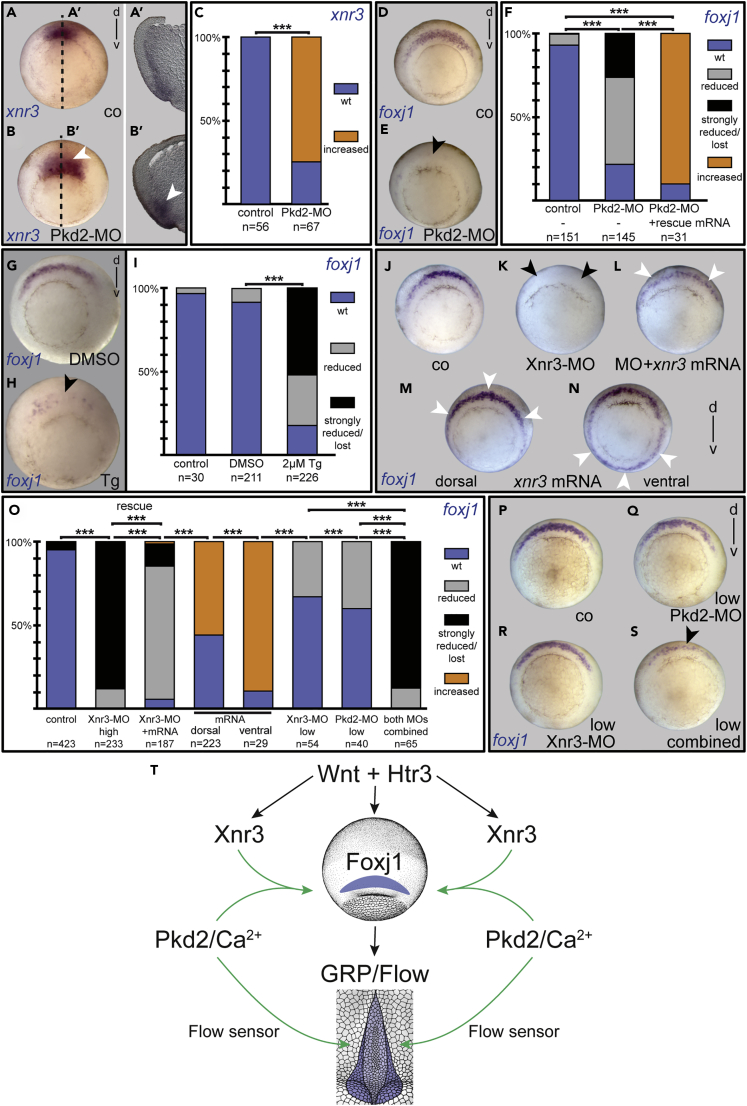
Next, we asked whether the GRP precursor tissue, the SM, was correctly specified during gastrulation. To that end, we analyzed SM marker genes xnr3 and foxj1. Both genes are known targets of canonical Wnt signaling; foxj1 is instrumental for SM and GRP function downstream of Wnt signaling (Glinka et al., 1996, Smith et al., 1995, Stubbs et al., 2008, Walentek et al., 2013). Remarkably, these genes responded differently to pkd2 knockdown: xnr3 was slightly upregulated, whereas foxj1 expression was reduced or absent (Figures 4A–4F). This effect was specific, as foxj1 expression was rescued (or even super-induced) upon co-injection of full-length pkd2 mRNA (Figures 4F and S4A–S4C). As reported, calcium spikes during gastrulation were dependent on intracellular calcium (Wallingford et al., 2001), and we treated embryos again with Tg. To test whether foxj1 expression was dependent on calcium dynamics as well, specimens were treated with brief pulses (maximum 20 min) at stage 9, when foxj1 expression is initiated before the start of gastrulation. Treated embryos developed without gastrulation defects but showed a lack of pitx2c expression in more than 60% of cases, reminiscent of pkd2 morphants (Figures S4D–S4F). When such specimens were analyzed at the onset of gastrulation (st. 10.5), foxj1 was reduced or absent as well, confirming a requirement of both calcium-dependent signals and Polycystin-2 for foxj1 induction (Figures 4G–4I). Surprisingly, in our hands, similar incubations during the initiation of foxj1 expression (late blastula st. 9 until early gastrula st. 10.5) using the calcium-chelator BAPTA-AM did not alter foxj1 expression (Figures S4G–S4I). Accordingly, such treated embryos did not show LR axis alterations at tail bud stages, as shown by wild-type pitx2c expression (Figure S4J). However, embryos incubated in BAPTA-AM from late blastula (st. 9) to late gastrula stages (st. 12.5), i.e., additionally covering the later sensitive time window of nodal1 initiation in the lateral GRP (cf. Figures S3J–S3P), again developed laterality defects by misexpression of pitx2c (Figure S4J). These results suggested that Polycystin-2/Tg and BAPTA-AM have different effects on the process of foxj1 induction. Yet, in sum, these experiments are in line with a function for pkd2 and specific calcium changes in the specification of the SM as the LRO precursor tissue.
Figure 4.
Polycystin-2 and Xnr3 Synergize to Induce the LRO Precursor Tissue of the Superficial Mesoderm
(A–F) Increased xnr3 (A–C) and reduced foxj1 expression (D–F) in stage 10.5 pkd2 morphants (B and E) as compared with control specimens (A and D). Dotted lines in A and B indicate plane of histological sections shown in A′, and B′, respectively. Note that foxj1 expression was rescued upon co-injection of a full-length pkd2 mRNA (F).
(G–I) Reduced foxj1 expression in early gastrula stages (st. 10.5) following Tg treatment (G, DMSO control embryo; H, Tg treated specimen; I, quantification of results).
(J–S) xnr3 and pkd2 synergize SM foxj1 induction. Loss of xnr3 resulted in attenuated foxj1 expression (K and O) compared with control uninjected specimens (J and O), which was highly efficiently rescued by co-injection of full-length xnr3 mRNA (L and O). Both dorsal (M) and ventral (N) overexpression of xnr3 mRNA caused an increase of endogenous or induction of ectopic foxj1, respectively (M–O). Injections of reduced MO doses of Xnr3-MO (O, R; 2 × 0.6pmol) or Pkd2-MO (O, Q; 2 × 0.75pmol) caused mild reductions of foxj1 expression. Co-injection of low doses of MOs resulted in strong inhibitory effects (O and S).
(T) Schematic depiction of known (black) and proposed (green) interactions required for SM specification. For details refer to main text. Increase of expression highlighted with white arrowheads and decrease with black arrowheads. Numbers in parentheses indicate the number of embryos analyzed for each condition.
See also Figures S4 and S5.
pkd2 Synergizes with xnr3 to Induce foxj1 in the Superficial Mesoderm
Little is known about early signaling pathways that set up the SM. We and others previously demonstrated that canonical Wnt signaling, which depends on type 3 serotonin receptor signaling (Htr3) in the SM, was required for the induction of both xnr3 and foxj1 (Beyer et al., 2012). In that light, the above-mentioned up-regulation of xnr3 in pkd2 morphants (Figures 4A–4C) argued against the participation of Polycystin-2 as part of the Wnt signaling module upstream of xnr3 and foxj1. To verify this, we analyzed whether pkd2 was required for Wnt-dependent secondary axis induction. Twinning was induced by ventral injection of wnt8 mRNA, in the presence or absence of Pkd2-MO. Inhibition of Polycystin-2 did not affect the frequency of secondary axis formation (Figures S5A–S5C), nor did it affect endogenous organizer gene expression (not shown). Polycystin-2, thus, should not be part of upstream canonical Wnt signaling but control foxj1 induction in the SM independently.
The opposing effects of pkd2 knockdown on foxj1 and xnr3 made us wonder whether the effect on foxj1 was mediated through Xnr3. This nodal-related gene is unusual, as it does not interact with TGF-β-type receptors but interacts with Fgf receptor 1 (Fgfr1); MO-mediated xnr3 knockdown specifically inhibits brachyury expression (Yokota et al., 2003). Besides serving as an SM marker gene, this Xenopus-specific factor has not been investigated for a possible function in the context of LR asymmetry. To test whether Xnr3 played a role in laterality determination, we targeted the Xnr3-MO to the dorsal midline (organizer and SM). Knockdown resulted in loss of dorsal brachyury expression, as described previously (Figures S5D–S5F; Yokota et al., 2003). Strikingly, when such specimens were analyzed for foxj1, expression in the SM was also lost or strongly reduced as compared with control embryos (Figures 4J, 4K, and 4O). This phenotype was specific to loss of Xnr3 function, as reintroduction of full-length xnr3-mRNA, which is insensitive to the Xnr3-MO, was able to rescue the observed reduction of foxj1 very efficiently (Figures 4L and 4O). Morphants that were raised further revealed the previously reported convergent extension defects (Yokota et al., 2003) and altered pitx2c expression (data not shown).
Conversely, when xnr3 was overexpressed in the dorsal SM, an increase of foxj1 expression was observed, specifically in the more lateral part of the expression domain, the future lateral GRP, demonstrating that Xnr3 was able to enhance foxj1 expression (Figures 4M and 4O). Interestingly, injecting xnr3 mRNA into the ventral side was sufficient to induce foxj1 expression ectopically, demonstrating its role in SM fate specification (Figures 4N and 4O). This could indicate a potential role of the well-characterized ventral Bmp signaling pathway in preventing the activation of foxj1 in the ventral superficial layer, as it has been shown that Xnr3 itself has a dorsalizing effect by inhibiting Bmp signaling (Hansen et al., 1997, Haramoto et al., 2004). Thus, to finally test whether xnr3 and pkd2 synergized in SM foxj1 induction, we performed epistasis experiments. MO doses for both genes were reduced such that individually they did not result in a strong reduction of foxj1 expression. Combined injection of Pkd2-MO and Xnr3-MO, however, resulted in a strong reduction or loss of foxj1, establishing a genetic interaction of these genes in SM foxj1 induction and, thus, in setting up a functional LRO (Figures 4O–4S). Interestingly, although exogenously introduced pkd2 mRNA caused an increase of foxj1 expression (Figure S4C), it was not sufficient to rescue foxj1 expression in xnr3 morphant specimens (data not shown).
In summary, our data demonstrate that in general terms, pkd2 is a conserved determinant of LR symmetry breakage in Xenopus. Surprisingly, however, pkd2 is strictly required upstream of leftward flow to set up the LRO during gastrulation, together with xnr3 (Figure 4T). Polycystin2 in the future SM cells could provide a crucial signal for specification. However, the protein of such cell-autonomous function should be of maternal origin, particularly because zygotic mRNA was not detected in the LR-relevant tissues, SM and GRP. Alternatively, Pkd2 could act non-cell autonomously in the deep tissue to induce foxj1 in the superficial layer, i.e., indirectly. It remains to be seen which type of activating signal could mediate this induction. Based on our timed Tg treatments at the blastula or late gastrula stages (Figures 4G–4I and S3J–S3L), and the corresponding central or lateral Pkd2-MO injections (Figures 3M and 4E, respectively), which resulted in either loss of foxj1 or loss of nodal1, respectively, the following conclusion can be drawn. These differentiable results from two different time points of LR symmetry breakage imply that both Pkd2 function and intracellular calcium changes are necessary at two separate steps of LRO induction and sub-functionalization in Xenopus (Figure 4T). It remains to be seen which other signaling pathways could be required for each of these steps.
In any way, we propose that in evolutionary terms, this may represent an ancestral Polycystin-2 function in amphibian embryos, which undergo a basal mode of vertebrate gastrulation and thus LRO development (Blum et al., 2009, Cooper and Virta, 2007). Interestingly, zebrafish morphant but not mutant embryos display early mesendodermal defects after maternal pkd2 knockdown, reminiscent of SM loss in Xenopus (Schottenfeld et al., 2007). It is tempting to speculate that Fgf signaling represents the common denominator in this context, as Xnr3 binds and signals via the Fgfr1 (Yokota et al., 2003). In zebrafish, Fgfr1 signaling affects foxj1 expression, LRO ciliogenesis, and flow (Neugebauer et al., 2009). In mouse embryos, early loss of Fgfr1-mediated signaling results in gastrulation defects, but pkd2-knockout mice do not display impaired LRO formation or flow (Yamaguchi et al., 1994, Yoshiba et al., 2012). Treatment with an Fgfr1 inhibitor, however, blocked nodal expression in lateral LRO cells (Oki et al., 2010) in much the same way as observed upon loss of pkd2 in Xenopus. Thus, LRO induction, although still dependent on Fgfr1, may have become independent of (maternal) pkd2 in modern bony fish and mammals. The later role of Polycystin-2 in flow sensing at the lateral LRO cells may very well be conserved in amphibians as well. The loss of LRO and flow in pkd2 morphants precluded the analysis in the context of the present study. The novel role ascribed to Polycystin-2 in Xnr3-/FGF-dependent LRO morphogenesis adds to the long list of functions of this factor that is conserved in animals from nematodes to humans.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
M.B. was funded by the Deutsche Forschungsgemeinschaft (BL285/9-2). J.K. and M.G. were recipients of a Ph.D. fellowship from the Landesgraduiertenförderung Baden-Württemberg. We thank Susanne Bogusch, Anna Schäfer, Sabrina Kurz, and Verena Andre for technical assistance, Kathrin Ott for help with double axis assay and IF experiments, and C. Kintner, A. Brivanlou, and J. Heasman for plasmids.
Author Contributions
P.V., M.B., and A.S. conceived, designed, and supervised the experiments; P.V., J.K., I.S., M.T., M.G., T.T., and T.B. performed and analyzed the experiments; P.V. and A.S. interpreted results; and P.V. wrote the original and the revised manuscript with the help of M.B. and final input of all authors.
Declaration of Interests
The authors declare no competing interest.
Published: April 27, 2018
Footnotes
Supplemental Information includes Transparent Methods, five figures, and one video and can be found with this article online at https://doi.org/10.1016/j.isci.2018.03.011.
Supplemental Information
References
- Beyer T., Danilchik M., Thumberger T., Vick P., Tisler M., Schneider I., Bogusch S., Andre P., Ulmer B., Walentek P. Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr. Biol. 2012;22:33–39. doi: 10.1016/j.cub.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Bisgrove B.W., Snarr B.S., Emrazian A., Yost H.J. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left–right axis. Dev. Biol. 2005;287:274–288. doi: 10.1016/j.ydbio.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Blum M., Feistel K., Thumberger T., Schweickert A. The evolution and conservation of left-right patterning mechanisms. Development. 2014;141:1603–1613. doi: 10.1242/dev.100560. [DOI] [PubMed] [Google Scholar]
- Blum M., Schweickert A., Vick P., Wright C.V.E., Danilchik M.V. Symmetry breakage in the vertebrate embryo: when does it happen and how does it work? Dev. Biol. 2014;393:109–123. doi: 10.1016/j.ydbio.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum M., Weber T., Beyer T., Vick P. Evolution of leftward flow. Semin. Cell. Dev. Biol. 2009;20:464–471. doi: 10.1016/j.semcdb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Busch T., Köttgen M., Hofherr A. TRPP2 ion channels: critical regulators of organ morphogenesis in health and disease. Cell Calcium. 2017;66:25–32. doi: 10.1016/j.ceca.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Cooper M.S., Virta V.C. Evolution of gastrulation in the ray-finned (actinopterygian) fishes. J. Exp. Zool. 2007;308B:591–608. doi: 10.1002/jez.b.21142. [DOI] [PubMed] [Google Scholar]
- Essner J.J., Amack J.D., Nyholm M.K., Harris E.B., Yost H.J. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- Futel M., Leclerc C., Le Bouffant R., Buisson I., Néant I., Umbhauer M., Moreau M., Riou J.-F. TRPP2-dependent Ca2+ signaling in dorso-lateral mesoderm is required for kidney field establishment in Xenopus. J. Cell Sci. 2015;128:888–899. doi: 10.1242/jcs.155499. [DOI] [PubMed] [Google Scholar]
- Glinka A., Delius H., Blumenstock C., Niehrs C. Combinatorial signalling by Xwnt-11 and Xnr3 in the organizer epithelium. Mech. Dev. 1996;60:221–231. doi: 10.1016/s0925-4773(96)00624-7. [DOI] [PubMed] [Google Scholar]
- Grimes D.T., Burdine R.D. Left-right patterning: breaking symmetry to asymmetric morphogenesis. Trends Genet. 2017;33:616–628. doi: 10.1016/j.tig.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C.S., Marion C.D., Steele K., George S., Smith W.C. Direct neural induction and selective inhibition of mesoderm and epidermis inducers by Xnr3. Development. 1997;124:483–492. doi: 10.1242/dev.124.2.483. [DOI] [PubMed] [Google Scholar]
- Haramoto Y., Tanegashima K., Onuma Y., Takahashi S., Sekizaki H., Asashima M. Xenopus tropicalis nodal-related gene 3 regulates BMP signaling: an essential role for the pro-region. Dev. Biol. 2004;265:155–168. doi: 10.1016/j.ydbio.2003.09.015. [DOI] [PubMed] [Google Scholar]
- McGrath J., Somlo S., Makova S., Tian X., Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Namigai E.K.O., Kenny N.J., Shimeld S.M. Right across the tree of life: the evolution of left-right asymmetry in the Bilateria. Genesis. 2014;52:458–470. doi: 10.1002/dvg.22748. [DOI] [PubMed] [Google Scholar]
- Neugebauer J.M., Amack J.D., Peterson A.G., Bisgrove B.W., Yost H.J. FGF signalling during embryo development regulates cilia length in diverse epithelia. Nature. 2009;458:651–654. doi: 10.1038/nature07753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S., Tanaka Y., Okada Y., Takeda S., Harada A., Kanai Y., Kido M., Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Oki S., Kitajima K., Meno C. Dissecting the role of Fgf signaling during gastrulation and left-right axis formation in mouse embryos using chemical inhibitors. Dev. Dyn. 2010;239:1768–1778. doi: 10.1002/dvdy.22282. [DOI] [PubMed] [Google Scholar]
- Pennekamp P., Karcher C., Fischer A., Schweickert A., Skryabin B., Horst J., Blum M., Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr. Biol. 2002;12:938–943. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- Sarmah B., Latimer A.J., Appel B., Wente S.R. Inositol polyphosphates regulate zebrafish left-right asymmetry. Dev. Cell. 2005;9:133–145. doi: 10.1016/j.devcel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Sáenz-Ponce N., Santillana-Ortiz J.-D., del Pino E.M. The gastrocoel roof plate in embryos of different frogs. Differentiation. 2011;83:S62–S66. doi: 10.1016/j.diff.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Schottenfeld J., Sullivan-Brown J., Burdine R.D. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development. 2007;134:1605–1615. doi: 10.1242/dev.02827. [DOI] [PubMed] [Google Scholar]
- Schweickert A., Vick P., Getwan M., Weber T., Schneider I., Eberhardt M., Beyer T., Pachur A., Blum M. The nodal inhibitor coco is a critical target of leftward flow in Xenopus. Curr. Biol. 2010;20:738–743. doi: 10.1016/j.cub.2010.02.061. [DOI] [PubMed] [Google Scholar]
- Schweickert A., Weber T., Beyer T., Vick P., Bogusch S., Feistel K., Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr. Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Shinohara K., Kawasumi A., Takamatsu A., Yoshiba S., Botilde Y., Motoyama N., Reith W., Durand B., Shiratori H., Hamada H. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat. Commun. 2012;3:622. doi: 10.1038/ncomms1624. [DOI] [PubMed] [Google Scholar]
- Shiratori H., Hamada H. TGFβ signaling in establishing left-right asymmetry. Semin. Cell. Dev. Biol. 2014;32:80–84. doi: 10.1016/j.semcdb.2014.03.029. [DOI] [PubMed] [Google Scholar]
- Smith W.C., McKendry R., Ribisi S., Harland R.M. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell. 1995;82:37–46. doi: 10.1016/0092-8674(95)90050-0. [DOI] [PubMed] [Google Scholar]
- Stubbs J.L., Oishi I., Izpisúa-Belmonte J.C., Kintner C. The forkhead protein Foxj1 specifies node-like cilia in Xenopus and zebrafish embryos. Nat. Genet. 2008;40:1454–1460. doi: 10.1038/ng.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan-Brown J., Schottenfeld J., Okabe N., Hostetter C.L., Serluca F.C., Thiberge S.Y., Burdine R.D. Zebrafish mutations affecting cilia motility share similar cystic phenotypes and suggest a mechanism of cyst formation that differs from pkd2 morphants. Dev. Biol. 2008;314:261–275. doi: 10.1016/j.ydbio.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C.J., Vogan K.J. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- Takao D., Nemoto T., Abe T., Kiyonari H., Kajiura-Kobayashi H., Shiratori H., Nonaka S. Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left–right axis formation. Dev. Biol. 2013;376:23–30. doi: 10.1016/j.ydbio.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Thastrup O., Cullen P.J., Drøbak B.K., Hanley M.R., Dawson A.P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc. Natl. Acad. Sci. USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisler M., Wetzel F., Mantino S., Kremnyov S., Thumberger T., Schweickert A., Blum M., Vick P. Cilia are required for asymmetric nodal induction in the sea urchin embryo. BMC Dev. Biol. 2016;16:28. doi: 10.1186/s12861-016-0128-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran U., Zakin L., Schweickert A., Agrawal R., Doger R., Blum M., De Robertis E.M., Wessely O. The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development. 2010;137:1107–1116. doi: 10.1242/dev.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick P., Schweickert A., Weber T., Eberhardt M., Mencl S., Shcherbakov D., Beyer T., Blum M. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev. Biol. 2009;331:281–291. doi: 10.1016/j.ydbio.2009.05.547. [DOI] [PubMed] [Google Scholar]
- Walentek P., Schneider I., Schweickert A., Blum M. Wnt11b is involved in cilia-mediated symmetry breakage during Xenopus left-right development. PLoS One. 2013;8:e73646–e73649. doi: 10.1371/journal.pone.0073646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford J.B., Ewald A.J., Harland R.M., Fraser S.E. Calcium signaling during convergent extension in Xenopus. Curr. Biol. 2001;11:652–661. doi: 10.1016/s0960-9822(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T.P., Harpal K., Henkemeyer M., Rossant J. fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- Yokota C., Kofron M., Zuck M., Houston D.W., Isaacs H., Asashima M., Wylie C.C., Heasman J. A novel role for a nodal-related protein; Xnr3 regulates convergent extension movements via the FGF receptor. Development. 2003;130:2199–2212. doi: 10.1242/dev.00434. [DOI] [PubMed] [Google Scholar]
- Yoshiba S., Hamada H. Roles of cilia, fluid flow, and Ca2+ signaling in breaking of left-right symmetry. Trends Genet. 2014;30:10–17. doi: 10.1016/j.tig.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Yoshiba S., Shiratori H., Kuo I.Y., Kawasumi A., Shinohara K., Nonaka S., Asai Y., Sasaki G., Belo J.A., Sasaki H. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science. 2012;338:226–231. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S., Zhao L., Brueckner M., Sun Z. Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr. Biol. 2015;25:556–567. doi: 10.1016/j.cub.2014.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.