Supplemental Digital Content is available in the text.
Key Words: consumer engagement, underserved populations, primary health care, patient-centered outcomes research
Abstract
Background:
Strategies to engage patients to improve and enhance research and clinical care are increasingly being implemented in the United States, yet little is known about best practices for or the impacts of meaningful patient engagement.
Objective:
We describe and reflect on our patient stakeholder groups, engagement framework, experiences, and lessons learned in engaging patients in research, from generating proposal ideas to disseminating findings.
Setting:
The ADVANCE (Accelerating Data Value Across a National Community Health Center Network) clinical data research network is the nation’s largest clinical dataset on the safety net, with outpatient clinical data from 122 health systems (1109 clinics) in 23 states.
Results:
Patients stakeholders codeveloped the ADVANCE engagement framework and its implementation in partnership with network leaders. In phase I of ADVANCE, patients were involved with designing studies (input on primary outcome measures and methods) and usability testing (of the patient portal). In phase II, the network is prioritizing research training, dissemination opportunities, an “ambassador” program to pair more experienced patient stakeholders with those less experienced, and evaluation of engagement activities and impacts.
Discussion:
The ADVANCE framework for patient engagement has successfully involved a diverse group of patients in the design, implementation, and interpretation of comparative effectiveness research. Our experience and framework can be used by other organizations and research networks to support patient engagement activities.
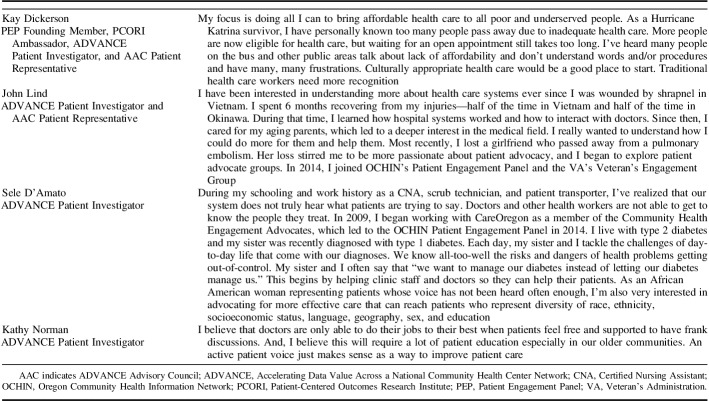
“We are all patients—you have the whole community to draw from for your patient advisors. Look to the people who you respect. Reach out to those who inspire you personally to find individuals willing to meaningfully give back from their life experience, because these patients have important opinions on this work. Involve community leaders, scientists, nurses, doctors, clinic managers, educators. Ask for their recommendations. The individuals you find will be a joy to work with and will drive great improvements. Traditional medicine will only recognize breakthroughs after they have been thoroughly studied and documented, and I chafe at the pain and loss caused by that delay. Having a small voice in the world of medical research enables me to participate where benefit will eventually be brought to the greatest number of people.” (ADVANCE Patient Investigator Lynn Robbins)
INTRODUCTION
As consumers of health care and stakeholders in clinical research, the importance of patients’ “real-world” insights has led to a burgeoning movement to engage patients in health care decision-making, quality improvement initiatives and more recently, research. The body of literature speaks to a need to clearly define the scope of “meaningful patient engagement” and how it can be effectively implemented. Limited research exists around identifying and recruiting patients, equitable compensation processes and best practices in providing research guidance.1–8 Furthermore, knowledge gaps remain on building an effective engagement infrastructure to address power differentials for patients joining research teams, as well as providing and testing engagement tools, role descriptions, and resources to aid in this revisioning of patient-centered outcomes research (PCOR).9,10
Founded in 2001, Oregon Community Health Information Network (OCHIN) is a nonprofit, community-based health information technology collaborative that serves 97 health systems (Federally Qualified Health Centers, community health centers [CHCs], critical access hospitals, and rural hospitals) in 18 states across the nation, linking 618 clinics with 4927 providers serving >2.2 million patients11–15 in 1 common electronic health record. OCHIN leads the Accelerating Data Value Across a National Community Health Center Network (ADVANCE) clinical data research network (CDRN) in partnership with Health Choice Network and Fenway Health. The network reaches 23 states and is the largest clinical dataset on the safety net in the nation. It has a truly national footprint, and engages patients, clinicians, health systems, and researchers.
CHCs, which comprise the majority of the ADVANCE network, have a long history of engaging patients on their community-based governing boards.16 Before ADVANCE, OCHIN researchers had developed mechanisms (eg, community retreats, focus groups, patient interviews, and project advisory councils) for engaging patients and communities for specific research projects. However, OCHIN had not explicitly structured research governance in a way that provided ongoing structure for direct patient engagement across projects and organizational services.17 Through ADVANCE, our network is able to build on the CHC tradition of patient engagement in a new era of PCOR.
Driven by a goal to fundamentally change the health research landscape, the Patient-Centered Outcomes Research Institute (PCORI) funded several research networks, like ADVANCE, to enable big data comparative effectiveness research that is driven by patient priorities.18–20 In this paper, we describe and reflect on our patient stakeholder groups, our engagement framework and our experience in engaging patients and other stakeholders in research, from generating proposal ideas to disseminating findings.
DESCRIPTION OF ENGAGEMENT GROUPS AND ACTIVITIES
The ADVANCE network began phase I in 2014 through an award from PCORI as a partner in their development of PCORnet: The National Patient-Centered Clinical Research Network. ADVANCE specifically contributes to this network as it brings vulnerable and diverse Federally Qualified Health Center patient populations to PCORnet’s PCOR. PCORI defines PCORnet as “a large, highly representative, national ‘network of networks’ that collects data routinely gathered in a variety of health care settings, including hospitals, doctors’ offices, and community clinics. By engaging a variety of stakeholders—patients, families, providers, and researchers—PCORnet empowers individuals and organizations to use these data to answer practical questions that help patients, clinicians, and other stakeholders make informed health care decisions.”21
Phase II of ADVANCE through PCORnet seeks to expand on its phase I goals of expanding our stakeholder engagement and community-academic partnerships; integrating outpatient, hospital, and community-level data into a single data management system; and build on our “community laboratory” of Federally Qualified Health Centers jointly created by our patient, clinician, and health system leader stakeholders. Our phase II project summary describes this effort as such: “The mission of the ADVANCE CDRN—a team of organizations from around the country—is to learn about how to improve the health of safety net patients, including people living in poverty, with little or no insurance … In the past, vulnerable patient populations have not been included in many studies. As a network of safety net clinics, ADVANCE is able to help bridge this gap. Patients, caregivers, and clinicians are helping guide the ADVANCE research plan, develop study questions and materials, and share findings. By taking part in ADVANCE projects, people have an opportunity to improve care for themselves, their families, and their communities.”22
The ADVANCE CDRN engages patients and other stakeholders through 4 groups: the OCHIN Patient Engagement Panel (PEP), the Clinic and Patient Engagement Workgroup (CAPE), the Community Research Outreach and Dissemination Program (CROP-D) and finally, the ADVANCE Advisory Council (AAC), which oversees all research conducted using the ADVANCE network, includes members from each of the ADVANCE data partners as well as patient (2) and clinician (1) stakeholders. ADVANCE data partners include the following organizations: OCHIN, Legacy Health, Health Choice Network, Kaiser Permanente Northwest Center for Health Research, Fenway Health, CareOregon Medicaid Managed Care Plan, Oregon Health and Science University, and the Robert Graham Center.22
OCHIN PEP
Building on these early efforts, OCHIN established a PEP to include patient voices and perspectives. Appendix 1, supplemental Digital Content 1, http://links.lww.com/MLR/B456, describes the timeline of the PEP as part of the development of the OCHIN Practice-based Research Network (PBRN) and ADVANCE research network. The details of the steps we took to establish the PEP and our lessons learned are described in a prior publication.23 Our founding PEP members codeveloped the selection process of additional members, compensation policy, recruitment methodologies, and health literacy review to materials. During ADVANCE phase I, the work of PEP was generally expanded to support research capacity building and the ADVANCE engagement framework was developed (see Fig. 1).
FIGURE 1.

ADVANCE Engagement Rubric. ADVANCE indicates Accelerating Data Value Across a National Community Health Center Network.
Currently, there are 18 active patient advisors on the PEP, representing diverse backgrounds and lived experiences across multiple states, urban/rural locations, races/ethnicities, ages, sex, and sexual orientations. Patient advisors provide ongoing guidance to improve research recruitment, retention, and transparency. Through ADVANCE, PEP members have increased their involvement as coinvestigators directly advising on prepared and/or submitted proposals (from 3 to 9 members advising on >22 projects). One member also successfully received 2 PCORI Pipeline to Proposals Tier One and Tier Two awards and has been building a separate patient-led collaborative research team, which now is poised to begin developing the team’s first comparative effectiveness research funding proposal(s).24
CAPE Workgroup
In June 2014 of ADVANCE phase I, the CAPE workgroup was created. The CAPE is a tactical group for identified operational, clinical, and informatics staff from ADVANCE organizations (eg, OCHIN; Health Choice Network; Fenway Health; Legacy; OHSU), OCHIN health systems clinical oversight, research team partners, and select patients from the PEP. The workgroup provides feedback on an ongoing basis and learn about the challenges and barriers around increasing patient portal adoption rates, enhancing patient engagement (via research projects, patient outreach strategies, and additional tools), building shared communication tools, and sharing best practices and success. Patient, clinician and health system leader advisors provide ongoing guidance to overcome barriers in expanding patient portal adoption and use, such as colearnings around health literacy, technology access in the safety net, Spanish-language resources, patient and clinic-facing instructional guides, and patient portal workflow development.
CROP-D
ADVANCE network infrastructure funding has allowed OCHIN to build the CROP-D as part of its operations model to support and extend its engagement work with stakeholders. Established toward the end of ADVANCE phase I, the CROP-D is tasked with: coordinating stakeholder engagement workgroups; developing protocols and tools to support member recruitment and participation in research projects; collecting data on member organizations’ research priorities and involvement; and disseminating research activities. The CROP-D is staffed from a variety of OCHIN departments and roles, including an Engagement Coordinator, a Site Principal Investigator, a Practice Facilitator, a Research Associate and an Account Manager.
PBRN
THE ADVANCE PBRN, established in 2007, is housed at OCHIN to maximize its independence and focus on community-based research. Members include network clinicians and operational staff, patient advisors, and research leadership to provide evaluation and assessment for our research endeavors. Members review proposals, share models and methodologies, and disseminate findings.
AAC
Early in ADVANCE phase I, the AAC was created to review and approve ADVANCE-specific policies, serve as the review board for any potential proposal, project, or data request utilizing ADVANCE, and develop and prioritize the ADVANCE research agenda. This model of shared decision-making is similar to that of the OCHIN and Health Choice Network community Boards of Directors. To ensure that ADVANCE is well integrated and attached to OCHIN’s formal governance structure, the Advisory Council is integrated directly into the organizational structure of the OCHIN Board of Directors, as encouraged by OCHIN’s bylaws for all projects of significance to OCHIN’s members. The Advisory Council includes representatives from each of the ADVANCE data partners (further described above), as well as patient and clinician representatives.
ADVANCE Engagement Framework
ADVANCE currently uses a 3-level framework to guide and prioritize our strategies for engagement, jointly developed from our early PEP work and our patient, clinician and health system leader stakeholders (Fig. 1). We actively recruit patients as investigators to advise and inform all aspects of ADVANCE (engagement level 1). We expanded the PEP, created a CAPE workgroup, and recruited patient representatives for the AAC and PBRN (engagement level 2). To further increase our ability to reach, inform, and engage more patients (engagement level 3), patient advisors offer ongoing guidance for the development and implementation of enhanced electronic tools for patients (eg, patient portals, websites, social media). Each of these groups serves an important purpose in bringing the voices of our key patient stakeholders into institutional strategies and leadership structures, and OCHIN has a dedicated budget for patient engagement activities.
IMPACT OF ENGAGEMENT
Through the initial creation of our engagement groups and framework described above through ADVANCE phase I, we developed the structure in which to involve a diverse group of patients in the design, implementation, and interpretation of comparative effectiveness research. As we were awarded phase II funding, we were able to build further on our stakeholder-identified engagement priorities of training, dissemination, and our ambassador program.
PHASE I ENGAGEMENT
Designing Studies
In the PEP and CAPE through level 2 of our engagement framework, patient investigators reviewed and edited insurance support tools from a patient perspective, engaging in 4 project phases: proposal development; adapting study methods; understanding the context and tool testing; and implementation.25 PEP guidance has resulted in a change of a proposal’s primary outcome measure following stakeholder investigator calls and proposal reviews, as well as guidance from presentation at the PEP. ADVANCE patient investigator guidance (through level 1) resulted in changes to proposal methodology, such as the addition of domains and enhanced frequency of patient-reported outcome (PRO) measures to include pretreatment and posttreatment. In addition, through this same study, patient investigator guidance on the level of burden that patients with the condition of study, shared by the patient investigator, might be willing to accept (from a 5 to a 40-min PRO screening) supported a confirmation of retaining the initial length of the screening. This was augmented by the patient investigator stating that “those with a hard-to-treat disease would be more than willing to spend the extra time if they knew they were potentially contributing to better cures.”
Usability Testing
Patient advisors codeveloped ADVANCE engagement level 3 activities. These included a clinical health survey (also assessing research interest) conducted across OCHIN clinics via patient portal; survey data included over 13,280 patient respondents, a response rate of 20% of all active patients (signed in at least once) on the portal. Patient advisors conducted rigorous usability testing, resulting in recommendations to make the survey more visible and its purpose more relevant for patients. Patient advisors have also guided layout and workflows for new PRO tools such as Screening, Brief Intervention, Referral to Treatment (SBIRT),26 Patient Health Questionnaire (PHQ),27 and the Patient-reported Outcomes Measurement Information System (PROMIS).28 These advisors are contributing to the development of best practices to increase patient portal adoption and other patient engagement communication strategies (eg, text messaging, social media). For example, to capture valid patient responses, a patient investigator advised on ADVANCE and the SBIRT tool within the patient portal. This specific guidance resulted in the removal of the tool’s scoring layout from the patient portal view and created a new best practice.
PHASE II ENGAGEMENT
On the basis of the feedback from ADVANCE’s community-academic partnerships and a proposal developed and approved by PEP members, the AAC-approved engagement priorities are as follows: training, ambassador program, dissemination, and evaluation. These priorities are shaping ADVANCE engagement activities throughout phase II.
Training
This priority includes training resources for all stakeholders. In response, ADVANCE has purchased the CITI Program Training for Responsible Conduct of Research29 for utilization by all stakeholder groups. The CROP-D is currently working with the PEP to facilitate their completion of the CITI training module and certification. Through an environmental scan of the available research trainings recommended from PCORI, our stakeholders and research staff, we assessed several options and determined that CITI training most closely aligned with our gaps in understanding, as well as our requirements for lnstitutional Review Board and human subject’s protection processes. PEP patient leaders are also codeveloping training resources) in partnership with OCHIN’s training subject matter experts and partner PCORnet CDRNs to be provided via web (websites, videos) and in person.
Ambassador Program
Our PEP and PBRN engagement structures are expanding their outreach through utilization of an ambassador program. Working with the Oregon Clinical and Translational Research Institute (OCTRI) and its framework for advocacy within community advisory boards, we have begun to recruit more PEP/PBRN members and coinvestigators and broadened our outreach to patients in the larger communities in which OCHIN member sites serve. We recently used this peer-to-peer program to engage a non-ADVANCE affiliated patient with a specific opioid-treatment background into an ADVANCE research proposal as a new patient coinvestigator. In this case, an established ADVANCE patient investigator co-led the successful recruitment effort for this role in collaboration with the network’s engagement coordinator.
This veteran patient investigator provided initial outreach for an introductory phone call between the prospective patient investigator and the research proposal’s principal investigator and team and continued support to build rapport and trust. In addition, through the veteran patient investigator’s prior experiences around development of proposal materials (letter of support, biosketch), they were able to provide guidance in the new patient investigator’s completion of these items. Although the proposal was unfortunately not funded, the inclusion of this new patient investigator, who shared personal experiences living with this condition, provided valuable guidance on the proposal’s methodology and recruitment strategies, and powerfully strengthened the significance section of the final submission.
Dissemination
Within the engagement priority of enhanced dissemination, the ADVANCE team focused on efficient and effective ways to widely disseminate PBRN research findings. This is seen by our network stakeholders as a critical component for accelerating the translation of research into practice. Social media strategies, including podcasts and blogging, have the potential to augment the reach of research beyond traditional publication venues.30 The founding patient member of the PEP was a featured patient ambassador author on the PCORI national blog, where she described the influence that PCORI has had on the work of ADVANCE and her perspectives on clinical research. In the post, the patient member and caregiver talks about how she got involved in her local health system, then state policy, and most recently ADVANCE as a part of PCORnet.31 Although development continues in these expanded dissemination efforts, we learned that they require thoughtful efforts—finding stories for blogs and podcasts that complement scholarly publications and using these social media posts to communicate findings about recently published papers to broad community and patient audiences.
Patient coauthors have advised, guided, and been published on accepted manuscripts, including key reviews and contributions from PEP member coauthors with lived experience of the condition of study.32 Patient investigators have presented at national and international conferences and coauthored research proposals and manuscripts.33,34 ADVANCE patient and clinician leaders recently acted as copresenters in the 2016 OCHIN Learning Forum (a national conference), with 5 PEP members and 2 PBRN steering committee members supporting 5 panels covering the impacts of the Affordable Care Act, stakeholder engagement, patient portal adoption, and enhancement and the OpenNotes movement, which provides patient access to visit notes through their patient portal.35
Evaluation
The ADVANCE 3-level engagement framework, developed with key input from many different stakeholders, serves as a guide for evaluating our patient engagement approaches. The network has been developing formal measures of engagement to track our activities and their impacts. Through an iterative process with the CROP-D and OCHIN research leadership, a number of process and board-reported measures were determined to evaluate our progress with engagement efforts. These measures include: the number of OCHIN member organizations actively involved in research projects, tracking the involvement of stakeholder workgroups in research activities (eg, the number of proposals and projects reviewed in partnership with stakeholder groups, the number of clinician and patient investigators named on research proposals and projects), and the centralization of tools and protocols for clinic and patient recruitment. In addition, the CROP-D is responsible for reaching the engagement-related funder milestones for the ADVANCE network.
Our ADVANCE team and stakeholders continually look to refine our metrics on what constitutes “successful engagement” and how we approach this process differently based on the audience. Our stakeholder (eg, patient, clinician, health system leader) engagement is evaluated within our ADVANCE team through both process measures (eg, attendance; number of coinvestigators) as well as outcome measures, such as documenting patient testimonials on the value of research engagement (Table 1), tracking patient guidance provided to aspects of network activities, and in what capacity feedback has impacted projects throughout the research lifecycle.
TABLE 1.
ADVANCE Patient Investigator Testimonials
For the development of outcome measures, patient advisors are asked to complete quarterly surveys soliciting the value of research engagement, identify any barriers and make recommendations to enhance their roles within the ADVANCE network. The development of these metrics are influenced through the PCORI Engagement Rubric, which provides key examples of “successful engagement” activities through each step of the research lifecycle.36
DISCUSSION
ADVANCE has successfully involved a diverse group of patients in the design, implementation, and interpretation of our research, making progress in 3 levels of engagement. As noted by our patient investigators, we have seen the importance of continuing to expand and improve on this work. Our PEP founding member and coauthor Kay Dickerson states, “My focus is doing all I can to bring affordable health care to all poor and underserved people. As a Hurricane Katrina survivor, I have personally known too many people pass away due to inadequate health care.” ADVANCE patient investigator and coauthor Sele D’Amato further illustrates the need for this work, stating, “As an African American woman representing patients whose voice has not been heard often enough, I’m also very interested in advocating for more effective care that can reach patients who represent diversity of race, ethnicity, socioeconomic status, language, geography, sex, and education.”
Through our phase II priorities, we further expanded our engagement of stakeholders through our ambassador model, building on the diversity of the network’s patient advisor base. With enhanced training resources and research partner mentorship, we have able to build the skills, experience, and expertise of our patient investigators, advisors and patient members of our PEP, CAPE, and other engagement groups. Our codeveloped engagement polices around compensation, patient investigator roles, and PEP member responsibilities bring an enhanced level of respect to these positions and also streamline our network’s ability to extend these roles to project-specific engagement opportunities.
Future Work
Using our 3 engagement priorities of training, the ambassador program and dissemination, future directions in meaningful patient engagement for our network will include the development of mechanisms to engage more patients with minimal burden (eg, brief online surveys), shared strategies to enhance working across a national collaborative, more consistent, relevant, and ongoing training for patient coinvestigators, and expanding toward more comprehensive and active collection of PROs via patient portals and other bidirectional communication methods to provide “real-time” support for clinical care. These additional efforts may further increase patient engagement in research that impacts health care delivery and health outcomes.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.lww-medicalcare.com.
Footnotes
Supported by the National Institutes of Health National Library of Medicine (grant no. RC4 1001482); the Patient-Centered Outcomes Research Institute (PCORI), PFA Cycle I Contract (2012), Health Systems; the Health Resources and Services Administration (grant no. UB2HA20235); and the Oregon Health & Science University Department of Family Medicine. This work was also supported through a PCORI Award (CDRN-1306–04716) for development of the National Patient-Centered Clinical Research Network, known as PCORnet. All phases of this study were also supported by PCORI Award (308). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of the PCORI, its Board of Governors or Methodology Committee or other participants in PCORnet.
The authors declare no conflict of interest.
REFERENCES
- 1.Westfall JM, VanVorst RF, Main DS, et al. Community-based participatory research in practice-based research networks. Ann Fam Med. 2006;4:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boote JD, Twiddy M, Baird W, et al. Supporting public involvement in research design and grant development: a case study of a public involvement award scheme managed by a National Institute for Health Research (NIHR) Research Design Service (RDS). Health Expect. 2015;18:1481–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams R, Shelley B, Sussman A. The marriage of community-based participatory research and practice-based research networks: can it work?—a Research Involving Outpatient Settings Network (RIOS Net) study. J Am Board Fam Med. 2009;22:428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domecq JP, Prutsky G, Elraiyah T, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullins CD, Abdulhalim AM, Lavallee DC. Continuous patient engagement in comparative effectiveness research. JAMA. 2012;307:1587–1588. [DOI] [PubMed] [Google Scholar]
- 6.Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect. 2015;18:1151–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank L, Forsythe L, Ellis L, et al. Conceptual and practical foundations of patient engagement in research at the patient-centered outcomes research institute. Qual Life Res. 2015;24:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA. 2012;307:1583–1584. [DOI] [PubMed] [Google Scholar]
- 9.Marlett N, Shklarov S, Marshall D, et al. Building new roles and relationships in research: a model of patient engagement research. Qual Life Res. 2015;24:1057–1067. [DOI] [PubMed] [Google Scholar]
- 10.Duncan J. Building infrastructure to engage patients, families and members in research within a learning health organization. J Patient Cent Res. 2015;2:133–134. [Google Scholar]
- 11.Devoe JE, Sears A. The OCHIN community information network: bringing together community health centers, information technology, and data to support a patient-centered medical village. J Am Board Fam Med. 2013;26:271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVoe JE, Gold R, Spofford M, et al. Developing a network of community health centers with a common electronic health record: description of the Safety Net West Practice-based Research Network (SNW-PBRN). J Am Board Fam Med. 2011;24:597–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVoe JE, Likumahuwa S, Eiff MP, et al. Lessons learned and challenges ahead: report from the OCHIN Safety Net West practice-based research network (PBRN). J Am Board Fam Med. 2012;25:560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krist AH, Green LA, Phillips RL, et al. Health information technology needs help from primary care researchers. J Am Board Fam Med. 2015;28:306–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeVoe JE, Gold R, Cottrell E, et al. The ADVANCE network: accelerating data value across a national community health center network. J Am Med Inform Assoc. 2014;21:591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright B, Martin GP. Mission, margin, and the role of consumer governance in decision-making at community health centers. J Health Care Poor Underserved. 2014;25:930–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angier H, Wiggins N, Gregg J, et al. Increasing the relevance of research to underserved communities: lessons learned from a retreat to engage community health workers with researchers. J Health Care Poor Underserved. 2013;24:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selby J, Beal A, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. J Am Med Assoc. 2012;307:1583–1584. [DOI] [PubMed] [Google Scholar]
- 19.Fleurence R, Curtis L, Platt R, et al. Launching PCORnet, a national patient-centered clinical research network. J Am Med Inform Assoc. 2014;21:578–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleurence R, Beal A, Sheridan S, et al. Patient-Powered Research Networks aim to improve patient care and health research. Health Aff. 2014;33:1212–1219. [DOI] [PubMed] [Google Scholar]
- 21.PCORnet. 2017. Available at: www.pcornet.org. Accessed June 5, 2017.
- 22.Accelerating data value across a National Community Health Center Network (ADVANCE). 2017. Available at: www.pcori.org/research-results/2015/accelerating-data-value-across-national-community-health-center-network. Accessed June 6, 2017.
- 23.Arkind J, Likumahuwa-Ackman S, Warren N, et al. Lessons learned from developing a patient engagement panel: an OCHIN report. J Am Board Fam Med. 2015;28:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henson-Apollonio V. Setting the stage for patient involvement: connections patients with periodontal disease—tier II. 2015. Available at: www.pcori.org/research-results/2016/setting-stage-patient-involvement-connecting-patients-periodontal-disease-tier. Accessed May 17, 2017.
- 25.Yamauchi M, Carlson MJ, Wright BJ, et al. Does health insurance continuity among low-income adults impact their children’s insurance coverage? Matern Child Health J. 2013;17:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Screening, Brief Intervention, Referral to Treatment (SBIRT). 2017. Available at: www.sbirtoregon.org. Accessed June 29, 2017.
- 27.Welcome to the Patient Health Questionnaire (PHQ) screeners. 2017. Available at: www.phqscreeners.com. Accessed June 29, 2017.
- 28.Patient Reported Outcomes Measurement Information System (PROMIS). 2017. Available at: www.phqscreeners.com. Accessed June 29, 2017.
- 29.CITI Program. 2016. Available at: www.citiprogram.org. Accessed May 10, 2016.
- 30.Hoang JK, McCall J, Dixon AF, et al. Using social media to share your radiology research: how effective is a blog post? J Am Coll Radiol. 2015;12:760–765. [DOI] [PubMed] [Google Scholar]
- 31.Dickerson K. Guiding research and policy to improve healthcare for everyone. 2014. Available at: www.pcori.org/blog/guiding-research-and-policy-improve-healthcare-everyone. Accessed May 9, 2016.
- 32.Nichols GA, McBurnie M, Paul L, et al. The high prevalence of diabetes in a large cohort of patients drawn from safety net clinics. Prev Chronic Dis. 2016;13:160056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeVoe J, Angier H, Likumahuwa S, et al. Use of qualitative methods and user-centered design to develop customized health information technology tools within federally qualified health centers to keep children insured. J Ambul Care Manage. 2014;37:148–154. [DOI] [PubMed] [Google Scholar]
- 34.Angier H, Marino M, Sumic A, et al. Innovative methods for parents and clinics to create tools for kids’ care (IMPACCT Kids’ Care) study protocol. Contemp Clin Trials. 2015;44:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leveille SG, Walker J, Ralston JD, et al. Evaluating the impact of patients’ online access to doctors’ visit notes: designing and executing the OpenNotes project. BMC Med Inform Decis Mak. 2012;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.PCORI Engagement Rubric. 2016. Available at: www.pcori.org/sites/default/files/Engagement-Rubric.pdf. Accessed October 25, 2016.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Website, www.lww-medicalcare.com.