Abstract
Introduction:
HIV-Tuberculosis (TB) co-infection remains an important cause of mortality in sub-Saharan Africa. Clinical trials have reported early (within 2 weeks of TB therapy) antiretroviral therapy (ART) reduces mortality among HIV-TB co-infected research participants with low CD4 counts, but this has not been consistently observed. We aimed to evaluate the current World Health Organization (WHO) recommendations for ART in HIV-TB co-infected patients on mortality in routine clinical settings.
Methods:
We compared two cohorts before (2008–2010) and after (2012–2013) policy change on ART timing after TB and examined the effectiveness of early versus delayed ART on mortality in HIV-TB co-infected participants with CD4 ≤100 cells/µL. We used inverse probability censoring-weighted Cox models on baseline characteristics to balance the study arms and generated hazard ratios for mortality.
Results:
Of 356 participants with CD4 counts ≤100 cells/µL, 180 were in the delayed while 176 were in the early ART cohorts. Their median age (32.5 years vs 32 years) and baseline CD4 counts (26.5 cells/µL vs 26 cells/µL) respectively were similar. There was no difference in mortality rates of both cohorts. The risk of death increased in participants with a positive Cryptococcal antigen (CrAg) test in both the early ART cohort (aHR = 2.6, 95%CI, 1.0 –6.8; P=0.045) and the delayed ART cohort (aHR = 4.2, 95%CI, 1.9–9.0; P<0.001
Conclusions:
Early ART in patients with HIV-TB co-infection was not associated with reduced risk of mortality in routine care. Asymptomatic Cryptococcal antigenemia increased the risk of mortality in both cohorts.
Keywords: Adult, HIV, Tuberculosis, Antiretroviral Therapy, Mortality
Introduction
HIV-Tuberculosis (TB) co-infection remains a leading cause of mortality in sub-Saharan Africa despite increasing availability of antiretroviral therapy (ART) [1]. Randomized trials have [2] reported a reduction in mortality or incidence of AIDS-defining illness among HIV-TB co-infected research participants with low CD4 counts who start ART within two weeks of TB treatment initiation [3-5]. As a result the World Health Organization (WHO) guidelines have been revised[6] to recommend early ART, particularly in HIV-TB co-infected patients with CD4 counts <50 cells/µL [2, 7, 8]. A few observational studies, most of which are retrospective, demonstrate this beneficial effect of ART on outcomes of HIV-TB co-infected patients with low CD4 counts [9-12] while other randomized studies have shown limited or no benefit of early versus delayed ART initiation [13-15]. Additional observational studies are important since concurrent HIV-TB treatment is associated with a high risk of complications like tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS), drug toxicities, and unmasking of other opportunistic infections, which may further influence clinical outcomes. Some of these complications may justify delayed start of ART in selected HIV-TB co-infected patients who are at high risk of poor outcomes.
Most randomized studies have compared mortality outcomes of two distinct HIV-TB co-infected populations starting ART early (<2 weeks) or late (>8 weeks) after initiation of TB treatment. Few studies have reported the outcomes for patients who start ART between 2 and 8 weeks after the start of TB treatment [3-5].
We aimed to evaluate the effect of starting ART early (2 weeks ± 1 week) versus starting ART late (≥3 weeks) after starting TB treatment on mortality outcomes of HIV-TB co-infected participants.
Methods
We examined the effect of early versus delayed ART on mortality from two prospective cohort studies in a high burden HIV-TB setting before [16, 17] and after the WHO guideline change on timing of ART initiation. The delayed ART initiation cohort run from January 7, 2008 to July 30, 2010 at the Mulago TB Treatment Centre while the early ART initiation cohort involved participants attending the HIV-TB clinic of Infectious Disease Institute within the same Mulago Hospital Complex from January 13, 2012 to December 17, 2013. The studies were conducted by the same clinical team that used similar case definitions for TB diagnosis and for classification of study outcomes.
Participant enrollment and study procedures
Study participants were enrolled from the Mulago HIV-TB clinic and the Infectious Disease Institute, HIV-TB clinic in Kampala, Uganda. The inclusion criteria for enrollment were: HIV-TB co-infected, ART-naïve adults who were 18 years or older with CD4 counts ≤100 cells/µL and were willing to participate in the study. Participants who had elevated liver transaminases greater than five times or were unable to return for study visits were excluded from the study.
History and physical examination were done for all participants who consented to participate in the study. The age (years), sex, weight, and height were determined and body mass index calculated (kg/m2). Investigations to confirm the diagnosis of TB and HIV were done. All participants had sputum examination for acid fast bacilli (AFB), a chest X-ray, and abdominal ultrasound scan performed. TB was diagnosed as bacteriologically positive if they were AFB sputum smear positive, had a positive Lowenstein-Jensen culture (or Mycobacterium Growth Inhibition Tube (MGIT) technique in the Early ART cohort, or M. tuberculosis detected on Xpert MTB/RIF (Cepheid, Sunnyvale, CA); the Xpert MTB/RIF technology was available only at the time of the 2012–2013 cohort. TB was confirmed by a positive result on microscopy, Xpert or culture; and extrapulmonary TB was diagnosed according to criteria recommended by WHO [18]. A diagnosis of Kaposi’s sarcoma was made on histological examination of biopsies of suspected skin lesions. Cryptococcal meningitis was diagnosed during hospitalization in patients who had suggestive clinical symptoms and had lumbar punctures and cerebral spinal fluid positive for Cryptococcus.
Hemoglobin level (g/dL) was determined at baseline (ART start) and the participants were classified according to WHO clinical staging. Serum cryptococcal antigen (CrAg) test was also performed at enrollment among asymptomatic participants. CrAg testing was by latex agglutination in 2008–2010, and by lateral flow assay (Immy Inc., Norman, Oklahoma) in 2012–2013. Participants with a positive serum CrAg were treated with fluconazole (400mg once daily in the delayed ART cohort and 800mg in the early ART cohort – consistent with revised WHO guidelines for pre-emptive treatment for HIV persons with a positive serum CrAg) [19]
TB treatment was provided using standard treatment regimens recommended by the National Tuberculosis Programme which included ethambutol (E), Isoniazid (H), rifampicin (R) and pyrazinamide (Z) for the intensive phase of two months followed by either four months of RH or six months of EH.
Antiretroviral therapy:
In the delayed ART cohort 2008–2010, ART was started at ≥3weeks after starting TB treatment while in the early ART cohort 2012–2013, ART was started 2 weeks ± 1 weeks after starting TB treatment in participants with CD4 counts <100 cells/µL. In both periods ART in TB patients consisted of triple therapy: a backbone of two nucleoside reverse transcriptase inhibitors (zidovudine or stavudine with lamivudine) with a non-nucleoside reverse transcriptase inhibitor (efavirenz or nevirapine).
Follow up:
Participants’ were followed up at week 2, months 1, 3, 6 following ART initiation; and at TB treatment completion. Participants were encouraged to come for a sick (unscheduled) clinic visit if they developed any worsening of symptoms during follow up. The participants were evaluated during follow up for any treatment-related complications like drug toxicities, new opportunistic diseases or TB-IRIS. Participants who did not return for a follow up visit, were traced using locator information and contacts they provided to establish their vital status.
Study outcomes:
The main study outcome was mortality during follow up. Mortality data were either obtained from hospital or postmortem records if participants were hospitalized prior to death or from the relatives or close associates who were identified as the next of kin. Participants were censored when they left the study or when they completed TB therapy. Participants that developed TB-IRIS were evaluated with a standard questionnaire and investigations for a final diagnosis of definite/probable TB-IRIS or not TB-IRIS; adjudication was made by clinician consensus based on the above findings. Participants who developed cryptococcal meningitis were hospitalized and treated for two weeks with daily amphotericin infusion (1mg/kg) followed by fluconazole maintenance therapy.
Statistical Analysis
Delayed ART initiation was defined as ART initiation ≥3 weeks after TB treatment initiation, and early ART initiation was defined as ART initiation within 2 weeks (±1 week) of TB treatment initiation.
The primary analysis evaluated the effect of early versus delayed ART initiation on mortality comparing survival time from the date of starting TB treatment to either death, loss to follow-up or administrative censoring at the end of TB therapy. Since survival time was calculated from TB treatment initiation, deaths occurring before ART initiation were attributed to the respective ART initiation strategy for complete measure of the total mortality that would accrue from the respective ART initiation strategy.
Secondary analyses compared the effect of early versus delayed ART initiation on mortality in sub-populations of participants with baseline CD4 counts <50 cells/µL and 50–100 cells/µL, adjusted for age, sex, hemoglobin level, TB category (extrapulmonary or not), and serum CrAg status. We also compared mortality among patients with positive and negative CrAg tests.
In sensitivity analysis, we evaluated the effect of potential contamination between the two study arms by allowing a short wash-out effect (from day 15 to day 21) to preserve more statistical power in the study or a longer wash-out effect (from day 15 to day 49) comparable to the randomised clinical trials [4, 5, 13], by restricting the Early ART cohort to ≤2 weeks of ART initiation; and by restricting the Delayed ART cohort to >3 weeks and >7 weeks respectively.
In order to allow for precise estimation of the effect of timing of ART initiation on the mortality of the TB-HIV participants, we used inverse-probability censoring-weighted Cox models with robust sandwich variance estimates to balance the study population in the delayed and early arm for baseline characteristics, probability of censoring and survival time.
Ethics:
All participants provided informed consent and the study was approved by the School of Medicine, College of Medical Sciences and by the Uganda National Council of Science and Technology (Ref. HS 1068).
Results
Study Participants
Of 387 participants with HIV-TB co-infection, who initiated TB medication with baseline CD4 counts ≤100 cells/µL, 201 were in the Delayed ART cohort and 186 in the Early ART cohort. In the Delayed ART cohort, 21 participants were excluded from the analysis, including 6 missing baseline CrAg results and 15 initiated on ART within 3 weeks of starting TB medication, while in the Early ART cohort, 10 were excluded from the analysis, including, 2 participants missing the hemoglobin results, 2 missing their baseline CrAg results and 6 initiated on ART after 3 weeks of starting TB medication. Therefore, 356 participants were included in the analysis, including 180 in the Delayed ART cohort and 176 in the Early ART cohort (Figure 1). Overall 311 (87%) study participants started ART. Reasons for failure to start ART included death 17(38%) , study withdrawal 7(15%), moving away from study area 6(13%), loss to follow up 4(9%), interrupting ART or TB treatment or failure to keep study appointments 4(9%); deferring ART until completion of TB treatment 4(9%) or taking ART from another site 3(7%).
Figure 1.
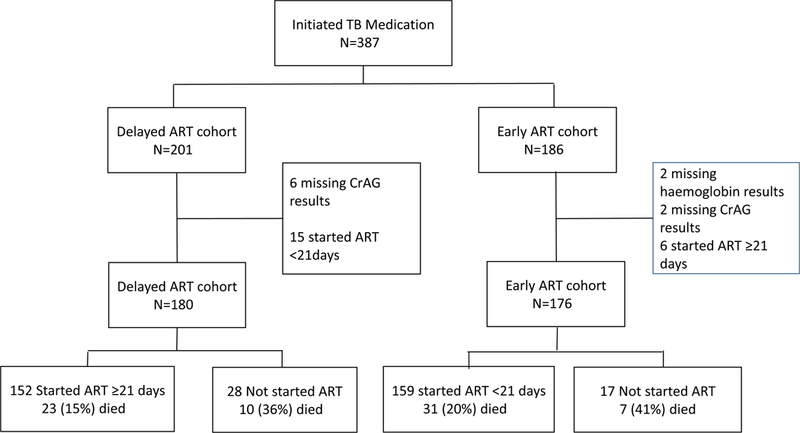
Flow chart for 387 study participants with HIV-TB co-infections enrolled in the study
The median age of study participants was similar, 32.5 years (IQR,27–39) and 32 years (IQR, 28–38) in the Delayed ART cohort and Early ART cohort respectively. Women comprised 47% and 48% in the Delayed ART cohort and Early ART cohort respectively. Participants’ median baseline CD4 counts was 26.5 cells/µL (IQR, 11–52) and 26 cells/µL (IQR, 11–48) in the Delayed ART cohort and Early ART cohort respectively. Of the participants who started ART 288(92.6%) had complete ART regimen information. In the Delayed ART cohort, majority of study participants were on stavudine 49(35.3%) and zidovudine-based 85(61.2%) regimens, while in the early ART cohort majority of the study participants were on tenofovir-based regimens 154(96.9%). At bivariate analysis WHO-staging IV (p<0.001), having extra-pulmonary TB (P<0.001), hemoglobin<8.0 g/dL (P=0.008), and a positive CrAg test (P=0.002) were significantly associated with Early ART cohort assignment (Table 1). After weighting, the two cohorts were more evenly distributed on the respective confounders (Table 1).
Table 1:
Socio-Demographic and Clinical Characteristics of Participants at Baseline
| Unweighted | Weighted* | ||||||
|---|---|---|---|---|---|---|---|
| Delayed | |||||||
| ART | Early ART | Delayed | Early ART | ||||
| Cohort | Cohort | P-value | ART Cohort | Cohort | P-value | ||
| Characteristics | N(%) | N(%) | N(%) | N(%) | |||
| Overall | 180(100%) | 176(100%) | 180(100%) | 176(100%) | |||
| Gender | |||||||
| Female | 96(53%) | 92(52%) | 0.841 | 88(49%) | 91(52%) | 0.584 | |
| Male | 84(47%) | 84(48%) | 92(51%) | 85(48%) | |||
| Age (years) | |||||||
| 18–24 | 21(12%) | 19(11%) | 0.272 | 22(12%) | 20(11%) | 0.418 | |
| 25–34 | 79(44%) | 90(51%) | 82(45%) | 88(50%) | |||
| 35–44 | 61(34%) | 44(25%) | 59(33%) | 46(26%) | |||
| 45–75 | 19(11%) | 23(13%) | 17(9%) | 23(13%) | |||
| CD4 counts (cells/µl) | |||||||
| 0–50 | 131(73%) | 133(76%) | 0.548 | 135(75%) | 131(74%) | 0.861 | |
| 51–100 | 49(27%) | 43(24%) | 45(25%) | 45(26%) | |||
| Haemoglobin (g/dL) | |||||||
| 0 – 8.0 | 59(33%) | 82(47%) | 0.008 | 76(42%) | 71(40%) | 0.677 | |
| 8.1 – 15.8 | 121(67%) | 94(53%) | 104(58%) | 105(60%) | |||
| WHO_Stage | |||||||
| III | 131(73%) | 89(51%) | <0.001 | 107(59%) | 108(61%) | 0.712 | |
| IV | 49(27%) | 87(49%) | 73(41%) | 68(39%) | |||
| Extra pulmonary TB | |||||||
| No | 137(76%) | 100(57%) | <0.001 | 115(64%) | 116(66%) | 0.680 | |
| Yes | 43(24%) | 76(43%) | 65(36%) | 60(34%) | |||
| CrAg Test result | |||||||
| Negative | 174(97%) | 155(88%) | 0.002 | 162(90%) | 162(92%) | 0.430 | |
| Positive | 6(3%) | 21(12%) | 18(10%) | 14(8%) | |||
Weighted using stabilized inverse probability weights
N=number, ART=antiretroviral therapy, WHO= World Health Organization, TB=tuberculosis, CrAg= cryptococcal antigen
Mortality in HIV-TB
A total of 69 (19.4%) participants died during the study. In the delayed ART arm, mortality was 18.3% (33/180) with 5.5% (10/180) mortality before starting ART and 12.8% (23/180) after starting ART. In the early ART arm, mortality was 21.6% (38/176); with 4.0% (7/176) mortality before starting ART and 17.6% (31/176) after starting ART (Figure 1). Overall, the median time of death was 74 (IQR, 42–147) days after starting TB treatment. The overall crude mortality incidence among all participants was 28.4 per 100 person years (95%CI, 21.3–38.6), (Table 2) with 25.2 per 100 person years (95%CI, 16–41.9) and 32.6 per 100 person years (95%CI, 22.9–47.6) in the Delayed ART and Early ART cohorts respectively.
Table 2:
Risk factors of death among HIV-TB co-infected patients comparing those initiated on Early or Delayed ART
| Characteristics | Cases/Person | Incidence/100 | Unadjusted (Weighted) | Adjusted (weighted) | ||
|---|---|---|---|---|---|---|
| years | person years (95% CI) |
HRs ( 95% CI) |
p-values |
HRs (95% CI) |
p-values |
|
| Overall | 69/240 | 28.42(21.3–38.6) | ||||
| Study | ||||||
| Delayed; CrAg-N | 23/128 | 17.3(11.3–27.6) | Ref | |||
| Delayed; CrAg-P | 13/9 | 146.51(63.5–373.7) | 6.07(2.6–14.1) | <0.001 | 4.17(1.9–9.0) | <0.001 |
| Early; CrAg-N | 31/96 | 31.67(21.6–47.6) | 1.62(0.9–2.8) | 0.092 | 1.54(0.9–2.8) | 0.154 |
| Early; CrAg-P | 4/8 | 44.89(18.2–122.6) | 2.41(0.9–6.3) | 0.072 | 2.63(1.0–6.8) | 0.045 |
| Gender | ||||||
| Female | 36/124 | 28.77(20.4–41.6) | Ref | Ref | ||
| Male | 33/117 | 28.05(17.6–47.4) | 0.97(0.5–1.7) | 0.902 | 0.65(0.4–1.1) | 0.112 |
| Age (years) | ||||||
| 18–24 | 15/23 | 66.12(30.3–166.6) | Ref | Ref | ||
| 25–34 | 24/116 | 20.03(13.3–31.2) | 0.33(0.1–0.7) | 0.006 | 0.30(0.1–0.6) | <0.001 |
| 35–44 | 23/78 | 28.67(17–52.1) | 0.48(0.2–1.1) | 0.084 | 0.39(0.2–0.7) | 0.003 |
| 45–75 | 9/25 | 33.1(16.3–74.1) | 0.54(0.2–1.4) | 0.218 | 0.59(0.2–1.4) | 0.228 |
| CD4 (cells/µl) | ||||||
| 0–50 | 58/178 | 32.46(23.6–45.7) | Ref | Ref | ||
| 51–100 | 11/62 | 16.74(9–34.1) | 0.54(0.2–1.4) | 0.218 | 0.59(0.2–1.4) | 0.228 |
| Haemoglobin(g/dL) | ||||||
| 0 – 8.0 | 35/91 | 37.97(24.4–62) | Ref | Ref | ||
| 8.1 – 15.8 | 34/149 | 22.58(15.8–33.2) | 0.62(0.4–1.1) | 0.093 | 0.77(0.5–1.3) | 0.327 |
| WHO Stage | ||||||
| III | 31/147 | 21.01(14.4–31.5) | Ref | Ref | ||
| IV | 38/93 | 40.13(26.4–63.6) | 1.89(1.1–3.3) | 0.021 | 1.79(1.1–2.9) | 0.017 |
| Extra pulmonary | ||||||
| TB* | ||||||
| No | 36/156 | 22.91(16.2–33.2) | Ref | |||
| Yes | 33/84 | 38.67(24.4–64.6) | 1.67(1.0–2.9) | 0.070 | ||
| TB-IRIS | ||||||
| Negative | 54/186 | 29(21.2–40.5) | Ref | |||
| Positive | 15/54 | 26.42(12.8–63.9) | 0.96(0.5–2.0) | 0.901 | ||
Delayed=ART initiation delayed beyond 21 days; Early=ART initiation within 21 days ; TB=tuberculosis
Results presented are weighted using stabilised inverse probability weights
HR=hazard ratio, CI=confidence intervals; CrAg -P=positive cryptococcal antigen test; CrAg-N = negative cryptococcal antigen test; CI=confidence interval; TB-IRIS = tuberculosis immune reconstitution inflammatory syndrome; ART= Antiretroviral therapy
Extra Pulmonary TB is collinear with WHO-Staging so it is excluded from the multivariate analysis
Causes of death
Thirty-nine (57%) of the 69 study participants who died had a documented cause of death from hospitalization or postmortem records. The identified causes of death in the delayed ART cohort included gastroenteritis with severe dehydration (n=4), Kaposi’s sarcoma (n=3), pneumonia (n=2), sepsis (n=2), TB-IRIS (n=2), TB (n=2), cryptococcal meningitis (n=1), pulmonary embolism (n=1), anaemia (n=1), drug induced hepatotoxicity (n=1) and renal failure (n=1). In the Early ART cohort ART cohort, the causes of death were cryptococcal meningitis (n=6), KS (n=2), drug-induced liver toxicity (n=2), pulmonary embolism (n=2), pneumocystis pneumonia (n=1), TB-IRIS (n=1) and sepsis (n=1).
TB-IRIS occurred in 68(19%) of the study participants. Majority of the TB-IRIS cases 45(66%) occurred in the delayed ART cohort and TB-IRIS was not predictive of mortality. Four of the six participants who died of cryptococcal meningitis in the Early ART cohort ART cohort had unmasking cryptococcal meningitis.
Predictors of mortality
WHO-stage IV was significantly associated with increased risk of death; compared to WHO-stage III (Adjusted Hazard Ratio (aHR)=1.79, 95%CI, 1.1– 2.9; P=0.017), and a positive serum CrAg test (aHR=2.95, 95%CI, 1.7–5.2; P<0.001) was significantly associated with increased risk of death. In the adjusted analysis, we found effect modification by CrAg status on the difference in mortality comparing early to delayed ART initiation. There was an increased risk of death in participants with a positive CrAg antigen test who received both delayed ART (aHR = 4.17, 95%CI, 1.9 – 9.0; P<0.001) and early ART (aHR = 2.63, 95%CI, 1.0 – 6.8; P=0.045) compared to participants who had a negative CrAg test who received delayed ART (Table 2). When we restricted the analysis to participants with baseline CD4 counts ≤50 cells/µL and a negative CrAg test, we still found a marginally significant increased risk of death in participants who initiated ART early compared to those who received delayed ART (aHR = 1.95, 95%CI, 1.0–3.9; P=0.054). Compared to participants who were CrAg negative in the delayed ART cohort, there was a five times increased risk of death in participants who had a positive CrAg test who received delayed ART (aHR = 4.78, 95%CI, 2.2 – 10.5; P<0.001) and a marginally significant increased risk of death in participants with a positive CrAg test who received early ART (aHR = 2.78, 95%CI, 1.0 – 7.9; P=0.053). When we restricted the analysis to participants with baseline CD4 counts 51–100 cells/µL, there were no significant differences in any of the strata (supplementary material, table 1s). In the sensitivity analysis, we found no significant differences in outcomes with or without the wash-out effect of 2–3 weeks or 2–7 weeks (supplementary material, table 2s and table 3s).
Age >25 years was associated with decreased risk of death; for participants aged 25 to 34 years (aHR=0.30, 95%CI, 0.1–0.6; P<0.001) and for participants aged 35 to 44 years (aHR=0.39, 95%CI, 0.2–0.7; P=0.03) (Table 2). Restricting to participants with CD4 count ≤50 cells/µL, we observed a similar association comparing participants aged 18–24 years old to participants aged 25 to 34 years (aHR=0.21, 95%CI 0.1–0.5; P<0.001), and participants aged 35 to 44 years (aHR=0.32, 95%CI 0.2–0.6; P=0.001). Similarly, WHO stage IV (aHR=1.92, 95%CI 1.2–3.3; P<0.017) remained predictive of mortality and CrAg positive result remained a significant effect modifier for the effect of timing of ART initiation (Table 3).
Table3:
Risk factors of death patients among HIV-TB co-infected patients with baseline CD4≤50 cells/µl comparing those initiated on Early or Delayed ART
| Characteristics | Incidence/100 | Unadjusted (weighted) | Adjusted (weighted) | |||
|---|---|---|---|---|---|---|
| Cases/Person | person years |
HRs ( 95% CI) |
p-values |
HRs (95% CI) |
p-values |
|
| years | (95% CI) | |||||
| Overall | 58/178 | 32.46(23.6–45.7) | ||||
| Study | ||||||
| Delayed; CrAg-N | 16/94 | 16.61(10–29.3) | Ref | |||
| Delayed; CrAg-P | 13/9 | 146.51(63.5–373.7) | 6.28(2.6–15.3) | <0.001 | 4.78(2.2–10.5) | <0.001 |
| Early; CrAg-N | 28/69 | 39.41(26.1–61) | 2.07(1.1–3.9) | 0.025 | 1.95(1.0–3.9) | 0.054 |
| Early; CrAg-P | 3/8 | 40.56(15.3–122.9) | 2.28(0.8–6.6) | 0.128 | 2.78(1.0–7.9) | 0.053 |
| Gender | ||||||
| Female | 30/91 | 33.07(22.6–49.6) | Ref | Ref | ||
| Male | 28/88 | 31.84(18.9–57.7) | 0.95(0.5–1.8) | 0.879 | 0.59(0.3–1.1) | 0.082 |
| Age (years) | ||||||
| 18–24 | 14/15 | 93.4(41.1–246.1) | Ref | Ref | ||
| 25–34 | 17/89 | 18.75(11.6–31.7) | 0.21(0.1–0.5) | <0.001 | 0.21(0.1–0.5) | <0.001 |
| 35–44 | 20/60 | 32.25(18.2–62.7) | 0.38(0.2–0.9) | 0.026 | 0.32(0.2–0.6) | <0.001 |
| 45–75 | 9/16 | 54.74(26.4–122.7) | 0.61(0.2–1.6) | 0.324 | 0.62(0.2–1.6) | 0.314 |
| Haemoglobin (g/dL) | ||||||
| 0 – 8.0 | 32/70 | 45.61(28.6–76.7) | Ref | Ref | ||
| 8.1 – 15.8 | 27/109 | 24.08(16–37.6) | 0.56(0.3–1.0) | 0.060 | 0.74(0.4–1.3) | 0.287 |
| WHO_Stage | ||||||
| III | 26/109 | 23.36(15.4–36.9) | Ref | Ref | ||
| IV | 33/69 | 46.86(29.6–77.9) | 1.99(1.1–3.6) | 0.024 | 1.92(1.1–3.3) | 0.017 |
| Extra pulmonary | ||||||
| TB* | ||||||
| No | 29/116 | 24.4(16.5–37.4) | Ref | |||
| Yes | 30/62 | 47.54(29.2–82.1) | 1.92(1.1–3.5) | 0.033 | ||
| TB-IRIS | ||||||
| Negative | 46/133 | 34.09(23.9–49.8) | Ref | |||
| Positive | 13/46 | 27.77(12.7–73.7) | 0.86(0.4–1.9) | 0.706 | ||
Delayed=ART initiation delayed beyond 21 days; Early=ART initiation within 21 days
Results presented are weighted using stabilised inverse probability weights
HR=hazard ratio, CI= confidence intervals; CrAg -P = positive cryptococcal antigen test; CrAg-N=negative cryptococcal antigen test; CI=confidence interval; TB-IRIS=tuberculosis immune reconstitution inflammatory syndrome; ART= Antiretroviral therapy
Extra Pulmonary TB is collinear with WHO-Staging so it is excluded from the multivariate analysis
Discussion
Starting ART among HIV-TB co-infected patients during TB treatment is crucial as it has been associated with reduced mortality [3–5, 14]. This reduction in mortality, especially among patients with CD4 counts <50 cells/µL, has been confirmed in clinical trials and systematic reviews [4, 8]. On the basis of this evidence WHO has recommended that ART should be started as an emergency in HIV-TB co-infected patients within 2 weeks of TB treatment initiation in patients with CD4 counts <50 cells/µL [20].
In this observational study, we saw no difference in mortality among participants with CD4 counts ≤50 cells/µL who were started on early versus delayed ART. We found a significant increase in mortality rates in participants with asymptomatic cryptococcal antigenemia in both early and delayed ART study arms. Our study findings corroborate those reported by Amogne et al. and Manosuthi et al. which did not show a reduction in mortality despite early ART initiation and indicated the role of comorbidities in diluting the treatment effect particularly in sub-Saharan Africa [13, 14]. Early ART has also not been shown to reduce mortality in participants with other categories of TB like TB meningitis as observed by Torok et al. [21]. For participants with CD4 counts 50–100 cells/µL, we found that no difference in mortality between the early and delayed ART groups. This is similar to what was reported in the clinical trials [3, 5]. Mfinanga et al. found no benefit of early ART in patients with CD4 counts >220 cells/µL and suggested that ART initiation in HIV-TB co-infected patients with high CD4 counts could be deferred until TB treatment is completed [22].
There are important differences between the studies on ART initiation in HIV/TB co-infected persons (Table 4). First, the patient populations studied had varied levels of median CD4 counts. Our study participants had severe CD4 count depletion (median CD4 of 24 cells/µL) comparable with the trial population of Blanc et al.[4] (median CD4 of 25 cells/µL). Most other studies had participants with higher CD4 counts and, in those studies, the effect of early ART on mortality in HIV/TB co-infected persons was less prominent. Second, the difference in prevalence and management of opportunistic infections may influence the final patient outcomes. For instance, despite having low CD4 counts, the CAMELIA study in Cambodia [4] had fewer deaths attributable to cryptococcal meningitis (2 out of 149) and KS (0 out of 149) compared to our study. Third, our study mainly focused on the primary outcome of mortality while some studies used a composite outcome of mortality and AIDS-defining illnesses. Fourth, there is a wide variation in the difference in the time to ART initiation in different studies. Most studies compared early ART (<2 weeks of TB treatment start) with delayed ART start (>8 weeks after TB treatment). In our study, ART was started relatively early after TB treatment in both cohorts (median 14 days in the Early ART cohort versus 44 days in the Delayed ART cohort).
Table 4:
Comparisons of outcomes of studies on ART initiation in HIV-TB co-infected persons
| Mortality / Serious Adverse Event | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline CD4 | Sample | Days to ART start | Mortality/ AIDS-defining Illness | ||||||
| attributed to: | |||||||||
| Study | cells/μL | size | (median, IQR) | incidence rate /100person-yr | |||||
| N(%) | |||||||||
|
Inclusion |
Median |
N |
Early |
Delayed |
Early |
Delayed |
Cryptococcus |
Kaposi’s sarcoma |
|
| CAMELIA[4] | <200 | 25 | 661 | 14 (14–15) | 56(56–57) | 8.28 | 13.77 | 10 (1.5) | 0 (0) |
| SAPIT[5] | <500 | 150 | 429 | 21(15–29) | 97(77–126) | 6.9 | 7.8 | 6 (1.4) | 1 (0.2) |
| STRIDE[3] | <250 | 77 | 809 | 10 (7–12) | 70(66–75) | 12.9** | 16.9** | 13 (1.6) | 11 (1.4) |
| 28(21–45)† | 11† | ||||||||
| TBHAART[13] | <200 | 72 | 478 | 7(4–48) | 19 | 6 (1.3) | 0 (0) | ||
| 56(48–75) | 7 | ||||||||
| TBHAART[22] | >220 | 367 | 1675 | <14* | ≤56* | 8.5** | 7.9** |
- |
5 (0.3) |
| TIME[14] | <350 | 43 | 156 | <28* | 28–56* | 8.76 | 7.25 | 9 (5.8) | 1 (0.6) |
| Present study | <=100 | 24 | 377 | 14 (14–16) | 44 (29–60) | 32.7 | 25.2 | 7 (10.1) | 5 (7.2) |
ART=Antiretroviral therapy; TB=tuberculosis;
exact duration not specified
composite mortality/AIDS-defining illnesses
ART was started at two time points (4 weeks and 8 weeks after TB treatment start) during the Delayed ART period.
An important difference between the two HIV-TB cohorts studies analyzed was the difference in ART regimens used. There was a country-wide change in the recommended NRTI backbone from a stavudine and zidovudine-based regimen (in the Delayed ART cohort) to a tenofovir-based ART regimen (in the Early ART cohort). Several studies and a Cochrane review have demonstrated that using a tenofovir-based regimen leads to superior viral suppression, immunological response and tolerability than a zidovudine or stavudine-based regimen [23-25]. As a result, we may have understimated the mortality attributable to ART in the Early ART cohort.
Our results indicate that positive serum CrAg is an independent predictor of mortality in HIV-TB co-infected patients have been reported previously in patients with HIV starting ART [26-28] and in HIV-TB cohorts [29]. Early identification of patients with cryptococcosis and pre-emptive treatment with fluconazole is cost-effective and may curb the excess mortality from cryptococcal disease [30, 31]. We also note that some of the subjects participants in the Early ART cohort “unmasked” cryptococcal meningitis despite having had a negative serum CrAg at study enrolment when starting TB therapy. These may represent missed diagnoses of subclinical cryptococcal antigenaemia in patients with advanced HIV immune suppression. Additional screening for cryptococcosis during the initial months of ART in patients with low CD4 counts may identify participants with cryptococcal antigenemia who may benefit from fluconazole or amphotericin treatment. The low incidence of cryptococcal meningitis in the study by Blanc et al[4] could be attributed to routine use of prophylactic fluconazole unlike in our study where it was only provided to participants with a positive serum CrAg at baseline. Lastly, the optimal time to ART initiation among patients with cryptococcal antigenemia has not been established. In the trial by Boulware at al., early ART was associated with increased mortality in patients with cryptococcal meningitis [32]. The known benefits of early ART in TB patients with CD4 counts <50 cells/µL seen in other studies may have been counteracted by the negative effects of early ART on patients with cryptococcal infection.
We also observed at least 10 per 100 person-years higher rates of mortality in our study compared to the randomized trials. Not surprisingly, WHO stage IV disease was also independently associated with increased mortality. This suggests that our study population may have had more advanced HIV diseases with more opportunistic infections and comorbidities leading to increased mortality. This in turn undercut the benefit of early ART initiation in patients with CD4 counts ≤50 cells/µL. There was a higher mortality among young adults aged 18 to 24 years co-infected with HIV-TB compared with older age groups. This may be due to delayed access to HIV and TB services by the young adults as a result of stigma and discrimination, non-disclosure and the lack of youth-friendly services in the public health sector. This has been reported in the context of challenges in access and adherence to ART [33].
Our study had some strengths and weaknesses. The study compared two well-characterized HIV-TB cohorts designed to study predictors of mortality. As observational studies, they were closer to a real-life situation. However, one cannot exclude bias in patient selection resulting in significant differences in the baseline populations (Table 1). We used inverse-probability censoring-weighted Cox models to address these baseline discrepancies. However, there could still be unmeasured confounders that could have led to poor model specification in generating the weights. It is also important to note that our results may only be generalizable to similar low income settings with a high burden of HIV-TB co-infection and opportunistic diseases like cryptococcal meningitis.
Conclusion
Early ART in patients with HIV-TB co-infection was not associated with reduced risk of mortality in these real world observational cohorts. Cryptococcal antigenemia in severely immunosuppressed AIDS patients with HIV-TB patients is associated with increased mortality in both early and delayed ART.
Supplementary Material
Acknowledgements
Authors: WW, VS, HMK, SRJ, RC and YMC conceived supervised the conduct of the study. WW wrote the first draft. WW, RS, AM, DM contributed to data collection. WW, HMK and RC supervised the conduct of the study. WW, VS, CH, SRJ and YMC conducted data analysis. All the authors critically reviewed and approved the manuscript.
Funding: We received funding for this work from European & Developing Countries Clinical Trials Partnership (EDCTP TA 10. 40200.019), additional support was provided to WW from the Fogarty International Center (D43TW009771) and the Flemish Interuniversity Council (VLIR-UOS) and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIAID/NIH). This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Other: We thank all the study participants, the study clinical staff: Proscovia Lwanga, Carol Olive Namujju, Jane Namaganda; Alfred Andama for the laboratory testing; Dr. Jabo Roy for the radiology reporting; Dr. Robert Lukande for the pathology work, the Amsterdam Institute for Global Health for data monitoring and Namawejje Lule Mariam for data management. We thank Dr. David Boulware for reading this manuscript and providing constructive comments. We also thank the Mulago Hospital administration and the National TB and Leprosy Unit; the Infectious Disease Institute, Clinical Services department for the support to conduct the study in the HIV-TB clinic and for the support to patient care in this study.
References:
- 1.World Health Organization. Global tuberculosis Report In. Geneva, Switzerland: World Health Organization; 2016. [Google Scholar]
- 2.Yan S, Chen L, Wu W, Fu Z, Zhang H, Li Z, et al. Early versus Delayed Antiretroviral Therapy for HIV and Tuberculosis Co-Infected Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One 2015; 10(5):e0127645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med 2011; 365(16):1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 2011; 365(16):1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med 2011; 365(16):1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO policy on collaborative TB/HIV activities: Guidelines for national programmes and other stakeholders In. Geneva, Swizerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 7.Odone A, Amadasi S, White RG, Cohen T, Grant AD, Houben RM. The impact of antiretroviral therapy on mortality in HIV positive people during tuberculosis treatment: a systematic review and meta-analysis. PLoS One 2014; 9(11):e112017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uthman OA, Okwundu C, Gbenga K, Volmink J, Dowdy D, Zumla A, et al. Optimal Timing of Antiretroviral Therapy Initiation for HIV-Infected Adults With Newly Diagnosed Pulmonary Tuberculosis: A Systematic Review and Meta-analysis. Ann Intern Med 2015; 163(1):32–39. [DOI] [PubMed] [Google Scholar]
- 9.Tabarsi P, Saber-Tehrani AS, Baghaei P, Padyab M, Mansouri D, Amiri M, et al. Early initiation of antiretroviral therapy results in decreased morbidity and mortality among patients with TB and HIV. J Int AIDS Soc 2009; 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanyerere HS, Mpunga J, Tweya H, Edginton M, Harries AD, Hinderaker SG, et al. Timing of antiretroviral therapy and effects on tuberculosis treatment outcomes in HIV-co-infected patients in Malawi. Public health action 2012; 2(4):174–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CH, Chen KJ, Tsai JJ, Lin YH, Cheng SH, Wang KF, et al. The impact of HAART initiation timing on HIV-TB co-infected patients, a retrospective cohort study. BMC Infect Dis 2014; 14:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han SH, Zhou J, Lee MP, Zhao H, Chen YM, Kumarasamy N, et al. Prognostic significance of the interval between the initiation of antiretroviral therapy and the initiation of anti-tuberculosis treatment in HIV/tuberculosis-coinfected patients: results from the TREAT Asia HIV Observational Database. HIV Med 2014; 15(2):77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amogne W, Aderaye G, Habtewold A, Yimer G, Makonnen E, Worku A, et al. Efficacy and Safety of Antiretroviral Therapy Initiated One Week after Tuberculosis Therapy in Patients with CD4 Counts < 200 Cells/muL: TB-HAART Study, a Randomized Clinical Trial. PLoS One 2015; 10(5):e0122587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manosuthi W, Mankatitham W, Lueangniyomkul A, Thongyen S, Likanonsakul S, Suwanvattana P, et al. Time to initiate antiretroviral therapy between 4 weeks and 12 weeks of tuberculosis treatment in HIV-infected patients: results from the TIME study. J Acquir Immune Defic Syndr 2012; 60(4):377–383. [DOI] [PubMed] [Google Scholar]
- 15.Sinha S, Shekhar RC, Singh G, Shah N, Ahmad H, Kumar N, et al. Early versus delayed initiation of antiretroviral therapy for Indian HIV-Infected individuals with tuberculosis on antituberculosis treatment. BMC Infect Dis 2012; 12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Worodria W, Massinga-Loembe M, Mazakpwe D, Luzinda K, Menten J, Van Leth F, et al. Incidence and predictors of mortality and the effect of tuberculosis immune reconstitution inflammatory syndrome in a cohort of TB/HIV patients commencing antiretroviral therapy. J Acquir Immune Defic Syndr 2011. [DOI] [PubMed]
- 17.Worodria W, Menten J, Massinga-Loembe M, Mazakpwe D, Bagenda D, Koole O, et al. Clinical spectrum, risk factors and outcome of immune reconstitution inflammatory syndrome in patients with tuberculosis-HIV coinfection. Antivir Ther 2012; 17(5):841–848. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents. Recommendations for HIV-prevalent and resource-constrained settings In. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 19.World Health Organization. Rapid Advice Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-infected Adults, Adolescents and Children In. Geneva, Swizerland: World Health Organization; 2011. [PubMed] [Google Scholar]
- 20.World Health Organization. WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders In. Geneva, Switzerland: World Health Organization; 2012. [PubMed] [Google Scholar]
- 21.Torok ME, Yen NT, Chau TT, Mai NT, Phu NH, Mai PP, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)--associated tuberculous meningitis. Clin Infect Dis 2011; 52(11):1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mfinanga SG, Kirenga BJ, Chanda DM, Mutayoba B, Mthiyane T, Yimer G, et al. Early versus delayed initiation of highly active antiretroviral therapy for HIV-positive adults with newly diagnosed pulmonary tuberculosis (TB-HAART): a prospective, international, randomised, placebo-controlled trial. Lancet Infect Dis 2014; 14(7):563–571. [DOI] [PubMed] [Google Scholar]
- 23.Dadi TL, Kefale AT, Mega TA, Kedir MS, Addo HA, Biru TT. Efficacy and Tolerability of Tenofovir Disoproxil Fumarate Based Regimen as Compared to Zidovudine Based Regimens: A Systematic Review and Meta-Analysis. AIDS research and treatment 2017; 2017:5792925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velen K, Lewis JJ, Charalambous S, Grant AD, Churchyard GJ, Hoffmann CJ. Comparison of tenofovir, zidovudine, or stavudine as part of first-line antiretroviral therapy in a resource-limited-setting: a cohort study. PLoS One 2013; 8(5):e64459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaulding A, Rutherford GW, Siegfried N. Tenofovir or zidovudine in three-drug combination therapy with one nucleoside reverse transcriptase inhibitor and one non-nucleoside reverse transcriptase inhibitor for initial treatment of HIV infection in antiretroviral-naive individuals. Cochrane Database Syst Rev 2010; (10):CD008740. [DOI] [PubMed]
- 26.Ganiem AR, Indrati AR, Wisaksana R, Meijerink H, van der Ven A, Alisjahbana B, et al. Asymptomatic cryptococcal antigenemia is associated with mortality among HIV-positive patients in Indonesia. J Int AIDS Soc 2014; 17:18821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, et al. Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health 2007; 12(8):929–935. [DOI] [PubMed] [Google Scholar]
- 28.Manabe YC, Nonyane BA, Nakiyingi L, Mbabazi O, Lubega G, Shah M, et al. Point-of-care lateral flow assays for tuberculosis and cryptococcal antigenuria predict death in HIV infected adults in Uganda. PLoS One 2014; 9(7):e101459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Worodria W, Massinga-Loembe M, Mazakpwe D, Luzinda K, Menten J, Van Leth F, et al. Incidence and predictors of mortality and the effect of tuberculosis immune reconstitution inflammatory syndrome in a cohort of TB/HIV patients commencing antiretroviral therapy. J Acquir Immune Defic Syndr 2011; 58(1):32–37. [DOI] [PubMed] [Google Scholar]
- 30.Jarvis JN, Harrison TS, Lawn SD, Meintjes G, Wood R, Cleary S. Cost effectiveness of cryptococcal antigen screening as a strategy to prevent HIV-associated cryptococcal meningitis in South Africa. PLoS One 2013; 8(7):e69288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, et al. Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or = 100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis 2010; 51(4):448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med 2014; 370(26):2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inzaule SC, Hamers RL, Kityo C, Rinke de Wit TF, Roura M. Long-Term Antiretroviral Treatment Adherence in HIV-Infected Adolescents and Adults in Uganda: A Qualitative Study. PLoS One 2016; 11(11):e0167492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


