Abstract
Laser immunotherapy (LIT) is a new anti-cancer therapy combining photothermal therapy (PTT) and immunostimulation, which eliminates the tumors by damaging tumor cells directly and promoting the release of damage-associated molecular patterns (DAMPs) to enhance tumor immunogenicity. To evaluate the effectiveness and safety of LIT for cutaneous squamous cell carcinoma (cSCC) and identify the correlations of temperature increase with the cell damage or DAMPs productions, the cell viability and the DAMPs productions of cSCC A431 cells treated by water-bath heating in different temperatures were investigated. And LIT with the optimal thermal effect for DAMPs production was performed on the mice bearing ultraviolet-induced cSCC and the patient suffering from a large refractory cSCC. The temperature increase in 45 – 50 °C causing around half of A431 cells to die directly had an optimal thermal effect for the productions of DAMPs, which could be further enhanced by local application of imiquimod, an immunoadjuvant. LIT eliminated most tumors and improved the survival rate of the cSCC mice (p < 0.05). A patient with cSCC was treated by LIT with significant tumor reduction after LIT increased the amounts of lymphocytes infiltrating in the tumor. No obviously adverse effect was found in the patient. LIT within optimal thermal effects is an effective and safe treatment modality for cSCC.
Keywords: cutaneous squamous cell carcinoma (cSCC), laser immunotherapy (LIT), photothermal (PTT), temperature gradient, imiquimod, damage-associated molecular patterns (DAMPs)
1. Introduction
Cutaneous squamous cell carcinoma (cSCC) is the second most common human skin cancer [1]. Left untreated, it may spread to other parts of the body, and it may ultimately lead to death [2]. Surgical excision is the first line of treatment for most cSCC, although other treatment modalities are also used depending upon the nature and site of the tumor and condition of individual participants [3, 4]. However, there are significant challenges when treating large, metastatic, and invasive cSCC, using conventional therapies [5].
The ultimate control of cancer has been suggested to lie in the host immune system [6]. Immunotherapy has been considered a promising treatment approach, and various strategies have been proposed, including dendritic cell-based vaccines, immune checkpoint therapy, cytokine therapy and immune-activating antibodies [7–9]. However, these immunotherapy strategies have yielded low response rates and most cancers still avoid or escape from immune control [10, 11]. Novel approaches are therefore needed to increase the efficacy of immunotherapy. Ideally, such novel approaches should not only destroy local tumors, but also at the same time achieve a potent systemic, tumor-specific immunological response to eradicate metastases at distant sites, with minimal adverse effects. Laser immunotherapy (LIT) composing of selective photothermal therapy (PTT) and a locally administered immunostimulant is a novel anti-tumor method, which has a synergistic effect of destroying the tumor and stimulating the anti-tumor immune responses [12, 13]. It has been used for breast cancer, melanoma and Rosai-Dorfman disease with promising outcomes [14–17].
PTT is the main component of LIT which induces a temperature gradient inside the tumors. Temperature increase can damage tumor cells through direct and indirect mechanism [18–20]. Direct mechanism is known as the necrosis and apoptosis of tumor cells. Indirect mechanism refers to tumor cells are eliminated by the immune response activated by PTT. The temperature increase in the tumor up-regulate the expression or release of tumor antigens, especially various damage-associated molecular patterns (DAMPs), such as heat shock proteins (HSP) 70, HSP 90 and high mobility group protein B1 (HMGB1), enhancing the tumor immunogenicity [21–24]. At the same time, the activity of macrophages, dendritic cells, T lymphocytes, B lymphocytes, and natural killer cells could also be enhanced by the temperature increase [25–29]. Finally, tumor cells are eliminated by the immune responses following the DAMPs expression.
Imiquimod, a unique toll-like receptor (TLR) agonist, was selected as the immunostimulant in the study to facilitate immunological stimulation. It has been approved by the FDA for the treatment of anogenital warts, actinic keratosis and superficial basal cell carcinoma [30]. Although the imiquimod monotherapy has a limited effectiveness for cSCC [31–33], it showed a strong immunological stimulating effect when combined with PTT for the treatment of melanoma patients, indicating that imiquimod is a good candidate as an immunostimulant in LIT for cSCC [16].
LIT has never been used for cSCC. Furthermore, the relationship between heat and the DAMPs expression is not conclusive, since the reported data are not consistent. It is still unclear what temperature range has optimal thermal effects on the enhancement of tumor immunogenicity in the LIT treatment for cSCC.
In this study, to gain the optimal thermal effects, we investigated the correlation between temperature increase and tumor cell death or DAMPs release/expression, with or without imiquimod. We evaluated the effectiveness of LIT with the optimal thermal effects for the ultraviolet (UV)-induced cSCC in SKH-1 mice. Finally, we translated this novel anti-tumor method to the clinic to treat a patient with a large refractory cSCC.
2. Results
2.1. Effects of Thermal Treatment on A431 Cell Activity
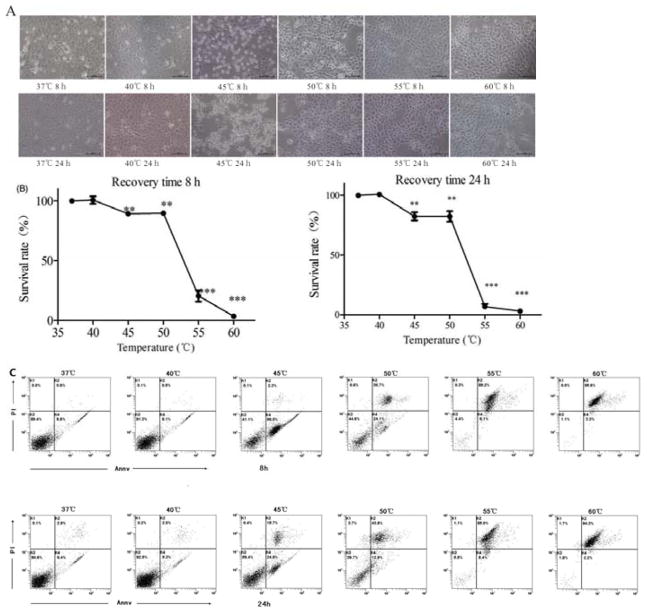
After different thermal treatments (10 minutes), the A431 cells were placed at 37 °C. The morphology (Figure 1A), the viability (Figure 1B) and the percentage of apoptosis/necrosis (Figure 1C) were detected at 8 h and 24 h. Temperature increase caused death of A431 cells in a temperature-dependent manner. The cells in 37 °C (control group) and 40 °C group grew normally, adhering to the wall with the morphological integrity. The survival rates of cells in 40 °C were 100.7%±4.4% at 8 h and 100.8%±2.4 % at 24 h. Most cells in 45 °C group became spheres and floated up, having a lower survival rate (89.8%±1.2% at 8 h, 82.4%±4.9% at 24 h) than cells in control group (P < 0.05). Most cells in 50 °C adhered tightly to the wall with white bubbles inside the cells. The survival rates were 91.5%±1.5 % at 8 h, and 82.4%±6.3% at 24 h. All of the cells in 55 °C and 60 °C adhered the wall tightly with white bubbles. The survival rates were only 24.0%±4.6% at 8 h, 7.0%±3.0% at 24 h in 55 °C, 3.2%±0.2% at 8 h and 3.1%±1.0% at 24 h in 60 °C. The percentages of apoptosis/necrosis were shown in Figure 1C. Most cells were alive in 37 °C and 40 °C group. Only 5.3% –9.8% cells appeared apoptosis. Most cells in 55 °C and 60 °C group died. 85.0%–95.6% cells appeared necrosis. Around 30%–60% cells died in 45 °C and 50 °C group, with an apoptosis percentage of 12.9% –56.5% and a necrosis percentage of 2.3%–43.8%.
Figure 1.
Temperature increase caused death of A31 cells in a temperature-dependent manner. A431 cells were treated under different temperatures (37 °C to 60 °C) for 10 minutes and recovered for 8 h or 24 h at 37 °C, and then imaged by inverted microscope (A), analyzed by CCK8 assay (B), and analyzed by flow cytometry with Annexin V/PI staining (C). Statistical analysis was performed by t-test; *P < 0.05, **P < 0.01, ***P<0.001, ns=not statistically significant.
2.2. Effects of Thermal Treatment on the Extracellular Release of DAMPs from Tumor Cells
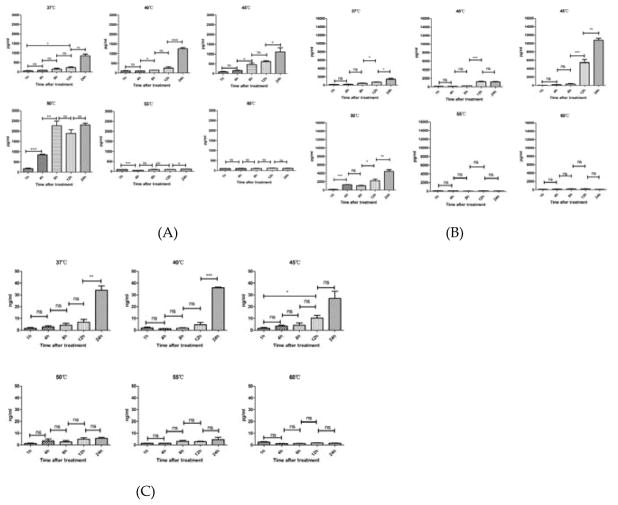
The extracellular releases of HSP70, HSP90, and HMGB1 in the supernatant of A431 cell culture were measured by enzyme linked immunosorbent assay (ELISA) after different temperature treatments (37 °C, 40 °C, 45 °C, 50 °C, 55 °C, and 60 °C) for 10 min. As shown in Figure 2, A431 cells released HSP70, HSP90 and HMGB1 after treatment. The productions of HSP70, HSP90 and HMGB1 increased along with the time in the range of 37 °C – 50 °C. Cells at 50 °C released the maximum amount of HSP70 while cells in 45 °C released the maximum amount of HSP90 and HMGB1.
Figure 2.
Temperature increase affected extracellular release of damage-associated molecular patterns (DAMPs) from A431 cells in a temperature-dependent manner. The release of heat shock proteins (HSP) 70 (A), HSP90 (B), and high mobility group protein (HMGB1) (C) from heat-stimulated A431 cells at different time points (1 h to 24 h) after the 10-minute heat treatments, at 37 °C, 40 °C, 45 °C, 50 °C, 55 °C, and 60 °C, analyzed using enzyme linked immunosorbent assay (ELISA) assay. Statistical analysis was performed by t-test; *P < 0.05, **P < 0.01, ***P < 0.001, ns=not statistically significant. Means ± SD are shown from 3 independent experiments.
2.3. Effects of Thermal Treatment on the Intracellular Expression of DAMPs in Tumor Cells
To determine intracellular changes of DAMPs, the expressions of HSP70, HSP90, and HMGB1 of A431 cells treated by different temperatures (37 °C, 40 °C, 45 °C, 50 °C, 55 °C, 60 °C) for 10 minutes were analyzed by western blot analysis. As shown in Figure 3, the expressions of both HSP70 and HSP90 at 45 °C were the highest in all temperature groups. And the expressions of HMGB1 at 45 °C was similar to as in 37 °C or 40 °C groups, which was higher than in 50°C, 55°C or 60 °C groups. HSP 70, HSP 90 and HMGB1 were down-regulated in high temperature group (50°C, 55°C, 60°C). The expressions of HSP70 and HSP90 increased gradually along within the 24 h-recovery time. However, the expressions of HMGB1 did not change in 24 h.
Figure 3.
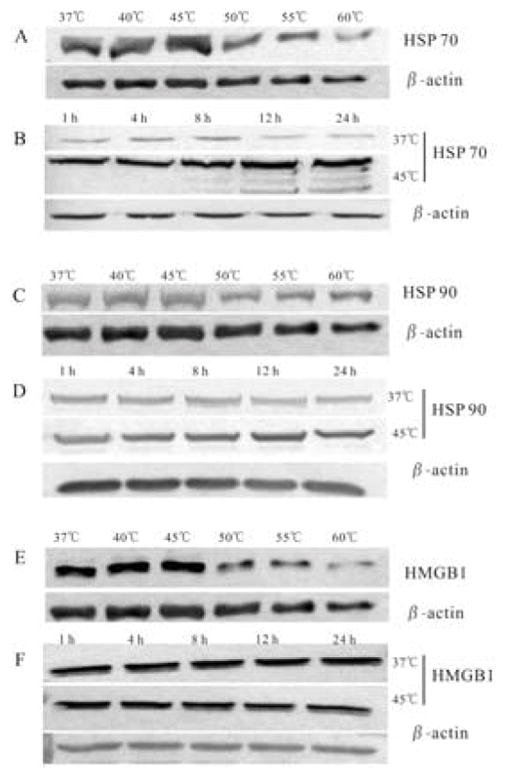
Temperature increase affected intracellular expression of damage-associated molecular patterns (DAMPs) from A431 cells in a temperature-dependent manner. The expressions of heat shock proteins (HSP) 70 (A, B), HSP90 (C, D), and high mobility group protein (HMGB1) (E, F) in heat-stimulated A431 cells at 8 h after 10-minute heat treatment at 37 °C, 40 °C, 45 °C, 50 °C, 55 °C, and 60 °C, and at different time points after heat treatment (1 h to 24 h), at 37 °C and 45 °C, analyzed by western blotting.
2.4. Effects of Imiquimod on Proliferation of A431 Cells and DAMPs Release
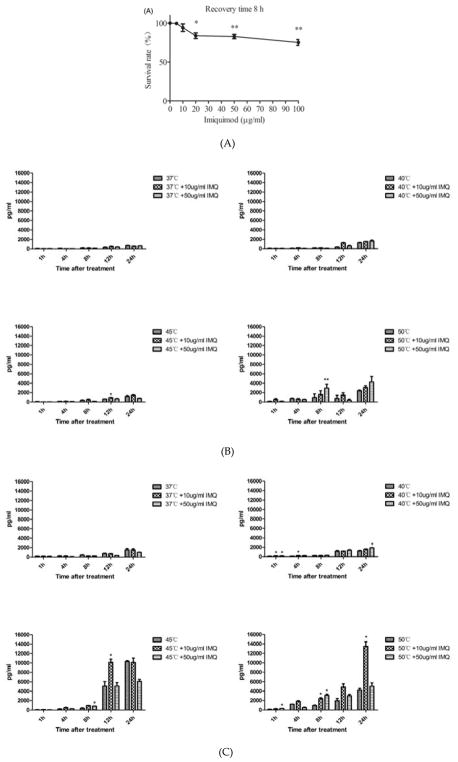
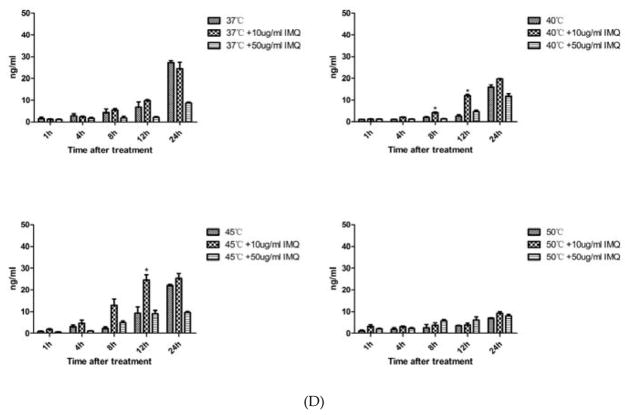
Since imiquimod could directly affect keratinocytes viability and it has been used for the treatment of cSCC. We investigated its effects on A431 cells. When the A431 cells were incubated with imiquimod, cell death occurred in a dose-dependent manner (Figure 4A). ELISA results (Figure 4B, 4C, 4D) showed that both 10 μg/ml and 50 μg/ml imiquimod increased the release of HSP70, HSP90 and HMGB1 after heat treatment. Generally, A431 cells treated by the combination of imiquimod and heat treatment released a maximum amount of DAMPs in a temperature range of 45°C –50°C.
Figure 4.
Effects of imiquimod on the viability of A431 cells and release of damage-associated molecular patterns (DAMPs). (A) Imiquimod directly killed A431 cell in a dose-dependent manner. Cells were incubated with imiquimod at different concentrations (0 μg/ml to 100 μg/ml) for 24 hours before being subjected to CCK8 assay. Statistical analysis was performed by t-test; (5, 10, 20, 50 or 100 μg/ml vs. 0 μg/ml). (B–D) Release of heat shock protein (HSP) 70, HSP90, and high mobility group protein B1 (HMGB1) from A431 cells treated under different temperature, with or without imiquimod. A431 cells were incubated with imiquimod at different concentrations (10 μg/ml and 50 μg/ml) for 24 h, followed by a 10-min heat treatment under different temperatures (37°C to 50 °C), and then recovered at 37 °C with imiquimod. The supernatant was collected and anlyzed by ELISA assay. Statistical analysis was performed by t-test; (temperature + IMQ (Imiquimod) group vs temperature group). Means ± SD are shown from 3 independent experiments.*p < 0.05, **p < 0.01.
2.5. cSCC Tumors in SKH-1 Mice Induced by UV Irradiation
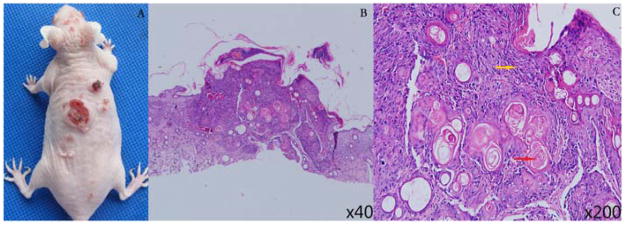
UV irradiation on SKH-1 mice lasted for 5 months until papules of 1–4 mm in diameter appeared and maintained for two weeks. After the UV irradiation, the papules continued to grow to various size of cauliflower-type lesions and some tumors began to develop surface erosion and ulcers, as shown in Figure 5A. The lesion was proved as cSCC by histopathological examination, showing a large number of atypical cells and keratin pearls in the dermis (Figure 5B, C).
Figure 5.
Cutaneous squamous cell carcinoma (cSCC) tumors in SKH-1 mice induced by ultraviolet (UV) irradiation. (A) Representative photograph of UV-treated SKH-1 hairless mice, inducing cSCC tumors with various sizes. The histological examination showed that a large number of atypical cells (→), keratin pearl (→) in the tissue at 40× (B) and 200× (C), which were the characteristic of cSCC tumors.
2.6. Anti-tumor Activity of LIT with Optimal Thermal Effects for UV-induced cSCC Mice
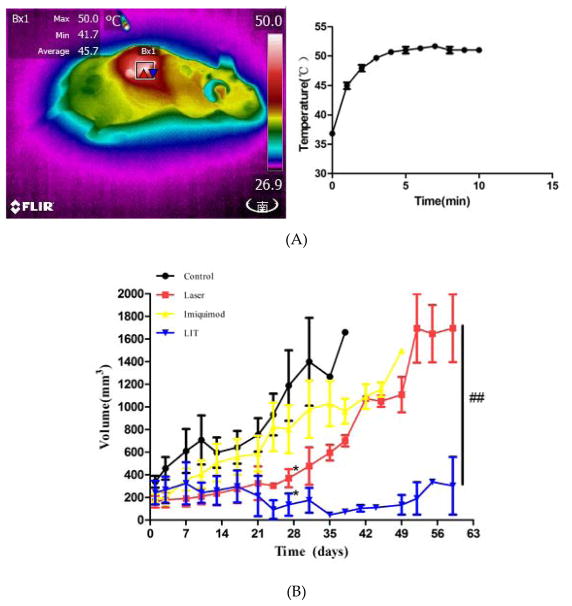
During laser irradiation, the superficial temperatures of mouse tumor were monitored to maintain the temperature in the range of 45°C –50°C to produce the maximum of DAMPs. (Figure 6A). The temperature in LIT group increased from 36 °C during irradiation and reached a saturation of around 50 °C in about 3 minutes.
Figure 6.
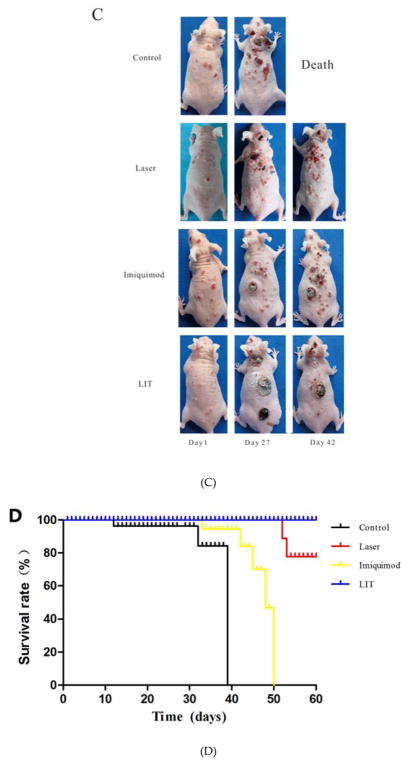
Anti-tumor effect of laser immunotherapy (LIT) in treating cutaneous squamous cell carcinoma (cSCC) in SKH-1 mice. (A) Temperature in tumor tissue during laser treatment. Left: Thermographic image showing the temperature of the tumor in one mouse with 808-nm laser illumination. Right: The temperature increase curve of the tumors with 808-nm laser illumination. (B) Tumor volume changes in different treatment groups. Statistical analysis for tumor volume changes was performed by t-test (day 27: Laser, or LIT group vs. Control group,*p < 0.05; Day 60: LIT group vs. Laser group, ##p < 0.01). (C) Representative photographs of mice at different times after treatments. (D) Survival rates of cSCC tumor bearing mice in different treatment groups.
Tumor growth was observed after the treatments (LIT, laser, imiquimod, and untreated control) ended (day 0). The results of the tumor volume from day 0 to day 60 are shown in Figure 6B. On day 27, the tumor volumes of mice in the untreated control group and imiquimod group increased significantly, which were higher than before treatment (P < 0.05). However, there was no significant increase in LIT group and laser group (P > 0.05) compared with before treatment. The tumor volumes of mice in LIT group and laser group were significantly lower than that in the control group (P < 0.05). The tumor volumes of mice in laser group increased along with imiquimod and control group after day 27. On day 60, only the tumor volumes of mice in LIT group had no difference from before treatment (P > 0.05), which were much lower than laser group (P < 0.05).
The representative photographs of mice after treatments are shown in Figure 6C. The tumors in control, imiquimod and laser groups became larger and more numerous with time. In 4 weeks after treatments, mice in control and imquimod groups had more cSCC lesions and the old lesions became obviously larger than before treatment. The number and volume of tumors in laser group increased mildly. The tumors in the mice treated by LIT disappeared in 4 weeks without obviously relapse. Six weeks after treatments, all mice in control group died. The numbers and volumes of tumors in laser group and imiquimod group increased obviously. However, there were no apparent increases in the numbers or volumes of tumors in LIT group.
The survival rates of mice in 60 days are shown in Figure 6D. All mice died within 40 days in the control group, and within 50 days in the imiquimod group. In the laser group, two mice died on day 52 and day 53, respectively. No mice in LIT group died within 60 days. The survival rate of LIT group was remarkablely higher than the control, imiquimod groups and laser group (P < 0.05).
Compared to control group, no obvious side effect was observed in imquimod, laser and LIT groups.
2.7. LIT with Optimal Thermal Effects for Patient with Refractory cSCC
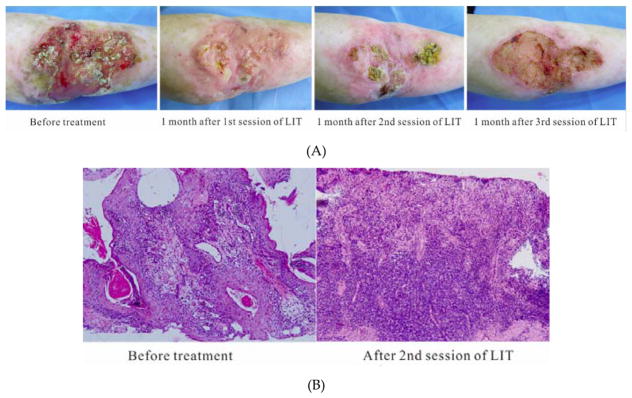
A 63-year old woman suffering from refractory cSCC was treated by a 10-week cycle of LIT with optimal thermal effects for three sessions (Figure 7A, B). During the treatment, the mean temperature on the tumor surface was controlled around 45 °C – 50 °C (Figure 7C). After one time of laser irradiation, the irradiated region appeared erythema immediately. A week later, the irradiated regions appeared crusts. After 1 session of LIT treatment, the oozing and bleeding disappeared. The surface of ulceration became dry and skin was growing from the edge to the center. The area of tumor was much smaller than before treatment. The skin temperature around the lesion returned to normal. After 2 sessions, the area of tumor became smaller further and the nodules disappeared or became flat (Figure 7A). The pale skin surrounding the tumor became ruddy. The motion ability of the right elbow joint recovered. Histopathology showed that there were more infiltrating lymphocytes in the upper and medial dermis than before treatment (Figure 7B), indicating that anti-tumor immune responses were enhanced by the LIT. After 3 sessions of LIT treatment, the tumor area was reduced furtherly and most nodules disappeared, leaving a superficial ulcer with a dry surface (Figure 7A). The updated blood routine showed her hemoglobin increased to 92 g/l. Besides the erythema and crusts, no other adverse reactions, such as edema, erosion, scarring or pain, was recorded.
Figure 7.
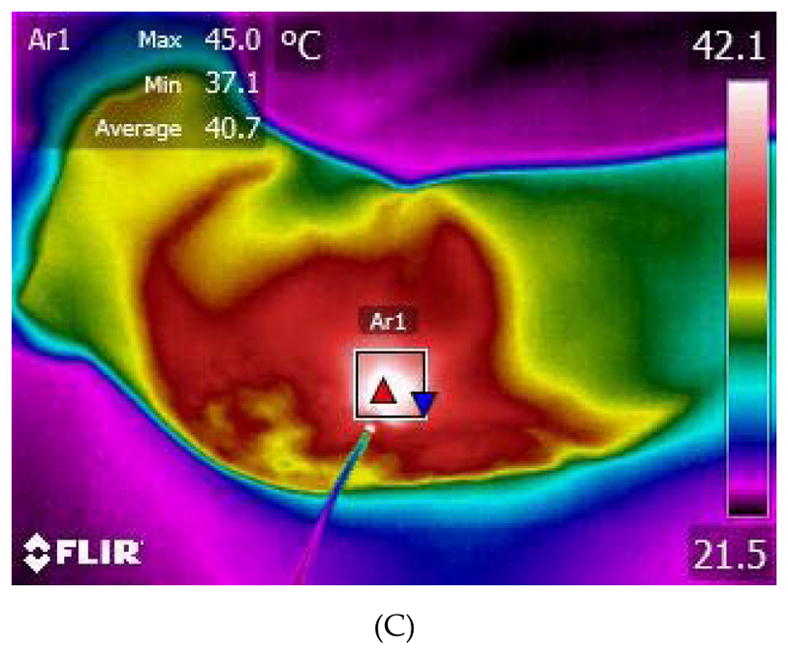
Laser immunotherapy (LIT) with optimal thermal effects for the patient with refractory cutaneous squamous cell carcinoma (cSCC) in clinic. (A) Representative photographs of a patient with cSSC before and after LIT treatments. (B) Histopathology of the patient before and after 2 sessions of LIT treatments at 100×. (C) A thermographic image of the patient being treated by laser.
3. Discussion
Cutaneous squamous cell carcinoma (cSCC) typically manifested as a spectrum of progressively advanced malignancies, ranging from a precursor actinic keratosis to squamous cell carcinoma in situ, invasive squamous cell carcinoma, and finally metastatic squamous cell carcinoma [34]. However, for large, invasive, and metastatic cSCC, traditional treatments, such as surgery, chemotherapy, and radiotherapy, were often unsatisfactory [35, 36]. In addition, the traditional treatments had many disadvantages, including destruction of normal tissue structure and function, dose limitation, and high recurrence rate [37, 38].
Laser immunotherapy (LIT) is a novel approach using a combination of PTT and immunological stimulation, imiquimod [39]. The temperature increase generated by PTT has an important role in killing tumor cells and activating immune response. We investigated the correlation between temperature increase and tumor cell death or DAMPs release/expression using the 10-minutes water-bath heating in vitro in the study. The results showed that temperature increase killed A31 cells in a temperature-dependent manner (Figure 1). Temperatures in the range of 37 °C– 40 °C had no obvious effect on killing A431 cells. Temperatures in the range of 45 °C –50 °C induced around half of cells to appear apoptosis or necrosis. A temperature increase of 55 °C –60 °C induced most A431 cell to be necrotic. The temperature increase of more than 45 °C was able to induce A431 cells to do die [19]. The temperature increase also promoted the extracellular release and the intracellular expression of DAMPS. Cells heated at 45°C released the maximum amount of HSP90 and HMGB1 and expressed the maximum amount of HSP90 and HMGB1. Cells heated at 50 °C released the maximum amount of HSP70. Therefore, the temperature increase of 45 °C – 50 °C could enhance the maximum amount of DAMPs releasing or expressing after PTT, considered as the optimal thermal effects temperature. DAMPs induced by temperature increase could furtherly activated immunogenic host response [25, 40, 41]. These signs were suggestive of programmed cell death-related damage signals which may lead to immunogenic tumor cell death (ICD) and ultimately to the activation of potent anticancer immunity [42–45]. ICD requires antigen linked spatiotemporal DAMP signals including the cell surface translocation of HSP70 and HSP90 followed by the passive release of HMGB1 protein from the nucleus during the late apoptotic stage [46–48]. Heat shock proteins are a family of conserved chaperones induced by cell stress including oxidative stress, irradiation, chemotherapeutic drugs, and heat and electromagnetic field [49]. In the cytoplasm, overexpressed HSP70 and HSP90 can inhibit apoptosis and act as a cytoprotector maintaining protein homeostasis [50, 51]. However, extracellular HSP can directly boost the immune response [52, 53]. Studies have demonstrated that local temperature increase could induce release of HSP from tumor cells[41]. HMGB1 represents a late signal of ICD with diverse roles. In the nucleus, it acts as a non-histone chromatin binding protein interacting with the minor groove of DNA and regulatory molecules such as p53, NF-κB, and steroid hormone receptors [54]. Upon cell stress, HMGB1 is released either from necrotic or apoptotic cells [48]. Extracellular HMGB1 can be a cytokine-like activator of macrophages, a chemotactant for neutrophils and a promoter of dendritic cell maturation through TLR 2/4 [47, 55], thereby activating innate immunity and leading to acquired responses. From the above, the temperature between 45 °C – 50 °C could induce the maximum DAMPs, having an optimal thermal effect for the ICD of cSCC cells.
Salamatus et al. found that temperature at 40 °C stimulated HSP70 release strongly [56]. As temperature increasing, this rapid secretion pathway of HSP 70 became progressively inhibited. When the temperature reached 55 °C, the active secretion would be abolished. In our study, it was 45 °C – 50 °C not 40 °C released the maximum HSPs. It was because that A431 cells were allowed to recover at 37 °C for 24 h after a 10-minutes heating. In the cooling process, cells continued to release HSPs due to a concomitant damage to the plasma membrane [56].
Application of imiquimod was also an important component of LIT. We further investigated the effect of the imiquimod dose on proliferation of A431 cells and DAMPs release during heat treatment. Our results showed that imiquimod could directly kill A431 cells in a dose-dependent manner (as shown in Figure 4A) [57, 58]. Besides, low concentration imiquimod could further increase the release of HSP70, HSP90 and HMGB1 during the heat treatment at 45 °C – 50 °C, which suggests that imiquimod could enhance the optimal thermal effect of temperature increase in promoting DAMPs release (as shown in Figure 4).
Then, we used LIT with optimal thermal dose (45°C –50 °C) to treat UV-induced cSCC in immune competent SKH-1 mice. The UV-induced cSCC could mimic the human skin squamous cell carcinoma perfectly [59]. In the study, the imiquimod monotherapy could only slow down the tumor growth and improve survival time slightly, which were consistent with the clinical reports of topical imiquimod treatment for cSCC [60, 61]. PTT monotherapy could also slow down the tumor growth and significantly improve survival time, which were consistent with the reports that PTT could kill squamous cell carcinoma and other cancer cells [62]. However, PTT monotherapy had no effect on the untreated tumors, which suggested that PTT had a limited systemic effect. Compared to imiquimod or PTT monotherapy, LIT could not only destroy local tumors, but also at the same time induced a potent systemic, tumor-specific immunological response to slow, and sometimes eradicate, untreated tumors at distant sites, as shown in Figure 6A–D. Our therapeutic effects, along with the lack of noticeable side effects, demonstrated that LIT was a safe and effective treatment approach for cSCC, especially for multiple, large, late-stage metastatic, or other inoperable cSCCs.
In the clinical practice, we used LIT with optimal thermal effect to treat an old woman suffering from a large refractory cSCC with the oozing and bleeding. After 3 sessions of the 10-week cycle of LIT, the area of tumor was much smaller than before treatment and most tumor nodules disappeared, leaving a superficial ulcer with a dry surface. In addition, the temperature and color of the skin around the lesion returned to normal. The motion ability of the right elbow joint recovered. The moderate anemia restored to normal. Meanwhile, the histopathology showed that there were more infiltrating lymphocytes in the upper and medial dermis than before the treatment (Figure 7B), indicating that LIT successfully activated the anti-tumor immune responses. The tumor cells were killed by the ICD caused by LIT. During or after treatment, only the temporary erythema and crusts were observed. No other obvious adverse reaction was recorded on the patient. The above results suggested LIT was an effective and safe method for cSCC in the clinic. Unfortunately, the tumor wasn’t eliminated completely by LIT in this short-term therapeutic scheme. It might be because the area was too large to be eliminated in a short-term. And the local anti-tumor immune responses of the patient were diminished by the age, anemia and cervical spondylopathy.
4. Materials and Methods
4.1. Chemicals and Reagents
High glucose Dulbecco’s modified eagle medium (DMEM), phosphate buffer saline (PBS), and penicillin/streptomycin were obtained from HyClone™ (Utah, USA). Fetal bovine serum (FBS) was obtained from Gibco (Billings, USA). Rabbit monoclonal anti-actin and rabbit monoclonal anti-HMGB1 (Cell Signaling Technology, Leiden, Netherlands), mouse monoclonal anti-HSP70, and rabbit polyclonal anti-HSP90 (Abcam, Cambridge, United Kindom) were used. Human HSP70 ELISA Kit (Ebioscience™, California, USA), human HSP90 ELISA Kit (Ebioscience™, California, USA), and human HMGB1 ELISA Kit (Shino-Test, Tokyo, Japan) were also used. In addition, FITC Annexin V Apoptosis Detection Kit I (BD, New Jersey, USA) and Chromogenic Western Blot Immunodetection Kit (Thermo Scientific, Massachusetts, USA) were also used. Imiquimod lyophilized powders were purchased from Invivogen(California, USA) and imiquimod creams were purchased from Sichuan Med-shine Pharmaceutical (Chengdu, China) were used.
4.2. Cell Culture
Human cSCC cell line A431 was purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. The cells were maintained in high glucose DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin antibiotics at 37 °C in an atmosphere of 5% CO2.
4.3. Heat Treatment of Cells
A431 cells were randomly divided into six heat treatment groups: 37 °C, 40 °C, 45 °C, 50 °C, 55 °C and 60 °C. The A431 cells (2×106/dish) growing in 60 mm dish were placed in a water bath with different temperature settings. The heating time was 10 min. Then, the A431 cells were cultured at 37 °C in an atmosphere of 5% CO2 after replacement of equal fresh medium for different durations (the recovery time). At designated time points, supernatants and heat-treated A431 cells were collected for different experiments.
4.4. Measurement of Cell Viability
The heat-treated A431 cells were observed with an inverted microscope at 8 h and 24 h to determine the cell morphological change. In addition, the heat-treated A431 cells (2×104/well) were seeded in 96-well plates immediately and incubated for 8 h and 24 h at 37 °C before being subjected to CCK8 assay to detect cell proliferation. Each group had 5 wells and the experiment was repeated three times. Survival rate = (OD treatment group-OD blank group)/(OD 37°C control group-OD blank group) × 100%. The cells (5×105 cells/group) were also collected at 8 h and 24 h after heat treatment and then washed in PBS, followed by incubation with FITC-AnnexinV and PI for 20 min at room temperature in the dark. After incubation, the cells were analyzed with flow cytometer to detect the percentage of cell apoptosis and necrosis.
4.5. Quantification of the Extracellular Release of DAMPs (HSP70, HSP90 and HMGB1)
In order to evaluate the release of HSP70, HSP90 and HMGB1 in response to temperature increase, the supernatants of the A431 cells were collected and centrifuged at different time points (1 h, 4 h, 8 h, 12 h and 24 h) after heat treatment with different temperature settings. Then, the supernatants were analyzed using ELISA-based HSP70, HSP90 and HMGB1 detection kits according to the manufacturer’s instructions.
4.6. Intracellular Expression of DAMPs (HSP70, HSP90 and HMGB1)
To determine the effect of temperature increase on intracellular expressions of HSP70, HSP90 and HMGB1, A431 cells were heat-treated with different temperature settings and were measured at 8 h after heat-treated. The correlation of DAMPs expression with time was also investigated via the measurement in 45 °C at different recovery time points (1 h, 4 h, 8 h, 12 h and 24 h). The proteins of heat-treated A431cells were lysed with radioimmunoprecipitation assay (RIPA) buffer (Tris base 50 mM, NaCl 150 mM, NP40 1%, sodium deoxycholate 0.25%, EDTA 1 mM) containing a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). The protein concentration was measured using a BCA protein assay kit (Pierce, Rockford, Illinois, USA). Equal amounts of proteins were separated by SDS-polyacrylamide gel electrophoresis on 8% gels and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, California, USA). The membranes were blocked with blocking solution according to the manufacturer’s instructions of the Chromogenic Western Blot Immunodetection Kit and then were incubated with primary antibodies (anti-HSP70, anti-HSP90 and anti-HMGB1) overnight at 4 °C. The membranes were then washed three times (15 min each time) with antibody wash solution, and were incubated with secondary antibody for 1 h at room temperature. The color reaction was developed using chromogenic substrate.
4.7. Effect of Imiquimod on Proliferation of A431 Cells
Aqueous solution of imiquiod was prepared using the powder of imiquimod. A431 cells (2×104/well) were incubated with imiquimod of different concentrations (0 μg/ml, 5 μg/ml, 10 μg/ml, 20 μg/ml, 50 μg/ml, and 100 μg/ml) for 24 h before being subjected to CCK8 assay. Each group had 5 wells and the experiment was repeated three times.
4.8. Effect of Imiquimod on Release of DAMPs (HSP70, HSP90 and HMGB1) by A431 Cells During Heat Treatment
A431 cells (2×106/dish) were seeded in 60 mm dish and incubated with 10 μg/ml or 50 μg/ml imiquimod for 24 h before being subjected to heat treatment with different temperature settings. Then, the A431 cells were continuously incubated with 10 μg/ml or 50 μg/ml imiquimod. The supernatants were collected and analyzed using ELISA-based HSP70, HSP90 and HMGB1 detection kits according to the manufacturer’s instructions, at determined time points (1 h to 24 h) after the heat treatment.
4.9. cSCC Tumors in SKH-1 Mice Induced by UV Irradiation
Immune competent SKH-1 mice (female, 6–8 weeks old, hairless) were purchased from Shanghai Public Health Clinical (Shanghai certificate number 2015–0002, China). cSCC on the back of mice were induced by solar-simulated ultraviolet irradiation (SS-03B type UV-B phototherapy instrument, SIGMA, Shanghai, China), as described previously[63]. When the papules arose in the irradiated back and grew up to a size of 1–4 mm in diameter, a histopathological examination was performed to confirm the lesion was the cSCC.
4.10. LIT with Optimal Thermal Effects for UV-induced cSCC Mice
Mice bearing UV-induced cSCC were randomly divided into four groups: LIT, laser, imiquimod, and untreated control (5 mice per group). Mice in LIT group were irradiated by an 808-nm laser (New industry photoelectric technology Co., Changchun, China) once every two weeks for a total of three times, and imiquimod cream was topically applied once a day for three days before and after each laser irradiation. Mice in laser group only received irradiation of the 808-nm laser as above. Mice in imiquimod group only received topical application of imiquimod cream as in the LIT group. Mice in control group were not given any treatment. Laser light was delivered to the tumor noninvasively using a fiber optic delivery system (Pioneer Optics Company, USA). The power density at the treatment area, which encompassed the tumor and 2 to 5 mm of the surrounding skin, was 1 W/cm2 for a treatment duration of 10 min, based on the previous protocol developed by Chen and co-workers [15] and the results in cell experiment in our study. The therapeutic targets were three largest tumors on the back. During laser irradiation, mice were anesthetized with an intraperitoneal injection of 7% hydrate of chlorine aldehyde. The surface temperature of the treated tumor was monitored by a thermal imaging camera (T420, FLIR, Oregon, USA) during laser irradiation to ensure the superficial temperatures of tumor were in the range of 45°C –50°C. If temperatures were higher than 55 °C, the irradiation should be discontinued until the temperatures dropped to 45 °C. After 3 times of treatment (day 0), the mice were observed daily for a period of 60 days, and the tumor were measured twice a week. Tumor volume was calculated using the formula: V=1/2ab2 (mm3) (a: length, b: width). Mice with a maximum tumor diameter >15 mm were euthanized, following the guidelines on Administration of Lab Animals.
4.11. LIT with Optimal Thermal Effects for One Patient with Refractory cSCC in clinic
A 63-year old woman presented with previously untreated bleeding ulcer on the right elbow came to our hospital. The patient suffered 30 years ago from neck pain and numbness of the right upper extremity. The patient was diagnosed as cervical spondylopathy and treated with a surgery. One year after the surgery, the numbness of right upper extremity became more serious due to damage of the nerve with cervical spondylopathy. The skin on her right upper extremity, especially her right forearm, became pale with a low skin temperature. Gradually, her right elbow developed a bleeding ulcer. In the past 30 years, the lesion grew larger and larger without any improvements on bleeding or ulceration. In April of 2014, the patient came to our hospital. Clinical investigation showed a ulcer of about 6×9 cm on her right elbow, accompanied with oozing, bleeding and multiple nodules covered by crust. The skin surrounding the ulcer was pale with a low skin temperature. The right elbow joint suffered from stiffness and loss of motion. A blood routine test showed her hemoglobin was only 53 g/l. A biopsy revealed the diagnosis of skin squamous cell carcinoma, grade I. The patient refused surgical excision in consideration of the large area of the ulcer. We treated the patient with LIT after a consent form was signed in June 2014.
A 10-week cycle of LIT with optimal thermal effects was carried out with the following steps: (1) imiquimod cream was topically applied to the surface of tumor every other day for 10 weeks. (2) The tumor were irradiated by an 808-nm laser once every two weeks for four times, and the first laser irradiation was carried out at the end of week 2. The power density at the treatment area, which encompassed of 2 cm2 the skin lesions total for 3 to 4, was also 1 W/cm2 for a treatment duration of 10 min. During laser irradiation, the superficial temperatures of tumor were monitored within the range of 45 °C – 50 °C. If temperatures were higher than 55 °C, the irradiation would be discontinued until the temperatures dropped to 45°C. The patient was treated with the 10-week cycle of LIT for three sessions. The treatment interval was one month. A histopathological examination was performed again after 2 sessions of LIT.
4.12. Statistical Analyses
Data were analyzed with Spass13.0 software and presented as mean ± standard deviation. All statistical analyses were performed using t-test except survival rates were compared using Mantel-Cox logrank test. P < 0.05 was considered statistically significant.
5. Conclusions
In conclusion, the temperature in the range of 45 °C – 50 °C could induce the maximum DAMPs, having an optimal thermal effect in inducing the ICD of cSCC cells. Imiquimod could improve the effect of PTT for generating DAMPs. The LIT composing of PTT with the optimal thermal effect and imiquimod was an effective and safe method for the treatments of cSCC either in mice or in the patient. Our results provided the basic understanding and pilot study of PTT in inducing immune response in cSCC treatment, particularly when used in combination with an immunostimulant, paving the way to further develop LIT into an effective clinical treatment modality for cSCC, especially for multiple, large, late-stage metastatic, or other inoperable cSCCs.
Acknowledgments
This work was funded in part by the National Natural Science Foundation of China (81472796, 81472538, 81601601), the Joint Project of New Frontier Technology of Shanghai Shen-kang Hospital Development Center (SHDC12015123), Yang Fan Program of Science and Technology Commission of Shanghai Municipality (15YF1410700), Multicenter Clinical Study of Early Diagnosis and Treatment for Facial Non-melanoma Skin Cancers, the Sub-topic of Key Project of Natural Science Foundation of Shanghai (15411950302), the grants from the US National Institutes of Health (R21 EB015509, RS20132225-106, R01 CA205348), and the Oklahoma Center for Advancement of Science and Technology (HR16-085).
Abbreviations
- cSCC
Cutaneous squamous cell carcinoma
- DAMPs
Damage-associated molecular patterns
- LIT
Laser immunotherapy
- PTT
Photothermal therapy
- HSP
Heat shock protein
- HMGB1
High mobility group protein B1
- UV
Ultraviolet
- TLR
Toll-like receptors
- ELISA
Enzyme linked immunosorbent assay
- ICD
Immunogenic tumor cell death
- IMQ
Imiquimod
Footnotes
Supplementary Materials: Supplementary materials can be found at www.mdpi.com/link.
Author Contributions: Wei R. Chen, Xiuli Wang and Lei Shi conceived and designed the experiments. Min Luo, Lei Shi, Fuhe Zhang and Haiyan Zhang performed the experiments. Feifang Zhou, Bo Wang, Peiru Wang Degang Yang and Guolong Zhang reviewed the study proposal and served as scientific advisor. Xiuli Wang, Wei R. Chen, Linglin Zhang and Haiyan Zhang contributed reagents and materials; Min Luo, Lei Shi, Feifang Zhou and Bo Wang analyzed the data; Min Luo, Lei Shi, Feifang Zhou Wei R. Chen, Xiuli Wang wrote and revised the paper.
Conflicts of Interest: The authors declare no conflict of interest.
References
- 1.Dotto GP, Rustgi AK. Squamous Cell Cancers: A Unified Perspective on Biology and Genetics. Cancer cell. 2016;29(5):622–637. doi: 10.1016/j.ccell.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. Journal of the American Academy of Dermatology. 2013;68(6):957–66. doi: 10.1016/j.jaad.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Ji J, Zhang H, Fan Z, Zhang L, Shi L, Zhou F, Chen WR, Wang H, Wang X. Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget. 2015;6(42):44688–702. doi: 10.18632/oncotarget.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parikh SA, Patel VA, Ratner D. Advances in the management of cutaneous squamous cell carcinoma. F1000prime reports. 2014;6:70. doi: 10.12703/P6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchinson L. Skin cancer: Setting the stage for cutaneous SCC. Nature reviews Clinical oncology. 2014;11(2):63. doi: 10.1038/nrclinonc.2014.2. [DOI] [PubMed] [Google Scholar]
- 6.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey M, Chang AL, Miska J, Wainwright DA, Ahmed AU, Balyasnikova IV, Pytel P, Han Y, Tobias A, Zhang L, Qiao J, Lesniak MS. Dendritic Cell-Based Vaccines that Utilize Myeloid Rather than Plasmacytoid Cells Offer a Superior Survival Advantage in Malignant Glioma. Journal of immunology (Baltimore, Md: 1950) 2015;195(1):367–76. doi: 10.4049/jimmunol.1401607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benci JL, Xu B, Qiu Y, Wu TJ, Dada H, Twyman-Saint Victor C, Cucolo L, Lee DS, Pauken KE, Huang AC, Gangadhar TC, Amaravadi RK, Schuchter LM, Feldman MD, Ishwaran H, Vonderheide RH, Maity A, Wherry EJ, Minn AJ. Tumor Interferon Signaling Regulates a Multigenic Resistance Program to Immune Checkpoint Blockade. Cell. 2016;167(6):1540–1554. e12. doi: 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. International Journal of Molecular Sciences. 2014;15(1):927–43. doi: 10.3390/ijms15010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zindl CL, Chaplin DD. Immunology. Tumor immune evasion. Science. 2010;328(5979):697–8. doi: 10.1126/science.1190310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruella M, Maus MV. Catch me if you can: Leukemia Escape after CD19-Directed T Cell Immunotherapies. Comput Struct Biotechnol J. 2016;14:357–362. doi: 10.1016/j.csbj.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WR, Carubelli R, Liu H, Nordquist RE. Laser immunotherapy: a novel treatment modality for metastatic tumors. Molecular biotechnology. 2003;25(1):37–44. doi: 10.1385/MB:25:1:37. [DOI] [PubMed] [Google Scholar]
- 13.Chen WR, Liu H, Ritchey JW, Bartels KE, Lucroy MD, Nordquist RE. Effect of different components of laser immunotherapy in treatment of metastatic tumors in rats. Cancer research. 2002;62(15):4295–9. [PubMed] [Google Scholar]
- 14.Zhou F, Wu S, Song S, Chen WR, Resasco DE, Xing D. Antitumor immunologically modified carbon nanotubes for photothermal therapy. Biomaterials. 2012;33(11):3235–42. doi: 10.1016/j.biomaterials.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Ferrel GL, Guerra MC, Hode T, Lunn JA, Adalsteinsson O, Nordquist RE, Liu H, Chen WR. Preliminary safety and efficacy results of laser immunotherapy for the treatment of metastatic breast cancer patients. Photochem Photobiol Sci. 2011;10(5):817–21. doi: 10.1039/c0pp00306a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X, Naylor MF, Le H, Nordquist RE, Teague TK, Howard CA, Murray C, Chen WR. Clinical effects of in situ photoimmunotherapy on late-stage melanoma patients: a preliminary study. Cancer Biol Ther. 2010;10(11):1081–7. doi: 10.4161/cbt.10.11.13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Shi L, Luo M, Chen J, Wang B, Zhang F, Keyal U, Bhatta AK, Chen WR, Wang X. Successful treatment of Rosai-Dorfman disease using in situ photoimmunotherapy. Indian journal of dermatology, venereology and leprology. 2017;83(3):332–336. doi: 10.4103/ijdvl.IJDVL_356_16. [DOI] [PubMed] [Google Scholar]
- 18.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nature reviews. Cancer. 2014;14(3):199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 19.Zhang HG, Mehta K, Cohen P, Guha C. Hyperthermia on immune regulation: a temperature’s story. Cancer letters. 2008;271(2):191–204. doi: 10.1016/j.canlet.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. The Journal of surgical research. 2005;127(2):208–23. doi: 10.1016/j.jss.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 21.Ito A, Shinkai M, Honda H, Wakabayashi T, Yoshida J, Kobayashi T. Augmentation of MHC class I antigen presentation via heat shock protein expression by hyperthermia. Cancer immunology, immunotherapy: CII. 2001;50(10):515–22. doi: 10.1007/s00262-001-0233-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.den Brok MH, Sutmuller RP, van der Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer research. 2004;64(11):4024–9. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Ji J, Zhang H, Fan Z, Zhang L, Shi L, Zhou F, Chen WR, Wang H, Wang X. Stimulation of dendritic cells by DAMPs in ALA-PDT treated SCC tumor cells. Oncotarget. 2015 doi: 10.18632/oncotarget.5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz FE, Dantas E, Cabrera M, Benitez CA, Delpino MV, Duette G, Rubione J, Sanjuan N, Trevani AS, Geffner J. Fever-range hyperthermia improves the anti-apoptotic effect induced by low pH on human neutrophils promoting a proangiogenic profile. Cell death & disease. 2016;7(10):e2437. doi: 10.1038/cddis.2016.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frey B, Weiss EM, Rubner Y, Wunderlich R, Ott OJ, Sauer R, Fietkau R, Gaipl US. Old and new facts about hyperthermia-induced modulations of the immune system. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2012;28(6):528–42. doi: 10.3109/02656736.2012.677933. [DOI] [PubMed] [Google Scholar]
- 26.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Critical reviews in oncology/hematology. 2002;43(1):33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 27.Repasky EA, Evans SS, Dewhirst MW. Temperature matters! And why it should matter to tumor immunologists. Cancer immunology research. 2013;1(4):210–6. doi: 10.1158/2326-6066.CIR-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans SS, Repasky EA, Fisher DT. Fever and the thermal regulation of immunity: the immune system feels the heat. Nature reviews Immunology. 2015;15(6):335–49. doi: 10.1038/nri3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meinander A, Soderstrom TS, Kaunisto A, Poukkula M, Sistonen L, Eriksson JE. Fever-like hyperthermia controls T Lymphocyte persistence by inducing degradation of cellular FLIPshort. Journal of immunology (Baltimore, Md: 1950) 2007;178(6):3944–53. doi: 10.4049/jimmunol.178.6.3944. [DOI] [PubMed] [Google Scholar]
- 30.Cui S, Yin D, Chen Y, Di Y, Chen H, Ma Y, Achilefu S, Gu Y. In vivo targeted deep-tissue photodynamic therapy based on near-infrared light triggered upconversion nanoconstruct. ACS nano. 2013;7(1):676–88. doi: 10.1021/nn304872n. [DOI] [PubMed] [Google Scholar]
- 31.Sharma SK, Mroz P, Dai T, Huang YY, St Denis TG, Hamblin MR. Photodynamic Therapy for Cancer and for Infections: What Is the Difference? Isr J Chem. 2012;52(8–9):691–705. doi: 10.1002/ijch.201100062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song S, Zhou F, Chen WR, Xing D. PDT-induced HSP70 externalization up-regulates NO production via TLR2 signal pathway in macrophages. FEBS Lett. 2013;587(2):128–35. doi: 10.1016/j.febslet.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki M, Kawakami N, Kawai K, Kanekura T. Cutaneous mucormycosis in bone marrow transplantation recipients. Eur J Dermatol. 2012;22(4):578–9. doi: 10.1684/ejd.2012.1770. [DOI] [PubMed] [Google Scholar]
- 34.Ratushny V, Gober MD, Hick R, Ridky TW, Seykora JT. From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. The Journal of clinical investigation. 2012;122(2):464–72. doi: 10.1172/JCI57415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA dermatology. 2016;152(4):419–28. doi: 10.1001/jamadermatol.2015.4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manyam BV, Gastman B, Zhang AY, Reddy CA, Burkey BB, Scharpf J, Alam DS, Fritz MA, Vidimos AT, Koyfman SA. Inferior outcomes in immunosuppressed patients with high-risk cutaneous squamous cell carcinoma of the head and neck treated with surgery and radiation therapy. Journal of the American Academy of Dermatology. 2015;73(2):221–7. doi: 10.1016/j.jaad.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 37.Barlow BR, Marshall RV, Wofford JD. Giant cutaneous squamous cell carcinoma requiring emergent embolization. JAAD case reports. 2016;2(3):216–8. doi: 10.1016/j.jdcr.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burton KA, Ashack KA, Khachemoune A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. American journal of clinical dermatology. 2016;17(5):491–508. doi: 10.1007/s40257-016-0207-3. [DOI] [PubMed] [Google Scholar]
- 39.Chen WR, Adams RL, Carubelli R, Nordquist RE. Laser-photosensitizer assisted immunotherapy: a novel modality for cancer treatment. Cancer letters. 1997;115(1):25–30. doi: 10.1016/s0304-3835(97)04707-1. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi T, Kakimi K, Nakayama E, Jimbow K. Antitumor immunity by magnetic nanoparticle-mediated hyperthermia. Nanomedicine (London, England) 2014;9(11):1715–26. doi: 10.2217/nnm.14.106. [DOI] [PubMed] [Google Scholar]
- 41.Werthmoller N, Frey B, Ruckert M, Lotter M, Fietkau R, Gaipl US. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2016;32(1):23–30. doi: 10.3109/02656736.2015.1106011. [DOI] [PubMed] [Google Scholar]
- 42.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochimica et biophysica acta. 2010;1805(1):53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Zitvogel L, Kepp O, Kroemer G. Decoding cell death signals in inflammation and immunity. Cell. 2010;140(6):798–804. doi: 10.1016/j.cell.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Palm NW, Medzhitov R. Pattern recognition receptors and control of adaptive immunity. Immunological reviews. 2009;227(1):221–33. doi: 10.1111/j.1600-065X.2008.00731.x. [DOI] [PubMed] [Google Scholar]
- 45.Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends in immunology. 2011;32(4):157–64. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 46.D’Eliseo D, Manzi L, Velotti F. Capsaicin as an inducer of damage-associated molecular patterns (DAMPs) of immunogenic cell death (ICD) in human bladder cancer cells. Cell stress & chaperones. 2013;18(6):801–8. doi: 10.1007/s12192-013-0422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krysko O, Love Aaes T, Bachert C, Vandenabeele P, Krysko DV. Many faces of DAMPs in cancer therapy. Cell death & disease. 2013;4:e631. doi: 10.1038/cddis.2013.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sangiuliano B, Perez NM, Moreira DF, Belizario JE. Cell death-associated molecular-pattern molecules: inflammatory signaling and control. Mediators of inflammation. 2014;2014:821043. doi: 10.1155/2014/821043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binder RJ. Functions of heat shock proteins in pathways of the innate and adaptive immune system. Journal of immunology (Baltimore, Md: 1950) 2014;193(12):5765–71. doi: 10.4049/jimmunol.1401417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rozenberg P, Kocsis J, Saar M, Prohaszka Z, Fust G, Fishelson Z. Elevated levels of mitochondrial mortalin and cytosolic HSP70 in blood as risk factors in patients with colorectal cancer. International journal of cancer. 2013;133(2):514–8. doi: 10.1002/ijc.28029. [DOI] [PubMed] [Google Scholar]
- 51.Soudry E, Stern Shavit S, Hardy B, Morgenstern S, Hadar T, Feinmesser R. Heat shock proteins HSP90, HSP70 and GRP78 expression in medullary thyroid carcinoma. Annals of diagnostic pathology. 2017;26:52–56. doi: 10.1016/j.anndiagpath.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Horvath I, Vigh L. Cell biology: Stability in times of stress. Nature. 2010;463(7280):436–8. doi: 10.1038/463436a. [DOI] [PubMed] [Google Scholar]
- 53.Andocs G, Meggyeshazi N, Balogh L, Spisak S, Maros ME, Balla P, Kiszner G, Teleki I, Kovago C, Krenacs T. Upregulation of heat shock proteins and the promotion of damage-associated molecular pattern signals in a colorectal cancer model by modulated electrohyperthermia. Cell stress & chaperones. 2015;20(1):37–46. doi: 10.1007/s12192-014-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nature reviews Cancer. 2012;12(12):860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 55.Ladoire S, Hannani D, Vetizou M, Locher C, Aymeric L, Apetoh L, Kepp O, Kroemer G, Ghiringhelli F, Zitvogel L. Cell-death-associated molecular patterns as determinants of cancer immunogenicity. Antioxidants & redox signaling. 2014;20(7):1098–116. doi: 10.1089/ars.2012.5133. [DOI] [PubMed] [Google Scholar]
- 56.Mambula SS, Calderwood SK. Heat induced release of Hsp70 from prostate carcinoma cells involves both active secretion and passive release from necrotic cells. International journal of hyperthermia: the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group. 2006;22(7):575–85. doi: 10.1080/02656730600976042. [DOI] [PubMed] [Google Scholar]
- 57.Schon M, Bong AB, Drewniok C, Herz J, Geilen CC, Reifenberger J, Benninghoff B, Slade HB, Gollnick H, Schon MP. Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod. Journal of the National Cancer Institute. 2003;95(15):1138–49. doi: 10.1093/jnci/djg016. [DOI] [PubMed] [Google Scholar]
- 58.Sohn KC, Li ZJ, Choi DK, Zhang T, Lim JW, Chang IK, Hur GM, Im M, Lee Y, Seo YJ, Lee JH, Kim CD. Imiquimod induces apoptosis of squamous cell carcinoma (SCC) cells via regulation of A20. PloS one. 2014;9(4):e95337. doi: 10.1371/journal.pone.0095337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Orazio J, Jarrett S, Amaro-Ortiz A, Scott T. UV radiation and the skin. International Journal of Molecular Sciences. 2013;14(6):12222–48. doi: 10.3390/ijms140612222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin-Garcia RF. Imiquimod: an effective alternative for the treatment of invasive cutaneous squamous cell carcinoma. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al.] 2005;31(3):371–4. doi: 10.1111/j.1524-4725.2005.31093. [DOI] [PubMed] [Google Scholar]
- 61.Wester A, Eyler JT, Swan JW. Topical imiquimod for the palliative treatment of recurrent oral squamous cell carcinoma. JAAD case reports. 2017;3(4):329–331. doi: 10.1016/j.jdcr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickerson EB, Dreaden EC, Huang X, El-Sayed IH, Chu H, Pushpanketh S, McDonald JF, El-Sayed MA. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer letters. 2008;269(1):57–66. doi: 10.1016/j.canlet.2008.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang H, Li J, Lv T, Tu Q, Huang Z, Wang X. Therapeutic and immune effects of 5-aminolevulinic acid photodynamic therapy on UVB-induced squamous cell carcinomas in hairless mice. Experimental dermatology. 2013;22(5):362–3. doi: 10.1111/exd.12132. [DOI] [PubMed] [Google Scholar]