Abstract
OBJECTIVE:
To compare birth weight and weight gain in HIV-exposed, uninfected infants up to 24 months old, who enrolled in the Malawian national HIV care clinic (HCC) programme either before or after Option B+ (OB+) was implemented.
DESIGN, SETTING and PARTICIPANTS:
HIV-exposed infants enrol in the HCC programme as soon as possible after birth and are followed up to at least 24 months old. This analysis includes HIV-exposed, uninfected (HEU) infants with recorded birth weight, date of birth, gender and at least one follow-up weight measurement from 21 health facilities in central and southern Malawi (January 2010 - December 2014). Weight-for-age z-scores (WAZ) were derived and compared by birth period using linear regression at birth and mixed effects models for postnatal weight gain up to 24 months old.
RESULTS:
Of 6,845 HEU infants included in this study, 88.5% were born after OB+. The proportion of infants exposed in-utero to combination antiretroviral therapy (ART) significantly increased after OB+ was implemented, and infants were exposed to ART for a longer time. There was no significant difference in WAZ at birth (p = 0.654) among HEU infants by birth period, but postnatal weight gain was faster among HEU infants born in the Option B+ period than infants born pre-Option B+.
CONCLUSION:
Birth weight was not affected by longer exposure to ART during pregnancy after OB+ was introduced, when weight gain in HEU infants was faster, possibly because their mothers were in better health.
Keywords: HIV-exposed uninfected infant, Option B+, weight-for-age z-scores, weight gain
INTRODUCTION
Scaling up prevention of mother-to-child transmission (PMTCT) interventions has drastically lowered the number of HIV-infected children worldwide. Without intervention, the likelihood of mother-to-child transmission (MTCT) of HIV ranges from 15% to 45%, but effective PMTCT interventions can reduce this risk to under 5% [1]. In Malawi, PMTCT coverage remained sub-optimal until 2011, when the Ministry of Health (MoH) pioneered implementation of universal lifelong combination antiretroviral therapy (ART) for pregnant and breastfeeding women regardless of their CD4 cell count or clinical stage (“Option B+” (OB+)) [2]. As a consequence of the scale-up of OB+, ART coverage in pregnant and breastfeeding women has increased and MTCT risk of HIV declined [3] resulting in a growing number of HIV-exposed, uninfected (HEU) children.
Although scaling up of PMTCT has greatly improved maternal health, there is concern that in-utero exposure to antiretrovirals may negatively affect birth outcomes, growth and development of infants born to HIV-infected mothers [4–6]. Some studies reported that in-utero antiretroviral exposure is associated with low birth weight [6–8]; others found no such association [9–12]. Some studies found differences in weight gain [13–17] between HEU infants and healthy controls. A systematic review published in 2014 was inconclusive about the effect of in-utero HIV and antiretroviral exposure on postnatal weight gain of HIV-exposed infants [4].
Although some studies have examined the association between in-utero ART exposure and adverse birth outcomes in the OB+ era [18,19], no study has investigated if OB+ affects the growth of HEU infants. We investigated the impact of OB+ on postnatal weight gain of Malawian HEU infants in the first 24 months of their lives under programmatic circumstances.
METHODS
Study setting
Malawi’s MoH provides integrated services for HIV-exposed infants in the national HIV care clinic (HCC) programme under Integrated HIV Management guidelines [20] as previously described [21]. Programme data suggest that most known HIV-exposed infants are enrolled and managed in this programme with regular clinic follow-up from 6 weeks after birth [20,22].
Study design and inclusion criteria
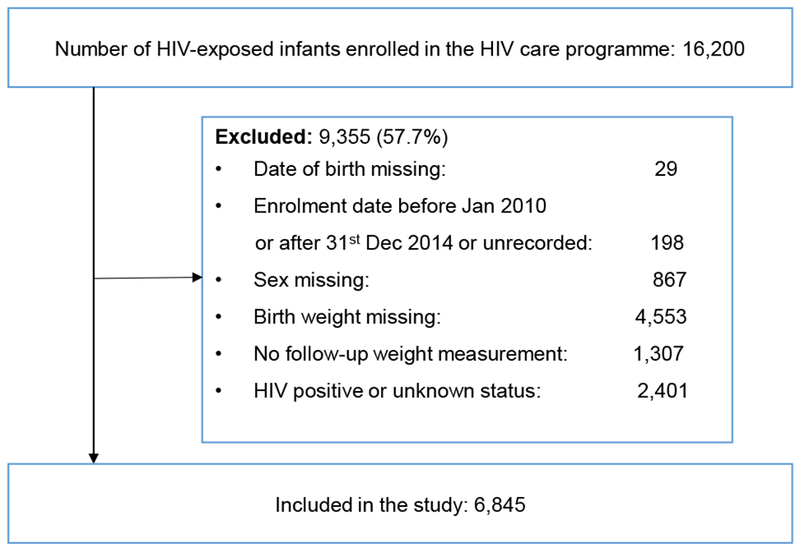
We included infants born to an HIV-infected woman, who enrolled in the HCC programme between January 2010 and December 2014 at any of the 21 large health facilities in central and southern Malawi that participated in our study to evaluate the implementation of OB+ (www.umoyoplus.org) (Figure 1). The facilities included 13 district hospitals, two central hospitals, three faith based hospitals, and three large health centres. All infants who had a negative HIV test result were included. HIV-infected infants diagnosed positive at any point during follow-up and infants with unknown HIV status were excluded. We also excluded infants with missing birth date, birth weight or sex, and infants with no follow-up weight measurement. Infants were included in the analysis from enrolment until the last recorded follow-up visit.
Figure 1: Study participants.
Data collection, preparation and management
Registration and follow-up data for HIV-exposed infants enrolled in the HCC programme are recorded on standardized paper-based treatment cards stored at health facilities. At each visit, trained health care workers measure infant’s weight with a mechanical scale that is calibrated daily [23]. Routinely collected data were digitized from the treatment cards, followed by double data entry and cleaning. Records were de-duplicated using probabilistic linkage [21].
Definitions and outcomes
Programmatic outcomes at the end of follow-up were defined as under follow-up, died, discharged uninfected, transferred out to another health facility, or lost to follow-up (LTFU). LTFU was defined as missing a clinic appointment for more than 60 days and not returning to care. ART was defined as a combination of at least three antiretroviral drugs. Before OB+, women eligible for ART received a combination of stavudine, lamivudine and nevirapine. Women not eligible for ART received zidovudine monotherapy during pregnancy followed by zidovudine + lamivudine dual therapy or single-dose nevirapine during labour. During OB+, all women received a combination of tenofovir, lamivudine and efavirenz.
We defined birth period as pre-Option B+ (pre-OB+) or Option B+ (OB+) using the date each facility switched to OB+. Infants born within a few months of the health facility’s switch to OB+ were classified into the OB+ group, although they were not necessarily exposed to OB+ from the beginning of pregnancy. We converted birth weight and weight measurements into age- and sex-adjusted z-scores, based on the WHO 2006 standard [24].
The primary outcome was postnatal weight gain in the first 24 months of life, assessed with weight-for-age z scores (WAZ). We also assessed factors associated with birth weight (WAZ at birth).
Statistical analyses
Baseline characteristics by birth period were compared with Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables. We used linear regression to investigate differences in WAZ at birth, and mixed effects models to examine changes in WAZ over time (with a random effect on the infant and fixed effects on the intercept and explanatory variables). We used a third order polynomial transformation for age (in months) because weight gain is not linear. We tested the effect of covariates on WAZ at birth and during follow-up, and for an interaction between the polynomial transformation of age and birth period, with a likelihood ratio test. We also did stratified analyses by birth period to identify predictors of postnatal weight gain in each birth period. All available weight measurements from enrolment up to the last follow-up visit (including infants who were LTFU, transferred out or who died) were included in the analysis. All mixed effects models were adjusted for the polynomial transformation of age. In multivariable models, we adjusted for infant’s gender, birth weight (<2.5kg, ≥2.5kg), type of health facility (health centre, faith-based hospital, district hospital, central hospital), and infant antiretroviral exposure during pregnancy or labour (none, mono- or dual-therapy, ART <4 weeks, or ART ≥4 weeks at any stage).
Results from models are presented as differences in WAZ with 95% confidence intervals. All analyses were performed in STATA version 14 (Stata Corporation, College Station, Texas, USA) and p-values <0.05 were considered significant.
The Malawi National Health Sciences Research Committee and the Cantonal Ethics Committee of Bern granted ethical approval.
RESULTS
Of the 16,200 infants enrolled in the HCC programme between February 2010 and May 2015, 9,355 infants were excluded, of whom 1,318 (14.1%) were born during the pre-OB+ and 8,008 (85.6%) during the OB+ period. This resulted in a study population of 6,845 HEU infants with a median follow-up of 11.3 months (interquartile range (IQR): 5.1-17.0 months). (Figure 1). Baseline characteristics of included and excluded infants were significantly different except for birth weight and sex (Appendix, Table A1).
Baseline characteristics by birth period
A total of 784 (11.5%) HEU infants were born in the pre-OB+ period and 6,061 (88.5%) in the OB+ period (Table 1). By the end of follow-up, 33.4% (n=2,285) of HEU infants were LTFU, 0.4% (n=27) had died, and 1.9% (n=127) had transferred to another facility. More HEU infants were LTFU in the pre-OB+ group (48.3%) than in the OB+ group (31.5%); rates of LTFU were 3.5 per 100 person-months (379/10756) in the pre-OB+ group and 2.7 per 100 person-months (1906/70298) in the OB+ group. Infants in the OB+ group were younger at enrolment, more likely exposed to ART in-utero (85.1% vs 64.1%) and ART exposure was longer (74.1% vs 42.2% on ART for ≥4 weeks), than those in the pre-OB+ group.
Table 1:
Characteristics of HIV-exposed, uninfected children by birth period
| Child birth period |
||||
|---|---|---|---|---|
| All infants (n = 6,845) | Pre Option B+ (n=784; 11.5%) | Option B+ (n=6,061; 88.5%) | P-value## | |
| Age at enrolment (months), median (IQR) | 2.0 (1.0-3.0) | 4.0 (2.0-9.0) | 2.0 (1.0-2.0) | < 0.0001 |
| Gender, n (%) | ||||
| Female | 3,455 (50.5) | 391 (49.9) | 3,064 (50.6) | 0.720 |
| Male | 3,390 (49.5) | 393 (50.1) | 2,997 (49.4) | |
| PEP at birth, n (%) | ||||
| No | 534 (7.8) | 136 (17.3) | 398 (6.6) | < 0.0001 |
| Yes | 5,999 (87.6) | 596 (76.0) | 5,403 (89.1) | |
| Unknown | 312 (4.7) | 52 (6.6) | 260 (4.3) | |
| Birth weight, n (%) | ||||
| <2.5kg | 823 (12.0) | 97 (12.4) | 726 (12.0) | 0.749 |
| ≥2.5kg | 6,022 (88.0) | 687 (87.6) | 5,335 (88.0) | |
| Birth weight (kg), median (IQR) | 3.0 (2.7-3.4) | 3.0 (2.8-3.4) | 3.0 (2.7-3.4) | 0.210 |
| In-utero antiretroviral exposure, n (%)1 | ||||
| No antiretrovirals | 464 (6.8) | 109 (13.9) | 355 (5.9) | < 0.0001 |
| Mono- or dual-therapy at any stage | 125 (1.8) | 125 (15.9) | -- | |
| ART< 4 weeks at any stage | 837 (12.2) | 172 (21.9) | 665 (11.0) | |
| ART ≥ 4 weeks at any stage | 4,820 (70.4) | 331 (42.2) | 4,489 (74.1) | |
| Unknown | 599 (8.8) | 47 (6.0) | 552 (9.1) | |
| Mother’s postpartum status | ||||
| No ART | 177 (2.6) | 84 (10.7) | 93 (1.5) | < 0.0001 |
| ART | 6,276 (91.7) | 590 (75.3) | 5,686 (93.8) | |
| ART with interruptions# | 364 (5.3) | 102 (13.0) | 262 (4.3) | |
| Died | 9 (0.1) | 3 (0.4) | 6 (0.1) | |
| Unknown | 19 (0.3) | 5 (0.6) | 14 (0.2) | |
| Follow-up duration (months), median (IQR) | 11.3 (5.1-17.0) | 12.0 (6.7-17.7) | 11.2 (4.9-16.9) | 0.004 |
| Outcome at the end of follow-up, n (%) | ||||
| Lost to follow-up* | 2,285 (33.4) | 379 (48.3) | 1,906 (31.5) | < 0.0001 |
| Discharged | 1,337 (19.5) | 362 (46.2) | 975 (16.1) | |
| Dead | 27 (0.4) | 6 (0.8) | 21 (0.4) | |
| Transferred out | 127 (1.9) | 13 (1.7) | 114 (1.9) | |
| Under follow-up | 3,069 (44.8) | 24 (3.1) | 3,045 (50.2) | |
| Facility type, n (%) | ||||
| Central hospital | 637 (9.3) | 100 (12.8) | 537 (8.9) | < 0.0001 |
| Health centre | 1,003 (14.7) | 60 (7.7) | 943 (15.6) | |
| Faith based hospital | 625 (9.1) | 105 (13.4) | 520 (8.6) | |
| District hospital | 4,580 (66.9) | 519 (66.2) | 4,061 (67.0) | |
ART was defined as a combination of at least three antiretroviral drugs. Before OB+, women eligible for ART received a combination of stavudine, lamivudine and nevirapine. Women not eligible for ART received zidovudine monotherapy during pregnancy followed by zidovudine + lamivudine dual therapy or single-dose nevirapine during labour. During OB+, all women received a combination of tenofovir, lamivudine and efavirenz.
ART: Antiretroviral therapy; PEP: Post exposure prophylaxis (children received zidovudine (AZT) in pre-Option B+ and nevirapine (NVP) during Option B+); IQR: Interquartile range.
Represents women who missed some ART visits to the health facility
Infants who missed a clinic appointment for more than 60 days and did not return to care
P values from Wilcoxon rank-sum tests (continuous variables) and Chi-square tests (categorical variables).
Factors affecting birth weight
There were no statistically significant differences in WAZ at birth among infants born before and after introduction of OB+ (Table 2). Infants enrolled at a health centre or district hospital had significantly higher WAZ at birth than infants enrolled at a central hospital. Exposure to antiretrovirals during pregnancy or labour, irrespective of regimen or duration, had no significant effect on WAZ at birth.
Table 2:
Linear regression analysis of weight-for-age z-scores (WAZ) at birth, birth weight; n=6,845
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-value* | β | 95% CI | P-value* | |
| Birth period | ||||||
| Pre Option B+ | 0 | 0.590 | 0 | 0.654 | ||
| Option B+ | −0.029 | −0.133; 0.075 | −0.027 | −0.145; 0.091 | ||
| Gender | ||||||
| Male | 0 | 0.928 | 0 | 0.801 | ||
| Female | 0.003 | −0.063; 0.069 | −0.009 | −0.077; 0.060 | ||
| In-utero antiretroviral exposure | ||||||
| No antiretrovirals | 0 | 0.163 | 0 | 0.188 | ||
| Mono- or dual-therapy at any stage | 0.131 | −0.142; 0.404 | 0.156 | −0.133; 0.444 | ||
| ART <4weeks at any stage | −0.090 | −0.247; 0.067 | −0.091 | −0.248; 0.066 | ||
| ART ≥4weeks at any stage | −0.096 | −0.228; 0.036 | −0.093 | −0.226; 0.040 | ||
| Facility type | ||||||
| Central hospital | 0 | 0.0003 | 0 | 0.0002 | ||
| Health centre | 0.259 | 0.120; 0.398 | 0.281 | 0.141; 0.421 | ||
| Faith based hospital | 0.022 | −0.132; 0.176 | 0.025 | −0.132; 0.182 | ||
| District hospital | 0.153 | 0.037; 0.269 | 0.138 | 0.022; 0.255 | ||
ART: Antiretroviral therapy; CI: Confidence interval
P values from likelihood ratio test
If the analysis was stratified by birth period, associations were similar (Appendix, Tables A2a and A2b). In the adjusted analysis the association between type of facility and WAZ at birth was however only present in the OB+ group.
Factors affecting postnatal weight gain
The interaction between polynomial transformation of age and birth period was not significant in univariable analysis (p-value 0.287) and was therefore not included in the model. Postnatal WAZ was higher in the OB+ group (difference 0.211, 95% CI 0.117–0.306; p<0.0001) irrespective of age after adjusting for gender, birth weight, facility type and maternal antiretroviral exposure (Table 3 and Figure 2). Other predictors of WAZ over time included a birth weight of ≥2.5kg and female gender. Infant’s exposure to antiretrovirals during pregnancy or labour, regardless of type of regimen or length of exposure, had no significant impact on postnatal WAZ in the univariable analysis. Infants exposed to ART for ≥4 weeks during pregnancy had significantly lower WAZ in the first 24 months of life in adjusted analyses. However, when we stratified the analysis by birth period (Appendix, Tables 3a and 3b), only infant gender and type of health facility had a significant effect on WAZ in the OB+ period, and there was no effect of type and duration of antiretroviral exposure on WAZ in either birth period.
Table 3:
Mixed-effects model of weight-for-age z-scores (WAZ) over time (age: 0-24 months)
| Univariable* |
Multivariable** |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | P-value# | β | 95% CI | P-value# | |
| Birth period | ||||||
| Pre Option B+ | 0 | <0.0001 | 0 | <0.0001 | ||
| Option B+ | 0.180 | 0. 095; 0.265 | 0.211 | 0.117; 0.306 | ||
| Gender | ||||||
| Male | 0 | 0.0001 | 0 | <0.0001 | ||
| Female | 0.108 | 0.054; 0.162 | 0.132 | 0.078; 0.186 | ||
| Birth weight | ||||||
| <2.5kg | 0 | <0.0001 | 0 | <0.0001 | ||
| ≥2.5kg | 1.093 | 1. 013; 1.172 | 1.108 | 1.025; 1.191 | ||
| In-utero antiretroviral exposure | ||||||
| No antiretrovirals | 0 | 0.851 | 0 | 0.042 | ||
| Mono- or dual-therapy at any stage | 0.025 | −0.200; 0.251 | 0.180 | −0.050; 0.410 | ||
| ART <4 weeks at any stage | 0.054 | −0.078; 0.185 | 0.040 | −0.085; 0.165 | ||
| ART ≥4 weeks at any stage | −0.018 | −0.128; 0.092 | −0.056 | −0.162; −0.050 | ||
| Facility type | ||||||
| Central hospital | 0 | <0.0001 | 0 | <0.0001 | ||
| Health centre | −0.050 | −0.163; 0.064 | −0.071 | −0.181; 0.040 | ||
| Faith based hospital | −0.290 | −0.415; −0.165 | −0.290 | −0.413; −0.166 | ||
| District hospital | −0.098 | −0.192; −0.005 | −0.135 | −0.226; −0.044 | ||
ART: Antiretroviral therapy; CI: Confidence interval; p-value from a likelihood ratio test
The univariable model assessed the association between WAZ and each variable individually with inclusion of the polynomial transformation of age.
The multivariable model assessed the association between WAZ and birth period adjusting for all independent variables and the polynomial transformation of age.
P values from likelihood ratio test
Figure 2:
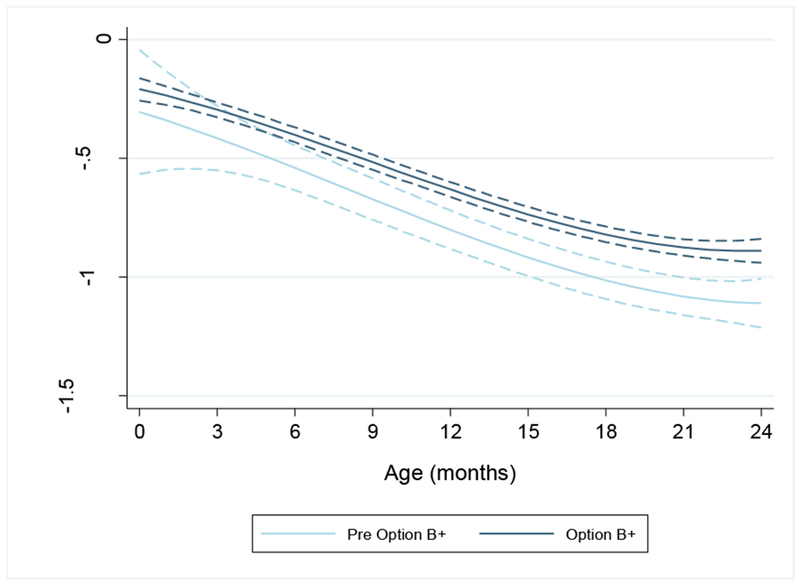
Predicted z-scores from univariable mixed effects model for weight-for-age by birth period (i.e. pre Option B+ and Option B+) adjusted for the polynomial transformation of age (months). Dotted lines represent 95% confidence intervals
DISCUSSION
The proportion of infants exposed to ART during pregnancy or labour increased significantly after OB+ was implemented, and infants were exposed to ART for a longer time. WAZ was similar at birth among HEU infants born before and after OB+, but postnatal weight gain was higher in HEU infants born in the OB+ period than infants born in the pre-OB+ period.
Prolonged exposure to ART, more common in the OB+ period, may increase adverse pregnancy outcomes like preterm delivery and low birth weight [25]. The European collaborative study found an association between prematurity and ART exposure, for all types of regimens [9]. Some studies from resource-limited settings found that lower birth weight was associated with in-utero ART exposure [8,26,27] but did not seem to impair postnatal growth [8,27,28].
We found that neither the type of antiretroviral regimen, nor length of in-utero ART exposure was associated with WAZ at birth in HEU infants consistent with other African studies [12,14,29]. Most infants in our study were exposed to ART in-utero, so a potential negative effect of ART was probably counter balanced by the favourable immunological and virological response to ART in the pregnant woman, resulting in improved maternal health that benefits infants’ outcomes. However, we had no data on CD4 cell counts and viral loads during pregnancy.
Disentangling the exact effect of the birth period, the type and length of ART exposure, and the CD4 cell count on WAZ at birth and longitudinal weight gain was not possible. Since we compared the pre-OB+ period with the OB+ period, the number of drugs and the types of antiretroviral regimens depended on the birth period. During the pre-OB+ period, women who were on combination ART had low CD4 cell counts or were in an advanced stage of the disease. The association of being on ART ≥4 weeks with slower weight gain may therefore be driven by observations in the pre-OB+ period. However, we could not confirm this hypothesis in stratified analyses, possibly because of the low sample size.
Since we did not capture the date of ART initiation, we also do not know if the women had started ART pre-conception, in the first trimester, or in the last two trimesters. This is a relevant limitation because a study from Brazil showed that birth weight was lower in infants with exposure to ART in the first trimester than later exposure or no exposure to ART in-utero [30]. Discrepant findings between studies may be related to differences in the populations studied, variations in antenatal and postnatal care and contrasting study designs (e.g. adjustment for different confounders and different selection criteria).
A number of additional factors were associated with postnatal WAZ. Contrary to other studies [31–35], we found no significant differences in WAZ at birth between boys and girls. However, girls had higher postnatal WAZ than boys, in agreement with a study done in South Africa [31]. HEU infants with normal weight at birth (≥2.5kg) had higher postnatal WAZ than those with low birth weight (<2.5kg) consistent with previous studies [17,36,37].
We faced several limitations. The first was our inability to determine if OB+ itself spurred improvement in postnatal weight gain, or if other factors were responsible. In developing countries, poverty, socio-economic conditions, malnutrition, and poor health affect the growth and development of children under the age of five [38]. Socio-economic, environmental and immunological factors are also associated with under-nutrition in children born to HIV-infected mothers [17,39,40]. We did not adjust for these factors because we had no data. Second, in routine clinical care LTFU is often high. Weight measurements are biased by LTFU, because children with impaired weight gain are more likely to be LTFU and to die. Therefore, we anticipate that weight gain was particularly overestimated in the pre OB+ group. Third, we only included large health facilities, so our findings may not be representative of infants from smaller rural health centres. Fourth, birth length was not recorded, and height was sporadically recorded during follow-up visits, so we could not compare length/height-for-age and weight-for-length/height z-scores over time. Fifth, we determined birth period according to infant’s date of birth. When a clinic implemented OB+, all women attending antenatal care, including those who were already on zidovudine prophylaxis, were instructed to switch to triple life-long ART at their next visit. In practice, however, the transition to OB+ may have taken some time, and infants born soon after the switch to OB+ were not exposed to the policy for the entire period. Therefore, inclusion of HEU infants born in the transition period in the OB+ group underestimates the positive effect of OB+ on weight gain. Finally, we excluded a large number of infants due to missing data whose characteristics were different from those included.
Our study has a number of strengths. We believe we are the first to assess weight gain in routine care during the first 24 months of life for HEU infants in the OB+ era. Our study had a large sample size, and followed HEU infants over a long period. Our study facilities were geographically diverse and included a variety of clinical settings.
Our finding of better postnatal weight gain among HEU infants born in the OB+ period is encouraging considering the widespread implementation of OB+. To confirm our findings, future studies should include small health centres and account for socio-economic characteristics, infant feeding practices, and maternal disease progression. OB+ increased exposure to ART during pregnancy. The increase did not affect birth weight, and seems to have improved postnatal weight gain in HEU infants, possibly because OB+ also improved mothers’ health.
Supplementary Material
Acknowledgements
We would like to thank the health facility staff and the staff from the Ministry of Health and the Christian Health Association of Malawi (CHAM), who supported data collection at the sites. We thank the data entry team (Abigail Nkukumila, Alick Momba, Ashton Mwechumu, Bazaliel Chimosola (deceased), Chikondi Milanzi, Enock Chauwa, Florence Kamunga, Gomezgani Nyasulu, Imelda Banda (deceased), Jestina Mhango, Kondwani Nyirenda, Lloyd Mkomela, Memory Dzonzi, Monica Chimbaza, Olivia Kamanga, Racheal Gonani, Takondwa Zidana, Zelipher Ziyenda), who diligently entered our data into an electronic database. Finally, we thank Baobab Health Trust for the support provided during the course of this study.
Declaration of interests
Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number U01AI069924. Additional support was provided by the Bill and Melinda Gates Foundation (Global Health Grant OPP1090200), The United States Agency for International Development- Partnerships for Enhanced Engagement in Research Health (PEER Health) grant AID OAA-A-11-0012. OK was supported by a professorship grant from the Swiss National Science Foundation (grant number 163878). The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsors.
References
- 1.World Health Organization. Mother-to-child transmission of HIV [Internet]. WHO; [cited 2017 Oct 17]. Available from: http://www.who.int/hiv/topics/mtct/about/en/ [Google Scholar]
- 2.Schouten EJ, Jahn A, Midiani D, Makombe SD, Mnthambala A, Chirwa Z, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. The Lancet. 2011;378(9787):282–4. [DOI] [PubMed] [Google Scholar]
- 3.Chimbwandira F, Mhango E, Makombe S, Midiani D, Mwansambo C, Njala J, et al. Impact of an innovative approach to prevent mother-to-child transmission of HIV-Malawi, July 2011-September 2012. Morb Mortal Wkly Rep. 2013;62(8):148–151. [PMC free article] [PubMed] [Google Scholar]
- 4.Jao J, Abrams EJ. Metabolic Complications of in utero Maternal HIV and Antiretroviral Exposure in HIV-exposed Infants Pediatr Infect Dis J. 2014;33(7):734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darak S, Darak T, Kulkarni S, Kulkarni V, Parchure R, Hutter I, et al. Effect of Highly Active Antiretroviral Treatment (HAART) During Pregnancy on Pregnancy Outcomes: Experiences from a PMTCT Program in Western India. AIDS Patient Care STDs. 2013;27(3):163–70. [DOI] [PubMed] [Google Scholar]
- 6.Ekouevi DK, Coffie PA, Becquet R, Tonwe-Gold B, Horo A, Thiebaut R, et al. Antiretroviral therapy in pregnant women with advanced HIV disease and pregnancy outcomes in Abidjan, Côte d’Ivoire. AIDS Lond Engl. 2008. September 12;22(14):1815–20. [DOI] [PubMed] [Google Scholar]
- 7.Briand N, Le Coeur S, Traisathit P, Karnchanamayul V, Hansudewechakul R, Ngampiyasakul C, et al. Growth of human immunodeficiency virus-uninfected children exposed to perinatal zidovudine for the prevention of mother-to-child human immunodeficiency virus transmission. Pediatr Infect Dis J. 2006;25(4):325–332. [DOI] [PubMed] [Google Scholar]
- 8.Powis KM, Smeaton L, Ogwu A, Lockman S, Dryden-Peterson S, van Widenfelt E, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr 1999. 2011;56(2):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Collaborative Study. Exposure to antiretroviral therapy in utero or early life: the health of uninfected children born to HIV-infected women. J Acquir Immune Defic Syndr 1999. 2003;32(4):380–7. [DOI] [PubMed] [Google Scholar]
- 10.Cotter AM, Garcia AG, Duthely ML, Luke B, O’Sullivan MJ. Is antiretroviral therapy during pregnancy associated with an increased risk of preterm delivery, low birth weight, or stillbirth? J Infect Dis. 2006. May;193(9):1195–201. [DOI] [PubMed] [Google Scholar]
- 11.Tuomala RE, Shapiro DE, Mofenson LM, Bryson Y, Culnane M, Hughes MD, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346(24):1863–70. [DOI] [PubMed] [Google Scholar]
- 12.Szyld EG, Warley EM, Freimanis L, Gonin R, Cahn PE, Calvet GA, et al. Maternal antiretroviral drugs during pregnancy and infant low birth weight and preterm birth. AIDS Lond Engl. 2006;20(18):2345–53. [DOI] [PubMed] [Google Scholar]
- 13.Rosala-Hallas A, Bartlett JW, Filteau S. Growth of HIV-exposed uninfected, compared with HIV-unexposed, Zambian children: a longitudinal analysis from infancy to school age. BMC Pediatr. 2017;17(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siberry GK, Williams PL, Mendez H, Seage GR, Jacobson DL, Hazra R, et al. Safety of tenofovir use during pregnancy: early growth outcomes in HIV-exposed uninfected infants. AIDS Lond Engl. 2012;26(9):1151–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paul ME, Chantry CJ, Read JS, Frederick MM, Lu M, Pitt J, et al. Morbidity and mortality during the first two years of life among uninfected children born to human immunodeficiency virus type 1-infected women: the women and infants transmission study. Pediatr Infect Dis J. 2005;24(1):46–56. [DOI] [PubMed] [Google Scholar]
- 16.Makasa M, Kasonka L, Chisenga M, Sinkala M, Chintu C, Tomkins A, et al. Early growth of infants of HIV-infected and uninfected Zambian women. Trop Med Int Health. 2007;12(5):594–602. [DOI] [PubMed] [Google Scholar]
- 17.Muhangi L, Lule SA, Mpairwe H, Ndibazza J, Kizza M, Nampijja M, et al. Maternal HIV infection and other factors associated with growth outcomes of HIV-uninfected infants in Entebbe, Uganda. Public Health Nutr. 2013;16(9):1548–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rempis EM, Schnack A, Decker S, Braun V, Rubaihayo J, Tumwesigye NM, et al. Option B+ for prevention of vertical HIV transmission has no influence on adverse birth outcomes in a cross-sectional cohort in Western Uganda. BMC Pregnancy Childbirth. 2017;17(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chagomerana MB, Miller WC, Pence BW, Hosseinipour MC, Hoffman IF, Tweya H, et al. PMTCT Option B+ Does Not Increase Preterm Birth Risk and May Prevent Extreme Prematurity: A Retrospective Cohort Study in Malawi. J Acquir Immune Defic Syndr. 2017;74(4):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ministry of Health Malawi. Clinical Management of HIV In Children and Adults [Internet]. 2014. [cited 2017 Dec 8]. Available from: http://apps.who.int/medicinedocs/documents/s18802en/s18802en.pdf
- 21.Haas AD, van Oosterhout JJ, Tenthani L, Jahn A, Zwahlen M, Msukwa MT, et al. HIV transmission and retention in care among HIV-exposed children enrolled in Malawi’s prevention of mother-to-child transmission programme. J Int AIDS Soc. 2017;20(1):21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ministry of Health Malawi. Integrated HIV Program Report: October -December 2014 [Internet]. 2014. [cited 2017 Dec 8]. Available from: https://www.hiv.health.gov.mw/cha_repository/file.php/92/92.pdf
- 23.Ministry of Health (MOH). Guidelines for Community-Based Management of Acute Malnutrition. 2nd Edition [Internet]. 2016. [cited 2017 Dec 11]. Available from: https://www.fantaproject.org/sites/default/files/resources/Malawi-CMAM-Guidelines-Dec2016.pdf
- 24.Leroy Jef L (2011). zscore06: Stata command for the calculation of anthropometric z-scores using the 2006 WHO child growth standards https://EconPapers.repec.org/RePEc:boc:bocode:s457279.
- 25.Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206(11):1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Njom Nlend AE, Nga Motazé A, Moyo Tetang S, Zeudja C, Ngantcha M, Tejiokem M. Preterm Birth and Low Birth Weight after In Utero Exposure to Antiretrovirals Initiated during Pregnancy in Yaoundé, Cameroon. PloS One. 2016;11(3):e0150565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibb DM, Kizito H, Russell EC, Chidziva E, Zalwango E, Nalumenya R, et al. Pregnancy and Infant Outcomes among HIV-Infected Women Taking Long-Term ART with and without Tenofovir in the DART Trial. PLOS Med. 2012;9(5):e1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Viganò A, Mora S, Giacomet V, Stucchi S, Manfredini V, Gabiano C, et al. In utero exposure to tenofovir disoproxil fumarate does not impair growth and bone health in HIV-uninfected children born to HIV-infected mothers. Antivir Ther. 2011;16(8):1259–66. [DOI] [PubMed] [Google Scholar]
- 29.van der Merwe K, Hoffman R, Black V, Chersich M, Coovadia A, Rees H. Birth outcomes in South African women receiving highly active antiretroviral therapy: a retrospective observational study. J Int AIDS Soc. 2011;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofer CB, Keiser O, Zwahlen M, Lustosa CS, Frota ACC, de Oliveira RH, et al. In Utero Exposure to Antiretroviral Drugs: Effect on Birth Weight and Growth Among HIV-exposed Uninfected Children in Brazil. Pediatr Infect Dis J. 2016;35(1):71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morden E, Technau K-G, Giddy J, Maxwell N, Keiser O, Davies M-A. Growth of HIV-Exposed Uninfected Infants in the First 6 Months of Life in South Africa: The IeDEA-SA Collaboration. PLOS ONE. 2016;11(4):e0151762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Natchu UCM, Liu E, Duggan C, Msamanga G, Peterson K, Aboud S, et al. Exclusive breastfeeding reduces risk of mortality in infants up to 6 mo of age born to HIV-positive Tanzanian women. Am J Clin Nutr. 2012;96(5):1071–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and Health Outcomes of HIV-Exposed and Unexposed Children in a PMTCT Cohort in Malawi. PLoS ONE. 2012;7(10):e47337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taha TE, Dadabhai SS, Rahman MH, Sun J, Kumwenda J, Kumwenda NI. Trends in birth weight and gestational age for infants born to HIV-infected, antiretroviral treatment-naive women in Malawi. Pediatr Infect Dis J. 2012;31(5):481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Homsy J, Moore D, Barasa A, Were W, Likicho C, Waiswa B, et al. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-Infected women on highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr 1999. 2010;53(1):28–35. [DOI] [PubMed] [Google Scholar]
- 36.Arpadi S, Fawzy A, Aldrovandi GM, Kankasa C, Sinkala M, Mwiya M, et al. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009;90(2):344–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel D, Bland R, Coovadia H, Rollins N, Coutsoudis A, Newell M-L. Breastfeeding, HIV status and weights in South African children: a comparison of HIV-exposed and unexposed children. AIDS. 2010;24(3):437–445. [DOI] [PubMed] [Google Scholar]
- 38.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ram M, Gupte N, Nayak U, Kinikar AA, Khandave M, Shankar AV, et al. Growth patterns among HIV-exposed infants receiving nevirapine prophylaxis in Pune, India. BMC Infect Dis. 2012;12(1):282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkatesh KK, Lurie MN, Triche EW, De Bruyn G, Harwell JI, McGarvey ST, et al. Growth of infants born to HIV-infected women in South Africa according to maternal and infant characteristics. Trop Med Int Health TM IH. 2010;15(11):1364–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


