Abstract
Objectives:
To investigate whether gender is associated with three recommended stages of the HIV care continuum and whether gender modifies known associations between level of alcohol use and HIV care among US veterans.
Design:
Retrospective cohort.
Methods:
Veterans Aging Cohort Study data were used to identify Veterans Health Administration (VA) patients with HIV and AUDIT-C alcohol screening 2/1/2008–9/30/2014. Modified Poisson regression models estimated the relative risk and predicted prevalences of engagement in HIV care (documented CD4 cells/μl or viral load copies/mL lab values), ART treatment (≥1 prescription), and viral suppression (HIV RNA <500 copies/mL) in the year following AUDIT-C (1) for women compared to men, and (2) for each level of alcohol use compared to nondrinking among women and among men. A multiplicative interaction between gender and alcohol use was tested.
Results:
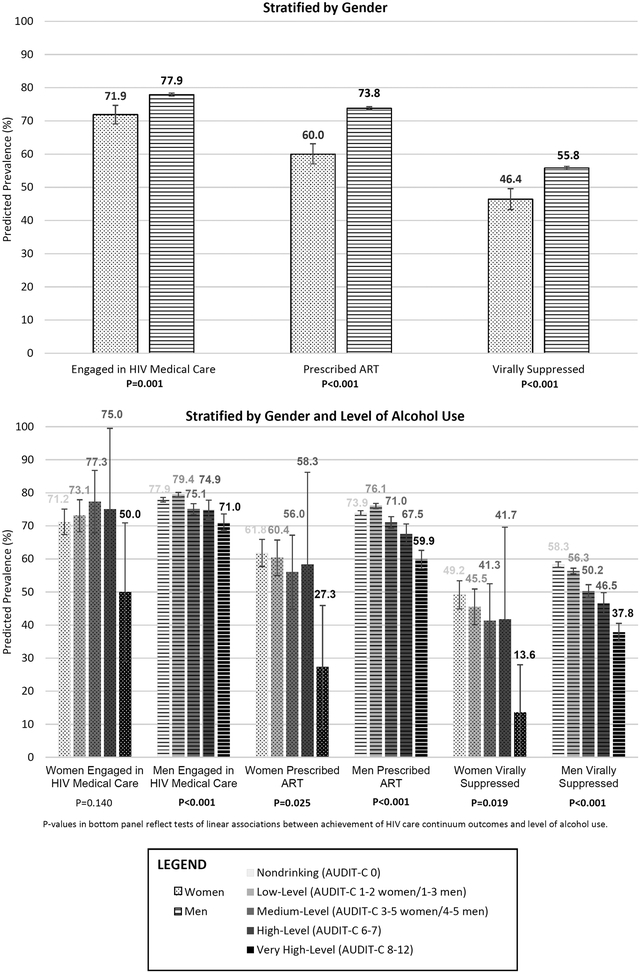
Among 33,224 patients, women (n=971) were less likely than men (n=32,253) to receive HIV care (p-values<0.001). Respective predicted prevalences for women and men were 71.9% (95% CI: 69.1–74.7%) and 77.9% (77.5–78.4%) for engagement, 60.0% (57.0–73.14%) and 73.8% (73.4–74.3%) for ART treatment, and 46.4% (43.3–49.6%) and 55.8% (55.3–56.3%) for viral suppression. Although the interaction between gender and alcohol use was not statistically significant, stratified analyses suggested worse outcomes for women than men at higher levels of alcohol use.
Conclusions:
In this large national cohort, women were less likely than men to be engaged in HIV medical care, prescribed ART, and virally suppressed. Interventions to improve HIV care for women are needed at all levels of alcohol use.
Keywords: Veterans, Women, HIV, Viral Load, Alcohol Drinking, Continuity of Patient Care, Social Determinants of Health
INTRODUCTION.
Despite medical advances that help improve health and slow transmission, HIV is common in the US [1]. To effectively treat and prevent HIV, the Joint United Nations Programme on HIV and AIDS [2] recommends monitoring care targets considered essential, collectively referred to as the HIV care continuum [3]. These include: diagnosis, linkage to HIV care, engagement in care, treatment with antiretroviral therapy (ART), and achievement of viral suppression [2, 4].
In the US, HIV primarily affects men [1]. However, incidence in women rose in the last two decades [5], and, though now declining [1], women make up ~25% of people with HIV (PWH) [1]. For heterosexual acquisition, women are physiologically more susceptible to HIV than men [5, 6] and have increased exposure to social determinants of health that may increase treatment barriers and disease susceptibility [7, 8].
Women may also be more vulnerable than men to the impact of modifiable behavioral factors such as alcohol use that adversely affect HIV-related care and outcomes [9]. Whether gender modifies known associations between level of alcohol use and HIV care among US veterans is unknown. Understanding gender-specific effects of alcohol use on receipt of HIV treatment and outcomes may help develop targeted interventions to increase engagement in HIV care and, ultimately, improve HIV-related outcomes for women and men.
As the nation’s largest integrated health care system and largest provider of HIV care, the Veterans Health Administration (VA) is an ideal laboratory in which to address these issues [4, 10, 11]. In a national cohort of PWH receiving VA care we: (1) examine gender differences in HIV care continuum outcomes, and (2) assess whether gender modifies associations between level of alcohol use and HIV care continuum outcomes.
METHODS
Data Source and Study Cohort.
We used national VA electronic health records (EHR) data from the Veterans Aging Cohort Study (VACS) [10, 12] to identify all patients aged 25–84 with documented HIV and alcohol screening between 2/1/2008, when screening results became widely available [13], and 9/30/2014. International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes determined HIV status based on two outpatient or one inpatient diagnosis code(s) for AIDS (042) and/or HIV infection (V08) [10, 14].
Measures.
Gender.
Gender was measured dichotomously as man or woman based on EHR documentation at the time of VACS enrollment.
Level of Alcohol Use.
Level of alcohol use was measured with the Alcohol Use Disorders Identification Test Consumption (AUDIT-C). The AUDIT-C is administered annually to >90% of established VA outpatients, with results documented in the EHR [15]. AUDIT-C scores range from 0–12 points; higher scores reflect greater consumption, probability of alcohol use disorder [16], and risk of mortality [17]. The first documented AUDIT-C after VACS enrollment was categorized into five levels of alcohol use: non-drinking (AUDIT-C 0), low (1–3 men/1–2 women), medium (4–5 men/3–5 women), high (6–7), and very high (8–12).
Outcomes.
Three HIV care continuum outcomes were measured in the year following AUDIT-C: 1) engagement in HIV care based on any documentation of CD4 (cells/μl) or HIV viral load (copies/mL) lab values [18]; 2) on ART, defined as having filled ≥1 prescription for any antiretroviral medication [18]; and 3) viral suppression measured as having HIV RNA <500 copies/mL [19] on the first available measure following AUDIT-C (<500 copies/mL has remained a target threshold over time even as assays have gotten more sensitive) [20].
Covariates.
Covariates included age at the time of alcohol screening (<50, 50–65, >65); race/ethnicity (black, Hispanic, white, and other/unknown) based on EHR documentation; year of AUDIT-C screening; and dichotomous measures of any mental health disorder (depressive disorder, anxiety, or serious mental illness including schizophrenia, bipolar, and schizoaffective disorders), alcohol use disorder (abuse, dependence, or alcohol-related conditions including cirrhosis), and drug use disorder (opioid, stimulant, cannabis, or other) based on ICD-9-CM codes in the year prior to AUDIT-C.
Analyses.
First, we compared cohort characteristics and level of alcohol use by gender using chi square tests. Next, we used modified Poisson regression models with robust error variances to estimate relative rates of HIV care continuum outcomes for women compared to men. Poisson was used because outcomes were expected to be common [21, 22]. Because we hypothesized a priori that gender would modify associations between level of alcohol use and HIV care outcomes, we also fit models that additionally included level of alcohol use and a multiplicative interaction between gender and alcohol use to estimate gender-specific effects of alcohol use. Non-drinking (AUDIT-C=0) was the referent category because research suggests this level is associated with the lowest level of risk for PWH [9]. We tested whether the relationship between level of alcohol use and each outcome was linear using orthogonal polynomial contrast tests. All models were first unadjusted and then adjusted for all covariates. Unadjusted models were considered primary because they reflect existing differences; adjusted models were fit to account for measured factors that may drive gender and alcohol-related differences in HIV care. To understand the clinical implications of differences in outcomes, we estimated predicted prevalences based on the unadjusted models using recycled predictions.
Because past-year non-drinking does not differentiate between lifetime abstainers and “sick-quitters,” [23] we conducted sensitivity analyses excluding PWH reporting no alcohol use (AUDIT-C=0), with low-level drinking as the referent.
Analyses were conducted using Stata 14 software [24]. Study procedures were approved by VA Connecticut Healthcare System IRB.
RESULTS
Study Cohort Characteristics
Among 39,239 VA PWH alive at the start of the study period, 33,224 (85%) had AUDIT-C screening and were included in analyses. Of these, 971 were women and 32,253 were men. Sixty-four percent were 50 years and older, 48% were black race/ethnicity, and 36% reported any mental health disorder. Nearly half (47%) reported non-drinking and 39%, 8%, 3%, and 4% reported low-, medium-, high-, and very high-level drinking, respectively. On average, women were younger than men, more likely to be black race/ethnicity, have mental health disorders, and less likely to report medium-, high-, and very high-level drinking (Supplemental File 1).
Gender and HIV Care Continuum
Women were less likely than men to be engaged in HIV medical care, on ART, and virally suppressed (Table 1). Figure 1 shows the predicted prevalence of PWH receiving each element of the HIV care continuum by gender. Results were similar after adjustment.
TABLE 1.
Associations Between Gender, Level of Alcohol Use and 3 Measures of the HIV Care Continuum in a Large National Cohort of US Veterans Health Administration Patients with HIV and Documented AUDIT-C Screening Between February 2008 and September 2014 (Women = 971, Men = 32,253)
| Unadjusted | Adjusted* | |||||||
|---|---|---|---|---|---|---|---|---|
| RR | [95% CI] | p-value | Test for Linear Trend† | aRR | [95% CI] | p-value | Test for Linear Trend† | |
|
Engaged in HIV Medical Care N=25,833 (698 for women, 25,135 for men) | ||||||||
| WOMEN (relative to men) | 0.92 | [0.89,0.96] | <0.001 | N/A | 0.90 | [0.86,0.94] | <0.001 | N/A |
| Level of Alcohol Use‡ | ||||||||
| Non-drinking (0) | -- | ref | . | 0.14 | -- | ref | . | 0.13 |
| Low-Level (1–2 women) | 1.03 | [0.94,1.12] | 0.56 | 1.04 | [0.96,1.14] | 0.31 | ||
| Medium-Level (3–5 women) | 1.09 | [0.95,1.24] | 0.23 | 1.09 | [0.96,1.24] | 0.17 | ||
| High-Level (6–7) | 1.05 | [0.76,1.47] | 0.76 | 1.08 | [0.78,1.49] | 0.66 | ||
| Very High Level (8–12) | 0.70 | [0.46,1.07] | 0.10 | 0.72 | [0.47,1.08] | 0.11 | ||
| MEN (referent) | -- | -- | . | N/A | -- | -- | . | N/A |
| Level of Alcohol Use§ | ||||||||
| Non-drinking (0) | -- | ref | . | <0.01 | -- | ref | . | <0.01 |
| Low-Level (1–3 men) | 1.02 | [1.01,1.03] | 0.00 | 1.02 | [1.01,1.03] | 0.00 | ||
| Medium-Level (4–5 men) | 0.96 | [0.94,0.99] | 0.00 | 0.96 | [0.94,0.98] | 0.00 | ||
| High-Level (6–7) | 0.96 | [0.92,1.00] | 0.05 | 0.95 | [0.91,0.98] | 0.00 | ||
| Very High Level (8–12) | 0.91 | [0.88,0.95] | 0.00 | 0.89 | [0.86,0.93] | 0.00 | ||
|
Prescribed ART N=24,400 (583 for women, 23,817 for men) | ||||||||
| WOMEN (relative to men) | 0.81 | [0.77,0.86] | <0.001 | N/A | 0.81 | [0.77,0.85] | <0.001 | N/A |
| Level of Alcohol Use‡ | ||||||||
| Non-drinking (0) | -- | -- | . | 0.03 | -- | -- | . | 0.04 |
| Low-Level (1–2 women) | 0.98 | [0.87,1.09] | 0.68 | 1.00 | [0.90,1.12] | 0.99 | ||
| Medium-Level (3–5 women) | 0.91 | [0.73,1.12] | 0.36 | 0.91 | [0.73,1.12] | 0.36 | ||
| High-Level (6–7) | 0.94 | [0.58,1.53] | 0.82 | 0.93 | [0.57,1.52] | 0.77 | ||
| Very High Level (8–12) | 0.44 | [0.22,0.88] | 0.02 | 0.46 | [0.23,0.91] | 0.03 | ||
| MEN (referent) | -- | -- | . | N/A | -- | -- | . | N/A |
| Level of Alcohol Use§ | ||||||||
| Non-drinking (0) | -- | ref | . | <0.01 | -- | ref | . | <0.01 |
| Low-Level (1–3 men) | 1.03 | [1.01,1.04] | 0.00 | 1.02 | [1.00,1.03] | 0.02 | ||
| Medium-Level (4–5 men) | 0.96 | [0.94,0.99] | 0.00 | 0.95 | [0.93,0.98] | 0.00 | ||
| High-Level (6–7) | 0.91 | [0.87,0.96] | 0.00 | 0.91 | [0.87,0.95] | 0.00 | ||
| Very High Level (8–12) | 0.81 | [0.77,0.85] | 0.00 | 0.82 | [0.78,0.86] | 0.00 | ||
|
Viral Suppression N=18,449 (451 for women, 17,998 for men) | ||||||||
| WOMEN (relative to men) | 0.83 | [0.78,0.89] | <0.001 | N/A | 0.85 | [0.79,0.91] | <0.001 | N/A |
| Level of Alcohol Use‡ | ||||||||
| Non-drinking (0) | -- | ref | . | 0.02 | -- | ref | . | <0.01 |
| Low-Level (1–2 women) | 0.93 | [0.80,1.07] | 0.30 | 0.96 | [0.83,1.12] | 0.62 | ||
| Medium-Level (3–5 women) | 0.84 | [0.63,1.12] | 0.23 | 0.88 | [0.66,1.16] | 0.35 | ||
| High-Level (6–7) | 0.85 | [0.43,1.66] | 0.63 | 0.87 | [0.46,1.66] | 0.68 | ||
| Very High Level (8–12) | 0.28 | [0.10,0.80] | 0.02 | 0.31 | [0.11,0.86] | 0.02 | ||
| MEN (referent) | -- | -- | . | N/A | -- | -- | . | N/A |
| Level of Alcohol Use§ | ||||||||
| Non-drinking (0) | -- | ref | . | <0.01 | -- | ref | . | <0.01 |
| Low-Level (1–3 men) | 0.97 | [0.95,0.99] | 0.00 | 0.99 | [0.97,1.01] | 0.27 | ||
| Medium-Level (4–5 men) | 0.86 | [0.83,0.90] | 0.00 | 0.88 | [0.85,0.92] | 0.00 | ||
| High-Level (6–7) | 0.80 | [0.74,0.86] | 0.00 | 0.82 | [0.77,0.89] | 0.00 | ||
| Very High Level (8–12) | 0.65 | [0.60,0.70] | 0.00 | 0.68 | [0.63,0.73] | 0.00 | ||
Adjusted for race/ethnicity, gender, fiscal year of AUDIT-C screening, age, and any mental health and non-alcohol substance use disorders
Linear trend tested using orthogonal polynomial contrasts
Ns = 539, 523, 75, 12, and 22 for no alcohol use, low-, mild-, moderate-, and high-severity alcohol use
Ns = 14907, 12662, 2610, 873, and 1201 for no alcohol use, low-, mild-, moderate-, and high-severity alcohol use
Figure 1.
Percent Achieving HIV Care Continuum Outcomes by Gender and Alcohol Use among US Veterans Health Administration Patients with HIV and Documented AUDIT-C Screening Between February 2008 and September 2014 (Women=971; Men=32,253).
Alcohol Use and HIV Care Continuum by Gender
There was no statistically significant interaction between level of alcohol use and HIV care continuum outcomes (in primary analyses, interaction term p-values were 0.30, 0.43 and 0.60 for engagement in HIV care, receipt of ART, and viral suppression, respectively). However, gender-stratified models suggested possible variability. Among women, very high-level drinking was associated with decreased likelihood of ART and viral suppression (but not engagement in HIV medical care) relative to non-drinking. Other levels of alcohol use were not associated with outcomes although numbers of women in these groups were small and confidence intervals wide (Table 1). Among men, relative to non-drinking, low-level drinking was associated with slightly increased likelihood of engaging in HIV medical care and ART while all other levels of alcohol use were associated with decreased likelihood of care continuum outcomes (Table 1).
Level of alcohol use was significantly inversely associated with all care continuum outcomes for both genders except engagement in HIV care among women. At every level of alcohol use, women had lower rates of HIV care continuum outcomes than men (Figure 1). All results were similar after adjustment.
Results of sensitivity analyses were comparable to primary results, although confidence intervals among women were much larger, likely due to reduced sample size (not shown).
DISCUSSION
In this large national cohort of PWH, gender disparities in key markers of effective HIV care were observed. Women were less likely than men to meet each HIV care continuum target, with fewer than half virally suppressed. At every level of alcohol use, women appeared to have poorer HIV care continuum outcomes than men. Further, the magnitude of reduction in rates of target care associated with very high-level drinking compared to non-drinking was much greater in women compared to men.
Gender-specific findings regarding level of alcohol use and HIV care continuum outcomes are concerning. Women with HIV may experience social vulnerabilities that increase alcohol-related risk and interfere with receipt of HIV care. In this cohort of women, almost two-thirds were black and half had clinically-identified mental health disorders. Both black race and mental health disorders are associated with stigma and discrimination that adversely influence HIV outcomes [1, 25–27]. Very high-level drinking adds synergistic risk [28, 29]. Women may also experience metabolic vulnerabilities whereby they suffer negative consequences of alcohol consumption faster and to a greater degree than men [30]. Such consequences have been documented for cognition, motor functioning, and reproductive health [30]. This is the first study to our knowledge to suggest that women with HIV and higher-level alcohol use may experience more severe HIV-related outcomes than men with the same conditions. Targeted interventions [31] may be needed to improve care and outcomes for women with HIV, particularly those with multiple and intersecting identities and comorbid conditions that increase risk.
This study has several limitations. First, power to detect associations between alcohol use and HIV care continuum outcomes among women may have been limited by smaller numbers of women with higher levels of alcohol use. Second, the EHR does not capture sexual orientation and may misclassify gender identity for some patients. Third, alcohol use may have been underreported by patients [13, 32] and underestimated by clinically documented screening [33]. Fourth, the reference category (AUDIT-C=0) includes both PWH with lifetime abstinence and those who became abstinent due to declining health. The latter may be medically complex patients for whom additional barriers to HIV care exist [34]. However, sensitivity analyses with low-level drinking as the referent suggested similar patterns. Finally, although we studied a national cohort, findings may not be generalizable to non-VA populations. While racially and ethnically diverse, VA PWH may be older on average than PWH in the general population. Additionally, care that women with HIV receive at VA may differ from non-VA care (e.g., care by Ryan White services). Women receiving non-VA care may experience even greater disparities, given that VA is an integrated system with free HIV care. Alternatively, as a minority group in a health system traditionally serving men, women may face more barriers within VA.
This large nationally representative study builds on previous research by examining gender disparities across HIV care continuum outcomes [35] and provides further evidence [9] that alcohol use negatively impacts HIV care. Consistent with VA’s commitment to continuous quality improvement, findings have been shared with HIV and women’s health policymakers, and VA initiatives to address gender disparity in the care continuum are underway. Continued monitoring in VA and further research in other settings is needed. Services targeting women, regardless their level of alcohol use, may be needed to optimize HIV care.
Supplementary Material
ACKNOWLEDGEMENTS
This study was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21AA022866–01; Williams/Bradley PIs) and COMpAAAS/Veterans Aging Cohort Study (U24-AA020794, U01-AA020790, U01-AA020795, U01-AA020799; U10 AA013566). Dr. Williams is supported by a Career Development Award from VA Health Services Research & Development (CDA 12–276).
Dr. Williams served as principal investigator of the study, guiding all stages of study design, analysis, interpretation and presentation. Dr. Justice serves as the principal investigator and Dr. Fiellin leads the Alcohol and Behavior Core of the Veterans Aging Cohort Study (VACS), which collected and provided study data and contributed structured review of results and interpretation. Ms. Matson wrote the manuscript in collaboration with Dr. Williams, revising it in accordance with contributions from all other authors. Dr. McGinnis completed all study analyses; Dr. Bensley and Ms. Frost contributed to literature review; all authors contributed to interpretation of findings, drafting and iterative review of the manuscript, and all authors approved the final manuscript.
Conflict of Interest and Source of Funding
This study was funded by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21AA022866–01; Williams/Bradley PIs) and COMpAAAS/Veterans Aging Cohort Study (U24-AA020794, U01-AA020790, U01-AA020795, U01-AA020799; U10 AA013566). Dr. Williams is supported by a Career Development Award from VA Health Services Research & Development (CDA 12–276).
The funders of this study had no role in study design or data collection. An employee of the funder (K. Bryant) is a co-author on this manuscript and helped guide analysis, interpretation and presentation of data and participated in the decision to submit the manuscript for publication. For the remaining authors, none were declared.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: Views presented in the manuscript are those of the authors and do not reflect those of the University of Washington, the Department of Veterans Affairs, or the United States Government.
REFERENCES
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016 https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf. 2017.
- 2.Joint United Nations Programme on HIV/AIDS. 90–90-90—an ambitious treatment target to help end the AIDS epidemic. http://www.unaids.org/en/resources/documents/2014/90-90-90. 2014.
- 3.Centers for Disease Control and Prevention. Understanding the HIV Care Continuum. https://www.cdc.gov/hiv/pdf/library/factsheets/cdc-hiv-care-continuum.pdf. 2017.
- 4.Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med 2015; 175(4):588–596. [DOI] [PubMed] [Google Scholar]
- 5.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science 2005; 308(5728):1582–1583. [DOI] [PubMed] [Google Scholar]
- 6.Floridia M, Giuliano M, Palmisano L, Vella S. Gender differences in the treatment of HIV infection. Pharmacol Res 2008; 58(3):173–182. [DOI] [PubMed] [Google Scholar]
- 7.Logan TK, Cole J, Leukefeld C. Women, sex, and HIV: social and contextual factors, meta-analysis of published interventions, and implications for practice and research. Psychol Bull 2002; 128(6):851–885. [DOI] [PubMed] [Google Scholar]
- 8.Wyrod R Gender and AIDS Stigma In: Stigma, Discrimination and Living with HIV/AIDS: A Cross-Cultural Perspective. Liamputtong P (editor): Springer; 2015. [Google Scholar]
- 9.Williams EC, Hahn JA, Saitz R, Bryant K, Lira MC, Samet JH. Alcohol use and Human Immunodeficiency Virus (HIV) Infection: current knowledge, implications, and future directions. Alcohol Clin Exp Res 2016; 4 (10):2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fultz SL, Skanderson M, Mole LA, Gandhi N, Bryant K, Crystal S, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care 2006; 44(8 Suppl 2):S25–30. [DOI] [PubMed] [Google Scholar]
- 11.Department of Veterans Affairs. HIV/Hepatitis C QUERI Strategic Plan. http://www.queri.research.va.gov/about/strategic_plans/hiv.pdf. 2010.
- 12.Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, et al. Veterans Aging Cohort Study (VACS): Overview and description. Med Care 2006; 44(8 Suppl 2):S13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley KA, Lapham GT, Hawkins EJ, Achtmeyer CE, Williams EC, Thomas RM, et al. Quality concerns with routine alcohol screening in VA clinical settings. J Gen Intern Med 2011; 26(3):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams EC, Lapham GT, Bobb JF, Rubinsky AD, Catz SL, Shortreed SM, et al. Documented brief intervention not associated with resolution of unhealthy alcohol use one year later among VA patients living with HIV. J Subst Abuse Treat 2017; 78:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley KA, Williams EC, Achtmeyer CE, Volpp B, Collins BJ, Kivlahan DR. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care 2006; 12(10):597–606. [PubMed] [Google Scholar]
- 16.Rubinsky AD, Dawson DA, Williams EC, Kivlahan DR, Bradley KA. AUDIT-C scores as a scaled marker of mean daily drinking, alcohol use disorder severity, and probability of alcohol dependence in a U.S. general population sample of drinkers. Alcohol Clin Exp Res 2013; 37(8):1380–1390. [DOI] [PubMed] [Google Scholar]
- 17.Justice AC, McGinnis KA, Tate JP, Braithwaite RS, Bryant KJ, Cook RL, et al. Risk of mortality and physiologic injury evident with lower alcohol exposure among HIV infected compared with uninfected men. Drug Alcohol Depend 2016; 161:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prevention CfDCa. Understanding the HIV Care Continuum. In; 2014.
- 19.Saag MS, Holodniy M, Kuritzkes DR, O’Brien WA, Coombs R, Poscher ME, et al. HIV viral load markers in clinical practice. Nat Med 1996; 2(6):625–629. [DOI] [PubMed] [Google Scholar]
- 20.McGinnis KA, Fiellin DA, Tate JP, Cook RL, Braithwaite RS, Bryant KJ, et al. Number of drinks to “feel a buzz” by HIV status and viral load in men. AIDS Behav 2016; 20(3):504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004; 159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 22.Greenland S Avoiding power loss associated with categorization and ordinal scores in dose-response and trend analysis. Epidemiology 1995; 6(4):450–454. [DOI] [PubMed] [Google Scholar]
- 23.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet 1988; 2(8623):1267–1273. [DOI] [PubMed] [Google Scholar]
- 24.StataCorp. Stata Statistical Software: Release 14. In. College Station, TX: StataCorp LP; 2014. [Google Scholar]
- 25.Illangasekare SL, Burke JG, Chander G, Gielen AC. Depression and social support among women living with the substance abuse, violence, and HIV/AIDS syndemic: a qualitative exploration. Womens Health Issues 2014; 24(5):551–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leserman J Role of depression, stress, and trauma in HIV disease progression. Psychosom Med 2008; 70(5):539–545. [DOI] [PubMed] [Google Scholar]
- 27.Oramasionwu CU, Hunter JM, Skinner J, Ryan L, Lawson KA, Brown CM, et al. Black race as a predictor of poor health outcomes among a national cohort of HIV/AIDS patients admitted to US hospitals: a cohort study. BMC infectious diseases 2009; 9:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan LE, Goulet JL, Justice AC, Fiellin DA. Alcohol consumption and depressive symptoms over time: a longitudinal study of patients with and without HIV infection. Drug Alcohol Depend 2011; 117(2–3):158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witbrodt J, Mulia N, Zemore SE, Kerr WC. Racial/ethnic disparities in alcohol-related problems: differences by gender and level of heavy drinking. Alcohol Clin Exp Res 2014; 38(6):1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nolen-Hoeksema S Gender differences in risk factors and consequences for alcohol use and problems. Clin Psychol Rev 2004; 24(8):981–1010. [DOI] [PubMed] [Google Scholar]
- 31.Chander G, Hutton HE, Lau B, Xu X, McCaul ME. Brief intervention decreases drinking frequency in HIV-infected, heavy drinking women: results of a Randomized Controlled Trial. J Acquir Immune Defic Syndr 2015; 70(2):137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams EC, Achtmeyer CE, Thomas RM, Grossbard JR, Lapham GT, Chavez LJ, et al. Factors Underlying Quality Problems with Alcohol Screening Prompted by a Clinical Reminder in Primary Care: A Multi-site Qualitative Study. J Gen Intern Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGinnis KA, Tate JP, Williams EC, Skanderson M, Bryant KJ, Gordon AJ, et al. Comparison of AUDIT-C collected via electronic medical record and self-administered research survey in HIV infected and uninfected patients. Drug Alcohol Depend 2016; 168:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crane HM, Nance RM, Merrill JO, Hutton H, Chander G, McCaul ME, et al. Not all non-drinkers with HIV are equal: demographic and clinical comparisons among current non-drinkers with and without a history of prior alcohol use disorders. AIDS Care 2017; 29(2):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backus L, Czarnogorski M, Yip G, Thomas BP, Torres M, Bell T, et al. HIV Care Continuum Applied to the US Department of Veterans Affairs: HIV Virologic Outcomes in an Integrated Health Care System. J Acquir Immune Defic Syndr 2015; 69(4):474–480. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.