Abstract
Objective
Compare the effects of a vaginal estradiol tablet and a vaginal moisturizer, each to placebo, on menopause-related quality of life and mood in postmenopausal women with moderate-severe vulvovaginal symptoms.
Methods
302 postmenopausal women enrolled in a 12-week, double-blind, placebo-controlled randomized trial were assigned to vaginal 10mcg estradiol tablet plus placebo gel (n=102), vaginal moisturizer plus placebo tablet (n=100), or dual placebo (n=100). We measured change from randomization to 12 weeks in total score of the Menopause-Specific Quality of Life Questionnaire (MENQOL). We also evaluated the 4 MENQOL domains, depressive symptoms as measured by the Patient Health Questionnaire 8 (PHQ-8), and anxiety symptoms as measured by the Generalized Anxiety Disorder questionnaire (GAD-7).
Results
Treatment with vaginal estradiol resulted in significantly greater improvement in total MENQOL scores compared to dual placebo (mean difference between arms −0.3 at 12 weeks (95% confidence interval [CI] −0.5, 0.0; p=0.01). A statistically significant group mean difference favoring vaginal estradiol was observed for the MENQOL sexual function domain (−0.4 at 12 weeks (95% CI −1.0, 0.1; p=0.005), but not for any of the other domains. Treatment with vaginal moisturizer did not provide greater improvement compared to placebo in total MENQOL scores (mean difference 0.2 at 12 weeks; 95% CI −0.1, 0.4; p=0.38) or in any of the MENQOL domains. Neither treatment group showed improvement compared with placebo in the PHQ-8 or GAD-7.
Conclusions
Treatment with low dose vaginal estradiol, but not vaginal moisturizer, modestly improved menopause-related quality of life and sexual function domain scores in postmenopausal women with moderate-severe vulvovaginal symptoms.
Keywords: clinical trials network, postmenopausal vulvovaginal symptoms, vaginal estrogen, vaginal moisturizer, quality of life, depressive symptoms, anxiety
INTRODUCTION
Bothersome vulvovaginal symptoms including lack of lubrication and discomfort or pain with vaginal intercourse are prevalent amongst postmenopausal women (1–3), with up to 75% reporting vaginal dryness and up to 40% reporting pain with intercourse.(2; 4) Moderate to severe vulvovaginal symptoms in postmenopausal women are associated with significant decreases in sexual functioning (5; 6) and in quality of life, comparable to the effects of other chronic conditions such as arthritis, asthma, and irritable bowel syndrome.(1)
Current treatment options for postmenopausal vulvovaginal symptoms include vaginal treatments(7; 8) such as vaginal estrogen creams, tablets, or rings; vaginal moisturizers; intravaginal prasterone (dehydroepiandrosterone, DHEA)(9); as well as the oral selective estrogen receptor modulator ospemifene.(11) Randomized, double-blind, placebo-controlled clinical trials have demonstrated that vaginal estrogen creams (12; 13), vaginal estrogen tablets (14–17), intravaginal prasterone (dehydroepiandrosterone, DHEA)(9) and ospemifene(10; 11) reduce vulvovaginal symptoms, although few of these trials have examined the effects of treatment on quality of life or mood. Limited data exists to support the use of vaginal moisturizers and no studies have examined their effects on quality of life.(18–20)
The Menopause Strategies: Finding Lasting Answers for Symptoms and Health (MsFLASH) clinical trials network recently completed a 3-arm double-blind trial of vaginal estradiol tablets, a vaginal moisturizer, and matching placebos for each to examine the efficacy of vaginal estradiol and vaginal moisturizer relative to placebo in improving genitourinary symptoms of menopause. The primary results of that trial showed no benefit over placebo for the vaginal estradiol tablet or moisturizer in reducing severity of most bothersome symptom (21). Herein we report the effects of vaginal estradiol tablet plus placebo gel or vaginal moisturizer plus placebo tablet vs. dual placebo on menopause-related quality of life (QOL), depressive symptoms, and anxiety.
METHODS
Study Design
A randomized, double-blind, placebo-controlled 12-week trial was conducted among women with moderate-severe vulvovaginal symptoms recruited at two sites: Kaiser Permanente Washington Health Research Institute in Seattle, WA and University of Minnesota in Minneapolis, MN. The protocol was approved by the institutional review board at each site and at the Data Coordinating Center. All women provided written informed consent.
The trial enrolled 302 women from April 2016 to February 2017. Women were eligible if they were 45–70 years old, ≥2 years since last menses, reported ≥1 moderate-severe symptom of vulvovaginal itching, pain, dryness, or irritation experienced at least weekly within the past 30 days; or pain with penetration at least once monthly. Exclusion criteria included: current vaginal infection, use of hormonal medication in past 2 months, use of antibiotics, vaginal moisturizer, probiotic, prebiotic or douche in past month, and chronic premenopausal vulvovaginal symptoms. Additional details of the trial design and methods were published previously.(21)
Treatment and study procedures
Women were recruited through direct mailings and Facebook ads targeted to women aged 50–70 years within 20 miles of the clinical sites. Randomization by permuted blocks of 9 and stratified by site was conducted in a secure web-based database, and implemented via a computerized inventory system for dispensing identical-appearing tablets in bottles and gel in tubes. Participants, study personnel, and clinical providers were blinded to treatment assignments.
Women were randomly assigned 1:1:1 to vaginal estradiol 10 mcg tablet (Vagifem) + placebo vaginal gel (hydroxyethyl cellulose); placebo vaginal tablet + vaginal moisturizer (Replens); or placebo tablet + placebo gel. The composition of the placebo vaginal gel, vaginal moisturizer, and placebo tablet have been described previously.(21)
Women were instructed to use the vaginal tablet (active or placebo) daily for two weeks, then twice weekly for the remaining 10 weeks. The vaginal gel (active or placebo) was to be used every three days throughout the trial. During the first two weeks, participants were advised to use tablet in the morning and gel in the evening. After that participants were instructed to use the products on alternate days.
Data Collection
Telephone contact at 1, 3 and 11 weeks post-randomization assessed protocol adherence and adverse events. Follow-up clinic visits were conducted 4 and 12 weeks post-randomization and included completion of questionnaires regarding menopause-specific quality of life, mood, and sexual function. At follow-up visits women were asked to bring medications; remaining pills were counted and gel tubes weighed to provide medication adherence estimates.
Outcomes/Measurements
Menopause quality of life was specified a priori as a secondary outcome, measured as change from baseline to 12 weeks in the total Menopause-Specific Qualify of Life questionnaire (MENQOL).(22). The MENQOL is a 29-item self-report measure of quality of life designed to capture information on the presence and bother of symptoms, feelings, and experiences in the 4 domains of vasomotor, physical, psychosocial and sexual functioning among midlife women in the menopause transition, total score range 1–8. For each item, women were asked to report if they had experienced that symptom or feeling in the past four weeks, and if they had, to rate bother on a scale of 0–6 corresponding to “not bothered at all” to “extremely bothered.” These two items were combined to create a score from 1 (not experiencing symptoms or feeling) to 8 (extremely bothered). Each domain score was the average of the item scores in that domain (higher scores indicated poorer quality of life). Validity, reliability, and responsiveness to change have been shown to be adequate to excellent.(22) In addition we evaluated mood using the 8-item version of the depression module of the Patient Health Questionnaire (PHQ-8), which asks responders to indicate how often they have been bothered by a variety of symptoms and feelings in the last two weeks. The PHQ-8 depression scale is scored continuously with a range from 0–24; accepted categories of depression severity are 0–4 normal, 5–9 mild, and ≥10 moderate to severe.(23; 24) Anxiety was evaluated using the 7-item Generalized Anxiety Disorder questionnaire (GAD-7), which also asks responders to indicate how often they have been bothered by specific symptoms or feelings in the previous two weeks. The GAD-7 score ranges from 0–21, with anxiety severity categorized as 0–4 normal, 5–9 mild, or ≥10 moderate to severe.(25)
Other measurements
As previously reported, the primary outcome of the trial was severity of the most bothersome symptom defined by the participant at trial enrollment as either vulvovaginal itching, pain, dryness, irritation, or pain with penetration. Severity was rated 0–3, signifying none, mild, moderate, or severe. At each visit, women completed a questionnaire about presence and severity of vulvovaginal symptoms. Demographic factors, smoking status, alcohol intake, and health status were assessed by questionnaire at baseline. Additional pre-specified secondary outcomes included sexual functioning assessed using the Female Sexual Function Index (FSFI) (26) and the Female Sexual Distress Scale – Revised (FSDS-R).(27)
Statistical analysis
A modified intent-to-treat analysis included all randomized participants who provided follow-up QOL, depressive symptoms, or anxiety symptoms data at week 4, week 12, or both, regardless of treatment adherence.
Linear regression models were fit to summarize total MENQOL score, the four MENQOL domain scores, the PHQ-8 score, and the GAD-7 score at both 4 and 12 weeks as a function of treatment assignment, baseline value of the outcome measure, visit, and clinical site. Robust standard errors were calculated using generalized estimating equations to adjust for correlation between repeated outcome measures. We hypothesized that the effect of treatment on MENQOL score might be modified by baseline characteristics, specifically those related to sexual function. We assessed the interaction between treatment assignment and each baseline characteristic [reporting pain with sex as the most bothersome symptom and baseline FSFI (< median; ≥ median)] within the repeated measures regression models. A sensitivity analysis evaluated intervention effects in models including only women who were adherent to treatment, defined as ≥80% medication use.
The planned sample size of the trial was determined by the primary trial endpoints. Reported p-values were based on the Wald statistic, with 2-sided p-value <0.05. Analyses were conducted using SAS Version 9.4 (SAS Institute, Cary, NC).
RESULTS
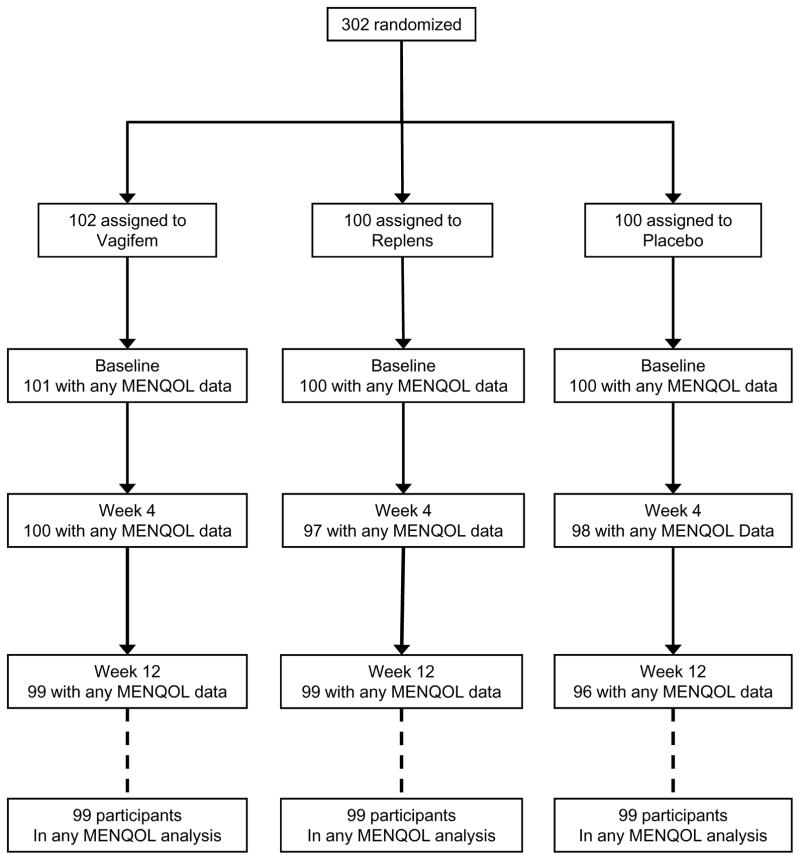
Three hundred two women were randomized to receive vaginal estradiol tablet plus placebo gel (n=102), placebo tablet plus vaginal moisturizer (n=100) or dual placebo (n=100) (Figure 1). Data on one or more domains of the MENQOL were available on 301 women (>99%) at baseline and 298 (99%) at four and/or twelve weeks of follow-up. Similarly for the PHQ-8 and GAD-7, data were available on 301 (>99%) women at baseline and 297 (97%) at follow-up.
Figure 1.
CONSORT Diagram: Randomization and Follow-up of Participants
Baseline characteristics were comparable between groups (Table 1). The mean age of study participants was 61 years; 88% were white and 85% were married or partnered. The most commonly reported most bothersome symptom was pain with vaginal penetration (182 [60%]). The majority of women (81%) were sexually active: 67% with a male partner, 1% with a female partner, and 44% self-stimulation. Mean (SD) total MENQOL score overall was 3.3 (SD 3.1); 70 women (23.3%) had PHQ-8 scores indicating mild depressive symptoms and 18 (6.0%) had scores indicating moderate-severe depressive symptoms; 70 (23.3%) had GAD-7 scores indicating mild anxiety and 28 (9.3%) had scores indicating moderate anxiety.
Table 1.
Baseline Characteristics Among Participants with Baseline Menopause Quality of Life (MENQOL) Data
| Vaginal estradiol | Vaginal moisturizer | Dual Placebo | |
|---|---|---|---|
| Baseline Characteristic | (n=101) | (n=100) | (n=100) |
| Age at screening (years), mean (SD) | 61 (4) | 61 (4) | 61 (4) |
| Age group, n (%) | |||
| <55 | 3 (3) | 0 (0) | 1 (1) |
| 55 to 59 | 35 (35) | 42 (42) | 47 (47) |
| 60 to 64 | 42 (42) | 41 (41) | 28 (28) |
| ≥65 | 21 (21) | 17 (17) | 24 (24) |
| Race/Ethnicity, n (%) | |||
| White | 87 (86) | 90 (90) | 90 (90) |
| African American | 7 (7) | 3 (3) | 2 (2) |
| Other / Unknown | 7 (7) | 7 (7) | 8 (8) |
| Body mass index (kg/m2), mean (SD) | 27 (5) | 26 (4) | 26 (6) |
| Body mass index (kg/m2), n (%) | |||
| <25 | 39 (39) | 44 (44) | 51 (51) |
| 25 to <30 | 37 (37) | 42 (42) | 26 (26) |
| ≥30 | 21 (21) | 14 (14) | 21 (21) |
| Education, n (%) | |||
| ≤ High school diploma / GED | 2 (2) | 3 (3) | 6 (6) |
| School after high school | 31 (31) | 27 (27) | 31 (31) |
| College graduate | 67 (66) | 70 (70) | 63 (63) |
| Marital Status, n (%) | |||
| Never married | 8 (8) | 2 (2) | 4 (4) |
| Divorced | 9 (9) | 8 (8) | 12 (12) |
| Widowed | 1 (1) | 0 (0) | 0 (0) |
| Married or like relationship | 83 (82) | 90 (90) | 84 (84) |
| Smoking, n (%) | |||
| Never | 66 (65) | 67 (67) | 66 (66) |
| Past | 31 (31) | 33 (33) | 32 (32) |
| Current | 4 (4) | 0 (0) | 2 (2) |
| Alcohol use (drinks/week), n (%) | |||
| 0 | 30 (30) | 31 (31) | 28 (28) |
| 1 to <7 | 50 (50) | 46 (46) | 53 (53) |
| ≥7 | 21 (21) | 23 (23) | 19 (19) |
| Sexually active, n (%) | |||
| Yes | 81 (80) | 80 (80) | 84 (84) |
| No | 20 (20) | 20 (20) | 16 (16) |
| MENQOL measure, mean (SD) | |||
| Total | 3.3 (1.2) | 3.2 (1.1) | 3.3 (1.0) |
| Physical | 3.0 (1.4) | 2.9 (1.0) | 3.0 (1.1) |
| Psychosocial | 2.7 (1.4) | 2.4 (1.1) | 2.5 (1.2) |
| Sexual function | 4.9 (2.0) | 4.9 (2.2) | 5.0 (2.0) |
| Vasomotor | 2.7 (2.0) | 2.6 (1.9) | 2.6 (1.7) |
| Depression (PHQ-8), n (%) | |||
| None (0–4) | 69 (68) | 75 (75) | 69 (69) |
| Mild (5–9) | 25 (25) | 22 (22) | 23 (23) |
| Moderate/Severe (≥10) | 7 (7) | 3 (3) | 8 (8) |
| Anxiety (GAD–7), n (%) | |||
| None (0–4) | 64 (63) | 75 (75) | 64 (64) |
| Mild (5–9) | 25 (25) | 21 (21) | 24 (24) |
| Moderate/Severe (≥10) | 12 (12) | 4 (4) | 12 (12) |
| Sexual Function (FSFI), mean (SD) | 15.2 (5.9) | 15.2 (6.5) | 16.1 (6.6) |
| Sexual Distress (FSDS-R), n (%) | |||
| Never/Rarely | 15 (15) | 12 (12) | 18 (18) |
| Occasionally | 33 (33) | 33 (33) | 33 (33) |
| Frequently/Always | 53 (53) | 54 (54) | 49 (49) |
| Most Bothersome Symptom, n (%) | |||
| Vulvar and/or vaginal itching | 10 (10) | 4 (4) | 6 (6) |
| Vulvar and/or vaginal soreness | 5 (5) | 7 (7) | 2 (2) |
| Vulvar and/or vaginal irritation | 7 (7) | 4 (4) | 8 (8) |
| Vaginal dryness | 22 (22) | 17 (17) | 23 (23) |
| Pain with vaginal penetration | 54 (54) | 68 (68) | 60 (60) |
| Self-reported health, n (%) | |||
| Excellent | 26 (26) | 27 (27) | 20 (20) |
| Very good | 41 (41) | 55 (55) | 47 (47) |
| Good | 33 (33) | 15 (15) | 30 (30) |
| Fair/Poor | 1 (1) | 3 (3) | 3 (3) |
Note: No significant differences observed between intervention and placebo groups
Abbreviations: MENQOL, Menopause Quality of Life questionnaire (scale 1–8; 1=best); PHQ-8, Patient Health Questionnaire 8 (range 0–24; 0=best); GAD-7, Generalized Anxiety Disorder 7 (range 0–21; 0=best); FSFI, Female Sexual Function Index (range 0–27; 27=best); FSDS-R, Female Sexual Distress Scale – Revised
At completion of the trial, among women with both baseline and follow-up MENQOL data, 83 of 100 (83%) women randomized to estradiol tablets returned their tablets for counting and were >80% adherent, compared to 80 of 99 (80%) women randomized to placebo tablets. 74 of 99 (74%) women randomized to vaginal moisturizer returned their gel for weighing and were >80% adherent, compared to 79 of 99 (79%) of women randomized to placebo gel.
Effect of Treatment Assignment on Total MENQOL Scale and Domain Subscales
Total MENQOL scores declined, i.e. menopause-related QOL improved, in all treatment groups (Table 2). Treatment with vaginal estradiol plus placebo gel resulted in significantly greater improvement in MENQOL scores compared to dual placebo (mean difference between groups at 12 weeks of −0.3 (95% confidence interval (CI) −0.5, 0.0; p=0.01) (Table 2). A statistically significant treatment group difference favoring the vaginal estradiol group was also observed for the sexual function domain (Table 2) (mean difference at 12 weeks of −0.4 (95% CI −1.0, 0.1; p=0.005) but not for any of the other domains (Table 2). Treatment with vaginal moisturizer plus placebo tablet did not result in greater improvement compared to dual placebo in total MENQOL scores (mean difference between groups at 12 weeks of 0.2; 95% CI −0.1, 0.4; p=0.38) or in any of the four domains of the MENQOL (Table 2).
Table 2.
Menopause Quality of Life (MENQOL) Outcomes, Change from Baseline to Weeks 4 and 12
| MENQOL Measures | Vaginal estradiol + Placebo gel | Vaginal moisturizer + Placebo tablet | Dual Placebo | Vaginal estradiol + Pbo vs. Dual Placebo | Vaginal moisturizer + Pbo vs. Dual Placebo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | Mean (95% CI) | N | Mean (95% CI) | N | Mean (95% CI) | Mean Difference (95% CI) | p-value* | Mean Difference (95% CI) | p-value* | |
| Total | 0.01 | 0.38 | ||||||||
| Baseline | 99 | 3.3 (3.1, 3.6) | 97 | 3.2 (3.0, 3.4) | 95 | 3.3 (3.1, 3.5) | 0.0 (−0.3, 0.3) | −0.1 (−0.4, 0.2) | ||
| Week 4 – baseline | 91 | −0.8 (−1.0, −0.6) | 89 | −0.5 (−0.6, −0.3) | 85 | −0.5 (−0.7, −0.4) | −0.3 (−0.5, 0.0) | 0.1 (−0.2, 0.3) | ||
| Week 12 – baseline | 89 | −1.0 (−1.2, −0.8) | 94 | −0.5 (−0.7, −0.4) | 86 | −0.7 (−0.9, −0.6) | −0.3 (−0.5, 0.0) | 0.2 (−0.1, 0.4) | ||
| Physical | 0.49 | 0.33 | ||||||||
| Baseline | 101 | 3.0 (2.7, 3.3) | 100 | 2.9 (2.7, 3.1) | 100 | 3.0 (2.8, 3.2) | 0.0 (−0.4, 0.3) | −0.2 (−0.4, 0.1) | ||
| Week 4 – baseline | 97 | −0.5 (−0.7, −0.3) | 95 | −0.2 (−0.3, 0.0) | 95 | −0.3 (−0.5, −0.1) | −0.2 (−0.4, 0.1) | 0.1 (−0.1, 0.4) | ||
| Week 12 – baseline | 96 | −0.5 (−0.7, −0.2) | 97 | −0.3 (−0.5, −0.1) | 95 | −0.5 (−0.7, −0.3) | 0.0 (−0.3, 0.3) | 0.2 (−0.1, 0.4) | ||
| Psychosocial | 0.59 | 0.69 | ||||||||
| Baseline | 101 | 2.7 (2.4, 3.0) | 98 | 2.4 (2.2, 2.6) | 99 | 2.5 (2.3, 2.8) | 0.2 (−0.2, 0.5) | −0.1 (−0.4, 0.2) | ||
| Week 4 – baseline | 97 | −0.2 (−0.4, 0.1) | 94 | −0.1 (−0.3, 0.1) | 96 | −0.1 (−0.3, 0.1) | −0.1 (−0.4, 0.2) | 0.0 (−0.3, 0.3) | ||
| Week 12 – baseline | 97 | −0.4 (−0.7, −0.2) | 96 | −0.2 (−0.4, 0.0) | 93 | −0.2 (−0.4, 0.0) | −0.2 (−0.6, 0.1) | 0.0 (−0.3, 0.3) | ||
| Sexual Function | 0.005 | 0.13 | ||||||||
| Baseline | 99 | 4.9 (4.5, 5.3) | 99 | 4.9 (4.5, 5.4) | 97 | 5.0 (4.6, 5.4) | −0.1 (−0.6, 0.5) | 0.0 (−0.6, 0.6) | ||
| Week 4 – baseline | 94 | −2.2 (−2.6, −1.8) | 94 | −1.5 (−1.9, −1.1) | 91 | −1.6 (−2.0, −1.2) | −0.7 (−1.2, −0.1) | 0.1 (−0.4, 0.7) | ||
| Week 12 – baseline | 92 | −2.3 (−2.7, −1.8) | 98 | −1.3 (−1.7, −0.9) | 90 | −1.8 (−2.2, −1.4) | −0.4 (−1.0, 0.1) | 0.5 (0.0, 1.1) | ||
| Vasomotor | 0.08 | 0.56 | ||||||||
| Baseline | 101 | 2.7 (2.3, 3.1) | 100 | 2.6 (2.3, 3.0) | 99 | 2.6 (2.3, 3.0) | 0.1 (−0.4, 0.6) | 0.0 (−0.5, 0.5) | ||
| Week 4 – baseline | 99 | −0.3 (−0.5, −0.1) | 96 | −0.2 (−0.5, 0.0) | 97 | −0.2 (−0.5, 0.1) | −0.1 (−0.4, 0.2) | 0.0 (−0.4, 0.3) | ||
| Week 12 – baseline | 98 | −0.7 (−1.0, −0.5) | 98 | −0.4 (−0.6, −0.2) | 95 | −0.3 (−0.5, −0.1) | −0.4 (−0.7, −0.1) | −0.1 (−0.4, 0.2) | ||
Abbreviations: MENQOL, menopause-specific quality of life (range 1–8; 1 = best); CI, confidence interval
p-values from contrasts comparing each treatment vs. placebo in a repeated measures linear model of outcome as a function of randomization assignment, baseline value of the outcome measure, visit week (4 or 12) and clinical site
Results were similar among adherent women (results not shown). We found no evidence of interactions between treatment assignment and selected characteristics (i.e. presence or absence of sexual function-related most bothersome symptom and baseline FSFI) on total MENQOL score or sexual function domain score (data not shown).
Effect of Treatment Assignment on Depressive Symptoms and Anxiety
The mean PHQ-8 score at baseline was 3.5 (SD 3.8). PHQ-8 scores improved in all treatment groups at week 12 but there were no statistically significant differences between vaginal estrogen and placebo gel vs. dual placebo or between vaginal moisturizer and placebo tablet vs. dual placebo (Table 3). Similarly, there were no statistically significant differences between either active arm vs. dual placebo in GAD-7 change from baseline scores at week 12.
Table 3.
Mood Outcomes, Change from Baseline to Week 12
| Measure | Vaginal estradiol + Placebo gel | Vaginal moisturizer + Placebo tablet | Dual Placebo | Vaginal estradiol + Pbo vs. Dual Placebo | Vaginal moisturizer + Pbo vs. Dual Placebo | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | Mean (95% CI) | N | Mean (95% CI) | N | Mean (95% CI) | Mean Difference (95% CI) | p-value* | Mean Difference (95% CI) | p-value* | |
| Depression (PHQ-8) | 0.63 | 0.83 | ||||||||
| Baseline | 101 | 3.5 (2.8, 4.2) | 100 | 3.2 (2.6, 3.7) | 100 | 3.8 (3.1, 4.5) | −0.3 (−1.3, 0.7) | −0.6 (−1.5, 0.3) | ||
| Week 12 – baseline | 97 | −0.5 (−1.2, 0.1) | 99 | −0.2 (−0.8, 0.4) | 96 | −0.5 (−1.0, 0.0) | −0.1 (−0.9, 0.7) | 0.3 (−0.5, 1.0) | ||
| Anxiety (GAD-7) | 0.17 | 0.99 | ||||||||
| Baseline | 101 | 4.1 (3.2, 5.0) | 100 | 2.9 (2.3, 3.6) | 100 | 4.1 (3.2, 4.9) | 0.0 (−1.2, 1.2) | −1.2 (−2.2, −0.1) | ||
| Week 12 – baseline | 98 | −1.2 (−1.8, −0.5) | 99 | 0.0 (−0.6, 0.7) | 96 | −0.6 (−1.4, 0.2) | −0.6 (−1.6, 0.4) | 0.6 (−0.4, 1.6) | ||
Abbreviations: CI, confidence interval; PHQ-8, Patient Health Questionnaire 8 (range 0–24, 0=best); GAD-7, Generalized Anxiety Disorder 7 (range 0–21; 0=best)
p-values from contrasts comparing each treatment vs. placebo in a linear model of week 12 outcome as a function of randomization assignment, baseline value of the outcome measure, and clinical site.
Results were similar among adherent women (data not shown).
DISCUSSION
In this double-blind placebo controlled randomized trial studying postmenopausal women with moderate-severe vulvovaginal symptoms, women in all three treatment groups had small improvements in total menopause-related quality of life over 12 weeks. Treatment with low dose vaginal estradiol plus placebo gel improved total quality of life and the sexual function domain of the MENQOL more than dual placebo. Treatment with a vaginal moisturizer and placebo tablet did not improve overall menopause-specific quality of life or any of its domains more than dual placebo. Compared with placebo, neither vaginal estrogen nor vaginal moisturizer had greater effects on depressive symptoms or anxiety symptoms.
Previous trials of vaginal treatments for postmenopausal vulvovaginal complaints have focused on vaginal symptoms and sexual function as outcomes; few report a measure of quality of life. Similar to the present study, randomized trials of the novel intravaginal agent, prasterone, and of an oral agent (bazedoxifene/conjugated estrogen), both in women with vulvovaginal symptoms, observed greater improvement in total MENQOL and sexual function domain scores relative to placebo. Treatment with bazedoxifene/conjugated estrogen resulted in a difference in the mean improvement in total MENQOL scores compared to placebo of −0.4 to −0.5, depending on the dose of the estrogen (0.45 mg or 0.625 mg); improvement in the sexual function domain was approximately 0.6–0.7 points greater with active treatment compared to placebo.(29) Similarly, treatment with intravaginal prasterone resulted in a mean difference in improvement in total MENQOL compared to placebo ranging from −0.2 to −0.55, depending on the dose of prasterone; the sexual function domain improved 0.6–1.2 (30) over placebo. Weisberg and colleagues compared the vaginal estradiol-containing ring (Estring) to a 25mcg vaginal estradiol tablet in an open-label, non-placebo-controlled study of women with vulvovaginal symptoms and did not observe effects on quality of life as measured by the Medical Outcomes Study Questionnaire Short Form (SF-36) or its subscales.(31) A non-randomized study of very low concentration estriol gel (0.005%) which also used the SF-36 index found improved QOL in women using estrogen over 12 weeks, primarily due to improvements in physical domains as opposed to mental domains.(32) A case series of a novel intravaginal estriol/progesterone suppositories reported improvement from baseline in MENQOL over 6 months, largely due to improvements in the sexual function domain, but this investigation is limited by the absence of a comparable control group.(33)
Although small, the magnitude of the effect of vaginal estradiol on total MENQOL in our study (−0.3 [95% CI −0.5, 0.0]) is similar to that observed for low dose oral 17-beta estradiol 0.5mg/day (−0.4 [95% CI −0.7, −0.2]) in a previous trial conducted in post-menopausal women with bothersome hot flashes.(28) In that trial, the improvement in total MENQOL was associated with changes in three of the four domains, with the exception of psychosocial.
Of note, neither low dose vaginal estradiol nor vaginal moisturizer had a greater impact than placebo on most bothersome vulvovaginal symptom severity or sexual functioning as measured by the Female Sexual Functioning Index (FSFI) in the primary trial analyses.(21) However, in this secondary analysis of trial data, we did observe a significantly larger improvement in the sexual function domain of the MENQOL among women assigned to vaginal estradiol and placebo gel. The three questions on the MENQOL regarding sexual functioning ask how much the responder has been bothered by a decrease in sexual desire, vaginal dryness during intercourse, and avoiding intimacy, thus improvements reflect feeling less bothered on these dimensions. In contrast, the FSFI’s 19 questions are more detailed and many ask responders to describe how often they felt sexual desire or interest, felt sexually aroused, were satisfied with their arousal during sexual activity, became lubricated during sexual activity, and experienced pain with vaginal penetration. These questions, which focus on frequency of sexual feelings, activity, and satisfaction, capture different aspects of sexual function than the MENQOL. The lack of alignment of the null treatment effects on the FSFI with modest treatment effects of vaginal estradiol on total and sexual MENQOL scores suggests that different information about sexual quality of life is detected when women are asked to provide a personal assessment of how much they are bothered by changes in sexual desire, vaginal dryness during intercourse and avoiding intimacy. The implication is that multiple approaches to measuring sexual quality of life are needed when assessing interventions to improve vaginal dryness or dyspareunia.
We did not observe effects of vaginal estradiol or vaginal moisturizer, as compared to placebo, on anxiety or depressive symptoms. The causal paths affecting anxiety and depression symptoms are complex and variable. Therapies directed at vaginal symptoms are most likely to improve symptoms such as dyspareunia or dryness, which in turn may improve sexual function. Twelve weeks of treatment with modest improvements in overall and sexual quality of life may not be sufficient to reverse longer term effects of sexual problems on mood or anxiety. Alternatively, the psychosocial underpinnings of depression and anxiety symptoms may not be related to sexual problems in many women, or the mood symptoms may be contributing to sexual problems (reverse causation). In addition, very few women in our trial had moderate or severe levels of depression or anxiety, making it difficult to measure improvements in these parameters.
Strengths of this trial include the use of reliable and valid measures of menopause-related quality of life and mood symptoms, use of placebos for both the vaginal estradiol and the vaginal moisturizer, and excellent participant retention and medication adherence. The study was not designed to compare the vaginal estradiol to vaginal moisturizer interventions directly.
CONCLUSIONS
Our results suggest that treatment with vaginal estradiol tablets in women with bothersome vulvovaginal symptoms improves overall menopause related quality of life more than placebo treatment. We did not observe a similar effect for a vaginal moisturizer. These results suggest that there may be modest benefit of treatment with vaginal estradiol tablets for improving aspects of sexual function captured in the MENQOL. Other considerations such as cost, ease of obtaining treatment, and personal preferences regarding vaginal treatment options may influence individual therapeutic choices.
Acknowledgments
Funding/Support: This study was funded by the National Institutes of Health/National Institute on Aging: R01 AG048209. The sponsor had no input in or control over the analysis of data, writing of manuscript or decision to publish. All of the opinions and conclusions reported herein are the authors’ own, and do not reflect the official position of the National Institute of Aging.
Footnotes
Author Contributions: Dr. Katherine Guthrie had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors made substantial contributions to the study and this manuscript. None were compensated for the manuscript preparation.
Trial registration: Clinical trials.gov: NCT02516202
Financial Disclosures: Dr. Mitchell is a consultant for Symbiomix Therapeutics. Dr. Reed receives grant support from Bayer Pharmaceuticals. Dr. LaCroix served on a scientific advisory board for Sermonix, Inc. All other authors have nothing to report.
References
- 1.DiBonaventura M, Luo X, Moffatt M, Bushmakin AG, Kumar M, Bobula J. The association between vulvovaginal atrophy symptoms and quality of life among postmenopausal women in the United States and Western Europe. J Womens Health (Larchmt ) 2015 Sep;24(9):713–22. doi: 10.1089/jwh.2014.5177. [DOI] [PubMed] [Google Scholar]
- 2.Minkin MJ, Reiter S, Maamari R. Prevalence of postmenopausal symptoms in North America and Europe. Menopause. 2015 Nov;22(11):1231–8. doi: 10.1097/GME.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 3.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. J Sex Med. 2009 Aug;6(8):2133–42. doi: 10.1111/j.1743-6109.2009.01335.x. [DOI] [PubMed] [Google Scholar]
- 4.Kingsberg SA, Wysocki S, Magnus L, Krychman ML. Vulvar and vaginal atrophy in postmenopausal women: findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey. J Sex Med. 2013 Jul;10(7):1790–9. doi: 10.1111/jsm.12190. [DOI] [PubMed] [Google Scholar]
- 5.Nappi RE, Kokot-Kierepa M. Women’s voices in the menopause: results from an international survey on vaginal atrophy. Maturitas. 2010 Nov;67(3):233–8. doi: 10.1016/j.maturitas.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Nappi RE, Palacios S, Particco M, Panay N. The REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) survey in Europe: Country-specific comparisons of postmenopausal women’s perceptions, experiences and needs. Maturitas. 2016 Sep;91:81–90. doi: 10.1016/j.maturitas.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013 Sep;20(9):888–902. doi: 10.1097/GME.0b013e3182a122c2. [DOI] [PubMed] [Google Scholar]
- 8.Sarri G, Davies M, Lumsden MA. Diagnosis and management of menopause: summary of NICE guidance. BMJ. 2015 Nov 12;351:h5746. doi: 10.1136/bmj.h5746. [DOI] [PubMed] [Google Scholar]
- 9.Labrie F, Archer DF, Koltun W, et al. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2016 Mar;23(3):243–56. doi: 10.1097/GME.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 10.Bachmann GA, Komi JO. Ospemifene effectively treats vulvovaginal atrophy in postmenopausal women: results from a pivotal phase 3 study. Menopause. 2010 May;17(3):480–6. doi: 10.1097/gme.0b013e3181c1ac01. [DOI] [PubMed] [Google Scholar]
- 11.Portman DJ, Bachmann GA, Simon JA. Ospemifene, a novel selective estrogen receptor modulator for treating dyspareunia associated with postmenopausal vulvar and vaginal atrophy. Menopause. 2013 Jun;20(6):623–30. doi: 10.1097/gme.0b013e318279ba64. [DOI] [PubMed] [Google Scholar]
- 12.Cardozo L, Bachmann G, McClish D, Fonda D, Birgerson L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: second report of the Hormones and Urogenital Therapy Committee. Obstet Gynecol. 1998 Oct;92(4 Pt 2):722–7. doi: 10.1016/s0029-7844(98)00175-6. [DOI] [PubMed] [Google Scholar]
- 13.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD001500. doi: 10.1002/14651858.CD001500.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M. Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol. 2008 Jan;111(1):67–76. doi: 10.1097/01.AOG.0000296714.12226.0f. [DOI] [PubMed] [Google Scholar]
- 15.Eriksen PS, Rasmussen H. Low-dose 17 beta-estradiol vaginal tablets in the treatment of atrophic vaginitis: a double-blind placebo controlled study. Eur J Obstet Gynecol Reprod Biol. 1992 Apr 21;44(2):137–44. doi: 10.1016/0028-2243(92)90059-8. [DOI] [PubMed] [Google Scholar]
- 16.Simon J, Nachtigall L, Gut R, Lang E, Archer DF, Utian W. Effective treatment of vaginal atrophy with an ultra-low-dose estradiol vaginal tablet. Obstet Gynecol. 2008 Nov;112(5):1053–60. doi: 10.1097/AOG.0b013e31818aa7c3. [DOI] [PubMed] [Google Scholar]
- 17.Simunic V, Banovic I, Ciglar S, Jeren L, Pavicic BD, Sprem M. Local estrogen treatment in patients with urogenital symptoms. Int J Gynaecol Obstet. 2003 Aug;82(2):187–97. doi: 10.1016/s0020-7292(03)00200-5. [DOI] [PubMed] [Google Scholar]
- 18.Biglia N, Peano E, Sgandurra P, et al. Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with urogenital atrophy: a preliminary study. Gynecol Endocrinol. 2010 Jun;26(6):404–12. doi: 10.3109/09513591003632258. [DOI] [PubMed] [Google Scholar]
- 19.Bygdeman M, Swahn ML. Replens versus dienoestrol cream in the symptomatic treatment of vaginal atrophy in postmenopausal women. Maturitas. 1996 Apr;23(3):259–63. doi: 10.1016/0378-5122(95)00955-8. [DOI] [PubMed] [Google Scholar]
- 20.Loprinzi CL, Abu-Ghazaleh S, Sloan JA, et al. Phase III randomized double-blind study to evaluate the efficacy of a polycarbophil-based vaginal moisturizer in women with breast cancer. J Clin Oncol. 1997 Mar;15(3):969–73. doi: 10.1200/JCO.1997.15.3.969. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell CM, Reed SD, Diem S, et al. Treating postmenopausal vulvovaginal symptoms: a randomized clinical trial of the efficacy of vaginal estradiol tablets, moisturizer and placebo. JAMA Intern Med. 2018 doi: 10.1001/jamainternmed.2018.0116. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hilditch JR, Lewis J, Peter A, et al. A menopause-specific quality of life questionnaire: development and psychometric properties. Maturitas. 1996 Jul;24(3):161–75. doi: 10.1016/s0378-5122(96)82006-8. [DOI] [PubMed] [Google Scholar]
- 23.Gilbody S, Richards D, Brealey S, Hewitt C. Screening for depression in medical settings with the Patient Health Questionnaire (PHQ): a diagnostic meta-analysis. J Gen Intern Med. 2007 Nov;22(11):1596–602. doi: 10.1007/s11606-007-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009 Apr;114(1–3):163–73. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Spitzer RL, Kroenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–7. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 26.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000 Apr;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 27.Derogatis L, Clayton A, Lewis-D’Agostino D, Wunderlich G, Fu Y. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med. 2008 Feb;5(2):357–64. doi: 10.1111/j.1743-6109.2007.00672.x. [DOI] [PubMed] [Google Scholar]
- 28.Caan B, LaCroix AZ, Joffe H, et al. Effects of estrogen and venlafaxine on menopause-related quality of life in healthy postmenopausal women with hot flashes: a placebo-controlled randomized trial. Menopause. 2015 Jun;22(6):607–15. doi: 10.1097/GME.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric. 2010 Apr;13(2):132–40. doi: 10.3109/13697130903305627. [DOI] [PubMed] [Google Scholar]
- 30.Labrie F, Archer D, Bouchard C, et al. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause. 2009 Sep;16(5):923–31. doi: 10.1097/gme.0b013e31819e85c6. [DOI] [PubMed] [Google Scholar]
- 31.Weisberg E, Ayton R, Darling G, et al. Endometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric. 2005 Mar;8(1):83–92. doi: 10.1080/13697130500087016. [DOI] [PubMed] [Google Scholar]
- 32.Caruso S, Cianci S, Amore FF, et al. Quality of life and sexual function of naturally postmenopausal women on an ultralow-concentration estriol vaginal gel. Menopause. 2016 Jan;23(1):47–54. doi: 10.1097/GME.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 33.Chollet JA, Carter G, Meyn LA, Mermelstein F, Balk JL. Efficacy and safety of vaginal estriol and progesterone in postmenopausal women with atrophic vaginitis. Menopause. 2009 Sep;16(5):978–83. doi: 10.1097/gme.0b013e3181a06c80. [DOI] [PubMed] [Google Scholar]