Abstract
Objectives:
Studies examining engagement in HIV care often capture cross-sectional patient status to estimate retention and identify predictors of attrition, which ignore longitudinal patient care-seeking behaviors. We describe the cyclical nature of (dis)engagement and re-entry into HIV care using the state transition framework.
Design:
We represent the dynamic patterns of patient care-retention using five states: Engaged in care, missed 1, 2, 3 or more expected visits, and deceased. Then we describe various care-seeking behaviors in terms of transitioning from one state to another (e.g., from disengaged to engaged). This analysis includes 31,009 patients enrolled in the CFAR Network of Integrated Systems (CNICS) in the US from 1996 to 2014.
Method:
Multi-state models for longitudinal data were used to identify barriers to retention and subgroups at higher risk of falling out of care.
Results:
The initial two years following primary engagement in care were a crucial time for improving retention. Patients who had not initiated antiretroviral therapy, with lower CD4 counts, higher viral load, or not having an AIDS defining illness were less likely to be retained in care.
Conclusion:
Beyond the individual patient characteristics typically used to characterize retention in HIV care, we identified specific periods of time and points in the care continuum associated with elevated risk of transitioning out of care. Our findings can contribute to evidence-based recommendations to enhance long-term retention in CNICS. This approach can also be applied to other cohort data to identify retention strategies tailored to each population.
Keywords: HIV care cascade, HIV care continuum, Retention in HIV care, Engagement in HIV care, State transition framework, Multi-state models
Introduction
The HIV care cascade (also known as the HIV care continuum) describes milestones in HIV care and is a useful model to monitor progress and identify needs for people living with HIV (PLWH) [1–3]. In most formulations, the model includes: identification of new cases, linkage to care, initiation of and adherence to antiretroviral therapy (ART) through retention in care, and sustained viral load (VL) suppression. The conceptual model provides a useful framework for evaluating the effectiveness of HIV care and developing strategies to improve health outcomes for PLWH, particularly by identifying factors associated with missing key milestones. For example, a recent CDC report demonstrated that approximately 86% PLWH in the US were aware of their HIV status, about 40% were engaged in care, 37% were prescribed ART, and 30% had undetectable VL [3]. From this summary, we can see that the largest loss in the continuum occurred during engagement in care.
Approaches examining the care cascade in this manner fall short in that they capture cross-sectional snapshots of binary patient status (e.g., retained in care: yes/no), aggregated within pre-defined time periods such as 6 months or 12 months, to examine retention rates and identify predictors of attrition in short-term periods [4–10]. Some studies acknowledge that longitudinal patient health-seeking behaviors are more complex than what is considered using traditional approaches, and thus should incorporate more dynamic processes of patient engagement, disengagement, and re-entry into care [11–14]. In the era of “Big Data,” clinical cohort data consisting of rich resources from electronic medical records (EMR) provide new opportunities to conduct comprehensive analyses of the HIV care cascade. While EMR and cohort studies usually do not contain information related to linkage to care, they do enable the examination of factors associated with movements in and out of care. Yet EMR data have not been fully used to study the dynamics of care retention partly due to a lack of coherent statistical methods that can handle longitudinal patient-level behaviors.
Recently, multi-state modeling approaches have become prominent in characterizing longitudinal patient dynamics along the phases in the HIV care cascade using cohort data [13, 15–17]. In the current analysis, we use the state transition framework developed by Lee at al. (2017) to describe the longitudinal process of engagement and retention in HIV care. We use data from the center for AIDS research (CFAR) Network of Integrated Clinical Systems (CNICS), one of the most comprehensive multisite HIV clinical databases in the US, to estimate the effects of known barriers to HIV care retention over time.
Methods
Study sample
CNICS is an integrated clinical database composed of EMR-based data from eight contributing CFAR sites: University of Alabama at Birmingham (UAB), Case Western Reserve University, University of California San Francisco, the University of Washington, the University of California San Diego, Fenway Health/Harvard University, University of North Carolina Chapel Hill, and Johns Hopkins University. Details can be found elsewhere [18]. PLWH become known to CNICS upon entry at CNICS-affiliated clinics to receive any HIV medical care or HIV testing. However, the CNICS cohort only includes patients with at least two primary care visits within 12 months since their initial linkage to CNICS care. As of December 31st, 2015, CNICS included data on 31,852 patients in HIV care including information on: demographics, HIV comorbidities, laboratory tests including CD4 and HIV-RNA, medications including ART, health care utilization, vital status, patient reported outcomes (PRO), ART resistance, and biologic specimens. Date of death was confirmed using the national death index (NDI) and social security death index data (SSDI).
We excluded from this analysis patients with first care visits before 1996, when ART became widely available, in order to examine the effect of initiating ART on care retention in the modern ART era. We also excluded those who entered the cohort within 200 days of the study end date, December 31, 2014. Hence this analysis included follow-up data from 31,009 patients who enrolled in HIV care in a CNICS-affiliated site from January 1996 through December 2014.
Model formulation and state definition
To ascertain patient-level engagement and retention status, individual follow up times were partitioned into roughly 6-month intervals reflecting the CNICS VL monitoring guidelines (VL monitoring twice a year at minimum), as VL is often used as a proxy measure for having had a care visit. We used a 200-day interval to allow for scheduling variations. Because of the inclusion criteria for CNICS requiring patients to have had at least two clinical visits within 12 months of enrollment in care, we defined time zero in this analysis as the date of the second visit, referred to here as baseline, to avoid artificially inflating engagement rates within the first 200-day time interval.
We defined longitudinal patterns of patient engagement in HIV care using five mutually exclusive states: Engaged in care (state=1), disengaged from care for one period (state=2), for two periods (state = 3), for three or more periods (state = 4), and deceased (state=5). The model captured patient-level transitions from and within states such as transitioning within engaged (retention), from engaged to disengaged, within disengaged, from disengaged to engaged (re-entry into care), and ultimately death (mortality). A graphical representation of the model is shown in Figure 1.
Figure 1. Graphical representation of retention aspects of care in the spanning of the HIV care continuum.
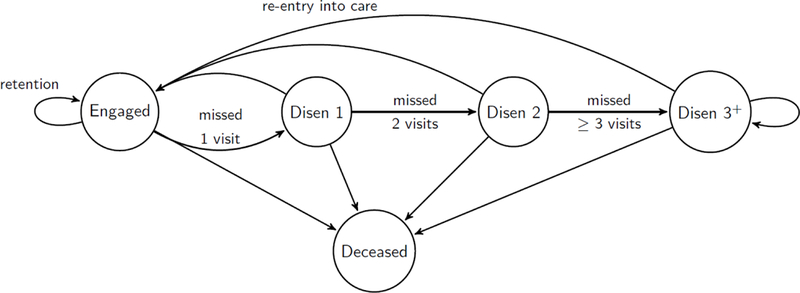
* Circles represent operational states that define the care retention. Engaged represents engaged in care state. Disen 1, 2, and 3+ represents disengaged for 1 interval (200 days; missed 1 expected clinical visit), 2 consecutive intervals (missed two visits), and 3 or more intervals (missed 3 or more visits), respectively. Arrows represent all possible one-step transitions within and between states and their practical meanings.
We assigned state membership at each interval end-point using the following algorithm: the individual patient’s state was defined as “engaged in care” if there was any record of a clinical encounter within the given 200-day time interval. If there was no clinic visit, the individual was disengaged from care unless he/she died within that time interval based on the date of death. Death was defined as an absorbing state, implying that no further transitions were allowed. All patients were considered engaged at baseline by the operational definition of the CNICS. Accordingly, transitions from states 2, 3, and 4 were examined starting 200 days, 400 days, and 600 days after baseline, respectively.
Statistical Analysis
Various transitions can be captured using conditional probabilities where t denotes time at each interval end point, k and j denote states, and St denotes state at time t. For example, represents the probability of retention at time t as it captures transitioning within the engaged state. Using these probabilities, first we estimated time-aggregated rates for each transition such as retention, engagement to disengagement, re-entry into care, etc. We created plots of estimated transition probabilities over time to provide a visual representation of temporal variations in transition dynamics.
State membership at each time t follows a multinomial distribution. Therefore, the discrete time multistate-model, formulated in terms of multinomial regression for repeated measures [15], was used to estimate the effects of covariates associated with state transitions while controlling for the effects of site, duration of follow-up, and calendar time, along with within-subject correlations. Specifically, we used the following model:
where αtjk is a time-specific intercept, xt is vector of covariates, and βjk represents relative risk ratios (RRR) which represent the effect of covariates on transitions from state j to k relative to the transition from state j to a reference state. Duration of follow-up and calendar year were fit using splines. We set transition to engagement in care as the reference. We used 1000 bootstrap samples for longitudinal data to estimate standard errors and confidence intervals for covariate effects.
We examined the effect of patient-level characteristics that are known to be associated with retention in HIV care from the published literature [6, 9, 12, 19–26], while controlling for site and temporal trends. Covariates included in multi-state models were as follows: CD4 count (<250, 250–500, >500) [19, 20, 26, 27], log10VL [9, 21], ART initiation (yes vs. no) [25, 26], ART initiation by calendar period associated with changes in WHO guidelines for ART eligibility (1996–2002, 2001–2003-2006, 2007–2010, and 2011+) [28–30], diagnosis of AIDS-defining illness (yes vs. no) [19, 22], race (White, African American (AA), others) [4, 6, 20, 22, 26], Hispanic ethnicity (yes vs. no) [4, 6, 20, 22], age (≤40yrs vs. >40yrs) [6, 20, 22, 24, 26], HIV risk factors by sex (men who have sex with men (MSM) who are non-injection drug users (non-IDU), MSM IDU, male heterosexual non-IDU, male heterosexual IDU, female heterosexual non-IDU, female heterosexual IDU, others) [4, 6, 22], site (UAB as reference), duration of follow-up, and calendar year. We considered an interaction between ART initiation and calendar periods reflecting changes in ART guidelines to examine the impact of changes in patient eligibility for ART initiation on care-retention. We also considered combinations of sex (at present) and HIV risk factors as a single covariate consisting of mutually exclusive categories. Information regarding injection drug use is coupled with sexual orientation in CNICS. Missing was considered as a valid category for each covariate and interpreted as “information never measured from clinic or never provided from the patient.” Covariates were time-fixed except for CD4, VL, ART, AIDS, duration of follow-up, and calendar year.
Throughout, we refer to an observed association as ‘statistically significant’ (at the 5% significance level) if the 95% confidence interval (CI) for RRR excludes 1.
Results
Sample Characteristics
Patients in the study sample were 81% male, 11% White, and 39% AA (Table 1). HIV acquisition risk factors were mostly MSM (51%) or heterosexual behaviors (30%). Median age at study entry was 39 years (interquartile range (IQR): 32–46). Median CD4 counts and log10VL at study entry were 348 (IQR:155–556), and 4.46 (IQR: 3.66–5.06), respectively.
Table 1.
Characteristics of 31,009 HIV-infected patients who were engaged in CNICS-affiliated HIV care between January 1st, 1996 and December 31st , 2014, at enrollment.
| Characteristic | Number (%) | Median (IQR) | % Missing |
|---|---|---|---|
| CD4 cell count (cells/ml3) | 11 | ||
| <250 | 7391 (23.8%) | ||
| 250−500 | 10586 (34.1%) | ||
| >=500 | 9612 (31%) | ||
| Log10 viral load | 4.46 (3.66, 5.06) | 13.6 | |
| <=3.5 | 10097 (32.6%) | ||
| 3.5−4.5 | 6060 (19.5%) | ||
| 4.5−5 | 4279 (13.8%) | ||
| >5 | 6268 (20.2%) | ||
| Initiated ART | 28303 (91.3%) | 8.7 | |
| AIDS-defining illness | 10550 (34%) | 18.6 | |
| Black race | 11961 (38.6%) | 3.3 | |
| Hispanic ethnicity | 3442 (11.1%) | 8.1 | |
| Age | 39 (32, 46) | ||
| Male sex | 25103 (81%) | 0 | |
| Sex and HIV risk factors | 3 | ||
| Male MSM non-IDU | 14226 (45.9%) | ||
| Male MSM IDU | 1623 (5.2%) | ||
| Male Heterosexual non-IDU | 3405 (11%) | ||
| Male Heterosexual IDU | 1014 (3.3%) | ||
| Female Heterosexual non-IDU | 4100 (13.2%) | ||
| Female Heterosexual IDU | 779 (2.5%) | ||
| Others | 5724 (18.5%) | ||
| Site | 0 | ||
| CWRU | 1908 (6.2%) | ||
| Fenway | 2394 (7.7%) | ||
| JHU | 4340 (14%) | ||
| UAB | 4732 (15.3%) | ||
| UCSD | 5857 (18.9%) | ||
| UCSF | 4458 (14.4%) | ||
| UNC | 3497 (11.3%) | ||
| UW | 3823 (12.3%) | ||
| Year at cohort entry | 0 | ||
| 1996−2000 | 6716 (21.7%) | ||
| 2001−2005 | 8622 (27.8%) | ||
| 2006−2010 | 8894 (28.7%) | ||
| 2011−2014 | 6776 (21.9%) |
Summary of transition rates and temporal trends
We calculated time-averaged, overall state transition probabilities (Table S-1 in supplemental data). Among patients engaged in CNICS care in a given 200-day interval, the rate of retention was 0.86, while disengagement was 0.13, and death was 0.01. Transition from disengaged states indicated the cyclic nature of engagement in care: among patients disengaged for 1, 2, and ≥3 intervals, 34%, 14%, and 3% returned to care, respectively. The per-interval mortality rate (aggregating values in the last column of Table S-1 in supplemental data) was about 0.05.
Figure 2 shows temporal trends in transition probabilities from either engaged in care or disengaged from care through 15 years of follow-up. Figure 2-1 indicates a sharp decrease in the probability of retention (green line) around the first year since baseline. Patients who were disengaged within the first year were at additional high risk of remaining disengaged in the following year, according to a sharp increase in the probability of continued disengagement (pink line) between year 1 and 2 (Figure 2-2). In addition, Figure 2-3 and 2-4 imply that patients disengaged for two or more intervals were much more likely to remain disengaged from care (pink lines) compared to returning to care (green lines). Mortality rates were relatively stable among care-engaged and disengaged patients and were constantly less than 0.02 per-interval time. In sum, Figure 2 demonstrates that the most critical period to retain CNICS patients in care are the first two years following enrollment. Patients with longer disengagement were at much higher risk of continued disengagement (conversely, much less likely to come back for care) than those having shorter disengagement for most of the follow-up period (data not shown). We did not observe a meaningful difference in mortality between care-engaged and disengaged patients.
Figure 2.
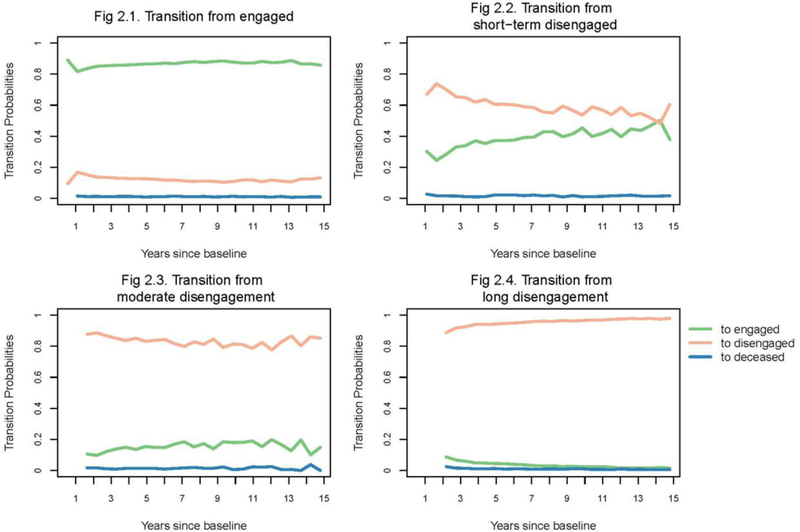
Temporal trends in state transition probabilities among care-engaged and care-disengaged patients over 15 years of follow-up period, presented in logit scale to amplify probabilities close to zero.
Effect of clinical characteristics on retention-related behaviors
Overall, clinical characteristics were significantly associated with transitions among patients who were engaged in CNICS care (Table 2); First, risk of transitioning from engaged to disengaged relative to remaining engaged in care among patients with CD4 counts 250–500, compared to those with CD4<250, was 0.87 (95% CI =[0.83,0.90]). Similarly, care-engaged patients with CD4 ≥ 500 were significantly less likely to disengage from care compared to those with CD4<250 (RRR=0.79; 95% CI=[0.76,0.83]). Therefore, patients with lower CD4 counts (<250) were significantly more likely to disengage from care rather than remain retained in care compared to those with higher CD4 counts. Among care-engaged patients with VL measurements, those with higher VL were more likely to disengage from care (RRR=1.26; 95% CI=[1.24,1.27]). Care-engaged patients who initiated ART were less likely to disengage from care (RRR=0.80; 95% CI=[0.75,0.85]). Among patients who initiated ART, those who initiated after 2006 were less likely to disengage from care compared to those who initiated in 1996–2002. Patients with CD4<250, higher VL, or no ART were also more likely to die in spite of their prior engagement with care, compared to patients with CD4 ≥ 250, lower VL, or ART. Among patients who initiated ART, mortality after engagement in care was significantly higher among those who initiated ART during 2003–2010 compared to those initiated in 1996–2002. However, no difference in mortality was found between patients who initiated in recent years (after 2010) compared to 1996–2002. Care-engaged patients with AIDS were less likely to disengage from care, but much more likely to die than patients without AIDS.
Table 2.
. Relative risk ratios (RRR) and 95% bootstrapped confidence intervals for effect of covariates on transitions from engaged in care to disengaged for 1 interval or death, relative to remaining engaged in care.
| Prior state | Engaged | |
|---|---|---|
| Current state | Disengaged | Death |
| CD4 counts (Ref: <250) | ||
| 250–500 | 0.87 (0.83, 0.90) | 0.28 (0.25, 0.30) |
| >500 | 0.79 (0.76, 0.83) | 0.17 (0.15, 0.19) |
| never measured | 4.67 (4.27, 5.16) | 2.09 (1.66, 2.66) |
| Viral load (VL) measured | 0.31 (0.29, 0.33) | 0.28 (0.23, 0.34) |
| log10 VL among measured | 1.26 (1.24, 1.27) | 1.27 (1.23, 1.30) |
| Initiated ART | 0.80 (0.75, 0.85) | 0.60 (0.53, 0.69) |
| Year initiated ART (Ref: 1996–200) | ||
| 2003–2006 | 1.03 (0.98, 1.09) | 1.14 (1.01, 1.29) |
| 2007–2010 | 0.91 (0.85, 0.98) | 1.24 (1.05, 1.49) |
| 2011+ | 0.79 (0.72, 0.87) | 1.25 (0.95, 1.64) |
| Missing ART information | 2.11 (1.95, 2.31) | 2.75 (2.30, 3.35) |
| AIDS-defining illness | 0.67 (0.65, 0.70) | 2.69 (2.50, 2.92) |
| Race (Ref: Caucasian) | ||
| African American | 1.02 (0.98, 1.06) | 0.90 (0.82, 0.98) |
| Others | 1.03 (0.97, 1.01) | 0.86 (0.71, 1.03) |
| Missing | 1.21 (1.09, 1.35) | 1.40 (1.04, 1.84) |
| Hispanic ethnicity | 0.79 (0.74, 0.84) | 0.64 (0.54, 0.75) |
| Missing ethnicity | 2.06 (1.83, 2.30) | 2.08 (1.62, 2.65) |
| Age>40yrs | 0.72 (0.70, 0.74) | 1.70 (1.57, 1.85) |
| Sex & HIV risk factors** (Ref: MSM NIDU) | ||
| MSM IDU | 1.02 (0.95, 1.09) | 1.43 (1.21, 1.69) |
| Male hetero NIDU | 1.10 (1.04, 1.15) | 1.41 (1.24, 1.61) |
| Male hetero IDU | 1.14 (1.05, 1.25) | 1.91 (1.62, 2.24) |
| Female hetero NIDU | 0.99 (0.95, 1.04) | 1.14 (0.99, 1.29) |
| Female hetero IDU | 0.94 (0.86, 1.03) | 1.62 (1.32, 1.92) |
| Others | 1.10 (1.05, 1.15) | 1.58 (1.42, 1.75) |
| Missing | 1.28 (1.16, 1.40) | 2.08 (1.69, 2.54) |
Factors that their 95% CI excludes 1 are highlighted in bold. All effects are adjusted by site, duration of follow-up, and calendar time (see supplemental data).
MSM represents men who sex with men; Hetero represents heterosexual contacts; IDU and NIDU represent injection drug users and none injection drug users, respectively.
Sex and HIV risk factors are combined. There is no main effect of sex as sex spans multiple risk factor categories. Also, injection drug use information is coupled with sexual orientation information to determine HIV risk factors in CNICS.
For time varying covariates (CD4, VL, ART, and AIDS), the most recently observed values obtained at last time engaged in care were used.
Clinical characteristics were, in general, significantly associated with transitions among patients who were disengaged from care as well (Table 3); among patients disengaged from care, those with higher CD4 (≥250) were less likely to die compared to those with lower CD4 counts. Among patients with VL measured, higher VL was consistently associated with continued disengagement and death. Initiating ART was negatively associated with continued disengagement and death among disengaged patients in most situations. Among patients who initiated ART, continued disengagement from care was significantly lower among those who initiated ART after 2006 compared to those who initiated in 1996–2002. In addition, mortality following long term disengagement from care (≥3 intervals) was significantly lower among those who initiated ART after 2010 compared to those who initiated in 1996–2002. Patients with AIDS were consistently less likely to continue to be disengaged, but more likely to die in most cases.
Table 3.
Relative risk ratios (RRR) and 95% bootstrapped confidence intervals for effect of covariates on transitions from disengaged states to continued disengagement or death, relative to remaining engaged in care.
| Prior state | Disengaged 1 | Disengaged 2 | Disengaged 3+ | |||
|---|---|---|---|---|---|---|
| Current state | Disengaged 2 | Death | Disengaged 3+ | Death | Disengaged 3+ | Death |
| CD4 counts (Ref: <250) | ||||||
| 250–500 | 0.97 (0.91, 1.05) |
0.38
(0.30, 0.48) |
1.18
(1.05, 1.33) |
0.51 (0.348, 0.739) |
1.19
(1.08, 1.32) |
0.63
(0.53, 0.75) |
| >500 |
0.83
(0.77, 0.89) |
0.18
(0.13, 0.24) |
1.15
(1.01, 1.30) |
0.26
(0.16, 0.40) |
1.10 (0.99, 1.23) |
0.49
(0.40, 0.59) |
| never measured |
5.39
(4.35, 6.87) |
2.68
(1.61, 4.56) |
3.22
(2.32, 4.78) |
1.26 (0.61, 2.60) |
2.94
(2.29, 3.94) |
1.50
(1.06, 2.14) |
| Viral load (VL) measured |
0.59
(0.51, 0.67) |
0.25
(0.16, 0.38) |
0.53
(0.40, 0.69) |
0.11
(0.05, 0.23) |
0.28
(0.22, 0.35) |
0.14
(0.10, 0.185) |
| log10 VL among measured |
1.04
(1.02, 1.06) |
1.27
(1.18, 1.35) |
0.96
(0.92, 0.99) |
1.24
(1.11, 1.41) |
1.06
(1.03, 1.10) |
1.26
(1.19, 1.33) |
| Initiated ART | 1.04 (0.92, 1.17) |
0.70
(0.49, 0.99) |
1.14
(0.95, 1.35) |
0.78
(0.49, 1.35) |
1.19
(1.02, 1.40) |
1.11 (0.87, 1.45) |
| Year initiated ART (Ref: 1996–2002) | ||||||
| 2003–2006 | 0.98 (0.89, 1.08) |
1.40
(1.03, 1.94) |
0.88 (0.75, 1.05) |
0.85 (0.52, 1.27) |
0.59
(0.52, 0.68) |
0.72
(0.56, 0.90) |
| 2007–2010 |
0.82 (0.73, 0.93) |
1.19 (0.76, 1.90) |
0.67
(0.54, 0.86) |
0.95 (0.48, 1.66) |
0.24
(0.20 0.29) |
0.35
(0.22, 0.53) |
| 2011+ |
0.62
(0.52, 0.74) |
0.60 (0.25, 1.36) |
0.36
(0.26, 0.50) |
0.67 (0.11, 1.92) |
0.05
(0.04, 0.07) |
0.17
(0.05, 0.34) |
| Missing ART information |
2.55
(2.18, 3.07) |
2.72
(1.62, 4.36) |
2.48
(1.93, 3.41) |
2.32
(1.25, 4.55) |
3.03
(2.39, 3.90) |
2.92
(2.07, 4.16) |
| AIDS-defining illness |
0.79
(0.73, 0.85) |
2.54
(2.03, 3.13) |
0.72
(0.64, 0.81) |
1.65
(1.16, 2.34) |
0.47
(0.41, 0.52) |
0.72
(0.59, 0.88) |
| Race (Ref: Caucasian) | ||||||
| African American |
0.84 (0.79, 0.90) |
0.712
(0.57, 0.90) |
0.77
(0.69, 0.86) |
0.92 (0.67, 1.31) |
0.72
(0.65, 0.78) |
0.94 (0.79, 1.10) |
| Others | 0.96 (0.87, 1.08) |
0.99 (0.61, 1.50) |
0.89 (0.74, 1.09) |
0.65 (0.24, 1.22) |
1.08 (0.93, 1.26) |
0.90 (0.59, 1.26) |
| Missing |
1.34
(1.11, 1.66) |
1.46 (0.48, 3.14) |
1.38
(1.00, 2.01) |
1.25 (0.22, 3.05) |
1.67
(1.26, 2.25) |
1.01 (0.50, 1.80) |
| Hispanic ethnicity | 0.96 (0.87, 1.07) |
0.50
(0.28, 0.79) |
0.90 (0.75, 1.08) |
0.52
(0.25, 0.91) |
0.98 (0.84, 1.15) |
0.71
(0.49, 0.98) |
| Missing ethnicity |
1.43
(1.20, 1.73) |
2.32
(1.20, 3.98) |
2.70 (2.03, 3.77) |
4.29
(1.74, 8.66) |
2.13
(1.74, 2.68) |
3.15
(2.14, 4.48) |
| Age>40yrs | 0.98 (0.92, 1.03) |
2.04 (1.66, 2.53) |
1.04 (0.94, 1.14) |
1.93
(1.42, 2.71) |
1.25
(1.16, 1.36) |
2.27
(1.92, 2.71) |
| Sex & HIV risk factors (Ref: MSM NIDU) | ||||||
| MSM IDU | 1.02 (0.90, 1.16) |
1.62
(1.05, 2.41) |
0.83 (0.69, 1.01) |
1.24 (0.65, 2.23) |
0.77
(0.65, 0.91) |
1.38
(1.01, 1.92) |
| Male hetero NIDU |
0.89 (0.80, 0.98) |
1.12 (0.80, 1.52) |
0.75
(0.64, 0.86) |
1.26 (0.77, 1.97) |
0.82
(0.72, 0.95) |
0.94 (0.72, 1.21) |
| Male hetero IDU | 1.12 (0.96, 1.28) |
1.97 (1.27, 2.99) |
0.75
(0.59, 0.97) |
1.77 (0.87, 3.31) |
0.63
(0.51, 0.77) |
1.17 (0.83, 1.63) |
| Female hetero NIDU | 1.01 (0.92, 1.10) |
1.07 (0.77, 1.46) |
0.86
(0.74, 0.99) |
1.43 (0.89, 2.33) |
0.81
(0.73, 0.92) |
0.73
(0.56, 0.94) |
| Female hetero IDU | 0.89 (0.76, 1.04) |
1.80
(1.10, 2.81) |
1.03 (0.78, 1.41) |
1.97 (0.78, 3.97) |
0.69
(0.55, 0.87) |
1.71
(1.18, 2.50) |
| Others | 1.07 (0.99, 1.16) |
1.49
(1.13, 1.97) |
0.90 (0.79, 1.02) |
1.37 (0.92, 2.10) |
0.78
(0.70, 0.87) |
1.38
(1.13, 1.69) |
| Missing |
1.25
(1.07, 1.48) |
1.52 (0.81, 2.53) |
1.21 (0.88, 1.72) |
3.89
(1.98, 7.66) |
1.76
(1.36, 2.32) |
2.93
(2.06, 4.37) |
Racial/Ethnicity disparity in retention
Among care-engaged patients, we observed no differences in disengagement from care by race. However, AA patients were less likely to die following engagement, compared to White patients (RRR=0.90; 95% CI=[0.82,0.98]). Non-Hispanic patients were more likely to disengage from care and die following engagement compared to patients reporting Hispanic ethnicity.
We observed a significant difference in the probability of continued disengagement by race. In general, AA patients were less likely to continue to be disengaged compared to White patients in CNICS.
Effect of age and HIV risk factors on retention
Among care-engaged patients, age and sex-HIV risk factors were significantly associated with transitions out of care: Older patients (age ≥ 40years) were less likely to disengage from care compared to younger patients, but had higher mortality rates. There was no effect of injection drug use on becoming disengaged among MSM, but IDU MSM had higher mortality than non-IDU MSM. Among male non-IDU, those identifying as heterosexual were more likely to disengage from care or die compared to MSM. Male heterosexual IDU were more likely to disengage or die compared to non-IDU MSM. No significant differences between non-IDU MSM and non-IDU female heterosexuals were observed. However, female heterosexual IDU were in general more likely to die compared to non-IDU MSM.
Discussion
This article presents a longitudinal examination of retention dynamics in the HIV care cascade. To our knowledge, this is among the first studies to make use of almost 20 years of cohort data from the modern ART era to examine temporal trends and factors associated with various transitions from care.
Our analysis shows the value of looking at temporal variations and some key factors to form a targeted policy or tailored monitoring/outreach plan to increase retention in HIV care. For example, our findings indicated that preventing early disengagement within the first two years following enrollment might be critical to prevent longer-term disengagement. This can inform development and calibration of existing retention-related efforts in CNICS which are similar to approaches described in [31]. More aggressive efforts early on to retain patients in care, such as developing a risk score that identifies patients who are at high risk of disengagement from care, and more intensive outreach for those who disengaged to re-engage them within the first two years post enrollment may enhance long-term retention rates in the CNICS cohort.
In addition to temporal trends, we identified that lower CD4 counts, higher VL, not initiating ART, and having an AIDS-defining illness was consistently associated with various care-disengagement outcomes in CNICS. Previous studies with shorter follow-up (<1 year) demonstrated that those with higher CD4 counts were less likely to be retained in care or more likely to miss scheduled appointments [19, 20, 26]. The discrepancy might be due to differing cohort eligibility criteria and durations of follow-up. Also, it is possible that our results show the consequences of better retention as they indicate only the associational relationship. Nonetheless, increased attention to monitoring the aforementioned subgroups of patients may maximize CNICS care efforts. From the effect of changes in ART eligibility guidelines, we observed that patients who initiated ART in recent years (2010 or later where the test and treat strategy became prominent) showed lower rates of disengagement from care. Our results therefore further support continued scale-up and implementation of the test and treat strategy, not only as part of HIV and AIDS prevention, but also as a strategy to improve care retention and reduce mortality among PLWH, as taking ART and the expansion of ART eligibility criteria were shown to be associated with better retention and lower mortality.
We also found reverse relationships in retention compared to findings from other studies [4, 32, 33]. We observed that care-engaged White patients were typically at higher risk of death compared to AA or patients in other racial groups, as well as at higher risk of continued disengagement once disengaged from care. However, the mortality results should be interpreted as exploratory because the CIs for those estimates were generally wide with upper 95% CIs close to 1 even within this large cohort.
Some of our findings reinforce existing evidence. For example, age and sex-HIV risk factor effects on retention in care reported here were consistent with existing evidence [34]; our findings therefore support the current recommendations that stress the needs of efforts to improve retention among younger patients and among male heterosexual PLWH. Regardless of sex and sexual orientation, people who inject drugs have increased risk of falling out of care including death.
There are several possible explanations for the differences in our findings compared to previous studies. In addition to cohort eligibility criteria and duration of follow-up, the differences might be partly attributable to our different time metric (200-day window) to capture engagement and retention in care. It is less strict than the usual definition of retention in care requiring at least 2 to 3 visits in a year [25]. Although there is no standard metric to define retention in HIV medical care [35–37], the 200-day window (6-months + two weeks) used here is a good representation of the retention indicator recommended by the US Department of Health Human Services and the Institute of Medicine [38, 39]. Also, note that our findings are based on analysis of multiple concurrent outcomes such as engagement, disengagement, and death whereas other analyses typically focused on single outcomes. Therefore, our analysis captures more comprehensive behaviors – the cyclic processes of engagement and retention in care - beyond simple retention, while accurately reflecting the current patient monitoring plans in the CNICS cohort. However, as we defined care visits based on VL measures, it is possible that some lab visits were misclassified as clinical visits with a doctor, leading to potentially overestimating retention in this study.
Our analysis was limited in that we did not control for some important patient-level characteristics such as substance abuse, depression, and insurance status, due to the quality of the available data. Kozak et al. (2012) demonstrated utility of PRO over electronic health records to determine substance abuse or depression status. However, CNICS included standardized (across sites) PRO measurements starting in 2000. The effect of insurance was not examined because individuals had multiple different insurance start dates without corresponding stop dates. Due to limited sample size and information, our analysis was not able to examine some small-scale minor groups that deserve more close attention such as transgender patients. Some demographic characteristics such as sex and race may be subject to misclassification due to variable data collection methods. Another limitation is that transferring to other clinics outside of the CNICS network is not captured in the CNICS database, which might result in inflation of some transition rates associated with disengagement. Finally, it should be noted that there are also barriers to using EMR such as inaccuracies in transcription, misclassification of some patient information, lack of standard terminology and practice to enter data across doctors, clinics, and/or sites.
In conclusion, our findings highlight the importance of modeling longitudinal patterns of engagement and retention in care to inform evidence-based approaches to improve care retention tailored to specific patient populations. Our approach may be used to develop prediction algorithms for identifying individuals who may be at higher risk of disengagement, loss, or death in other clinical cohorts or in national surveillance data.
Supplementary Material
Acknowledgements:
This research was supported by R01 AI108441 (PI: Hogan) and P30 AI042853 (Providence-Boston Center for AIDS Research); P30 AI094189 (JHU CFAR); R24 AI067039 (CFAR Network of Clinical Systems).
Contributor Information
Hana Lee, Department of Biostatistics, Brown University, Providence, USA.
Xiaotian K Wu, Department of Biostatistics, Brown University, Providence, USA.
Becky L Genberg, Department of Epidemiology, Johns Hopkins University, Baltimore, USA,.
Michael J Mugavero, School of Medicine, University of Alabama at Birmingham, Birmingham, USA.
Stephen R Cole, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, USA.
Bryan Lau, Department of Epidemiology, Johns Hopkins University, Baltimore, USA.
Joseph W Hogan, Department of Biostatistics, Brown University, Providence, USA.
References
- 1.The White House, O.o.N.A.P., National HIV/AIDS Strategy, Improving Outcomes: Accelerating Progress Along the HIV Care Continuum. 2013. [Google Scholar]
- 2.The White House, O.o.t.P.S., FACT SHEET: Accelerating Improvements in HIV Prevention and Care in the United States through the HIV Care Continuum Initiative. 2013. [Google Scholar]
- 3.Centers for Disease Control and Prevention, C., Understanding the HIV Care Continuum. 2017. [Google Scholar]
- 4.Giordano TP, et al. , Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials, 2009. 10(5): p. 299–305. [DOI] [PubMed] [Google Scholar]
- 5.Kozak MS, et al. , Patient Reported Outcomes in Routine Care: Advancing Data Capture for HIV Cohort Research. Clinical Infectious Diseases, 2012. 54(1): p. 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lucas GM, Chaisson RE, and Moore RD, Highly active antiretroviral therapy in a large urban clinic: Risk factors for virologic failure and adverse drug reactions. Annals of Internal Medicine, 1999. 131(2): p. 81-+. [DOI] [PubMed] [Google Scholar]
- 7.Marks G, et al. , Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS, 2010. 24(17): p. 2665–78. [DOI] [PubMed] [Google Scholar]
- 8.Thompson MA, et al. , Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Annals of Internal Medicine, 2012. 156(11): p. 817-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yehia BR, et al. , Barriers and facilitators to patient retention in HIV care. Bmc Infectious Diseases, 2015. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mugavero MJ, et al. , Beyond Core Indicators of Retention in HIV Care: Missed Clinic Visits Are Independently Associated With All-Cause Mortality. Clinical Infectious Diseases, 2014. 59(10): p. 1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebeiro P, et al. , Retention Among North American HIV-Infected Persons in Clinical Care, 2000–2008. Jaids-Journal of Acquired Immune Deficiency Syndromes, 2013. 62(3): p. 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugavero MJ, et al. , The State of Engagement in HIV Care in the United States: From Cascade to Continuum to Control. Clinical Infectious Diseases, 2013. 57(8): p. 1164–1171. [DOI] [PubMed] [Google Scholar]
- 13.Blitz S, et al. , The Use of Multistate Models to Examine Associations of Stress and Adherence With Transitions Among HIV Care States Observed in a Clinical HIV Cohort. J Acquir Immune Defic Syndr, 2017. 76(3): p. 303–310. [DOI] [PubMed] [Google Scholar]
- 14.Lesko CR, et al. , A longitudinal, HIV care continuum: 10-year restricted mean time in each care continuum stage after enrollment in care, by history of IDU. AIDS, 2016. 30(14): p. 2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H, et al. , A state transition framework for patient-level modeling of engagement and retention in HIV care using longitudinal cohort data. Statistics in Medicine, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powers KA and Miller WC, Building on the HIV Cascade: A Complementary “HIV States and Transitions” Framework for Describing HIV Diagnosis, Care, and Treatment at the Population Level. Jaids-Journal of Acquired Immune Deficiency Syndromes, 2015. 69(3): p. 341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillis J, et al. , A Multi-State Model Examining Patterns of Transitioning Among States of Engagement in Care in HIV-Positive Individuals Initiating Combination Antiretroviral Therapy. Jaids-Journal of Acquired Immune Deficiency Syndromes, 2016. 73(5): p. 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitahata MM, et al. , Cohort profile: The Centers for AIDS Research Network of Integrated Clinical Systems. International Journal of Epidemiology, 2008. 37(5): p. 948–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arici C, et al. , Factors associated with the failure of HIV-positive persons to return for scheduled medical visits. HIV Clin Trials, 2002. 3(1): p. 52–7. [DOI] [PubMed] [Google Scholar]
- 20.Catz SL, et al. , Predictors of outpatient medical appointment attendance among persons with HIV. AIDS Care, 1999. 11(3): p. 361–73. [DOI] [PubMed] [Google Scholar]
- 21.Cohen SM, et al. , HIV Viral Suppression Among Persons With Varying Levels of Engagement in HIV Medical Care, 19 US Jurisdictions. Jaids-Journal of Acquired Immune Deficiency Syndromes, 2014. 67(5): p. 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Israelski D, et al. , Sociodemographic characteristics associated with medical appointment adherence among HIV-seropositive patients seeking treatment in a county outpatient facility. Preventive Medicine, 2001. 33(5): p. 470–475. [DOI] [PubMed] [Google Scholar]
- 23.Mugavero MJ, et al. , Missed Visits and Mortality among Patients Establishing Initial Outpatient HIV Treatment. Clinical Infectious Diseases, 2009. 48(2): p. 248–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poole WK, et al. , A characterisation of patient drop outs in a cohort of HIV positive homosexual/bisexual men and intravenous drug users. Journal of Epidemiology and Community Health, 2001. 55(1): p. 66–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tedaldi EM, et al. , Retention in care within 1 year of initial HIV care visit in a multisite US cohort: who’s in and who’s out? J Int Assoc Provid AIDS Care, 2014. 13(3): p. 232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ulett KB, et al. , The Therapeutic Implications of Timely Linkage and Early Retention in HIV Care. Aids Patient Care and Stds, 2009. 23(1): p. 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giordano TR, et al. , Predictors of Retention in HIV Care Among a National Cohort of US Veterans. Hiv Clinical Trials, 2009. 10(5): p. 299–305. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization, Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2013. [PubMed] [Google Scholar]
- 29.World Health Organization, Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 2010. [PubMed] [Google Scholar]
- 30.World Health Organization, Antiretroviral therapy for HIV infection in adults and adolescents: Recommendations for a public health approach. 200640 [PubMed] [Google Scholar]
- 31.Higa DH, et al. , Interventions to improve retention in HIV primary care: a systematic review of U.S. studies . Curr HIV/AIDS Rep, 2012. 9(4): p. 313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulett KB, et al. , The therapeutic implications of timely linkage and early retention in HIV care . AIDS Patient Care STDS, 2009. 23(1): p. 41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mugavero MJ, et al. , Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis, 2009. 48(2): p. 248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National HIV Curriculum, Retention in HIV Care. 2018. [Google Scholar]
- 35.Horstmann E, et al. , Retaining HIV-infected patients in care: Where are we? Where do we go from here? Clin Infect Dis, 2010. 50(5): p. 752–61. [DOI] [PubMed] [Google Scholar]
- 36.Mugavero MJ, et al. , From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS, 2010. 24(10): p. 607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford TN, Sanderson WT, and Thornton A, A comparison study of methods for measuring retention in HIV medical care. AIDS Behav, 2013. 17(9): p. 3145–51. [DOI] [PubMed] [Google Scholar]
- 38.Services, U.D.o.H.a.H., Retention in HIV Medical Care. 2014. [Google Scholar]
- 39.Young B, The Importance of Retention in HIV Care. Medscape, 2014. [Google Scholar]
- 40.Kozak MS, et al. , Patient reported outcomes in routine care: advancing data capture for HIV cohort research. Clin Infect Dis, 2012. 54(1): p. 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


