Structured Abstract
Objective.
The FMR1 premutation is a common genetic abnormality, affecting ~1:150 women in the United States. Clinical neuropsychologists are becoming increasingly aware of their role in the clinical management of the FMR1 premutation, which is associated with risk for a range of cognitive, executive, neuromotor, and psychological impairments, including neurodegenerative disease. This study investigated atypical eye contact as a critical neuropsychological phenotype associated with the FMR1 premutation, and its potential interface with social anxiety.
Methods.
Thirty-eight women with the FMR1 premutation and 27 control women engaged in a 20-minute conversational sample with an examiner. Eye contact quality was coded from the videotaped samples by blinded coders. Mixed models tested group differences in eye contact during the beginning and the end of the conversation. Social anxiety and broad autism phenotype traits were tested as predictors of eye contact quality across the groups.
Results.
Women with the FMR1 premutation exhibited significantly reduced eye contact during both the beginning and the end of the social interaction, despite a “warm-up” effect where eye contact improved by the end of the interaction. Eye contact quality was not associated with social anxiety or broad autism phenotype traits.
Conclusions.
This study supports reduced eye contact as a phenotypic feature of the FMR1 premutation, which presents independent of social anxiety and the broad autism phenotype. These findings contribute to a growing understanding of the neuropsychological phenotype of the FMR1 premutation, which has public health implications given that >1 million individuals in the United States carry this genetic abnormality.
Keywords: fragile X premutation, eye gaze, social gaze, fragile X carriers, social phobia
Clinical neuropsychologists are becoming increasingly aware of their role in the management of Fragile X Mental Retardation-1 (FMR1) premutation syndromes. The FMR1 premutation is characterized by an expansion of 55–200 repeats of the CGG sequence on the FMR1 gene (Maddalena et al., 2001). This trinucleotide expansion is highly prevalent, affecting 1 in 151 females in the United States (Seltzer, Baker, et al., 2012), and is associated with risk for having a child affected by fragile X syndrome (Nolin et al., 2003). Neuropsychologists have a significant role in the management of the FMR1 premutation, as this genetic abnormality is associated with a neuropsychological phenotype characterized by a range of cognitive, social, and psychological issues that vary in penetrance and severity. Cognitive features of the FMR1 premutation in women include deficits in inhibitory control, attention, and working memory (Kraan et al., 2013). About 5% of females with the FMR1 premutation are affected by autism spectrum disorder, and expression of the broad autism phenotype (BAP) is also elevated in this group (Clifford et al., 2007; Losh et al., 2012; Schneider et al., 2016). Perhaps the best-characterized clinical consequence is psychological vulnerability, which includes rates of social anxiety disorder estimated at 6–18% in women (Bourgeois et al., 2011; Franke, Leboyer, Gansicke, & Weiffenbacj, 1998; Roberts et al., 2016). Additionally, 16% of females with the FMR1 premutation will develop Fragile X-Associated Tremor-Ataxia Syndrome (FXTAS), a late-onset neurodegenerative movement disorder characterized by tremor, ataxia, dementia, executive dysfunction, and mood disorders (Hall et al., 2014). Assessment and monitoring of FXTAS risk and decline in patients with the FMR1 premutation is also a critical role of the clinical neuropsychologist (as highlighted in a recent special issue in The Clinical Neuropsychologist, e.g., Hessl & Grigsby, 2016).
Although the FMR1 premutation is clearly associated with neuropsychological risk, our understanding the phenotypes associated with this genetic abnormality is nonetheless still emerging. A decade ago, the prevailing view was that individuals with the FMR1 premutation were “silent” carriers with no associated clinical features (e.g., Hagerman & Hagerman, 2002). Therefore, many aspects of the FMR1 premutation profile remain undefined, particularly in women. Atypical eye contact, in particular, has been largely unexplored, despite its critical role in social functioning. Atypical use of social gaze can be highly disruptive of social engagement, as eye gaze facilitates key social functions such as the ability to understand the mental states of others, the establishment of listener/speaker roles, and the perception of speaker intent and deception (Baron-Cohen, 1995; Bruner, 1983; Frith & Frith, 2001; Hanna & Brennan, 2007; Ho, Foulsham, & Kingstone, 2015; Kleinke, 1986; McCarthy & Lee, 2009; Tomasello & Tomasello, 2009; Vertegaal, Slagter, Van der Veer, & Nijholt, 2001). Attunement to social gaze is thought to represent an innate, biologically prepared skill that provides the foundation for the later development of adaptive social behavior, and is present in newborns as young as 1–2 days old (Farroni, Csibra, Simion, & Johnson, 2002; Farroni, Massaccesi, Menon, & Johnson, 2007; Grossmann, Johnson, Farroni, & Csibra, 2007). Impaired perception and use of eye gaze is closely tied with brain pathology, with a large network of brain areas implicated, including the superior temporal sulcus region, the amygdala, the fusiform gyrus, and some frontal and parietal areas (Itier & Batty, 2009). In the hands of a skilled neuropsychologist, information about a patient’s eye contact can lend insight into specific neuronal circuits underlying the patient’s neuropsychological profile.
While there is a paucity of research on eye contact in the FMR1 premutation, gaze avoidance is a hallmark feature of fragile X syndrome. Gaze avoidance in fragile X is thought to be rooted in social anxiety and is often most striking in response to initial interactions in novel social settings, but improves over time (Cohen et al., 1988; Roberts, Weisenfeld, Hatton, Heath, & Kaufmann, 2007). Some clinical case descriptions suggest that this profile of gaze avoidance may extend to the FMR1 premutation, such as a case study by Tassone et al. (2000) where “difficulty making eye contact” was described in a 9-year-old girl and a 33-year-old woman. One other study failed to detect differences between the eye contact of women with the FMR1 premutation and controls (Riddle et al., 1997). However, this study focused on clinician impressions of eye contact during a medical examination and the blunt “present/absent” scale utilized may have been inadequate for capturing subtle differences. Other evidence of altered use of social gaze comes from a recent study by Klusek et al. (2017), which found that women with the FMR1 premutation showed increased attention to averted gaze relative to controls during an experimental eye tracking paradigm. These studies underscore the need for focused investigation of eye contact in the FMR1 premutation. Understanding the eye gaze profile of the FMR1 premutation will contribute to the growing understanding of the clinical effects associated with this prevalent genetic abnormality and may have treatment implications. The present study contributes to our nascent understanding of the neuropsychological profile of the FMR1 premutation phenotype through characterization of atypical eye contact and its potential interface with social anxiety and the BAP.
Social anxiety is characterized by excessive fear of social scrutiny that often leads to avoidance of feared social situations (American Psychiatric Association, 2013). Eye gaze is one modality through which negative social evaluations are conveyed, and thus may represent a fear-relevant stimulus for individuals with social anxiety (Clark & Wells, 1995; Mogg, Philippot, & Bradley, 2004; Wirth, Sacco, Hugenberg, & Williams, 2010). Several studies have documented a connection between social anxiety symptoms and reduced eye contact, both in non-patient and social anxiety disorder samples (Daly, 1978; Farabee, Holcom, Ramsey, & Cole, 1993; Schneier, Rodebaugh, Blanco, Lewin, & Liebowitz, 2011). Additionally, delayed or deficient habituation to novel social stimuli—characteristic of shy or “slow to warm up” temperament— is associated with substantially heightened risk for social anxiety (Biederman et al., 2001; Clauss & Blackford, 2012; Fox, Henderson, Marshall, Nichols, & Ghera, 2005; Gladstone, Parker, Mitchell, Wilhelm, & Malhi, 2005; Hirshfeld et al., 1992; Rothbart, Ahadi, & Evans, 2000). Thus, we hypothesized women with the FMR1 premutation would show a profile of gaze avoidance that is most striking in the initial moments of a social interaction but improves given time to adjust to the social context. This hypothesized profile parallels the gaze avoidance profile of fragile X syndrome, which is characterized by prominent reductions in eye contact during the initial moments of social interaction, with improvement over time but failure to normalize to the level of controls (Cohen et al., 1988; Roberts, Weisenfeld, Hatton, Heath, & Kaufmann, 2007).
To explore alternative potential mechanisms of reduced eye contact in the FMR1 premutation, this study also examined the association between BAP traits and eye contact. The BAP is comprised of subclinical traits that mirror the core deficits of autism and reflect genetic vulnerability to the condition (Losh, Childress, Lam, & Piven, 2008). These traits are seen at increased rates in women with the FMR1 premutation (Losh et al., 2012; Schneider et al., 2016) and may influence the use of social gaze. Atypical eye contact is a hallmark feature of autism spectrum disorder, and differences in gaze behavior and processing extend to the BAP (Chen & Yoon, 2010; Losh & Piven, 2007). Thus, the present study sought to better characterize the neuropsychological profile of the FMR1 premutation through examination of eye contact quality and its association with BAP and social anxiety phenotypes. Our research questions were as follows:
Is eye contact reduced in women with the FMR1 premutation relative to control women, and is a “warm up effect” observed where eye contact improves with time? We hypothesized that women with the FMR1 premutation would exhibit reduced eye contact that is most salient in the initial minutes of interaction, before adjustment to the novel social context has taken place. It was expected that eye contact would improve by the end of the social interaction, but would nonetheless remain reduced relative to controls, suggesting failure to adjust to the social context.
Do social anxiety and BAP phenotypes relate to the quality of eye contact in women with the FMR1 premutation and controls? We hypothesized that reduced eye contact would be associated with elevated social anxiety symptoms and BAP traits.
Methods
Participants
Participants included 38 women with the FMR1 premutation and 27 neurotypical control women who were participating in a larger study on communication profiles in the FMR1 premutation, which has been described previously (e.g., Klusek et al., 2017). Women with the FMR1 premutation were recruited through their children who were participating in larger developmental studies of fragile X syndrome or the FMR1 premutation, or from the local community by word of mouth. The presence of the FMR1 premutation was confirmed via genetic testing conducted as part of the larger study. Although genetic testing for controls was beyond the scope of the present study, premutation alleles were ruled out in 70% of the control participants, who completed genetic testing through dual enrollment in a related pilot study. Control women were recruited from the local community through word of mouth and social media. This group consisted of women who were mothers of typically developing children, which allowed us to exclude women who had children with developmental delays potentially associated with fragile X syndrome. Typical development in children was defined as the absence of a diagnosed or treated developmental delay/disorder per maternal report and by scores below the threshold for autism spectrum disorder on the Social Communication Questionnaire (SCQ; Rutter, Bailey, & Lord, 2003). Four women initially recruited for the study were excluded: one because of elevated SCQ scores in her child, and three because of scores ≥ 1.5 standard deviations below the mean on the IQ Composite of the Kaufman Brief Intelligence Test-2 (Kaufman & Kaufman, 2004). The groups were similar in age, IQ, education level, and race; see Table 1 for demographic information.
Table 1.
Group characteristics
| Characteristic | Group |
||
|---|---|---|---|
|
FMR1 Premutation (n=38) |
Control (n=27) |
Test of Group Differences (p–value) |
|
| Age (years) | |||
| M (SD) | 43.38 (7.92) | 40.85 (8.27) | 0.217 |
| Range | 25.53–55.32 | 28.72–64.02 | |
| IQ1 | |||
| M (SD) | 105.23 (13.38) | 104.62 (11.48) | 0.865 |
| Range | 81.00–130.00 | 83.00–135.00 | |
| Highest Education Level (%) | |||
| Some high school | 2 | -- | |
| High school graduate/GED | 16 | 4 | |
| Some college/technical school | 24 | 15 | |
| Associates/technical degree | 5 | 11 | 0.319 |
| Bachelor’s degree | 21 | 29 | |
| Some graduate work | 8 | 4 | |
| Master’s degree | 21 | 22 | |
| Professional/advanced degree | 3 | 15 | |
| Race (%) | |||
| African American | 2.78 | 14.81 | 0.156 |
| Caucasian | 94.44 | 85.19 | |
| Other | 2.78 | -- | |
Note.
Measured with the IQ Composite of the Kaufman Brief Intelligence Test-2 (Kaufman & Kaufman, 2004).
Measures
Eye Contact
Eye contact was coded from a 20 minute conversational “life history” interview, which has been used extensively as a semi-structured conversational sample in studies of non-disordered adults (e.g., Klusek, Losh, & Martin, 2014; Klusek, McGrath, Abbeduto, & Roberts, 2016; Losh et al., 2012). Examiners introduced conversational topics around a standard template of open-ended topics, such as “What kinds of activities did you enjoy most as a child?”. Probe questions focused on the participants’ childhood and adult life prior to having children, and did not include questions about their children. While all participants received the same standard probe questions, the interviewer facilitated conversation around the standard topics by commenting on participants’ responses, asking follow-up questions, and offering information. The participants were not aware that the interaction would be coded for eye contact or other social behaviors. Each conversational sample was videotaped with both the participant and examiner visible on the screen.
Eye contact was rated from the first three minutes and last three minutes of the videotaped conversational sample. These time segments were chosen to obtain a measure of initial eye contact, when the social situation is most novel, and final eye contact after habituation has taken place. One summary code capturing the overall quality of eye contact was assigned for the initial and final observation points. The summary eye contact rating was represented by a 5-point scale, with a higher score denoting progressively worse eye contact. For example, a “1” indicated eye contact that was contextually appropriate and well integrated with speech, a “3” indicated eye contact that was mildly reduced, and a “5” indicated eye contact that was rare or significantly reduced. Each sample was coded by two trained raters who had no prior knowledge of group membership. Although the raters were initially blind, six women with the FMR1 premutation revealed their group membership during the interview; statistical analyses indicated that the inclusion of these samples did not influence study results and therefore the full sample was retained in final analyses (see Data Analysis). The raters were trained by independent coding of a series of practice files until they achieved 100% agreement with each other on three consecutive training files. After training, each rater scored each sample independently and consensus scores for each file were determined through discussion. Inter-rater reliability prior to consensus was ICC (3, 2) = 0.93 for initial eye contact and ICC (3, 2) = 0.92 for final eye contact.
Social Anxiety Symptoms
The Liebowitz Social Anxiety Scale-Self Report (LSAS-SR; Liebowitz, 1987) measured continuous social anxiety symptoms. This self-report scale consists of 24 items representing social situations such as, “talking with people you don’t know very well” and “looking at people you don’t know very well in the eyes.” Items capture trait social anxiety symptoms across a range of contexts, as opposed to state symptoms (i.e., a temporary response to a specific social event). Each item is scored on a 4-point scale based on ratings of fear/anxiety and avoidance behavior associated with each situation experienced within the last week. Scores are tallied to create a total score representing the severity of social anxiety symptoms. The LSAS-SR has high internal consistency of 0.95 and high agreement with the clinician-administered version (Fresco et al., 2001). A cut-off score of 30 has been proposed for differentiating individuals with social anxiety disorder from non-anxious controls (Rytwinski et al., 2009).
Social Anxiety Disorder
Current and lifetime clinical social anxiety disorder (social phobia) were also assessed in the FMR1 premutation sample. Social anxiety disorder in the FMR1 premutation was evaluated by a trained doctoral-level examiner with the Structured Clinical Interview for DSM-IV-TR (SCID; First & Gibbon, 2004), which is a semi-structured interview that diagnoses Axis I disorders according to DSM-IV criteria. The interviews were audio recorded and 20% were randomly selected and second-scored for inter-rater reliability by a licensed psychologist who was blind to group membership; percent agreement for the presence/absence of social anxiety disorder was 100%. Both lifetime and current occurrence of social anxiety disorder were queried, however, in this dataset all individuals who had a positive lifetime history of social anxiety disorder also met diagnostic criteria for current social anxiety. Therefore, current and lifetime disorder were collapsed into a single category in analyses. SCID data were missing for 4 participants due to time constraints. These data were not collected on controls because it was expected that too few controls would meet criteria for social anxiety disorder to yield meaningful analyses across dichotomized subgroups.
Broad Autism Phenotype Traits
The Broad Autism Phenotype Questionnaire (BAP-Q; Hurley, Losh, Parlier, Reznick, Piven, 2007; Sasson et al., 2013) was administered as an index of BAP symptoms. The BAP-Q was developed to identify the BAP in parents for family studies of autism spectrum disorder, and has good reliability, internal consistency, a validated factor structure, and evidence of convergent validity with clinical assessments of the BAP (Ingersoll, Hopwood, Wainer, & Donnellan, 2011; Hurley et al., 2007). The BAP-Q combines self and informant report to evaluate symptoms within the core domains of the BAP: aloofness, pragmatic language difficulties, and rigidity. Participants completed the self-report form and asked someone who “knew them well” to complete the informant-report form. Informants were provided a prepaid mailer to return the questionnaire directly to the researchers after completion. The questionnaire consists of 36 items scored on a 1–6 scale based on the frequency each feature is exhibited. The BAP-Q yields a best-estimate total score, which is an averaged summary score of the informant and participant reports. Higher scores are consistent with increased BAP traits; a cut-off score of 3.19 is recommended to identify females who are “positive” for the BAP (Sasson et al., 2013).
Procedure
Assessments were administered as part of a larger research protocol that lasted approximately three hours. About a week prior to the assessment, participants were mailed a packet including the LSAS-SR and BAP-Q forms. Participants were asked to bring the completed questionnaires with them to the assessment (a prepaid mailer was included in the packet for informants to mail the BAP-Q informant form directly to the researchers). The first hour of the assessment consisted of standardized cognitive testing. The life history interview followed completion of the cognitive assessments, and represented the first opened-ended, non-structured activity. The SCID was the final assessment administered in the protocol. All interviews were conducted by one of four trained, doctoral-level female examiners. It was not possible for the examiners to remain blind to group membership given the length of the protocol. However, examiners were blind to the participant’s LSAS-SR, BAP-Q and SCID results at the time of the interview. Informed participant consent was obtained and all procedures were approved by the Institutional Review Board of the University of South Carolina.
Data Analysis
Analyses were carried out using SAS 9.4 (SAS Insititute, 2012). First, descriptive statistics were computed and the data were examined for normal distribution. Right skewing was observed for the LSAS-SR total score. The Box Cox transformation technique (Box & Cox, 1964) was applied to find the optimal normalizing transformation and the LSAS-SR was transformed by λ= 0.50. Next, potential confounds were examined to determine covariates for the final models. No examiner effects were indicated by general linear models testing the effect of examiner on the quality of initial (p =.400) and final (p = .742) eye contact. Pearson correlations indicated that age was not associated with eye contact during either initial or final observation points, within the groups collapsed (p’s > .659) or across the groups (p’s > .521). The quality of eye contact was also not associated with IQ within groups (all p’s > .216) or across groups (p’s > .592). Based on these analyses, age, IQ, and examiner were not covaried in the final models.
To address the first research question, a mixed effects linear model tested group, condition, and their interaction as predictors. Race and education level were included as covariates, as it is possible that these demographic characteristics could account for variance in eye contact behavior. Then, the relationship between social anxiety and eye contact was tested using a mixed effects general linear model. The model included group, condition, the LSAS-SR total score, the LSAS-SR-by-group interaction, and the LSAS-SR-by-condition interaction as predictors, as well as the covariates race and education level. Next, a second mixed model tested current social anxiety disorder as a predictor of eye contact across conditions in the FMR1 premutation sample. The model included condition, the occurrence of social anxiety disorder, and their interaction as predictors. A final mixed effects model was fit to test the association between BAP symptoms and eye contact. This model included group, condition, BAP symptoms, the interactions between BAP symptoms and group and condition, as well as race and education level. For all models, condition (initial/final) was specified as a random effect, nested within participant. All models were estimated with a compound symmetry covariance structure, which was selected through examination of the AICC fit estimates and examination of the correlation matrices to confirm model assumptions. Cohen’s f 2 local effect sizes were computed as described in Selya, Rose, Dierker, Hedeker, & Mermelstein (2012). Cohen’s f 2 estimates the effect size of a single predictor within the context of a multivariate model, with values of 0.02, 0.15, and 0.35 generally indicative of small, medium, and large effects, respectively (Cohen, 1998). Finally, to test the potential influence of the six women who un-blinded themselves during the interview, each model was repeated with these participants omitted; inference across all solutions was identical and therefore these participants were retained in the final analysis.
Results
Descriptive Statistics
Descriptive statistics for each of the continuous predictor and outcome variables are presented in Table 2. To describe the proportion of women who exhibited clearly atypical gaze behavior, the percent of women who were assigned a “5” on the eye contact rating scale (marking atypical eye contact that was strikingly reduced or rare) was computed. Scores of “5” were assigned to 39% of the FMR1 premutation in the initial observation phase and to 18% in the final observation phase (in contrast to 11% in the initial observation and 4% in final observation for the controls). Eye contact scores of “1” (reflecting appropriate eye contact) were assigned to 26% (initial observation) and 37% (final observation) of the FMR1 premutation sample, and to 37% (initial observation) and 63% (final observation) of the control women. The groups did not differ significantly on the LSAS-SR (t [56.9] = 1.57, p = 0.121) or the BAP-Q (t [56.36] = 1.04, p = 0.301), although the women with the FMR1 premutation obtained higher mean scores on both measures. Seven women (20%) with the FMR1 premutation met diagnostic criteria for social anxiety disorder. The mean eye contact scores for the women with the FMR1 premutation who were positive for social anxiety disorder were M = 3.57, SD = 1.81 for initial and M = 2.57, SD = 0.79 for final ratings. The average scores of the women with the FMR1 premutation who were negative for social anxiety disorder were M = 3.22, SD = 1.69 for initial and M = 2.78, SD = 1.63 for final ratings.
Table 2.
Descriptive statistics
| Group | ||
|---|---|---|
| Variable |
FMR1 premutation (n=38) |
Control (n=27) |
| M (SD), range | M (SD), range | |
| Initial Eye Contact Score | 3.21 (1.71), 1.00–5.00 | 2.40 (1.45), 1.00–5.00 |
| Final Eye Contact Score | 2.66 (1.53), 1.00–5.00 | 1.70 (1.14), 1.00–5.00 |
| Liebowitz Social Anxiety Scale-Self Report Total Score (untransformed) |
39.48 (27.30), 2.00–115.00 | 29.62 (20.90), 0–82.00 |
| Liebowitz Social Anxiety Scale-Self Report Total Score (transformed) |
10.02 (4.25), 1.46–19.54 | 8.34 (4.00), 0–16.22 |
| Broad Autism Phenotype Questionnaire- Total Score | 2.59 (0.58), 1.56–4.08 | 2.47 (0.37), 1.82–3.28 |
Eye Contact across Groups and Initial and Final Observation Phases
The mixed model indicated a significant main effect of group (t [54] = −2.78, p = 0.008, 95% CI [−1.83, −0.30], f 2 = 0.06) and condition (t [63] = 3.09, p = 0.003, 95% CI [0.20, 0.91], f 2 = 0.16), with a non-significant group-by-condition interaction (t [63] = 0.54, p = 0.588, 95% CI [−0.40, 0.71], f 2 < 0.01). Thus, women with the FMR1 premutation were rated as having poorer eye contact than control women across both the beginning and end of the interview. A warm-up effect was observed in both groups where eye contact was poorer during the initial three minutes of conversation compared to the final three minutes; see Fig. 1.
Figure 1. Eye contact across groups and initial and final observation points.
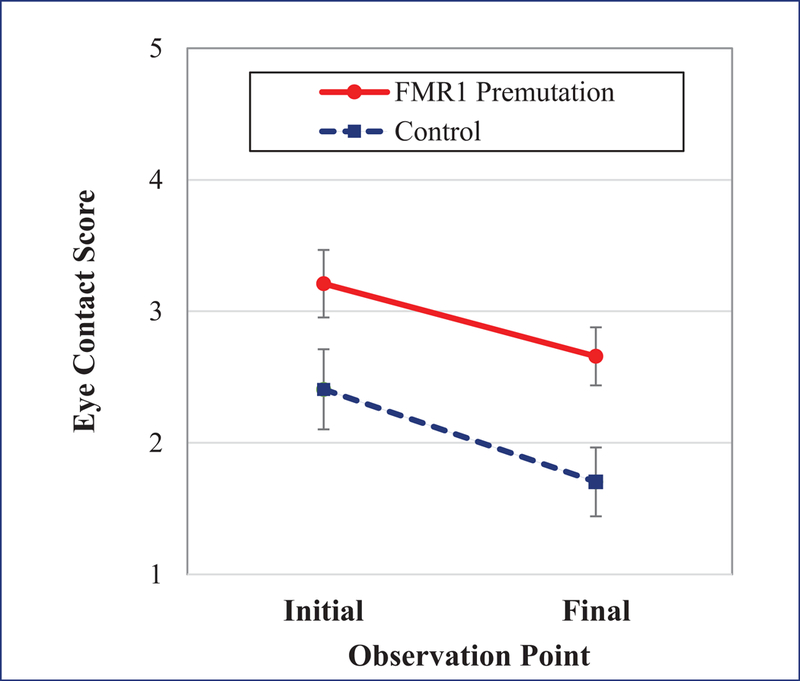
Note. A higher score denotes poorer eye contact. Eye contact was reduced in the FMR1 premutation relative to controls in both conditions. Both groups showed improved eye contact in the final minutes of the conversation relative to the initial minutes.
Relationships with Social Anxiety Symptoms and Diagnoses
First, a mixed model tested the relationship between social anxiety symptoms and eye contact across the groups. The effect of social anxiety symptoms on eye contact was not significant (t [46] = 1.11, p = 0.274, 95% CI [−0.07, 0.26], f 2 = 0.01). The interactions between social anxiety symptoms and group (t [46] = −0.82, p = 0.415, 95% CI [−0.26, 0.11], f 2 < 0.01), and social anxiety symptoms and condition (t [57] = −0.04, p = 0.966, 95% CI [−0.07, 0.7], f 2 < 0.01) were also not significant.
A second mixed model tested the impact of current social anxiety disorder on eye contact within the FMR1 premutation group. The presence of social anxiety disorder did not have a significant effect on eye contact (t [32] = 0.53, p = 0.603, 95% CI [−1.00, 1.70], f 2 = 0.02) and the interaction between social anxiety disorder and condition was also non-significant (t [32] = −1.17, p = 0.251, 95% CI [−1.52, 0.41], f 2 = 0.02).
Relationships with Broad Autism Phenotype Traits
The presence of BAP features did not account for significant variation in eye contact (t [47] = 0.35, p = 0.729, 95% CI [−1.34, 1.90], f 2 < 0.01). The interactions between BAP symptoms and group (t [47] = −0.38, p = 0.708, 95% CI [−2.08, 1.42], f 2 < 0.01), and BAP symptoms and condition (t [58] = 0.22, p = 0.827, 95% CI [−0.52, 0.64], f 2 < 0.01) were also not significant.
Discussion
This is the first study to quantify atypical eye contact in women with the FMR1 premutation during a conversational interaction. Contrary to hypotheses, reduced eye contact was not linked with either symptoms or clinical diagnoses of social anxiety, or with features of the BAP. This study informs the complex and varied clinical presentation of the FMR1 premutation, particularly in regard to key nonverbal social communication patterns and the disassociation of component features of the FMR1 premutation phenotype that may otherwise appear to be related and may map to disparate brain regions or biological pathways.
Through detailed, blinded evaluations of eye contact exhibited during a conversational interview, this study provides evidence that eye contact is reduced in women with the FMR1 premutation during social conversation with an unfamiliar examiner. Although the eye contact of the women with the FMR1 premutation improved by the end of the interaction, it did not normalize to the level of the control women. Thus, poor eye contact was sustained and cannot be attributed to initial shyness or wariness. The finding of atypical eye contact is consistent with imaging studies of the FMR1 premutation showing atypical activation patterns in the amygdala and superior temporal sulcus, which are areas involved in the perception and use of eye gaze (Hessl et al., 2007; Hessl et al., 2011; Itier & Batty, 2009). In men with the FMR1 premutation, reduced amygdala activation is associated with reduced expression of Fragile X Mental Retardation Protein (FMRP), the protein encoded by the FMR1 gene (Hessl et al., 2011). FMRP is mildly reduced in the FMR1 premutation and is thought to underlie certain aspects of aberrant brain function and behavior. Follow-up studies incorporating FMR1 molecular genetic measures in the study of eye gaze behavior may shed light on gene-brain-behavior relationships.
Contrary to our hypotheses, social anxiety was not associated with poor eye contact in the FMR1 premutation. This finding is inconsistent with some prior research suggesting a link between social anxiety and reduced eye contact in other groups, although methodological differences may explain divergent findings. For example, Farabee et al. (1993) also directly coded the eye contact of socially anxious individuals, but used a persuasive argument context where participants presented a controversial topic to an agreeing or disagreeing confederate. Eye contact was only reduced with the disagreeing confederate, suggesting that the impact of social anxiety on eye contact is most robust when the communication context is threatening. The life history conversational sample used in this study was intentionally designed to elicit conversation around neutral, easily discussed topics, so as to obtain a naturalistic sample that mimics real-life conversations. Social anxiety may have been more closely tied to eye contact in our sample with the FMR1 premutation had we used a more threatening sampling context. Notably, despite our use of a neutral communication context, eye contact was nonetheless reduced in the FMR1 premutation compared to controls. This suggests that eye contact is reduced in the FMR1 premutation even in communication contexts that are intended to be non-threatening, and that factors other than social anxiety likely underlie poor eye contact in the FMR1 premutation.
This study also examined an alternative hypothesis: that reduced eye contact in the FMR1 premutation presents as a consequence of elevated BAP features. Using a rating scale that has been shown to be sensitive to the BAP in family studies of autism spectrum disorder, we indexed BAP features in the FMR1 premutation and found no association with poor eye contact. In another study including an overlapping sample, Klusek et al. (2017) also documented an unexpected decoupling between pragmatic language deficits (a primary feature of the BAP) and attention to eye gaze indexed via an experimental eye tracking paradigm. Taken together, these studies demonstrate that atypical use of eye gaze in the FMR1 premutation is not associated with the BAP, as suggested by evidence gathered across a combination of experimental, direct-observation, and informant- and self-report measures.
Why, then, did the women with the FMR1 premutation show reduced eye contact? One possibility is that poor eye contact stems from executive deficits that are seen in a subset of women with the FMR1 premutation. Eye gaze represents a rich source of information that requires cognitive resources to monitor; some evidence suggests that gaze aversion can be used as a strategy to “free up” cognitive resources needed for task performance when the cognitive system is taxed (Doherty-Sneddon et al., 2002; Doherty-Sneddon & Phelps, 2005; Glenberg, 1997). Another possibility is that physiological arousal dysregulation underlies poor eye contact in the FMR1 premutation, given new evidence of aberrant neuroendocrine and autonomic stress regulation patterns in women with the FMR1 premutation (Klusek et al., 2017; Seltzer, Barker, et al., 2012). Direct gaze is associated with increased arousal response in other groups (Akechi et al., 2013; Hessl, Glaser, Dyer-Friedman, & Reiss, 2006; Nichols & Champness, 1971) and it is possible that gaze avoidance in the FMR1 premutation represents a coping mechanism to avoid negative arousal excitation. Follow-up studies are needed to achieve a better understanding of the mechanisms underlying atypical social gaze in the FMR1 premutation.
Aberrant use of eye contact could contribute to the social-cognitive phenotype of the FMR1 premutation. A number of studies have shown that the perception of eye contact facilitates cognitive and attentional processing that support social engagement. For example, adults performing visual search tasks show more efficient detection of faces and eyes when the targets display direct gaze (Conty, Tijus, Hugueville, Coelho, & George, 2006; Senju & Hasegawa, 2005; Senju, Hasegawa, & Tojo, 2005). Eye contact also enhances the retrieval of social-cognitive knowledge, such as the discrimination of emotional, facial, speech, and gender information (Adams & Kleck, 2003, 2005; Farroni et al., 2007; Guellai & Streri, 2011; Hood, Macrae, Cole‐Davies, & Dias, 2003; Macrae, Hood, Milne, Rowe, & Mason, 2002; Milders, Hietanen, Leppänen, & Braun, 2011). Women with the FMR1 premutation who spend less time participating in mutual gaze may miss out on some of these processing benefits. Follow-up studies are needed to delineate causal relationships between atypical eye contact and other social phenotypes of the FMR1 premutation, which may have implications for targeted intervention. Another critical next step of this work is to delineate the temporal emergence of eye gaze phenotypes in young children with the FMR1 premutation. It is unclear whether atypical eye contact in the FMR1 premutation reflects a neurodevelopmental feature present in early childhood, or perhaps atypical eye gaze is a later-emerging trait indicative of preclinical FXTAS. Better understanding of the developmental emergence of atypical eye gaze in women with the FMR1 premutation can inform the assessment and monitoring of FXTAS-related neurodegeneration, as well as the early identification and intervention for children who may be most at risk for poor social outcomes.
This study has a number of strengths, which include blinded, highly reliable direct-observation of eye contact from a semi-naturalistic communicative interaction. Our examination of both social anxiety symptoms as well as DSM-based clinical diagnoses in relation to eye gaze is also a strength, and our detected rate of social anxiety disorder in women with the FMR1 premutation was consistent with previous studies (e.g., Bourgeois et al., 2011; Franke et al., 1998; Roberts et al., 2016). Study limitations include the relative lack of racial diversity, which may limit generalization of findings. Although the life history conversational interview was designed to mimic naturalistic conversation, it should be noted that eye contact was observed during interaction with an examiner as part of a research protocol and results may not generalize to other communication partners or contexts. Additionally, while the coders who evaluated eye contact from the videotaped conversational samples were blind to group membership, it was not possible for the examiners to remain blind given the length of the research protocol. It should also be noted that the correlational study design limits our ability to determine causal directions. Although this study provides novel evidence of aberrant eye gaze in the FMR1 premutation, we were did not detect associations between atypical eye gaze and two hypothesized correlates: social anxiety and the broad autism phenotype. Thus, we were unable to identify mechanisms potentially driving atypical social gaze in the FMR1 premutation. Follow-up studies are needed to gain a better understanding of the underpinnings of this atypical social behavior.
Conclusion
In conclusion, this study provides novel insight into the FMR1 premutation phenotype, as it is the first to empirically examine eye contact quality in women with the FMR1 premutation compared to control women. We documented reduced eye contact in women with the FMR1 premutation compared to control women, which was not associated with BAP or social anxiety phenotypes. This study builds on prior clinical descriptions to empirically confirm reduced eye contact as a phenotypic feature of the FMR1 premutation. Findings add to a growing knowledge base concerning the social phenotype of the FMR1 premutation, which has substantial implications for public health given the high prevalence of this genetic condition in women and its association with risk across a variety of neuropsychological domains.
Acknowledgments
Funding Details
This work was partially supported by the National Institutes of Health; under Grant numbers F32DC013934, R01MH090194, R01HD024356, P30HD03110, U54HD079125; and by the University of South Carolina Magellan Scholar program.
Footnotes
Disclosure of Interest
The authors report no conflicts of interest.
Contributor Information
Jessica Klusek, Department of Communication Sciences and Disorders, Keenan Building, Room 358, 1229 Marion Street, University of South Carolina, Columbia SC 29208; phone: 803-777-5049; klusek@mailbox.sc.edu.
Alexis Ruber, Department of Psychology, 1512 Pendleton Street, University of South Carolina, Columbia SC 29208; phone: 803-777-5049; ARuber@email.sc.edu.
Jane E. Roberts, Department of Psychology, 1512 Pendleton Street, University of South Carolina, Columbia SC 29208; phone: 803-777-5676; jane.roberts@sc.edu
References
- Adams RB, & Kleck RE (2003). Perceived gaze direction and the processing of facial displays of emotion. Psychological Science, 14(6), 644–647 [DOI] [PubMed] [Google Scholar]
- Adams RB, & Kleck RE (2005). Effects of direct and averted gaze on the perception of facially communicated emotion. Emotion, 5(1), 3. [DOI] [PubMed] [Google Scholar]
- Akechi H, Senju A, Uibo H, Kikuchi Y, Hasegawa T, & Hietanen JK (2013). Attention to Eye Contact in the West and East: Autonomic Responses and Evaluative Ratings. PLOS ONE, 8(3), e59312. doi: 10.1371/journal.pone.0059312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association; (2013): Diagnostic and statistical manual of mental disorders 5th ed; DSM-5. American Psychiatric Publishing, Inc. [Google Scholar]
- Baron-Cohen S (1995). Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press. [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Hérot C, Friedman D, Snidman N, … Faraone SV (2001). Further evidence of association between behavioral inhibition and social anxiety in children. American journal of Psychiatry, 158(10), 1673–1679 [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, … Hagerman RJ (2011). Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. Journal of Clinical Psychiatry, 72(2), 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GE, & Cox DR (1964). An analysis of transformations. Journal of the Royal Statistical Society. Series B (Methodological), 211–252. [Google Scholar]
- Bruner J (1983). The acquisition of pragmatic commitments. The transition from prelinguistic to linguistic communication, 27–42. [Google Scholar]
- Chen FS, & Yoon JD (2011). Brief Report: Broader autism phenotype predicts spontaneous reciprocity of direct gaze. Jounral of Autism and Developmental Disorders, 41, 1131–1134. [DOI] [PubMed] [Google Scholar]
- Clark DM, & Wells A (1995). A cognitive model of social phobia. Social phobia: Diagnosis, assessment, and treatment, 41(68), 00022–00023 [Google Scholar]
- Clauss JA, & Blackford JU (2012). Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child and Adolescent Psychiatry, 51, 1066 – 75.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, & Loesch DZ (2007). Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders, 37(4), 738–747. doi: 10.1007/s10803-006-0205-z [DOI] [PubMed] [Google Scholar]
- Cohen IL, Fisch GS, Sudhalter V, Wolf-Schein EG, Hanson D, Hagerman R, Jenkins EC, & Brown WT (1988). Social gaze, social avoidance, and repetitive behavior in fragile X males: A controlled study. American Journal on Mental Retardation, 92, 436–446. [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates. [Google Scholar]
- Conty L, Tijus C, Hugueville L, Coelho E, & George N (2006). Searching for asymmetries in the detection of gaze contact versus averted gaze under different head views: a behavioural study. Spatial vision, 19(6), 529–545. [DOI] [PubMed] [Google Scholar]
- Daly S (1978). Behavioural correlates of social anxiety. British Journal of Clinical Psychology, 17, 117–120. [DOI] [PubMed] [Google Scholar]
- Doherty-Sneddon G, Bruce V, Bonner L, Longbotham S, & Doyle C (2002). Development of gaze aversion as disengagement from visual information, Developmental Psychology, 38, 438–445. [PubMed] [Google Scholar]
- Doherty-Sneddon G & Phelps FG, (2005). Gaze aversion: A response to cognitive or social difficulty? Memory & Cognition, 33, 727–733. [DOI] [PubMed] [Google Scholar]
- Edelmann RJ, & Baker SR (2002). Self‐reported and actual physiological responses in social phobia. British Journal of Clinical Psychology, 41(1), 1–14 [DOI] [PubMed] [Google Scholar]
- Farabee DJ, Holcom JML, Ramsey SL, & Cole SG (1993). Social Anxiety and Speaker Gaze in a Persuasive Atmosphere. Journal of Research in Personality, 27(4), 365–376. doi: 10.1006/jrpe.1993.1025 [DOI] [Google Scholar]
- Farroni T, Csibra G, Simion F, & Johnson MH (2002). Eye contact detection in humans from birth. Proceedings of the National Academy of Sciences, 99(14), 9602–9605. doi: 10.1073/pnas.152159999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farroni T, Massaccesi S, Menon E, & Johnson MH (2007). Direct gaze modulates face recognition in young infants. Cognition, 102(3), 396–404. doi: http://dx.doi.org/10.1016/j.cognition.2006.01.007 [DOI] [PubMed] [Google Scholar]
- First MB, & Gibbon M (2004). The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) In Segal MJHDL (Ed.), Comprehensive handbook of psychological assessment, Vol. 2: Personality assessment (pp. 134–143). Hoboken, NJ, US: John Wiley & Sons Inc. [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, & Ghera MM (2005). Behavioral inhibition: linking biology and behavior within a developmental framework. Annu. Rev. Psychol, 56, 235–262 [DOI] [PubMed] [Google Scholar]
- Franke P, Leboyer M, Gansicke M, & Weiffenbacj O (1998). Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Research, 90, 113–127 [DOI] [PubMed] [Google Scholar]
- Fresco D, Coles M, Heimberg RG, Liebowitz M, Hami S, Stein M, & Goetz D (2001). The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological medicine, 31(06), 1025–1035 [DOI] [PubMed] [Google Scholar]
- Frith U, & Frith C (2001). The biological basis of social interaction. Current Directions in Psychological Science, 10(5), 151–155 [Google Scholar]
- Gladstone GL, Parker GB, Mitchell PB, Wilhelm KA, & Malhi GS (2005). Relationship between self-reported childhood behavioral inhibition and lifetime anxiety disorders in a clinical sample. Depression and Anxiety, 22(3), 103–113. doi: 10.1002/da.20082 [DOI] [PubMed] [Google Scholar]
- Glenberg AM (1997). What memory is for. Behavioral & Brain Sciences, 20, 1–9. [DOI] [PubMed] [Google Scholar]
- Grossman P, Wilhelm FH, Kawachi I, & Sparrow D (2001). Gender differences in psychophysiological responses to speech stress among older social phobics: congruence and incongruence between self-evaluative and cardiovascular reactions. Psychosomatic Medicine, 63(5), 765–777 [DOI] [PubMed] [Google Scholar]
- Grossmann T, Johnson MH, Farroni T, & Csibra G (2007). Social perception in the infant brain: gamma oscillatory activity in response to eye gaze. Social Cognitive and Affective Neuroscience, 2(4), 284–291. doi: 10.1093/scan/nsm025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guellai B, & Streri A (2011). Cues for early social skills: direct gaze modulates newborns’ recognition of talking faces. PloS one, 6(4), e18610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, & Hagerman PJ (2002). The fragile X premutation: into the phenotypic fold. Current opinion in genetics & development, 12(3), 278–283 [DOI] [PubMed] [Google Scholar]
- Hall DA, Birch RC, Anheim M, Jønch AE, Pintado E, O’Keefe J, … Leehey MA (2014). Emerging topics in FXTAS . Journal of Neurodevelopmental Disorders, 6(1), 31. doi: 10.1186/1866-1955-6-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna JE, & Brennan SE (2007). Speakers’ eye gaze disambiguates referring expressions early during face-to-face conversation. Journal of Memory and Language, 57(4), 596–615. doi: http://dx.doi.org/10.1016/j.jml.2007.01.008 [Google Scholar]
- Hessl D, Glaser B, Dyer-Friedman J, & Reiss AL (2006). Social behavior and cortisol reactivity in children with fragile X syndrome. Journal of child psychology and psychiatry, and allied disciplines, 47(6), 602–610 [DOI] [PubMed] [Google Scholar]
- Hessl D, & Grigsby J (2016). Fragile X-associated tremor/ataxia syndrome: another phenotype of the fragile X gene. The Clinical Neuropsychologist, 30(6), 810–814. doi: 10.1080/13854046.2016.1186661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Rivera S, Koldewyn K, Cordeiro L, Adams J, Tassone F, … Hagerman RJ (2007). Amygdala dysfunction in men with the fragile X premutation. Brain, 130, 404–416 [DOI] [PubMed] [Google Scholar]
- Hessl D, Wang JM, Schneider A, Koldewyn K, Le L, Iwahashi C, … Rivera SM (2011). Decreased FMRP expression underlies amygdala dysfunction in carriers of the fragile X premutation. Biological Psychiatry, 70(9), 859–865. doi: 10.1016/j.biopsych.2011.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, … Kagan J (1992). Stable behavioral inhibition and its association with anxiety disorder. Journal of the American Academy of Child & Adolescent Psychiatry, 31(1), 103–111 [DOI] [PubMed] [Google Scholar]
- Ho S, Foulsham T, & Kingstone A (2015). Speaking and listening with the eyes: Gaze signaling during dyadic interactions. PLoS ONE, 10(8), e0136905. doi: 10.1371/journal.pone.0136905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn-Saric R, & McLeod DR (2000). Anxiety and arousal: physiological changes and their perception. Journal of Affective Disorders, 61(3), 217–224. doi: 10.1016/S0165-0327(00)00339-6 [DOI] [PubMed] [Google Scholar]
- Hood BM, Macrae CN, Cole‐Davies V, & Dias M (2003). Eye remember you: The effects of gaze direction on face recognition in children and adults. Developmental Science, 6(1), 67–71 [Google Scholar]
- Hurley R, Losh M, Parlier M, Reznick JS, & Piven J (2007). The broad autism phenotype questionnaire. Journal of Autism and Developmental Disorders, 37, 1679–1690. [DOI] [PubMed] [Google Scholar]
- Ingersoll B, Hopwood CJ, Wainer A, & Donnellan MB (2011). A comparison of three self-report measures of the broader autism phenotype in a non-clinical sample. Journal of Autism and Developmental Disorders, 41, 1646–1656. [DOI] [PubMed] [Google Scholar]
- Itier RJ, & Batty M (2009). Neural bases of eye and gaze processing: The core of social cognition. Neuroscience and biobehavioral reviews, 33(6), 843–863. doi: 10.1016/j.neubiorev.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AS, & Kaufman NL (2004). Kaufman Brief Intelligence Test, 2nd ed Los Angeles, CA: Pearson Assessments. [Google Scholar]
- Kleinke CL (1986). Gaze and eye contact: a research review. Psychological bulletin, 100(1), 78. [PubMed] [Google Scholar]
- Klusek J, LaFauci G, Adayev T, Brown WT, Tassone F, & Roberts J (2017). Cardiac autonomic function in women with the FMR1 premutation is associated with mRNA and CGG Expansion but not depression or anxiety. Journal of Neurodevelopmental Disorders, 9(1–16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Losh M, & Martin G (2014). Sex differences and within-family associations in the broad autism phenotype. Autism: International Journal of Research and Practice, 18, 106–116. doi: 10.1177/1362361312464529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, McGrath SE, Abbeduto L, & Roberts JE (2016). Pragmatic language features of mothers with the FMR1 premutation are associated with the language outcomes of adolescents and young adults with fragile X syndrome. Journal of Speech, Language, and Hearing Research, 59, 49–61. doi: doi: 10.1044/2015_JSLHR-L-15-0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Schmidt J, Fairchild AJ, Porter A, & Roberts JE (2017). Altered sensitvity to social gaze in the FMR1 premutation and pragmatic language competence. Journal of Neurodevelopemtnal Disoders, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan CM, Hocking DR, Bradshaw JL, Fielding J, Cohen J, Georgiou-Karistianis N, & Cornish KM (2013). Neurobehavioural evidence for the involvement of the FMR1 gene in female carriers of fragile X syndrome. Neuroscience & Biobehavioral Reviews, 37(3), 522–547. doi: http://dx.doi.org/10.1016/j.neubiorev.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Liebowitz MR (1987). Social phobia. Modern Problems of Pharmacopsychiatry, 22, 141–173 [DOI] [PubMed] [Google Scholar]
- Losh M, Klusek J, Martin GE, Sideris J, Parlier M, & Piven J (2012). Defining genetically meaninful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 159B(6), 660–668. doi: 10.1002/ajmg.b.32070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, & Piven J (2007). Defiing key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics: Part B, 147B, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M & Piven J (2007). Social-cognition and the broad autism phenotype: identifying genetically meaningful phenotypes. Journal of Child Psychology & Psychiatry, 48, 105–112. [DOI] [PubMed] [Google Scholar]
- Macrae CN, Hood BM, Milne AB, Rowe AC, & Mason MF (2002). Are you looking at me? Eye gaze and person perception. Psychological Science, 13(5), 460–464 [DOI] [PubMed] [Google Scholar]
- Maddalena A, Richards CS, McGinniss MJ, Brothman A, Desnick RJ, Grier RE, … Popovich, B. (2001). Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Genetics in Medicine, 3(3), 200–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss I, Wilhelm F, & Gross J (2004). Is there less to social anxiety than meets the eye? Emotion experience, expression, and bodily responding. Cognition and Emotion, 18(5), 631–642 [Google Scholar]
- McCarthy A, & Lee K (2009). Children’s knowledge of deceptive gaze cues and its relation to their actual lying behavior. Journal of Experimental Child Psychology, 103(2), 117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milders M, Hietanen JK, Leppänen JM, & Braun M (2011). Detection of emotional faces is modulated by the direction of eye gaze. Emotion, 11(6), 1456–1461. doi: 10.1037/a0022901 [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, & Bradley BP (2004). Selective attention to angry faces in clinical social phobia. Journal of Abnormal psychology, 113, 160–165. doi: 10.1037/0021-843X.113.1.160 [DOI] [PubMed] [Google Scholar]
- Nichols K, & Champness B (1971). Eye gaze and the GSR. Journal of Experimental Social Psychology, 7(6), 623–626 [Google Scholar]
- Nolin SL, Brown WT, Glicksman A, Houck JGE, Gargano AD, Sullivan A, … Sherman, S. L. (2003). Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. American Journal of Human Genetics, 72(2), 454–464. doi: http://dx.doi.org/10.1086/367713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond VP, McCroskey JC, & Payne SK (1991). Nonverbal behavior in interpersonal relations: Prentice Hall Englewood Cliffs, NJ.
- Riddle J, Cheema A, Sobesky W, Gardner S, Taylor A, Pennington B, & Hagerman R (1997). Phenotypic involvement in females with the FMR1 gene mutation. American Journal on Mental Retardation, 102(6), 590–601 [DOI] [PubMed] [Google Scholar]
- Roberts JE, Weisenfeld LAH, Hatton DE, Heath M, & Kaufmann WE (2007). Social approach and autistic behavior in children with fragile X syndrome. Journal of Autism and Developmental Disorders, 37, 1748–1760. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, McCary LM, Ford AL, Golden RN, & Bailey DB (2016). Trajectory and Predictors of Depression and Anxiety Disorders in Mothers with the FMR1 Premutation . Biological psychiatry, 79(10), 850–857. doi: http://dx.doi.org.pallas2.tcl.sc.edu/10.1016/j.biopsych.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, & Evans DE (2000). Temperament and personality: origins and outcomes. Journal of personality and social psychology, 78(1), 122. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, & Lord C (2003). SCQ: The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Rytwinski NK, Fresco DM, Heinberg RG, Coles ME, Liebowitz MR, Cissell S, et al. (2009). Screening for social anxiety disorder with the self-report version of the Liebowitz Social Anxiety Scale. Depression and Anxiety, 26, 34–38. [DOI] [PubMed] [Google Scholar]
- Insititute SAS. (2012). SAS 9.4 for Windows SAS Institue Inc., Cary, NC, USA [Google Scholar]
- Sasson NJ, Lam KS, Childress D, Parlier M, Daniels JL, & Piven J (2013). The broad autism phenotype questionnaire: Prevalence and diagnostic classification. Autism Research, 6, 134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbordone RJ, & Saul RE (2000). Neuropsychology for health care professionals and attorneys: CRC Press. [Google Scholar]
- Schneider A, Johnston C, Tassone F, Sansone S, Hagerman RJ, Ferrer S, et al. (2016). Broad autism spectrum and obsessive-compulsive symptoms in adults with the fragile X premutation. The Clinical Neuropsychologist, 30, 929–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneier FR, Rodebaugh TL, Blanco C, Lewin H, & Liebowitz MR (2011). Fear and avoidance of eye contact in social anxiety disorder. Comprehensive psychiatry, 52(1), 81–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, & Mandel D (2012). Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 159B(5), 589–597. doi: 10.1002/ajmg.b.32065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selya AS, Rose JS, Dierker LC, Hedeker D, Mermelstein RJ (2012). A practical guide to calculating Cohen’s f2, a measure of local effect size, from PROC MIXED. Frontiers in Psychology, 3, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Barker ET, Greenberg JS, Hong J, Coe C, & Almeida D (2012). Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychology, 31(5), 612–622. doi: 10.1037/a0026528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senju A, & Hasegawa T (2005). Direct gaze captures visuospatial attention. Visual Cognition, 12(1), 127–144. doi: 10.1080/13506280444000157 [DOI] [Google Scholar]
- Senju A, Hasegawa T, & Tojo Y (2005). Does perceived direct gaze boost detection in adults and children with and without autism? The stare-in-the-crowd effect revisited. Visual Cognition, 12(8), 1474–1496 [Google Scholar]
- Tassone F, Hagerman RJT, A. K., Mills, J. B., & Harris, S. W. (2000). Clinical involvement and protein expression in individuals with the FMR1 premutation. American Journal of Medical Genetics, 91, 144–152. doi: [DOI] [PubMed] [Google Scholar]
- Tomasello M, & Tomasello M (2009). Constructing a language: A usage-based theory of language acquisition: Harvard university press. [Google Scholar]
- Vertegaal R, Slagter R, Van der Veer G, & Nijholt A (2001). Eye gaze patterns in conversations: there is more to conversational agents than meets the eyes Proceedings of the SIGCHI conference on Human factors in computing systems (pp. 301–308): ACM. [Google Scholar]
- Wirth JH, Sacco DF, Hugenberg K, & Williams KD (2010). Eye Gaze as relational evaluation: Averted eye gaze leads to feelings of ostracism and relational devaluation. Personality and Social Psychology Bulletin, 36(7), 869–882. doi: 10.1177/0146167210370032 [DOI] [PubMed] [Google Scholar]


