Abstract
The gold standard for noninvasive blood pressure (BP) measurement, the Doppler technique does not provide systolic (SBP) and diastolic (DBP) pressure and may limit therapy outcomes. To improve patient care, we tested specifically designed Experimental BP (ExpBP) monitor and the Doppler technique by comparing non-invasive measures to the intra-arterial (I-A) BP in 31 end-stage heart failure patients (4 females) 2.6±3.4 days post-LVAD implantation (20 HeartMate II and 11 HeartWare). Bland-Altman (B-A) plots revealed that the ExpBP monitor overestimated mean arterial pressure (MAP) by 1.2 [4.8] mm Hg (mean difference [SD]), while the Doppler by 6.7 [5.8] mm Hg. The Exp BP SBP was overestimated by 0.8 [6.1] mm Hg and DBP by 1.9 [5.3] mm Hg compared to the respective I-A pressures. Both techniques achieved similar measurement reliability. In the measurement “success rate” expressed as a frequency (percent) of readable BP values per measurement attempts, Doppler accomplished 100% vs. 97%, 97% and 94% of successful detections of MAP, SBP and DBP provided by the ExpBP monitor. The ExpBP monitor demonstrated higher accuracy in the MAP assessment than the Doppler in addition to providing SBP and DBP in majority of subjects. Improved BP control may help to mitigate related neurologic adverse event rates.
Keywords: systolic, diastolic, non-invasive, monitor, LVAD
INTRODUCTION
Continuous-flow (CF) Left Ventricle Assist Device (LVAD) therapy, an established treatment modality for advanced heart failure (HF), is experiencing exponential growth due to increased durability and progressive engineering of the pumps1–3. In non-LVAD populations, the arterial blood pressure (BP) is easily obtained by auscultation or the oscillometric method, but in CF LVAD patients accurate BP assessment remains challenging due to a reduced pulse pressure4. Despite advancements in LVAD technology, a specific, clinically validated LVAD BP monitor is not currently available. Traditional automated oscillometric BP monitors are capable of successfully measuring BP in approximately 55–60% of cases regardless of the measurement accuracy, while manual auscultation allows BP assessment in less than 20% of LVAD measurements4. Currently, clinical management of LVAD patients relies on a Doppler BP method, which significantly overestimates mean arterial pressure5. Intracranial bleeding, one of the major adverse events of LVAD therapy, is associated with poor BP control3,6. Limited availability of Doppler-derived BP could also compromise adequate therapeutic response and negatively influence clinical outcomes7,8. Conversely, in patients with hypertension, the overestimation of MAP may contribute to antihypertensive drug overdosing with subsequent, impaired renal perfusion and a higher risk of falls due to underlying orthostatic hypotension.
In response to this clinical need, an experimental, non-invasive, brachial cuff blood pressure (ExpBP) monitor has been developed with algorithms customized for the altered hemodynamics of LVAD patients. Accordingly, we studied the validity, repeatability, and measurement “success rate” of the ExpBP monitor compared to the intra-arterial (I-A) BP. Secondly, we compared the non-invasive LVAD ambulatory “gold standard” Doppler technique to the I-A BP in the same population.
METHODS
Patients
A total of thirty one end-stage heart failure patients (4 females; age 63±10 years; BMI 28.6±6.0 kg.m2) indicated for durable mechanical cardiac support with an LVAD implanted at Mayo Clinic, Rochester, Minnesota were included in the study. The patients’ demographic information including medical history, cardiac risk factors, and related blood markers is illustrated in table 1A with the pump characteristics displayed in table 1B.
Table 1A.
Demographic characteristics of studied cohort.
| Variable n=31 |
Mean ± SD or “n” (%) |
|---|---|
| Age (years) | 63±10 |
| Male sex | 27 (84) |
| Weight (kg) | 88.8±24.5 |
| Height (m) | 1.75±0.11 |
| Body Mass Index (kg/m2) | 28.6±6.0 |
| Nonischemic Dilated CMP | 17 (55) |
| Ischemic CMP | 13 (41) |
| Complex CHD | 1 (3) |
| Diabetes | 14 (44) |
| Hypertension | 15 (47) |
| COPD | 7 (22) |
| Obstructive Sleep Apnea | 11 (34) |
| Chronic Kidney Disease | 18 (57) |
| Hiperlipidemia | 11 (34) |
| Atrial fibrilation | 4 (13) |
| Smoking | 6 (19) |
| HeartMate II | 20 (62) |
| HeartWare | 11 (34) |
| Erythrocytes (x 10(12)/L) | 3.5±0.8 |
| Hemoglobin (g/dL) | 10.4±2.2 |
| Platelets (x 10(9)/L) | 185±84 |
| Creatinine (mg/dL) | 1.6±0.9 |
| Blood Urea Nitrogen (mg/dL) | 33.9±19.3 |
| Potasium (mmol/L) | 4.3±0.5 |
CHD= Congenital Heart Disease, CMP= Cardiomyopathy, COPD= Chronic Obstructive Pulmonary Disease
Table 1B.
Device types implanted and settings at the time of BP assessment.
| Variable | HeartMate II n=20 Mean ± SD or “n” (%) |
HeartWare n=11 Mean ± SD or “n” (%) |
|---|---|---|
| Age, y | 67.2±8.0 | 56.7±9.1 |
| Male, % | 19 (95) | 8 (73) |
| Ischemic etiology % | 11 (55) | 2 (18) |
| LVAD Speed [RPM] | 9010±382 | 26667±198 |
| LVAD Power [Watts] | 5.2±0.9 | 4.1±1.3 |
| Pulsatility Index | 5.7±1.0 | N/A |
LVAD= Left Ventricle Assist Device, SD= standard deviation
Study design
The present single center prospective, non-randomized study was approved by Mayo Clinic institutional review board (IRB). To achieve the goal of the study, blood pressure was measured in patients supported by a continuous flow LVAD Heart Mate II (HM II) (Thoratec Corporation, CA) or HeartWare (HW) (HW Corp., MA) device at the Intensive Care Unit, 2.6±3.4 days after device implantation. Blood pressure was assessed in patients in a stable, supine position. The invasive BP was recorded continuously via the critical care monitor, Philips IntelliVue MP 50 (Phillips, the Netherlands) connected to a 20 Fr intra-arterial (I-A) catheter placed in the radial artery through disposable pressure transducer TruWaveTM PXMK2064 (Edwards Lifesciences, Irvine, CA). Prior to each recording, the system was flushed, zeroed, and the transducer was leveled at the phlebostatic axis9,10. For the non-invasive BP assessments, appropriately sized cuffs were chosen with the cuff placed either on the ipsilateral or contralateral arm based on clinical restrictions; however, BP was assessed non-invasively by the ExpBP monitor and the Doppler technique from the same extremity11. Doppler Flow Detector, Model 811-BTS (Parks Medical Electronics, Inc., Aloha, Oregon, U.S.A) with a calibrated phygmomanometer, Model Baum Pocket Aneroid (W. A. Baum Co. Inc., NY, USA) was used for detecting the Doppler BP as previously described4. Blood pressure was assessed in triplicate for each measurement method within one minute between measurements, obtaining I-A records from the continuous BP monitoring, followed by a non-invasive measurement using the ExpBP monitor, and finally, Doppler ultrasound.
The non-invasive Blood Pressure Measurement System
The prototype of the non-invasive BP (NIBP) measurement system was specifically designed for the LVAD population, consisting of the ExpBP monitor operated via the attached computer The device was developed on a standard oscillometric principle for non-invasive BP measurement12 with customized hardware and software to adapt for lower BP pulsatility (Supplemental Fig. 1). The device automatically measured oscillometric pulsations during cuff deflation at speed of 2 mm Hg/s, and was programmed to generate the most linear cuff deflation course, while minimizing artifacts caused by control valves. A standard pressure sensor (Freescale Semiconductor, type: MPXV5050GP) was used to measure the pressure in the cuff. The pressure signal from the sensor was filtered by an analog first-order low-pass antialiasing filter with a cut-off frequency of 140 Hz. Data were then digitized with a sampling frequency of 400 Hz.
Algorithms for Arterial Pressure Detection
The standard oscillometric BP method was applied to obtain MAP values12. In the device, the raw digitized pressure signal with oscillometric pulsations was filtered by a third order high pass Bessel filter with a cut-off frequency of 0.4 Hz to remove a slowly varying component of deflating cuff pressure. As a result the filtered signal contained only the superimposed rapid pressure oscillations (at a range about mm Hg). During the cuff deflation, peak-to-peak oscillation amplitude changes gradually increased, reaching a maximum and then decreasing (Fig. 1). It has been shown that the maximum peak-to-peak amplitude of oscillations occurs when the cuff pressure corresponds to the MAP12–15. The device used a MAP detection algorithm that employed a peak detector to find positive and negative peaks of oscillations based on polynomial fitting (Figure 1)16. At the time of a maximum absolute difference of envelopes, the cuff pressure corresponded to MAP, as described previously13,16. The SBP and DBP were obtained using height-based criteria17. The “successful measurement” was considered when the BP value was displayed for a given measurement attempt. The pulse pressure (PP) was calculated as the difference between SBP and DPB.
Figure 1.
Temporal characteristics of cuff pressure vs. measured oscillometric BP pulsations.
Statistical Analyses
According to the recommendations of American Heart Association for BP assessment, the average of the second and the third measurement was considered as a representative value for the calculation of non-invasive BP measures11. For the I-A BP measures, 30 second average values were used. Where appropriate, variables were summarized as mean (standard deviation) and frequency (percent) for continuous and categorical measurements, respectively. The Bland-Altman (B-A) plots were constructed for the evaluation of the methods agreement between the ExpBP monitor versus I-A BP and Doppler BP versus I-A, respectively, with the bias of ±95 % confidence intervals. Also, mean absolute difference and Pearson’s correlations were used to compare between methods. For the repeatability assessment, mean absolute difference between the second and third BP measurement for each pressure (MAP, SBP, DBP, PP) of a given method was performed with Pearson’s correlation coefficient calculated between obtained values. In addition, the “measurement success rate” was expressed as a percent of total measurement attempts. Data was analyzed using JMP Pro 10 (SAS, NC) statistical software package and Bland-Altman plots were carried out in GraphPad Prism version 7.00 (GraphPad Software, La Jolla California, USA). Significance was considered present when P<0.05.
RESULTS
The general overview of results, demonstrating means of BP values from all three methods, is illustrated in Figure 2. SBP, DBP, and MAP were assessed by using the ExpBP monitor with an additional calculation of the PP, compared to the Doppler technique, which generated only a single BP value.
Figure 2.
Comparison of blood pressure values between techniques.
Validation Analysis of the Experimental BP Monitor and Doppler Technique referenced to Intra-Arterial Pressures
The Bland-Altman analyses revealed a closer agreement of methods in the assessment of MAP between the ExpBP monitor and I-A pressure compared to the agreement in the MAP derived by the current gold standard, Doppler Technique and I-A MAP (Figure 3 A, 3B). Mean absolute differences (MAD) between ExpBP monitor MAP vs. I-A MAP and Doppler BP vs. I-A MAP, respectively were 3.9±1.1 mm Hg and 7.5±1.0 mm Hg, resp.
Figure 3.
Figure 3A. Agreement of methods for mean arterial pressure assessment delivered via the arterial line and the ExpBP monitor in Bland-Altman analysis and Pearson correlation coefficient.
Figure 3B. Agreement of methods for mean arterial pressure assessment delivered via the arterial line and the Doppler Technique in Bland-Altman analysis and Pearson correlation coefficient.
Additionally, there were strong Pearson correlations between I-A vs. ExpBP monitor SBP, DBP, and PP (r=0.84; p<0.01, r=0.80, p<0.01 and r=0.73, p<0.01). Compared to corresponding I-A pressures, B-A plots displayed that the ExpBP monitor overestimated SBP by 0.8 [−11.3+12.8] mm Hg, DBP by 1.9 [−8.5+12.3] mm Hg and underestimated PP by 1.3 [−13.3+10.8] mm Hg (Supplemental Fig. 2 and 3).
Measurements Repeatability
The I-A technique achieved the highest reliability expressed in Pearson correlations and MAD between second and third measurements; r=0.99 (p<0.01) and 1.0±1.2 mm Hg for MAP, r=0.98 (p<0.01) and 1.3+1.9 mm Hg for SBP, r=0.99 (p<0.01) and 0.7±0.8 mm Hg for DBP and lastly r=0.98 (p<0.01) and 1.0±1.7 mm Hg for PP.
For the ExpBP monitor, analyses have revealed the Pearson correlation of r= 0.98 (p<0.01) with MAD of 1.5±1.2 mm Hg between the second and the third measurement for MAP, r= 0.95 (p<0.01) with MAD of 2.4±2.1 mm Hg for SBP, r=0.94 (p<0.01) with MAD of 1.9±2.4 mm Hg for DBP and r=0.84 (p<0.01) with MAD of 2.9±3.1 mm Hg for PP.
The Doppler technique has displayed correlation between the 2nd and the 3rd measurement of r= 0.98 (p<0.01) with a 1.5±1.1 mm Hg of mean absolute difference between measurements, when rigorously performed by a single, trained observer.
Measurement Success Rate
In the CF LVAD population, problems commonly associated with the BP assessment using automated devices lead to remarkably a high number of “error” readings, when no values are provided by the BP monitor. In our study, an original approach was applied, allowing for an independent assessment of SBP and DBP from the ExpBP monitor after MAP was positively determined by the algorithm. This allowed for higher success rates for the MAP and SBP compared to DBP values. The Doppler technique achieved a high success rate in a critical care setting (Table 2).
Table 2.
Measurement success rates overview.
| Percent of Total Successful Blood Pressure Measurement Attempts | Percent of Patients with 3 Successful Blood Pressure Measurement Attempts | Percent of Patients with Successful 2nd and 3rd Blood Pressure Measurement Attemps | |
|---|---|---|---|
| Intra-Arterial BP | |||
| MAP | 100% | 100% | 100% |
| SBP | 100% | 100% | 100% |
| DBP | 100% | 100% | 100% |
| Exp. BP Monitor | |||
| MAP | 95% | 86% | 97% |
| SBP | 89% | 78% | 97% |
| DBP | 87% | 74% | 94% |
| Doppler Technique | |||
| Doppler BP | 99% | 97% | 100% |
Blood pressure was assessed three times by each method for a total of 31 subjects resulting in 93 measurement attempts by each method. BP=blood pressure, MAP= mean arterial pressure, SBP= Systolic blood pressure, DBP= diastolic blood pressure, Exp. BP Monitor= Experimental Blood Pressure Monitor
Sub-analysis of Doppler BP According to the Intra-Arterial Pulse Pressure
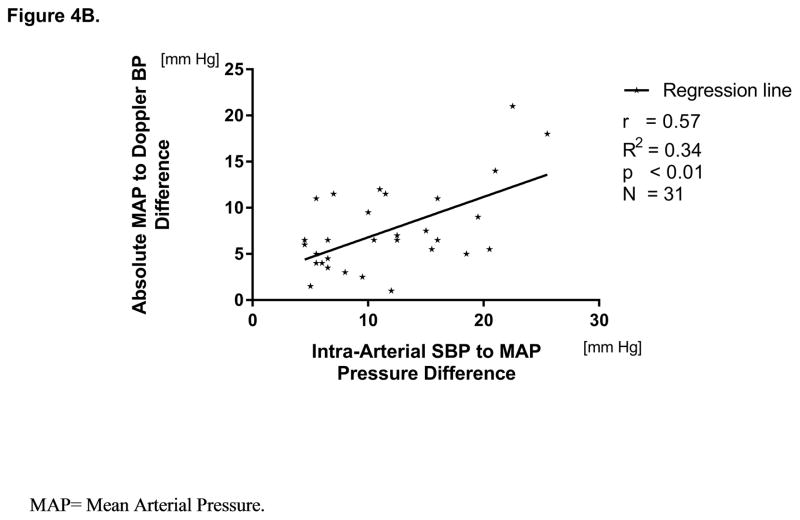
By analyzing the accuracy of Doppler measurements, the data revealed a significant positive relationship between the I-A MAP to Doppler BP difference and the I-A PP (r=0.55, p<0.01). Also, this relationship between the I-A MAP and the Doppler BP was significant if calculated for I-A SBP to I-A MAP pressure difference (r=0.57, P<0.01) (Figure 4A, 4B).
Figure 4.
Figure 4A. Linear Relationship between intra-arterial pulse pressure and absolute mean arterial pressure to Doppler pressure difference.
Figure 4B. Linear Relationship between intra-arterial systolic to mean arterial pressure and absolute mean arterial pressure to Doppler pressure difference.
DISCUSSION
In the presented work, we tested the performance of an experimental BP monitor specifically designed to overcome the challenging hemodynamic characteristics of patients supported by continuous-flow LVADs. The design allowed for the non-invasive measurement of the MAP, SBP and DBP in majority of CF LVAD patients at early post-implantation stage using the ExpBP monitor. Acquired BP values were in close agreement to the respective invasive BP values. In addition, the accuracy and reliability of the Doppler technique was tested by a single operator under rigorous conditions.
Performance of the Experimental BP Monitor in Respect to Regulatory Standards for BP Monitors
The narrowest ±95% limit of agreement in the B-A analysis was achieved by comparing the I-A to the ExpBP monitor MAP, followed by DBP and SBP. The unique algorithm used in the ExpBP monitor also allowed for the low bias of 1.2 [4.8] mm Hg (mean [SD]) in the MAP assessment, 0.8 [6.1] mm Hg for the SBP, and 1.9 [5.3] mm Hg for the DBP, respectively. According to the Revised British Hypertension Society (BHS) protocol defining procedures for validation of BP monitors for special groups and in special circumstances (pregnant, children, elderly subjects, etc.), BP is required to be assessed in 30 subjects from the particular studied population and evaluated against a reference method (including ausculatory method). To achieve the highest degree of accuracy (grade A), the difference in BP values obtained by a tested method and a reference method must be ≤ 15mm Hg in at least 95% of measurements, ≤ 10 mm Hg in at least 85%, and at least ≤ 5 mm Hg in at least 60% of measurements18. In categories ≤ 15 mmHg, ≤ 10 mmHg, and ≤ 5mm Hg the ExpBP monitor achieved 99%, 93% and 65% for SBP and 100%, 95% and 57% for DBP. The existing standard of the International Organization for Standardization ISO 81060-2:2013 allows for BP monitors validation in groups with special conditions. The norm defines pass/fail criteria based on BP mean difference between tested and invasive reference method ≤ 5 mmHg with standard deviation ± 8 mm Hg from all measurements (minimum of 150 measurement on 15 patients)19.
Further improvement of the accuracy using oscillometric principle in continuous-flow LVAD patients may be challenging due to a diminished pulse pressure, where movement artifacts, and particularly the low frequency respiratory-synchronous BP modulation, have relatively higher impact on the recorded signal compared to the non-LVAD population. This may generate a flatter oscillometric envelop (Figure 1), which in turn increases the level of uncertainty in the detection of the maximal peak. In response, this may negatively affect the accuracy of MAP assessment, and consequently, also impact calculations of SBP and DBP. Paradoxically, artifacts traditionally perceived to be challenging for the oscillometric method in non-LVAD population (arrhythmias, mainly atrial fibrillation) may have smaller effect on the accuracy or measurement success rate in the LVAD population due to the position of the pump by-passing the left ventricle (LV). In this setting, the atrial contribution to the final stroke volume is diluted between the pump and the LV output, which may be seen in a relatively less pronounced beat-to-beat stroke volume variability during uneven LV filling. Our data suggest that the BP assessment should not be significantly affected when the residual pulse pressure exceeds approximately 5 mm Hg. This may have positive clinical implications also for the aortic valve closure post-LVAD implantation, surgical procedure performed in indicated cases to prevent progression of the aortic insufficiency.
LVAD Specific Performance Assessment of the Experimental BP Monitor
Apart from the accuracy and reliability of BP assessment, a common problem of BP monitors not specifically designed for the LVAD patients is a remarkable rate of “error” readings, when no BP value is provided by an automated monitor4,5. In this study, the ExpBP monitor achieved over 90% of successful BP readings for the second and third measurement attempts for MAP, SBP, and DBP (table 2.), despite the majority of subjects receiving vasopressors at the time data collection. The success rates were maximized due to a unique feature to provide each of the BP values independently, even if the SBP or DBP was a missing value. Authors speculated that the ExpBP monitor might possibly achieve an even higher success rate in a stable CF LVAD population with a longer time post-LVAD implantation and no pharmacologically induced peripheral vasoconstriction. This needs to be confirmed in further studies.
Arterial Pulse Pressure and Pulsatility Index
Interpretations of calculated pulse pressure (PP= SBP-DBP) should be used with caution in potential attempts to use this information for the pump setting evaluation. The extrapolation of the value of pulsatility index (PI) provided by HeartMate pumps to the calculated PP remains challenging, since no significant correlation was found between the ExpBP monitor PP and PI (r=0.35, p=0.15, n=20) and also between the I-A PP and PI (r=0.28, p=0.23, n=20) in the dataset collected on subjects very shortly after the LVAD placement. Hence, further effort is required in technological research focused on developing more reliable methods for a precise assessment of the residual pulsatility to enable its clinical utilization beyond the traditional PI value provided by the HM device controllers, as stressed by Cheng et al. and others20,21. Promising improvements in hemodynamic monitoring of LVAD patients may be seen in future with clinical implementation of implantable hemodynamic sensors.
Overall, the current results suggest excellent performance of the ExpBP monitor in the assessment of SBP, DBP, and MAP compared to recently published data focused on testing monitors for BP measurement in the CF LVAD population5,22. Present work is in consideration with the idea of implementing automated BP monitors suitable to patients with CF physiology into clinical practice23.
Accuracy of the Doppler BP in Respect to the Arterial Pulse Pressure and Clinical Implications
The Doppler technique achieved very high measurement success rate and repeatability, in concordance with published literature4,5,8. Although the Doppler BP showed strong correlation with the I-A MAP, overall Doppler overestimated I-A MAP in average by 6.7 [5.8] mm Hg (Figure 3B) and underestimated SBP by 5.0 [6.6] mm Hg. Furthermore, the data revealed a positive relationship between the I-A PP and the I-A MAP to Doppler BP difference (Figure 4A and 4B). This data supports previous observations by Lanier et al. that that the accuracy of MAP assessment by the Doppler technique decreases with increased pulsatility 5. Thus, in patients with a higher PP, the Doppler may determine more closely I-A SBP rather than I-A MAP. This relationship may have an important clinical implication in managing post-LVAD hypertension based on the recommendation to maintain the Doppler BP between 70 to 80 mm Hg and not to exceed 90 mmHg24–26. Thus, with increased pulsatility, there is a risk of overdosing antihypertensive therapy because of significantly higher differences between the true MAP vs the measured Doppler BP. In turn, potential chronic hypoperfusion may contribute to impaired glomerular filtration pressure, increasing risk of orthostatic hypotension with syncope and higher risk of cranial trauma. Other than the inability to provide SBP and DBP, the limitation of the Doppler technique is in the need for a trained person to perform a measurement. Yet, despite the clinical importance, this limitation of the Doppler technique will remain unsolved until either another physiologic surrogate for the peripheral arterial pulsatility assessment will be implemented in the Doppler technique or until an LVAD specific automated BP monitors will overcome the limitations of the Doppler to provide only a single BP value. However, the remarkable success rate of Doppler technique may reserve a continuing position of this technique in the setting of hemodynamically challenging conditions (e.g. circulatory shock).
Study limitations
In the present study we tested the performance of the ExpBP monitor prototype. For clinical use, the final design of the monitor will require extended clinical validation. The BHS validation protocol requires specific distribution of BP values in the tested population, which was not appreciated in present study18. Since the ExpBP monitor was tested on critically ill subjects, the methodology was limited by certain inconsistency in non-invasive BP measurements being acquired on either ipsilateral or contralateral arm from the reference method (due to vein thrombosis, etc.). However, none of the subjects included in the study were to be found with a significant lateral BP difference. Moreover, although the BHS protocol does not recommend the BP to be assessed on the contralateral arm, the newer internationally valid ISO standard 81060-2 allows this approach after the lateral difference determination18,19. Lastly, the results from the Doppler technique could be positively or negatively biased, since the Doppler BP assessment was not blinded from the invasive BP.
CONCLUSION
The ExpBP monitor was able to assess SBP, DBP, and MAP in the majority of CF LVAD subjects supported either by axial or centrifugal pumps with good agreement to the intra-arterial BP. The independent displaying SBP and DBP after MAP determination may improve overall measurement success rates. Automated BP monitors specifically designed for the CF LVAD patients might simplify self- and home monitoring of BP, contribute to safer use of antihypertensive drugs, and in turn, may alleviate adverse outcomes associated with poor BP control.
Supplementary Material
Experimental BP monitor operated via computer with customized software on the left. Equipment for the synchronized recording of invasive and non-invasive BP signals on the right.
Agreement of methods for SBP assessment delivered via the arterial line and the experimental BP monitor in Bland-Altman analysis and Pearson correlation coefficient.
Agreement of methods for DBP assessment delivered via the arterial line and the experimental BP monitor in Bland-Altman analysis and Pearson correlation coefficient.
Acknowledgments
The authors would like to acknowledge Jan Havlik, PhD and Jan Dvorak, MSc from the Czech Technical University in Prague, Czech Republic for technical support and signal interpretation, Alex Carlson, senior technician from the Human Integrative and Environmental Physiology Laboratory for his technical support, and Jacob Schaefer, an undergraduate student at the University of Notre Dame for his contribution to this work.
Funding Sources
This study was supported by the Mayo Clinic Cardiovascular Prospective Project Award and the National Institutes of Health grant HL71478 (B.D.J.) and institutional resources for research by Czech Technical University in Prague, Czech Republic. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to disclose.
Literature
- 1.Heidenreich Pa, Albert NM, Allen La, et al. Forecasting the impact of heart failure in the united states a policy statement from the american heart association. Circ Hear Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th Official Adult Heart Transplant Report—2012. J Hear Lung Transplant. 2012;31:1052–1064. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Pagani FD, et al. INTERMACS ANNUAL REPORT Seventh INTERMACS annual report: 15,000 patients and counting. J Hear Lung Transplant. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Bennett MK, Roberts Ca, Dordunoo D, Shah A, Russell SD. Ideal methodology to assess systemic blood pressure in patients with continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2010;29(5):593–594. doi: 10.1016/j.healun.2009.11.604. [DOI] [PubMed] [Google Scholar]
- 5.Lanier GM, Orlanes K, Hayashi Y, et al. Validity and reliability of a novel slow cuff-deflation system for noninvasive blood pressure monitoring in patients with continuous-flow left ventricular assist device. Circ Hear Fail. 2013;6:1005–1012. doi: 10.1161/CIRCHEARTFAILURE.112.000186. [DOI] [PubMed] [Google Scholar]
- 6.Teuteberg JJ, Ewald GA, Adamson RM, et al. Risk assessment for continuous flow left ventricular assist devices: does the destination therapy risk score work? An analysis of over 1,000 patients. J Am Coll Cardiol. 2012;60(1):44–51. doi: 10.1016/j.jacc.2012.02.032S0735-1097(12)01058-3. [pii] [DOI] [PubMed] [Google Scholar]
- 7.Saeed O, Jermyn R, Kargoli F, et al. Blood Pressure and Adverse Events During Continuous Flow Left Ventricular Assist Device Support. Circ Hear Fail. 2015 doi: 10.1161/CIRCHEARTFAILURE.114.002000. [DOI] [PubMed] [Google Scholar]
- 8.Lampert B, Weaver S, Scanlon A, et al. Blood Pressure Control in Continuous Flow Left Ventricular Assist Devices – Efficacy and Impact on Adverse Events. J Hear Lung Transplant. 2012;31(4):S251. doi: 10.1016/j.healun.2012.01.750. [DOI] [PubMed] [Google Scholar]
- 9.Hollenberg SM. Hemodynamic monitoring. Chest. 2013;143(5):1480–1488. doi: 10.1378/chest.12-1901. [DOI] [PubMed] [Google Scholar]
- 10.Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40(12):1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickering TGT, Hall JJE, Appel LJL, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Cou. Hypertension. 2005;111(45):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 12.Posey JA, Geddes LA, Williams H, Moore AG. The meaning of the point of maximum oscillations in cuff pressure in the indirect measurement of blood pressure. 1. [Accessed July 16, 2017];Cardiovasc Res Cent Bull. 8(1):15–25. http://www.ncbi.nlm.nih.gov/pubmed/5357773. [PubMed] [Google Scholar]
- 13.Zheng D, Amoore JN, Mieke S, Murray A. Estimation of mean arterial pressure from the oscillometric cuff pressure: comparison of different techniques. Med Biol Eng Comput. 2011;49(1):33–39. doi: 10.1007/s11517-010-0694-y. [DOI] [PubMed] [Google Scholar]
- 14.BRONZINO JD. Biomedical Engineering Handbook. Trinity College, Hartford, Connecticut, USA: CRC press; 1999. [Google Scholar]
- 15.Sorvoja H, Myllylä R. NONINVASIVE BLOOD PRESSURE MEASUREMENT METHODS. [Accessed July 16, 2017];Mol Quantum Acoust. 2006 :27. https://pdfs.semanticscholar.org/8f83/e5a0c79b099a9eda08c9e822510ea9f393ed.pdf.
- 16.Fabian V, Havlik J, Dvorak J, et al. Differences in mean arterial pressure of young and elderly people measured by oscilometry during inflation and deflation of the arm cuff. Biomed Tech (Berl) 2016:1–11. doi: 10.1515/bmt-2015-0098. [DOI] [PubMed] [Google Scholar]
- 17.Forouzanfar M, Dajani HR, Groza VZ, Bolic M, Rajan S, Batkin I. Oscillometric blood pressure estimation: Past, present, and future. IEEE Rev Biomed Eng. 2015;8:44–63. doi: 10.1109/RBME.2015.2434215. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien Eoin, William L, De Swiet M, et al. The British Hypertension society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993:19. doi: 10.1097/00004872-199306000-00013. [DOI] [PubMed] [Google Scholar]
- 19.American National Standard ANSI/AAMI/ISA 81060-2. 2013. Non-Invasive Sphygmomanometers-Part 2: Clinical Validation of Automated Measurement Type. [Google Scholar]
- 20.Cheng A, Williamitis Ca, Slaughter MS. Comparison of continuous-flow and pulsatile-flow left ventricular assist devices : is there an advantage to pulsatility? 2014;3(6):573–581. doi: 10.3978/j.issn.2225-319X.2014.08.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards AL, Fitzmorris PS, Hairrell AO, et al. Mo2020 Low Pulsatility Index Is Associated With an Increased Hazard of Gastrointestinal Bleeding in Patients With Continuous-Flow Left Ventricular Assist Devices. Gastroenterology. 2015;148(4):S-770–S-771. doi: 10.1016/S0016-5085(15)32629-9. [DOI] [Google Scholar]
- 22.Castagna F, McDonnell BJ, Stöhr EJ, et al. Non-invasive measurement of peripheral, central and 24-hour blood pressure in patients with continuous-flow left ventricular assist device. J Hear Lung Transplant. 2017;36(6):694–697. doi: 10.1016/j.healun.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Markham DW, Drazner MH. Measuring nonpulsatile blood pressure: say goodbye to the Doppler? Circ Heart Fail. 2013;6(5):879–880. doi: 10.1161/CIRCHEARTFAILURE.113.000579. [DOI] [PubMed] [Google Scholar]
- 24.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Hear Lung Transpl. 2010;29(4 Suppl):S1–39. doi: 10.1016/j.healun.2010.01.011S1053-2498(10)00043-4. [pii] [DOI] [PubMed] [Google Scholar]
- 25.Park SJ, Milano CA, Tatooles AJ, et al. Outcomes in advanced heart failure patients with left ventricular assist devices for destination therapy. Circ Hear Fail. 2012;5(2):241–248. doi: 10.1161/CIRCHEARTFAILURE.111.963991. [DOI] [PubMed] [Google Scholar]
- 26.Rogers JG, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55(17):1826–1834. doi: 10.1016/j.jacc.2009.12.052S0735-1097(10)01022-3. [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental BP monitor operated via computer with customized software on the left. Equipment for the synchronized recording of invasive and non-invasive BP signals on the right.
Agreement of methods for SBP assessment delivered via the arterial line and the experimental BP monitor in Bland-Altman analysis and Pearson correlation coefficient.
Agreement of methods for DBP assessment delivered via the arterial line and the experimental BP monitor in Bland-Altman analysis and Pearson correlation coefficient.