Abstract
Following oil spills such as the Deepwater Horizon accident (DWH), contamination of seafood resources and possible increased health risks attributable to consumption of seafood in spill areas are major concerns. In this study, locally harvested finfish and shrimp were collected from research participants in southeast Louisiana and analyzed for polycyclic aromatic hydrocarbons (PAHs). PAHs are some of the most important chemicals of concern regarding oil spill-contaminated seafood resources during and following oil spills. Some PAHs are considered carcinogens for risk assessment purposes, and currently, 7 of these can be combined in life-time cancer risk assessments using EPA approaches. Most PAHs were not detected in these samples (minimum detection limits ranged from 1.2 to 2.1 PPB) and of those that were detected, they were generally below 10 PPB. The pattern of detected PAHs suggested that the source of these chemicals in these seafood samples was not a result of direct contact with crude oil. Life-time cancer risks were assessed using conservative assumptions and models in a probabilistic framework for the 7 carcinogenic PAHs. Life-time health risks modeled using this framework did not exceed a 1/10,000 cancer risk threshold. Conservative, health-protective deterministic estimates of the levels of concern for PAH chemical concentration and seafood intake rates were above the concentrations and intake rates modeled under this probabilistic framework. Taken together, consumption of finfish and shrimp harvested from southeast Louisiana following the DWH does not pose unacceptable life-time cancer risks from these 7 carcinogenic PAHs even for the heaviest possible consumers.
Keywords: consumption health risks, polycyclic aromatic hydrocarbons, oil spill
1. INTRODUCTION
During and following petroleum spills in marine environments, there are often immediate and lingering concerns regarding negative impacts on deep sea as well as estuarine resources and possible health risks associated with consuming finfish and shellfish harvested from affected areas.(1–24) This was certainly evident following the two largest marine petroleum spills in the United States (US), those being the Exxon Valdez tanker spill and the Deepwater Horizon (DWH) platform spill.(1–3, 8–17, 19–24) Because of such concerns, considerable resources from the responsible party or parties, health agencies, and academic researchers are then directed towards scrutinizing finfish and shellfish samples to determine if any spill-related chemicals have contaminated the seafood supply.(9–14, 17, 20, 22–25)
The general protocol used by health agencies including the Environmental Protection Agency (EPA) and the Food and Drug Administration (FDA) in the US involves collecting samples of commercial finfish and shellfish from coastal areas affected by the spill.(23) Areas with visible oil are immediately closed to commercial harvest, and samples from these areas are only collected after no oil is observed. In areas without visible oil, samples are systematically collected to undergo strict safety testing. Collected samples are first subject to organoleptic screening by trained experts, and areas from which those samples were collected that fail that screening process remain closed or are subsequently closed. Samples that pass the organoleptic screening are then subject to chemical analysis. While not the only chemicals or elements of concern in spilled oil (e.g. elements such as cadmium and vanadium), the polycyclic aromatic hydrocarbons (PAHs) are those compounds health experts consider to be of most concern based on toxicity and concentration.(19, 22, 26–28) Some of these PAHs, especially those with 4–6 aromatic rings, are known or suspected carcinogens.(26, 27) Areas from which those samples were collected that contain these PAHs at concentrations above defined, health-protective levels pose possible excessive health risks to consumers and are closed until all samples have concentrations at or below those deemed health-protective.
Following the DWH accident, a community-based participatory research project was initiated primarily based on the concerns of local and regional fisherfolk and consumers that the finfish and shellfish (primarily shrimp) they harvest, sell, and/or consume might be unsafe for consumption.(26, 22, 23, 29) While extensive analytical testing of finfish and shellfish was being conducted during and after the spill, there were questions about where the samples were being collected from for testing and who was doing the actual analytical testing.(22, 23) For example, four different laboratory methods were used by three different regulatory agencies for chemical analysis of seafood. Three methods based on gas chromatography followed by mass spectroscopy with or without extraction, and one based on a liquid chromatography fluorescence method, were used by three different regulatory agencies for PAH analysis of seafood in Louisiana.(30) The range of the limit of detection among these methods spanned three orders of magnitude. There were also concerns about the risk assessments that were developed and that those reported did not adequately address the potential risks to heavy finfish and shellfish consuming populations, susceptible groups (e.g. children), and life-time or long-term consumers.(22, 23) The analysis reported herein is part of a larger project collecting samples from harvesters as well as non-harvesting consumers to better address such concerns from local citizenry.
This report documents the results and outcomes of probabilistic cancer risk modeling of possible life-time exposure to PAHs in finfish and shellfish samples from southeast Louisiana collected after the DWH. Chemical levels of concern and intake rate levels of concern were deterministically estimated from the analytical data and are reported as well to provide a different contextual perspective on the results of the chemical analysis and risk output. A total of 42 PAHs comprised the analyte panel of which only a fraction (~17%) are presently useful for estimating long-term cancer health risks.(31) A rapid extraction and analysis method was used to identify and quantify PAHs in this panel. Limitations associated with this method, especially as more PAH analytes are included in the additive cancer risk model, are addressed in the discussion.
2. MATERIALS AND METHODS
2.1 Finfish and Shellfish Samples
One hundred women, a subset of a larger project, were recruited to participate in the project evaluating seafood (i.e. finfish and shellfish) safety following the DWH accident. Active recruitment began in 2012 and was completed in 2015. Informed consent was obtained from all participants, and all human subjects research was covered under the Tulane University IRB protocol #262504. Women were recruited from Orleans, Jefferson, St. Bernard, Plaquemines, Terrebonne, Lafourche, and Assumption parishes in southeast Louisiana. Details regarding study subjects can be found in Simon-Friedt et al.(23)
Each participant was asked to provide a finfish or shellfish sample that was ≥ 4 ounces (~114 grams) for chemical analysis of PAH content. PAHs were considered the compounds of most concern based on existing knowledge of toxicity and carcinogenicity as well as their concentration in crude oil, thus other compounds such as those in the dispersants were not included. Participants were asked to provide the type of finfish or shellfish that constituted the preferred and most often type consumed by the participant and/or other members of the household. In addition, if they reported consuming locally harvested finfish or shellfish, they were asked to consider providing that type in the context of the preferred or most often consumed. Samples were collected from each participant at their home address and assigned a unique identification number consistent with each subject’s ID number. Samples were kept in their original packaging in a cooler on frozen ice packs (−80°C) and transported back to Tulane University within 6 hours of collection and placed at −80°C until transport to the chemical analysis laboratory at Louisiana State University in Baton Rouge, LA (LSU-BR).
2.2 Polycyclic Aromatic Hydrocarbon Analysis
2.2.1 Tissue Extraction Method
Determination of polycyclic aromatic hydrocarbons (PAH) residues in seafood samples was carried out using QuEChERS-dSPE (http://www.restek.com/pdfs/805-01-001.pdf) extraction followed by gas chromatography/mass spectrometry (GC/MS) analysis (QuEChERS-dSPE is the “Quick Easy Cheap Effective Rugged Safe” extraction method using dispersive Solid Phase Extraction). This extraction method was initially designed by Restek for rapid screening of pesticides in fruits and vegetables and was extrapolated to PAH contamination in seafood samples for human consumption after the DWH.
Following same-day transport from Tulane University to LSU-BR in a cooler on frozen ice packs (−80°C), shellfish and finfish samples were logged, given a unique laboratory identification number, and stored frozen (−20°C) until extraction. Batches of nine (9) samples and one (1) method blank were extracted at a time. Samples were defrosted and ~15g of sample was homogenized in a cleaned and solvent-rinsed tissuemiser. Homogenized samples were then transferred into solvent-rinsed centrifuge tubes and the initial sample weight was recorded. Method blanks were 15g of pre-cleaned, anhydrous sodium sulfate. A total of 15mL of acetic acid/acetonitrile mix (1%/99%) was added to each sample and each sample was spiked with surrogate standard (i.e., 20ppm of 5α-androstane and phenanthrene-d10). Tubes were capped and vortexed for 1 min. One extraction salt packet (Restek, Q110 Q-sep) was added and the sample was immediately vortexed for another minute. All samples were then centrifuged for 10 min at 10,000 rcf. Supernatant was removed off the top of all sample tubes using a solvent-cleaned syringe and transferred to individual Q-sep dSPE vials (Restek, 26221 Q-sep dSPE tube). Samples were vortexed for 1 min and centrifuged for 1 min at >1,500 rcf. At this point, 1mL out of a total of 15mL of each sample supernatant was transferred into a GC/MS autosampler vial. Internal standard (i.e. 10ng of naphthalene-d8, acenaphthalene-d10, chrysene-d12, and perylene-d12) was added to each sample, the sample was capped, and analyzed by GC/MS.
2.2.2 Gas Chromatography/Mass Spectroscopy (GC/MS) Methodology
The GC/MS was an Agilent 6890A GC system configured with a 5% diphenyl/95% dimethyl polysiloxane high resolution capillary column (30 meter, 0.25 mm ID, 0.25 micron film) directly interfaced to an Agilent 5973 MS detector system. An Agilent 7693 Auto Injector was used for sample introduction into the GC/MS system. The injection temperature was set at 280°C and only high-temperature, low thermal-bleed septa were used in the GC inlet. The GC was operated in the temperature program mode with an initial column temperature of 60°C for 3 mins then increased to 280°C at a rate of 5°C/min and held for 3 mins. The oven was then heated from 280°C to 300°C at a rate of 1.5°C/min and held at 300°C for 2 mins. Total run time was 65.33 mins per sample. The interface to the MS was maintained at 300°C. The MS was operated in the selected ion monitoring (SIM) to maximize the detection of the target analytes in Table I. The instrument was operated such that the selected ions for each acquisition window are scanned at a rate greater than 1.4 scans/sec with a dwell time of 60 milli-seconds for each analyte within each grouping.
Table I.
Target polycyclic aromatic hydrocarbon analytes and their respective quantitation ions. The C# prefix refers to the number of alkyl groups on each respective parent compound (e.g. C2-Pyrene refers to all dialkyl Pyrenes).
| Analyte* | SIM Ion (m/z) |
Analyte* | SIM Ion (m/z) |
|---|---|---|---|
| Naphthalene-d8 | 136 | C1-Pyrene | 216 |
| Naphthalene | 127 | C2-Pyrene | 230 |
| C1-Naphthalene | 142 | C3-Pyrene | 244 |
| C2- Naphthalene | 156 | C4-Pyrene | 258 |
| C3- Naphthalene | 170 | Naphthobenzothiophene | 234 |
| C4- Naphthalene | 184 | C1- Naphthobenzothiophene | 248 |
| Acenaphthene-d10 | 164 | C2- Naphthobenzothiophene | 262 |
| Fluorene | 166 | C3- Naphthobenzothiophene | 276 |
| C1- Fluorene | 180 | Benz[a]anthracene | 228 |
| C2- Fluorene | 194 | Chrysene | 228 |
| C3- Fluorene | 208 | C1- Chrysene | 242 |
| Dibenzthiophene | 184 | C2- Chrysene | 256 |
| C1- Dibenzthiophene | 198 | C3- Chrysene | 270 |
| C2- Dibenzthiophene | 212 | C4- Chrysene | 284 |
| C3- Dibenzthiophene | 226 | Perylene-d12 | 264 |
| Phenanthrene | 178 | Benzo[b]fluoranthene | 252 |
| C1- Phenanthrene | 192 | Benzo[k]fluoranthene | 252 |
| C2- Phenanthrene | 206 | Benzo[e]pyrene | 252 |
| C3- Phenanthrene | 220 | Benzo[a]pyrene | 252 |
| C4- Phenanthrene | 234 | Perylene | 252 |
| Anthracene | 178 | Indeno[1,2,3-cd]pyrene | 276 |
| Chrysene-d12 | 240 | Dibenz[a,h]anthracene | 278 |
| Fluoranthene | 202 | Benzo[g,h,i]perylene | 276 |
| Pyrene | 202 |
Compounds in bold are the internal standards added prior to analysis
2.2.3 Quantitative Analysis
GC/MS-SIM data was processed by Agilent Chemstation™ Software using a customized data analysis method. The analysis method was run on each sample and resulted in raw peak integration data that was transferred to a spreadsheet program for calculation of quantitative concentrations. The concentration of specific target PAH analytes was determined by a 5-point calibration curve and internal standard method. Internal standards are always added to the sample extracts just prior to GC/MS analysis. A commercially available oil analysis calibration standard containing parent (non-alkylated) hydrocarbons was used to establish the 5-point calibration curve that resulted in an average response factor for each PAH analyte in the standard mixture. Alkylated homologs were quantified using the response factor of their respective parent PAH compound, and are therefore considered as semi-quantitative concentrations. This is the standard procedure since alkylated standards are not available for all isomers and homologs. Recovery of all analytes was estimated using a two hydrocarbon surrogate standards: 5α-androstane (alkanes) and phenanthrene-d10 (PAHs). Surrogate standards were added to each sample prior to the extraction process.
Pesticide/reagent grade dichloromethane (DCM) and hexane were obtained from Makron Fine Chemicals™ (Center Valley, PA) and the Q-Sep salt (Q110) and dSPE (#26221) materials (i.e., QuEChERS) were obtained through Restek (Bellefonte, PA). The A.C.S. certified sodium sulfate (anhydrous, 10–60 mesh) was purchased through Fisher Scientific (Waltham, MA).
2.2.4 Analytical Standards and Reference Oil Standard
A commercially prepared oil analysis standard (Absolute Standards, Hamden, CT) was used to prepare a five-point calibration standard. A continuing calibration standard (i.e., one point of the initial five-point calibration standard) was analyzed in each batch of samples, or each 12-hour period over which analyses were performed. The acceptance criterion for the continuing calibration standard was ± 20% of the average relative response factor calculated from the initial five-point curve. The internal standard solutions consisted of naphthalene-d8, acenaphthene-d10, chrysene-d12, and perylene-d12 in DCM at 1000µg/ml (AccuStandard Inc., New Haven, CT). The internal standards were purchased individually from AccuStandard Inc. (New Haven, CT) and were stored until they were combined into the internal standard solution. The surrogate standards included 5α-androstane (alkanes) and phenanthrene-d10 (aromatics) in DCM, and were also stored individually until the standard solution was made at 20ppm (AccuStandard Inc., New Haven, CT). Macondo 252 (MC252) source oil collected directly from the riser of the DWH oil rig was the reference oil standard used for all analyses. The reference oil standard was prepared by extracting 1g of pure oil in 40mL of solvent (or equivalent ratio of 1g:40mL, e.g. 0.50g:20mL). The laboratory reference oil was analyzed in each sample batch as an additional QA/QC sample.
2.2.5 Quality Assurance/Quality Control
Standard, good laboratory procedures were used in handing and analyzing all tissue samples. These include use of cleaned and solvent rinsed labware/glassware, vials, and syringes. Method extraction blanks were prepared and analyzed with each group of tissue sample extractions. Table II shows the method detection limits (MDL) of the QuEChERS-dSPE method. These values were obtained by conducting replicate analyses (n=7) of oyster samples spiked at a known concentration with the PAH oil analysis mixture. The methylated naphthalenes and phenanthrenes given in Table 2 are for reference purposes only because any alkyl homologs detected in a sample were integrated as groups of peaks, not as individual isomers.
Table II.
Method detection limits (MDLs) for polycyclic aromatic hydrocarbon analytes using the Quick Easy Cheap Effective Rugged Safe extraction method using dispersive Solid Phase Extraction (QuEChERS/dSPE method).
| PAH Compounds | MDL (ng/g) |
PAH Compounds | MDL (ng/g) |
|---|---|---|---|
| Naphthalene | 1.8 | 1-methylphenanthrene | 1.7 |
| Benzothiophene | 2.0 | 3,6-dimethylphenanthrene | 1.5 |
| 2-methylnaphthalene | 1.9 | Fluoranthene | 1.8 |
| Biphenyl | 1.7 | Pyrene | 1.6 |
| 2-ethylnaphthalene | 2.0 | Benzo[a]fluorene | 1.5 |
| Acenaphthylene | 1.9 | 1-methylpyrene | 1.7 |
| Acenaphthene | 2.0 | Benz[a]anthracene | 1.9 |
| Dibenzfuran-d8 | 1.9 | Chrysene | 1.5 |
| Dibenzfuran | 1.7 | 5-methylchrysene | 1.9 |
| Fluorene | 1.3 | Benzo[b]fluoranthene | 2.0 |
| Dibenzthiophene | 1.4 | Benzo[k]fluoranthene | 1.8 |
| Phenanthrene | 1.2 | Benzo[e]pyrene | 2.0 |
| Anthracene | 2.0 | Benzo[a]pyrene | 2.0 |
| Carbazole | 1.7 | Perylene | 1.9 |
| 4-methyldibenzthiophene | 1.8 | Indeno[1,2,3-cd]pyrene | 2.1 |
| 2-methylphenanthrene | 1.6 | Dibenz[a,h]anthracene | 2.0 |
| 2-methylanthracene | 1.8 | Benzo[g,h,i]perylene | 1.9 |
At the start of each analysis period, or every twelve hours, the mass spectrometer was tuned to PFTBA, the internal instrument standard. Laboratory analytical and reference standards, such as the continuing calibration standard and MC252 source oil, were analyzed with each sample batch. This standard operating procedure ensures quality assurance/quality control of the instrument conditions prior to sample analysis.
2.3 Health Risk Modeling
The Microsoft Excel for Windows (version 2013) add-in @Risk software (version 7.5.1; Palisade Corporation, Ithaca, NY) was used for all probabilistic risk modeling. @Risk allows for distributional properties to be assigned to formula variables that can then be used in computer simulations to better account for variability and uncertainty in the underlying model and data.(22) Both Monte Carlo and Latin Hypercube sampling methods during simulations were used in this study with each run comprising 10,000 iterations.
The life-time average daily dose formula was used for developing probabilistic health risk output (i.e. cancer risk probabilities). The formula and a description can be found in Wilson et al.(22) Variables included in this study were PAH concentrations, finfish and shellfish (shellfish were exclusively shrimp described in more detail below) intake rates, exposure duration, body weight, and averaging time (life-time). Constants included age-dependent adjustment factors (ADAFs) described in more detail below. Though subject to debate and preference, acceptable cancer risks can range from 1/1,000,000 up to 1/10,000. For this study, a 1/10,000 cancer risk was used as the threshold for discussion and comparative purposes.
2.3.1 PAHs and Concentrations Used in Health Risk Modeling
PAHs included in the health risk modeling were those considered carcinogens using EPA methods, benzo[a]pyrene, benz[a]anthracene, chrysene, benzo[b]fluoranthene, benzo[k]fluoranthene, indeno[1,2,3-c,d]pyrene, and dibenz[a,h]anthracene. Furthermore, these were selected because there are well-established relative tumor potencies used by the EPA for these selected PAHs using B[a]P as the tumor reference compound.(31) This allows risk assessors to determine a summed or aggregate B[a]P equivalent (B[a]PEQ) concentration for this entire group of PAHs that can then be used with the oral slope factor (OSF) for risk of dose-dependent cancer development for B[a]P. The OSF for B[a]P is 1.0 per mg/kg/day.(32) Distributional properties for these PAHs were problematic primarily because they were not detected in the majority of samples. Therefore, two different distributions were selected to model PAH concentrations under different, conservative assumptions. In one case, it was assumed that PAH concentrations were uniformly distributed (U-distributed) among either finfish or shellfish samples with either the minimum detection limit (MDL) or the highest detected concentration serving as the upper truncation limit and 0 serving as the lower truncation limit. In the other case, PAH concentrations were assumed to follow a triangular distribution (Tri-distributed) with 0 as the lower truncation limit, the MDL serving as the mode or most frequently occurring concentration, and the highest concentration detected, where possible, serving as the upper truncation limit.
2.3.2 Intake Rates of Seafood
Finfish and shrimp intake rates and resulting risk models were treated separately for each type of seafood. An intake rate of 45.2 grams/day (0.7 lbs/week) was used for shrimp and was based on our previous work with a local shrimping community that better represents heavy consumers of shrimp.(22) This rate is approximately three times that of the 90th percentile of the US population used by the FDA.(10–12) Finfish intake rates were developed using three possibilities all modeling different levels of heavy consumption. Both the FDA and EPA promote the consumption of 1–2 servings (4–8 ounces or 114–228 grams) of fish per week as a healthy intake rate.(33) Under one possibility, it was assumed that individuals in this population eat approximately twice this amount of fish (4 servings/week). Under this assumption, the intake rate on average was 64.9 grams/day (1 lb/week). Under the second possibility, it was assumed that individuals in this population are more similar to subsistence consumers.(34) Under this assumption, the intake rate suggested by the EPA is on average 142.4 grams/day (2.2 lbs/week). Under the third possibility, it was assumed that individuals in this population are some of the heaviest consumers observed and according to the EPA, the intake rate for such individuals would be on average 540 grams/day (8.3 lbs/week).(34, 35) Intake rates under all scenarios were assumed to be normally distributed and were modeled as such using a lower truncation limit of 0 and an assumed upper truncation limit of 2,272 grams/day (5 lbs/day). Risks from consuming both shrimp, at an average of 45.2 grams/day, and finfish, at either an average of 64.9, 142.4, or 540 grams/day, were combined for estimating overall probabilistic cancer risks. One other conservative assumption made in this study is that all PAH content consumed is absorbed from ingested shrimp and finfish.
2.3.3 Exposure Duration (in years)
Exposure duration (in years) was assumed to effectively last for a life-time with exposures beginning at the age of 1. This necessitated that exposure duration was almost always 1 year shorter than the averaging time or life-time. Exposure duration and life-time were assumed to be highly correlated, therefore simulations were conducted using an exposure duration × life-time correlation matrix with a conservative correlation coefficient set at 0.9 (90% correlation). This was also done to ensure that exposure duration did not exceed life-time in simulation iterations. Exposure duration was modeled as a normal distribution with an average of 76.7 years (SD=4 years), a lower truncation limit of 60 years, and an upper truncation limit of 99 years.
2.3.4 Body Weight (in kilograms)
Body weights were taken from the CDC NCHS database for the US.(36) An overall average of 81.7 kg (SD=21.3 kg) was used based on this database for both men and women in the US. The lower truncation limit of 40 kg was taken from the dataset on Vietnamese-Americans used by Wilson et al.(22) This was the lowest body mass reported in that study. An upper truncation limit of 106 kg was determined from averaging the 90th percentiles for both women and men in the CDC NCHS database. Body weight was modeled as a normal distribution.
2.3.5 Averaging Time (in years)
Averaging time or life-time was derived from the CDC NCHS for contemporary US lifespans.(37) An average life-time of 77.7 years (SD=4 years) was estimated by averaging life-times for women and men in all available ethnic and racial categories. A lower truncation limit of 60 years and an upper truncation limit of 100 years were used to reasonably bound life-time which was modeled as a normal distribution.
2.3.6 Age-Dependent Adjustment Factors for Probabilistic Cancer Risk Estimates
It was assumed that these high consumption levels and therefore possible exposures began after the first year of age. Based on previous information, it is unlikely that heavy seafood consumption is occurring from birth to the age of 1.(38) Because PAHs act through a common mutagenic mode of action, the EPA recommends that age-dependent adjustment factors (ADAFs) be applied to exposures that occur during childhood and adolescence for the purposes of risk assessment.(39, 40) An ADAF of 10 was applied to cancer risk estimates for exposures that occurred from the age of 1 to 2. An ADAF of 3 was applied to cancer risk estimates for exposures that occurred from the age of 2 to the age of 16.(39) This was done to account for increased sensitivity to mutagenic carcinogens during this period of rapid development that may increase the risk of developing cancer as an adult following chronic exposures.(39)
2.3.7 Levels of Concern
Chemical levels of concern (CLoCs) were determined by rearranging the cancer risk equation using a deterministic approach. This was done to determine the B[a]PEQ concentration at high intake rates of shrimp and finfish that would be necessary to exceed a 1/10,000 cancer risk.(21, 34) To be most conservative in this process, a minimum body weight of 40 kg was used as well as an exposure duration of 59 years and an averaging time of 60 years.
Intake Rate Levels of Concern (IRLoCs) were determined by rearranging the cancer risk equation using a deterministic approach. This was done to determine the mass quantities of shrimp and finfish at the modeled average PAH concentrations that would be required to ingest on a daily basis that would then result in health risks that exceed a 1/10,000 probability. Modeled averages were used for this analysis under the assumption that, on average, this would be the concentration of B[a]PEQ PAHs that would be consumed rather than, for example, a life-time, daily consumption of the maximum possible concentration of B[a]PEQ which was considered highly improbable. As was done for determining the CLoCs, a conservative minimum body weight of 40 kg, an exposure duration of 59 years, and an averaging time of 60 years was used. ADAFs of 10 and 3 were used to derive these deterministic estimates.
3. RESULTS
3.1 Finfish and Shellfish Samples
A total of 93 participants (93%) provided either a finfish or shellfish sample for analysis. Seventy-one samples of finfish and shellfish (93% of which were shrimp) were considered to be of local origin determined by package labeling, known source, or by participant report. They included 42 samples of shrimp and 25 samples of finfish. Four additional samples comprising 2 crawfish samples, 1 squid sample, and 1 crab sample were collected and analyzed. Because of small sample size, the latter 4 samples were not included in these analyses. Two shrimp samples were from imported shrimp and 1 shrimp sample was of unknown origin. These were not included in these analyses. Samples of finfish considered local in origin were catfish, pompano, croaker, flounder, black drum, speckled trout, or redfish as reported by the participant. An additional 19 samples of finfish were considered to be imports determined by package labeling or reported or known species. These included tilapia, swai, and salmon.
3.2 Polycyclic Aromatic Hydrocarbon Analysis
3.2.1 PAHs-General Results
Concentrations of PAHs as well as the number of PAHs detected were observed to be higher in locally harvested finfish than they were in shrimp though the levels detected in all samples were in the low parts per billion (usually <10 ppb). Importantly, the pattern of PAHs detected did not support crude oil as the source. With the exception of naphthalene, unsubstituted or non-alkylated PAHs were the only isomers detected in both shrimp and finfish. In the case of naphthalene, the alkyl isomers were at lower concentrations than the unsubstituted isomer and often were not detected even when the parent form was detected. Alkyl isomers of the 2-, 3-, 4-, and likely the 5- and 6-ringed PAHs are more abundant in crude oil than the unsubstituted parent isomer, thus these data suggest that the PAHs detected in the sampled seafood likely come from sources other than crude oil. If crude oil, fresh to moderately fresh, were the source of the PAHs in the tissues of these samples, one would expect a higher ratio of alkylated to unsubstituted PAHs in contrast to what was observed.
3.2.2 PAHs in Shrimp
Seven of the 42 PAHs (~17%) were detected in at least one sample of locally harvested shrimp. Of these 7 PAHs detected across all samples, 83% were unsubstituted (e.g. phenanthrene as opposed to alkyl- or methyl-phenanthrene). The only alkylated PAHs detected were isomers of naphthalene, and these were detected in just 19% of samples, far fewer than the unsubstituted parent compound found in 57% of samples.
Table III shows the PAHs detected, the number of samples with detectable concentrations for each PAH, and moment statistics regarding the levels.
Table III.
Polycyclic aromatic hydrocarbons detected in shrimp samples considered of local origin provided by study participants.
| PAH(s) | Number of Samples with Detects (% of total) |
Mean Concentration (PPB, ± SD) |
Geometric Mean Concentration (PPB) |
Maximum Concentration (PPB) |
|---|---|---|---|---|
| Naphthalene | 24 (57%) | 8.2 (7.8) | 6.1 | 36.6 |
| C1-Naphthalenes | 7 (17%) | 18.4 (22.8) | 10.4 | 61.3 |
| C2-Naphthalenes | 1 (2%) | 56.8 | 56.8 | 56.8 |
| Dibenzthiophene | 2 (5%) | 37.4 (14.0) | 36.0 | 47.3 |
| Phenanthrene | 8 (19%) | 2.2 (0.6) | 2.1 | 3.2 |
| Pyrene | 2 (5%) | 4.0 (1.7) | 3.8 | 5.2 |
| Chrysene | 2 (5%) | 1.8 (0.2) | 1.8 | 1.9 |
3.2.3 PAHs in Finfish
Fourteen of the 42 PAHs (~33%) were detected in at least one sample of locally harvested fish. Of these 14 PAHs detected across all samples, 79% were unsubstituted. The only alkylated PAHs detected were isomers of naphthalene and naphthobenzothiophene. Alkyl isomers of naphthalene were detected in 56% of samples, far fewer than the unsubstituted parent compound found in 88% of samples. Table IV shows the PAHs detected, the number of samples with detectable concentrations for each PAH, and moment statistics regarding the levels.
Table IV.
Polycyclic aromatic hydrocarbons detected in finfish samples considered of local origin provided by study participants.
| PAH | Number of Samples with Detects (% of total) |
Mean Concentration (PPB, ± SD) |
Geometric Mean Concentration (PPB) |
Maximum Concentration (PPB) |
|---|---|---|---|---|
| Naphthalene | 22 (88%) | 20.4 (67.5) | 6.0 | 322.0 |
| C1-Naphthalenes | 4 (16%) | 4.6 (1.2) | 4.5 | 5.7 |
| C2-Naphthalenes | 6 (24%) | 38.4 (18.1) | 32.0 | 61.1 |
| C3-Naphthalenes | 4 (16%) | 23.0 (21.3) | 18.2 | 61.1 |
| Fluorene | 6 (24%) | 3.3 (2.2) | 2.8 | 6.7 |
| Dibenzthiophene | 1 (4%) | 5.0 | 5.0 | 5.0 |
| Phenanthrene | 10 (40%) | 7.6 (8.6) | 5.4 | 30.9 |
| Anthracene | 1 (4%) | 5.8 | 5.8 | 5.8 |
| Fluoranthene | 1 (4%) | 8.7 | 8.7 | 8.7 |
| Pyrene | 2 (8%) | 3.3 (0.4) | 3.3 | 3.6 |
| C1-Naphthobenzothiophenes | 1 (4%) | 5.4 | 5.4 | 5.4 |
| Chrysene | 8 (32%) | 6.9 (9.6) | 3.2 | 24.4 |
| Dibenz[a,h]anthracene | 2 (8%) | 2.6 (0.4) | 2.6 | 2.9 |
| Benzo[g,h,i]perylene | 3 (12%) | 2.7 (0.2) | 2.7 | 2.9 |
3.3 Health Risk Modeling
3.3.1 General Results
Modeled health risks were driven primarily by the two most potent carcinogenic PAHs, benzo[a]pyrene and dibenz[a,h]anthracene (Tables V and VI). Based on the assumptions regarding seafood (i.e. shrimp and finfish) consumption, finfish consumption largely drove probabilistic health risks. Tri-distributed probabilistic health risks were virtually indistinguishable from U-distributed probabilistic health risks. Maximum health risks under each modeling exercise were below or just at a 1/10,000 risk threshold.
Table V.
Levels of carcinogenic polycyclic aromatic hydrocarbons in shrimp samples used for assessing life-time cancer health risks. Levels include the minimum detection limits (MDLs), maximum detected where possible, relative potency factor (RPF) adjusted levels, and distributional means.
| Concentration (in PPM) |
||||||
|---|---|---|---|---|---|---|
| PAH | Minimum Detection Limit |
Maximum Detected |
RPF- adjusted MDL |
RPF- adjusted Maximum |
U-distributed Mean |
Tri-distributed Mean |
| Benz[a]anthracene | 1.9E-3 | Not Detected | 1.9E-4 | N/A | 9.5E-5 | 1.3E-4 |
| Chrysene | 1.5E-3 | 1.9E-3 | 1.5E-6 | 1.9E-6 | 9.5E-7 | 1.1E-6 |
| Benzo[b]fluoranthene | 2.0E-3 | Not Detected | 2.0E-4 | N/A | 1.0E-4 | 1.3E-4 |
| Benzo[k]fluoranthene | 1.8E-3 | Not Detected | 1.8E-5 | N/A | 9.0E-6 | 1.2E-5 |
| Benzo[a]pyrene | 2.0E-3 | Not Detected | 2.0E-3 | N/A | 1.0E-3 | 1.3E-3 |
| Indeno[1,2,3-c,d]pyrene | 2.1E-3 | Not Detected | 2.1E-4 | N/A | 1.1E-4 | 1.4E-4 |
| Dibenz[a,h]anthracene | 2.0E-3 | Not Detected | 2.0E-3 | N/A | 1.0E-3 | 1.3E-3 |
| Totals | 4.6E-3 | 2.3E-3 | 3.1E-3 |
Table VI.
Levels of carcinogenic polycyclic aromatic hydrocarbons in finfish samples used for assessing life-time cancer health risks. Levels include the minimum detection limits (MDLs), maximum detected where possible, relative potency factor (RPF) adjusted levels, and distributional means.
| Concentration (in PPM) |
||||||
|---|---|---|---|---|---|---|
| PAH | Minimum Detection Limit |
Maximum Detected |
RPF- adjusted MDL |
RPF- adjusted Maximum |
U-distributed Mean |
Tri-distributed Mean |
| Benz[a]anthracene | 1.9E-3 | Not Detected | 1.9E-4 | N/A | 9.5E-5 | 1.3E-4 |
| Chrysene | 1.5E-3 | 2.4E-2 | 1.5E-6 | 2.4E-5 | 1.2E-5 | 8.6E-6 |
| Benzo[b]fluoranthene | 2.0E-3 | Not Detected | 2.0E-4 | N/A | 1.0E-4 | 1.3E-4 |
| Benzo[k]fluoranthene | 1.8E-3 | Not Detected | 1.8E-5 | N/A | 9.0E-6 | 1.2E-5 |
| Benzo[a]pyrene | 2.0E-3 | Not Detected | 2.0E-3 | N/A | 1.0E-3 | 1.3E-3 |
| Indeno[1,2,3-c,d]pyrene | 2.1E-3 | Not Detected | 2.1E-4 | N/A | 1.1E-4 | 1.4E-4 |
| Dibenz[a,h]anthracene | 2.0E-3 | 2.9E-3 | 2.0E-3 | 2.9E-3 | 1.5E-3 | 1.6E-3 |
| Totals | 5.5E-3 | 2.8E-3 | 3.4E-3 |
3.3.2 Life-time Cancer Risks Modeled Using U-distributed PAHs
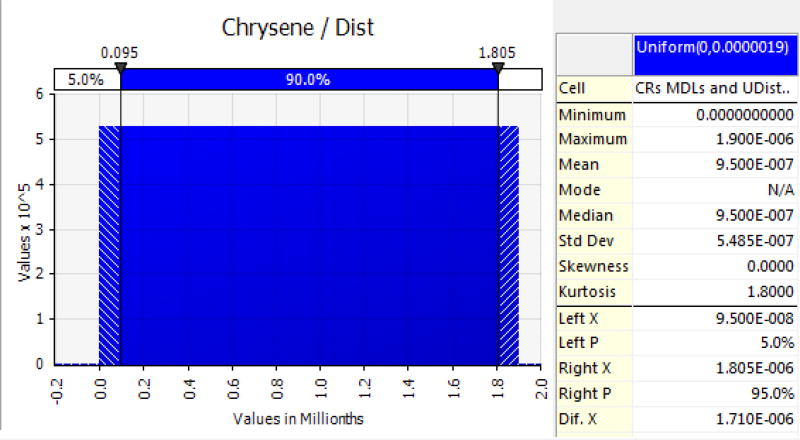
An example of a chrysene modeled as a U-distributed PAH in locally harvested shrimp is shown in Figure 1.
Figure 1.
Chrysene, as an example, modeled as a U-distributed PAH for estimating probabilistic life-time cancer risks from ingesting seafood (shrimp in this case) containing carcinogenic PAHs. Concentrations for all modeled PAHs, specifically those at the MDL or maximum, were adjusted to reflect tumor-inducing potency and reported as B[a]PEQ (e.g. chrysene at 0.001 relative to B[a]P).
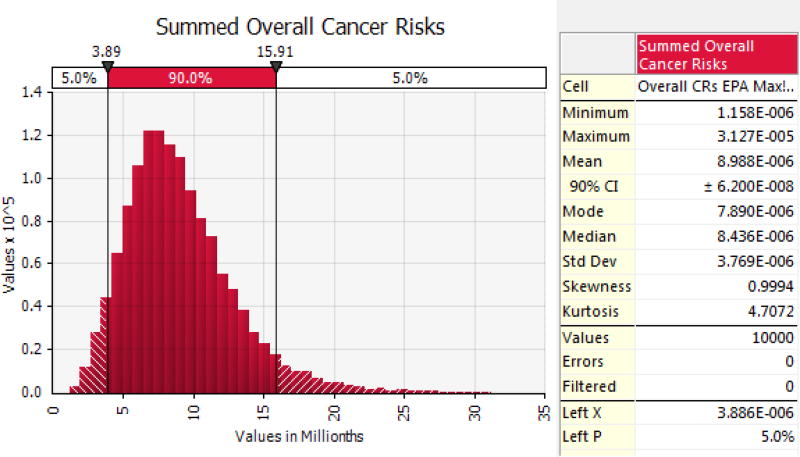
Table VII presents estimated life-time cancer risks modeled using U-distributed carcinogenic PAHs under explicit shrimp and finfish consumption assumptions. Cancer risks were estimated assuming heavy consumption of both shrimp and fish on a daily basis. An example of the probabilistic cancer risk output is shown in Figure 2.
Table VII.
Life-time cancer risks including the mean, median, and maximum under each of the shrimp and finfish consumptions scenarios modeling polycyclic aromatic hydrocarbon concentrations and B[a]PEQs under the assumption they follow U-distributions.
| Modeled Assumptions |
Mean (SDe) | Median (90%f) | Maximum |
|---|---|---|---|
| Shrimpa and Finfishb | 5.2E-6 (2,4E-6) | 4.8E-6 (2.1E-6 to 9.6E-6) | 2.0E-5 |
| Shrimpa and Finfishc | 9.0E-6 (3.8E-6) | 8.4E-6 (3.9E-6 to 1.6E-5) | 3.1E-5 |
| Shrimpa and Finfishd | 2.8E-5 (1.2E-5) | 2.7E-5 (1.1E-5 to 5.1E-5) | 1.0E-4 |
Shrimp consumption using a mean of 45.2 grams/day based on Wilson et al. (2015)
2X FDA Comsumption: Finfish consumption using twice the USFDA’s recommendation of 2 servings (8 ounces/week)
Subsistence: Finfish consumption using the USEPA’s subsistence estimate of 142.4 grams per day
Heaviest: Finfish consumption using the USEPA’s highest estimate of 540 grams/day
SD=standard deviation
90% contains from the 5%-ile to the 95%-ile)
Figure 2.
An example of probabilistic cancer risk output using U-distributed PAHs. This represents the combined life-time cancer risk distribution for consumers of shrimp (Wilson et al. 2015) and finfish (EPA subsistence).
3.3.3 Life-time Cancer Risks Modeled Using Tri-distributed PAHs
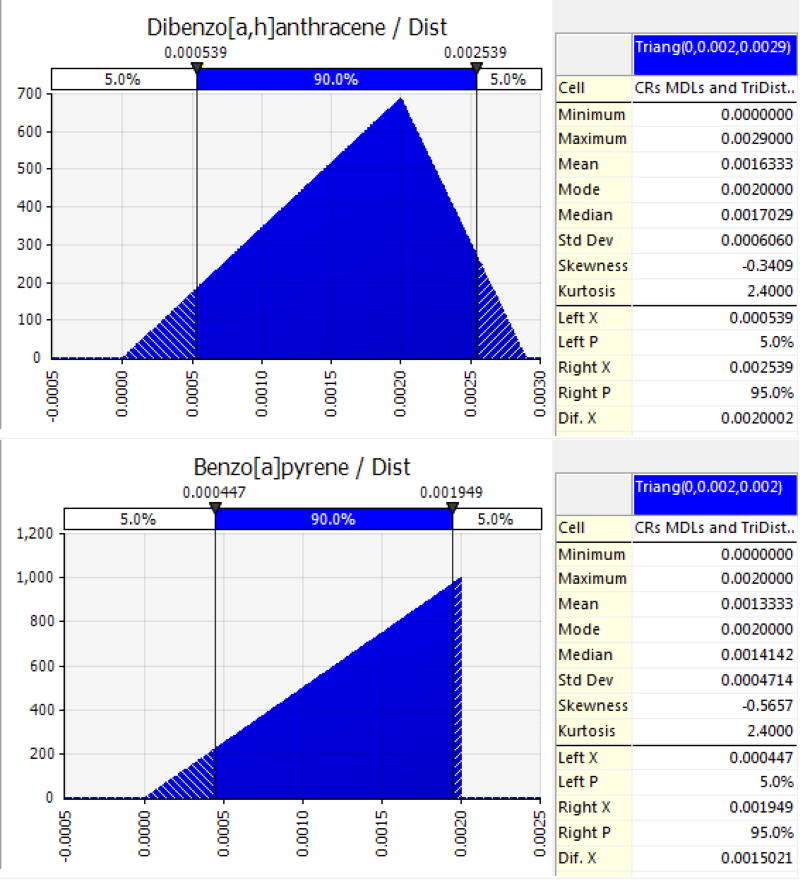
An example of a chrysene modeled as a Tri-distributed PAH in locally harvested finfish is shown in Figure 3.
Figure 3.
Dibenz[a,h]anthracene (detected) and benzo[a]pyrene (not detected), as examples, modeled as Tri-distributed PAHs for estimating probabilistic life-time cancer risks from ingesting seafood (finfish in this case) containing carcinogenic PAHs. Concentrations at the MDL were treated as the mode and the maximum for non-detects and were treated only as the mode for detects using the maximum detected as the distributional maximum. These were RPF-adjusted to reflect the tumor-inducing potency (e.g. dibenz[a,h]anthracene at 1.0) relative to B[a]P.
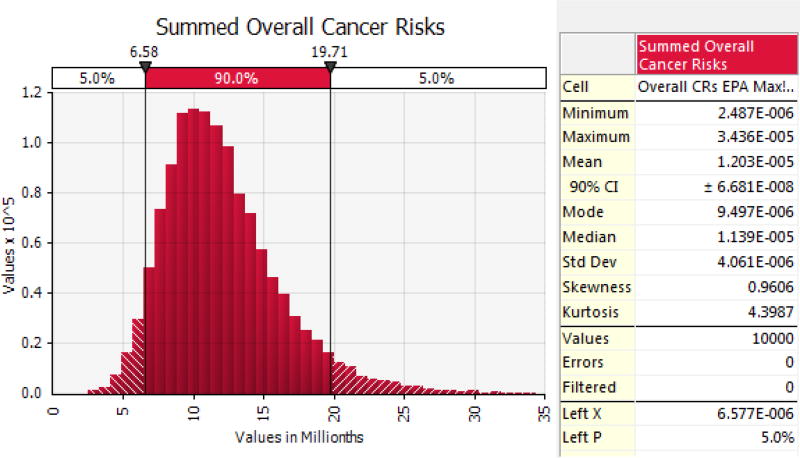
Table VIII presents estimated life-time cancer risks modeled using Tri-distributed carcinogenic PAHs under explicit shrimp and finfish consumption assumptions. Cancer risks were estimated assuming heavy consumption of both shrimp and fish on a daily basis. An example of the probabilistic cancer risk output is shown in Figure 4.
Table VIII.
Life-time cancer risks including the mean, median, and maximum under each of the shrimp and finfish consumptions scenarios modeling polycyclic aromatic hydrocarbon concentrations and B[a]PEQs under the assumption they follow triangular distributions.
| Modeled Assumptions |
Mean (SDe) | Median (90%f) | Maximum |
|---|---|---|---|
| Shrimpa and Finfishb | 7.0E-6 (2.7E-6) | 6.6E-6 (3.3E-6 to 1.2E-5) | 2.5E-5 |
| Shrimpa and Finfishc | 1.2E-5 (4.1E-6) | 1.1E-5 (6.6E-6 to 2.0E-5) | 3.4E-5 |
| Shrimpa and Finfishd | 3.8E-5 (1.2E-5) | 3.6E-5 (2.1E-5 to 6.1E-5) | 1.0E-4 |
Shrimp consumption using a mean of 45.2 grams/day based on Wilson et al. (2015)
2X FDA Consumption: Finfish consumption using twice the USFDA’s recommendation of 2 servings (8 ounces/week)
Subsistence: Finfish consumption using the USEPA’s subsistence estimate of 142.4 grams per day
Heaviest: Finfish consumption using the USEPA’s highest estimate of 540 grams/day
SD=standard deviation
90% contains from the 5%-ile to the 95%-ile)
Figure 4.
An example of probabilistic cancer risk output using Tri-distributed PAHs. This represents the combined life-time cancer risk distribution for consumers of shrimp (Wilson et al. 2015) and finfish (EPA subsistence).
3.3.4 Chemical Levels of Concern (CLoCs)
CLoCs are presented as B[a]PEQs in Table IX. Using the MDL as the maximum possible concentration for those PAHs that were not detected in any sample along with the maximum detected for those PAHs that were detected in at least one sample (chrysene in shrimp; chrysene and dibenz[a,h]anthracene in finfish), the maximum possible concentration of carcinogenic PAHs in B[a]PEQs based on the data in this study and for these samples was 4.6 PPB in shrimp and 5.5 PPB in finfish.
Table IX.
Deterministic Chemical Levels of Concern (CLoCs) using a cancer risk of 1/10,000, a body weight of 40 kg, an exposure duration of 59 years, and an averaging time of 60 years under the defined shrimp and finfish consumption scenarios. Age-dependent adjustment factors of 10 (1–2 years of age) and 3 (2–16 years of age) were used to develop these estimates.
| CLoCa (in PPB) | |
|---|---|
| Shrimp (45.2 grams/day) | 65.8 (12.0x maximum possible)b |
| Finfish (64.9 grams/day) | 45.8 (8.3x maximum possible)b |
| Finfish (142.4 grams/day) | 20.9 (3.8x maximum possible)b |
| Finfish (540 grams/day) | 5.5 (1.0x maximum possible)b |
benzo[a]pyrene equivalents (B[a]PEQs)
Maximum possible concentration of carcinogenic PAHs in B[a]PEQs
3.3.5 Intake Rate Levels of Concern (IRLoCs)
IRLoCs for the average B[a]PEQ concentration in shrimp and finfish are presented in Table X.
Table X.
Deterministic Intake Rate Levels of Concern (IRLoCs) developed from the average U-distributed and Tri-distributed modeled PAHsa using a cancer risk of 1/10,000, a body weight of 40 kg, an exposure duration of 59 years, and an averaging time of 60 years. Age-dependent adjustment factors of 10 (1–2 years of age) and 3 (2–16 years of age) were used to develop these estimates.
| Shrimp grams/day (lbs/day) |
Finfish grams/day (lbs/day) |
|
|---|---|---|
| U-distributed PAHs | 1,288.0 (2.8) | 1,073.6 (2.4) |
| Tri-distributed PAHs | 844.4 (1.9) | 767.7 (1.7) |
using average benzo[a]pyrene equivalent total PAH concentrations
4. DISCUSSION
4.1 PAHs in Shellfish and Finfish Samples
Samples provided in this study by our research participants were collected from 2012 through 2015. PAHs were at very low to non-detectable concentrations, with MDLs ranging from 1.2–2.1 PPB. Detected PAHs were generally below 10 PPB and in most samples few if any PAHs were detected. Of the PAHs that were detected, the majority were unsubstituted isomers and relatively few alkylated isomers were detected. Research indicated that our participants and likely most consumers and harvesters in the region were very concerned about the safety of their seafood and adjusted their diets based on that concern.(23) Consistent with other testing that was being done in samples from the Gulf Coast, chemical analysis as conducted in this study indicated that PAHs in these samples were low and not likely to result from any direct contact with crude oil despite the volume of oil spilled in the DWH accident.(9–13, 19, 22, 30) While no particularly good options for mitigating coastal impacts were evident, it could be argued that the use of dispersants in deep water in this situation, while damaging to deep water fauna and flora, may have prevented or reduced contamination of many commercial and recreational coastal seafood resources. Furthermore, based on the temporality of sample collection in our study, it may now be argued that PAH levels in the locally harvested seafood samples represent “background” or “between event” levels for future comparison and evaluation.
Locally harvested seafood is readily available from a variety of sources from local, independent markets to chain stores as well as a variety of other sources spanning those that are indirectly- or directly-sourced from harvesters. In this region and among our participants, the common practice is to regularly consume locally harvested shellfish, shrimp, and finfish.(23, 24) Because the participants providing samples in this study were primarily consumers, it could be argued that they were especially likely to report their sources accurately as they and their families were consuming this seafood as opposed to also generating revenue from the product. In this study, a key assumption was made that participants’ reporting the origin of their sample, local or imported, was accurate to the best of their knowledge. Self-reporting the source of the provided samples is acknowledged as a possible limitation in this study, but it is considered unlikely that misreporting of sample origin was common. For the few samples that were provided in vacuum-sealed packaging with product information including source, this was also considered to be reasonably accurate (e.g. where commercial packaging indicates the product was from China). Though not systematically evaluated, it has been the experience of this research team that both seafood harvesters and consumers in southeast LA, and likely elsewhere, are most interested in the quality and safety of their product and food.(23) The relatively sample size in this study is another limitation explicitly acknowledged in that it is possible the results are not readily generalizable to a larger geographic region. That said, the results reported here are consistent with those from previous research studies investigating PAH levels in finfish and shellfish and from large-scale monitoring projects during and following the DWH.(13, 19, 41) This suggests that the relatively small sample size can be considered representative especially of southeast LA.
Shellfish and seafood types other than shrimp and finfish were not represented in this evaluation of food safety. While seafood such as crabs and oyster are not as widely consumed, they are still extremely important from both a dietary and economic perspective. A very small number of samples were obtained representing seafood types other than shrimp and finfish limiting the cancer risk assessments to those two primary sources.
4.2 QuEChERS-dSPE Method
With respect to the PAHs (n=7) that were used for life-time cancer risk assessments in this study, the chemical analysis methods were considered sufficient. This is despite the number of samples below the MDLs (1.2–2.1 PPB). Under very conservative assumptions designed to address the number of censored values, albeit in crude but simplifying probabilistic modeling approaches, providing reasonable risk evaluations was still possible and considered appropriate. It is acknowledged that completely uncensored, accurate, and precise data is the absolute ideal for providing the best possible risk assessments. With environmental samples that have sub-PPB levels of PAHs this is often quite difficult without much more extensive and expensive extraction and concentration methods. Therefore, for the carcinogenic PAHs used for risk assessment purposes in this study, it is argued that the distributional modeling approaches should be considered appropriate, useful, and erring towards quite conservative and health protective (discussed further below).
Because the current assessment for life-time cancer risks for PAHs relies on the additive B[a]PEQs model, future assessments will require methods with lower MDLs, preferably in the low parts-per-trillion to accurately, quantitatively model health risks. This is because adding more PAHs, as well as alkyl-isomers of existing carcinogenic unsubstituted PAHs to this RPF-based model, will only drive cancer risks up. Assessments using quite conservative assumptions, such as those in this study, will then increasingly overestimate life-time cancer risks to untenable levels. Far fewer censored values (i.e. <30–40% censored values maximally) will be necessary to provide more accurate and precise metrics for distributionalizing PAH concentrations for use in more unbiased, objective probabilistic risk modeling.
4.3 Health Risks and Conclusions
Health risk modeling in this study used very conservative assumptions including high to extremely high intake rates of both shrimp and finfish on a daily basis for respective life-times with heavy consumption beginning at 1 year of age. Intake rates of shrimp were 3X that used in the USFDA assessment following the DWH accident, and intake rates of finfish modeled in this study exceeded the USFDA recommendation of at least 2 servings per week with each serving being 4 ounces/114 grams by 2X–16X.(10–12, 22) Exposure duration and life-time were assumed to be highly correlated such that no matter the duration of simulated life-time, exposure duration occurred for virtually the entire life-time with exception of the age interval birth to year 1. Body weights were simulated down to a life-time of 40 kg. ADAFs were applied to early childhood and adolescent exposures to further compensate for developmental sensitivities.(39) Finally, modeled distributions of PAHs were designed to conservatively account for censored values using the MDL as both the maximum and mode for those carcinogenic PAHs that were not detected at or above the MDL in any of the samples. For those carcinogenic PAHs that were detected (n≥1 sample), the MDL was used as the mode with maximum detected used as the distributional maximum.
Health risks met the cancer risk threshold only at the highest finfish intake rates of 540g/day on average. Simulations were examined in more detail to determine the characteristics that contributed to the high risk estimates. Table XI summarizes the properties of those at the highest risk and those at lowest risk under the highest finfish intake rate model using PAHs that followed conservative triangular distributions.
Table XI.
Risk simulation parameters for life-time average daily dose variables for the highest and lowest simulated risks under the most conservative modeled scenarios for life-time probabilistic cancer risks (out of 10,000 iterations).
| Highest Risk Simulations (n=5) |
Lowest Risk Simulations (n=5) |
|
|---|---|---|
|
| ||
| Average Risk (SDa, Range) | 1.0E-4 (3.9E-6, 9.5E-5 to 1.0E-4) | 8.8E-6 (6.7E-7, 8.0E-6 to 9.6E-6) |
|
| ||
| Average IRb of Shrimp (SD) | 56 g/day (26 g/day) | 21 g/day (7 g/day) |
|
| ||
| Average IR of Finfish (SD) | 550 g/day (19 g/day) | 546 g/day (10 g/day) |
|
| ||
| Average Body Weight (SD) | 42.7 kg (1.7 kg) | 93.1 kg (12.8 kg) |
|
| ||
| Average Exposure Duration (SD) | 74 yrs (4.3 yrs) | 77 yrs (2.6 yrs) |
|
| ||
| Average Life-time (SD) | 75 yrs (4.8 yrs) | 79 yrs (1.7 yrs) |
|
| ||
| Average B[a]PEQs | 3.6E-3 mg/kg (117%) | 2.8E-3 mg/kg (91%) |
| Shrimp (% of average) | 4.9E-3mg/kg (145%) | 9.5E-4 mg/kg (28%) |
| Finfish (% of average) | ||
|
| ||
| Average benzo[a]pyrene concentration, % of overall modeled maximum, % of MDL, or % of the overall modeled average B[a]PEQs | Shrimp-1.6E-3 mg/kg, 81%, 81%, 124% | Shrimp-1.2E-3 mg/kg, 60%, 60%, 92% |
| Finfish-2.0E-3 mg/kg, 98%, 98%, 150% | Finfish-2.8E-4 mg/kg, 14%, 14%, 22% | |
|
| ||
| Average dibenz[a,h]anthracene concentration, % of overall modeled maximum, % of MDL, or % of the overall modeled average B[a]PEQs | Shrimp-1.6E-3 mg/kg, 82%, 82%, 126% | Shrimp-1.2E-3 mg/kg, 61%, 61%, 94% |
| Finfish-2.5E-3 mg/kg, 88%, 128%, 159% | Finfish-2.7E-4 mg/kg, 9%, 14%, 17% | |
Qualitative evaluation of simulations representing those at the highest life-time risk compared to those with the lowest life-time risk provide additional insights into the modeled assessments. For example, those simulations indicating highest risks that are just at the 1/10,000 cancer risk threshold suggest that such individuals would weigh 40 kg for their entire life-time (~75 years with an exposure duration of 74 years), they would consume 56g shrimp and 550g finfish on a daily basis, and that B[a]PEQs concentrations in such shrimp and finfish would exceed modeled averages by 117% and 145% respectively on a daily basis. Considering the two most potent PAHs, benzo[a]pyrene and dibenz[a,h]anthracene, B[a]PEQs for each would exceed modeled averages in shrimp and finfish by 124% and 150% (B[a]P) and 126% and 159% (D[a,h]A) respectively. Though theoretically possible, these simulated values in reality are highly improbable.
The pattern of PAHs detected in the samples in this study do not support crude oil as the most likely source.(42) Assuming these samples can be considered representative, this suggests that contamination of locally harvested seafood at least from 2012 until 2015 by the DWH was simply not evident. While the health benefits of consuming shrimp and finfish as part of low-fat, low-cholesterol, high-protein diet have not been extensively discussed, this must be considered important and relevant to these conclusions.(43) Alternatives to shrimp and finfish in such a diet are highly unlikely to be as healthy and beneficial and effectively communicating the benefits and risks of seafood consumption in that context are critical.(43, 44) Coupled with the conservative health risk modeling used in this study, this provides further support for the conclusion that life-time consumption of locally harvested shrimp and finfish from southeast Louisiana remains part of a healthy, well-balanced diet and does not pose an unacceptable lifetime cancer risk to even heavy consumers.
Social Media Summary.
After the Deepwater Horizon oil spill, seafood safety was concerning to southeast Louisiana residents. We measured levels of oil chemicals in seafood under modeled scenarios and determined that eating seafood after the oil spill did not contribute to an increased cancer risk over a lifetime of heavy seafood consumption.
Acknowledgments
This work was supported by the National Institutes of Environmental Health Sciences [5U19ES020677], which aided the authors in the study design, collection, analysis, and interpretation of data, as well as writing of the manuscript. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS or the NIH. This work was partially supported by the Baton Rouge Area Foundation, which aided the authors in the analysis and interpretation of data and writing of the manuscript. We would like to thank David Gauthe and Donald Bogen at Bayou Interfaith Shared Community Organizing, and Daniel Nguyen at Mary Queen of Vietnam Community Development Corporation for their invaluable help and dedication in carrying out this work.
References
- 1.Saxton WL, Newton RT, Rorberg J, et al. Polycyclic aromatic hydrocarbons in seafood from the Gulf of Alaska following a major crude oil spill. Bulletin of Environmental Contamination and Toxicology. 1993;51(4):515–22. doi: 10.1007/BF00192166. [DOI] [PubMed] [Google Scholar]
- 2.Bolger M, Henry SH, Carrington CD. Hazard and risk assessment of crude oil contaminants in subsistence seafood samples from Prince William Sound. In: Rice SD, Spies RB, Wolfe DA, et al., editors. Proceedings of the Exxon Valdez Symposium. Vol. 18. Bethesda, Maryland: American Fisheries Society; 1996. pp. 837–43. [Google Scholar]
- 3.Field LJ, Fall JA, Nighswander TS, et al., editors. Evaluating and Communicating Subsistence Seafood Safety in a Cross-Cultural Context: Lessons Learned from the Exxon Valdez Oil Spill Pensacola. Florida: SETAC Press; 1999. p. 338. (SETAC Technical Publications) [Google Scholar]
- 4.Law RJ, Hellou J. Contamination of fish and shellfish following oil spill incidents. Environmental Geosciences. 1999;6(2):90–8. [Google Scholar]
- 5.Gilroy DJ. Derivation of shellfish harvest reopening criteria following the New Carissa oil spill in Coos Bay, Oregon. Journal of Toxicology and Environmental Health, Part A. 2000;60(5):317–29. doi: 10.1080/00984100050030109. [DOI] [PubMed] [Google Scholar]
- 6.Surís-Regueiro JC, Garza-Gil MD, Varela-Lafuente MM. The Prestige oil spill and its economic impact on the Galician fishing sector. Disasters. 2007;31(2):201–15. doi: 10.1111/j.1467-7717.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- 7.Garza MD, Prada A, Varela M, et al. Indirect assessment of economic damages from the Prestige oil spill: consequences for liability and risk prevention. Disasters. 2009;33(1):95–109. doi: 10.1111/j.1467-7717.2008.01064.x. [DOI] [PubMed] [Google Scholar]
- 8.Levin JL, Gilmore K, Carruth A, et al. An interview with Vietnamese fishermen of Louisiana in the wake of the oil spill. Journal of Agromedicine. 2010;15(4):337–42. doi: 10.1080/1059924X.2010.521109. [DOI] [PubMed] [Google Scholar]
- 9.Gohlke JM, Doke D, Tipre M, et al. A review of seafood safety after the Deepwater Horizon blowout. Environmental Health Perspectives. 2011;119(8):1062–9. doi: 10.1289/ehp.1103507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rotkin-Ellman M, Wong KK, Solomon GM. Seafood contamination after the BP Gulf oil spill and risks to vulnerable populations: A critique of the FDA risk assessment. Environmental Health Perspectives. 2011;120(2):157–61. doi: 10.1289/ehp.1103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickey RW. FDA risk assessment of seafood contamination after the BP oil spill. Environmental Health Perspectives. 2012;120(2):a54–a5. doi: 10.1289/ehp.1104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotkin-Ellman M, Solomon G. FDA risk assessment of seafood contamination after the BP oil spill: Rotkin-Ellman and Solomon respond. Environmental Health Perspectives. 2012;120(2):a55–a6. doi: 10.1289/ehp.1103695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia K, Hagood G, Childers C, et al. Polycyclic aromatic hydrocarbons (PAHs) in Mississippi seafood from areas affected by the Deepwater Horizon oil spill. Environmental Science & Technology. 2012;46(10):5310–8. doi: 10.1021/es2042433. [DOI] [PubMed] [Google Scholar]
- 14.Ylitalo GM, Krahn MM, Dickhoff WW, et al. Federal seafood safety response to the Deepwater Horizon oil spill. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(50):20274–9. doi: 10.1073/pnas.1108886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Genualdi S, DeJager L, Begley T. Assessments and improvements in methods for monitoring seafood safety in response to the Deepwater Horizon oil spill. Journal of Agricultural and Food Chemistry. 2013;61(14):3542–7. doi: 10.1021/jf305344z. [DOI] [PubMed] [Google Scholar]
- 16.Greiner AL, Lagasse LP, Neff RA, et al. Reassuring or risky: The presentation of seafood safety in the aftermath of the British Petroleum Deepwater Horizon oil spill. American Journal of Public Health. 2013;103(7):1198–206. doi: 10.2105/AJPH.2012.301093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sammarco PW, Kolian SR, Warby RAF, et al. Distribution and concentrations of petroleum hydrocarbons associated with the BP/Deepwater Horizon Oil Spill, Gulf of Mexico. Marine Pollution Bulletin. 2013;73(1):129–43. doi: 10.1016/j.marpolbul.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Cunha I, Neuparth T, Moreira S, et al. Management of contaminated marine marketable resources after oil and HNS spills in Europe. Journal of Environmental Management. 2014;135:36–44. doi: 10.1016/j.jenvman.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 19.Fitzgerald TP, Gohlke JM. Contaminant levels in Gulf of Mexico reef fish after the Deepwater Horizon oil spill as measured by a fishermen-led testing program. Environmental Science & Technology. 2014;48(3):1993–2000. doi: 10.1021/es4051555. [DOI] [PubMed] [Google Scholar]
- 20.Sammarco PW, Kaltofen M, Kolian S, et al. A response to Wilson et al. A critique of the manuscript: “ Distribution and concentrations of petroleum hydrocarbons associated with the BP/Deepwater Horizon oil spill, Gulf of Mexico”. Marine Pollution Bulletin. 2014;79(1–2):391–2. doi: 10.1016/j.marpolbul.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Wilson MJ, Wickliffe JK, Overton E. A critique of the manuscript: “Distribution and concentrations of petroleum hydrocarbons associated with the BP/Deepwater Horizon oil spill, Gulf of Mexico”. Marine Pollution Bulletin. 2014;79(1–2):389–90. doi: 10.1016/j.marpolbul.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 22.Wilson MJ, Frickel S, Nguyen D, et al. A targeted health risk assessment following the Deepwater Horizon Oil Spill: polycyclic aromatic hydrocarbon exposure in Vietnamese-American shrimp consumers. Environmental Health Perspectives. 2015;123:152–9. doi: 10.1289/ehp.1408684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simon-Friedt BR, Howard JL, Wilson MJ, et al. Louisiana residents’ self-reported lack of information following the Deepwater Horizon oil spill: Effects on seafood consumption and risk perception. Journal of Environmental Management. 2016;180:526–37. doi: 10.1016/j.jenvman.2016.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sathiakumar N, Tipre M, Turner-Henson A, et al. Post-deepwater horizon blowout seafood consumption patterns and community-specific levels of concern for selected chemicals among children in Mobile County, Alabama. International Journal of Hygiene and Environmental Health. 2017;220(1):1–7. doi: 10.1016/j.ijheh.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Lubchenco J, McNutt MK, Dreyfus G, et al. Science in support of the Deepwater Horizon response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(50):20212–21. doi: 10.1073/pnas.1204729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wickliffe J, Overton E, Frickel S, et al. Evaluation of polycyclic aromatic hydrocarbons using analytical methods, toxicology, and risk assessment research: Seafood safety after a petroleum spill as an example. Environmental Health Perspectives. 2014;122(1):6–9. doi: 10.1289/ehp.1306724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egyptian Journal of Petroleum. 2016;25(1):107–23. [Google Scholar]
- 28.Osuji LC, Onojake CM. Trace heavy metals associated with crude oil: A case study of Ebocha-8 oil-spill-polluted site in Niger Delta, Nigeria. Chemistry & Biodiversity. 2004;1(11):1708–15. doi: 10.1002/cbdv.200490129. [DOI] [PubMed] [Google Scholar]
- 29.Zilversmit L, Wickliffe J, Shankar A, et al. Correlations of Biomarkers and Self-Reported Seafood Consumption among Pregnant and Non-Pregnant Women in Southeastern Louisiana after the Gulf Oil Spill: The GROWH Study. International Journal of Environmental Research and Public Health. 2017;14(7):784. doi: 10.3390/ijerph14070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louisiana Seafood Safety and Monitoring Plan. Sample Results. 2015 Sep; (4/30/10-1/31/14) [Google Scholar]
- 31.USEPA. United States Environmental Protection Agency. D Office of Research and Development Washington; Jul, 1993. Provisional Guidance for Quantitative Risk Assessment of Polycyclic Aromatic Hydrocarbons. EPA/600/R-93/089. [Google Scholar]
- 32.IRIS. [8/24/2017];Benzo[a]pyrene (BaP) 2017 https://cfpub.epa.gov/ncea/iris2/chemicalLanding.cfm?substance_nmbr=136.
- 33. [8/24/2017];2017 EPA-FDA Advice about Eating Fish and Shellfish. https://www.epa.gov/fish-tech/2017-epa-fda-advice-about-eating-fish-and-shellfish.
- 34.USEPA. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories. United States Environmental Protection Agency. Oo Water; Nov, 2000. Risk Assessment and Fish Consumption Limits. EPA 823-B-00-008. [Google Scholar]
- 35.Harris SG, Harper BL. A Native American exposure scenario. Risk Analysis. 1997;17(6):789–95. doi: 10.1111/j.1539-6924.1997.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 36.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007–2010. Vital Health Stat. NCfH Statistics. 2012:252. [PubMed] [Google Scholar]
- 37.Murphy SL, Xu J, Kochanek KD. Deaths: Final Data for 2010. National Vital Statistics Reports. NCfH Statistics. 2013 May 8;:4. [PubMed] [Google Scholar]
- 38.USEPA. Child-Specific Exposure Factors Handbook. United States Environmental Protection Agency. 2008 Sep; EPA/600/R-06/096F. [Google Scholar]
- 39.USEPA. Supplemental Guidance for Assessing Susceptibility from Early-Life Exposure to Carcinogens. United States Environmental Protection Agency. 2005 Mar; EPA/630/R-03/003F. [Google Scholar]
- 40.USEPA. Guidelines for Carcinogen Risk Assessment. United States Environmental Protection Agency. 2005 Mar; EPA/630/P-03/001F. [Google Scholar]
- 41.2010 Gulf Oil Spill. Louisiana Department of Health and Hospitals. LDoHa Hospitals; Louisiana Seafood Safety and Monitoring Plan Sample Results (4/30/10-1/31/14) http://new.dhh.louisiana.gov/assets/oph/Center-EH/envepi/fishadvisory/Documents/BP_Report_September_2015_FINAL.pdf. [Google Scholar]
- 42.Overton EB, Ashton BM, Miles MS. Historical polycyclic aromatic and petrogenic hydrocarbon loading in Northern Central Gulf of Mexico shelf sediments. Marine Pollution Bulletin. 2004;49(7–8):557–63. doi: 10.1016/j.marpolbul.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 43.USFDA. [8/25/2017];Eating Fish: What Pregnant Women and Parents Should Know. 2017 doi: 10.1097/01.NAJ.0000515224.05161.d6. https://www.fda.gov/Food/ResourcesForYou/Consumers/ucm393070.htm. [DOI] [PubMed]
- 44.Verbeke W, Vanhonacker F, Frewer LJ, et al. Communicating risks and benefits from fish consumption: impact on Belgian consumers' perception and intention to eat fish. Risk Analysis. 2008;28(4):951–67. doi: 10.1111/j.1539-6924.2008.01075.x. [DOI] [PubMed] [Google Scholar]