Abstract
Objective
To assess the correlation between different pain symptoms and different domains of women's health-related quality of life (HRQoL).
Methods
Seventy-seven women with deep infiltrating endometriosis were successively enrolled between June 2011 and August 2013 while being prepared to undergo laparoscopy due to pain and/or infertility. We quantified the intensities of dysmenorrhea, deep dyspareunia, chronic pelvic pain, and dyschezia (menstrual and non-menstrual) using a 11-point visual analog scale (VAS: 0–10) and the validated full versions of the Short Form 36 (SF36) and Endometriosis Health Profile (EHP30) questionnaires to assess HRQoL. The pain symptoms were considered simultaneously in a hierarchical agglomerative clustering method (exploratory multivariate approach) and the associations among scores were tested by bivariate correlation.
Results
Dysmenorrhea showed the lowest similarity on to the multivariate cluster analysis and no statistically significant correlation with the other pain symptoms: deep dyspareunia (P=0.244), chronic pelvic pain (P=0.108), menstrual dyschezia (P=0.238), and non-menstrual dyschezia (P=0.380). Dysmenorrhea and chronic pelvic pain were the main symptoms correlated with all domains of the SF36 and the EHP30 (core instrument) questionnaires (P<0.05).
Conclusion
Dysmenorrhea and chronic pelvic pain were independent factors associated with HRQoL.
Keywords: Dysmenorrhea, Dyspareunia, Dyschezia, Pelvic pain, Woman's health
Introduction
Evaluations of the actual impact of pelvic pain on women's mental health and health-related quality of life (HRQoL) have not yet provided simple and conclusive results [1]. Differences among results may be caused by individual variations in the subjective perception of pain, the nature and intensity of the pain, and associations among different pain symptoms [2]. Somatization, depression, sensitivity, and anxiety levels were higher in endometriosis patients than in controls, whereas chronic pelvic pain was only recently termed a functional somatic syndrome [3,4].
Deep infiltrating endometriosis (DIE) infiltrates multiple pelvic organs to levels deeper than 5 mm [5]. It is considered the most severe form of endometriosis and poses the most difficult therapeutic problems since the most effective measures must be identified to resolve the specific issues that are most important to patients [6]. In these cases, although effective medical therapies for controlling DIE exist, radical surgical excision of the lesions is usually necessary to improve patient symptoms and quality of life [7,8].
In general, the two pain symptoms most frequently associated with endometriosis are dysmenorrhea and deep dyspareunia, which may occur independently [9]. Patients with endometriosis report experiencing more pain during intercourse than control patients; the stimulation of pain fibers due to fibrotic traction and pressure from endometriotic nodules filled with fibrotic tissue may play a role in this pathogenesis [10,11]. The considerable variability in patient descriptions of pain suggests that several different mechanisms are involved in endometriosis-related pain [12]. Although other conditions may impair sexual function, deep dyspareunia affects it severely, and findings of long-term follow-ups suggest that the surgical management of DIE in symptomatic patients can improve their sex lives [13]. Therefore, believing that endometriosis-related pain negatively affects HRQoL across countries and ethnicities, women have frequently undergone medical and surgical treatments for endometriosis that focus on mitigating the principal pain symptoms [14].
Quantitative approaches should ideally help physicians and patients discuss the potential benefits, risks, and costs of each treatment alternative [6]. Different tools have been used to assess and quantify HRQoL in women with endometriosis [15]. In the present study, we assessed the influence of different pain symptoms on different domains of women's quality of life using the validated full versions of both the Short Form 36 (SF36) and the Endometriosis Health Profile (EHP30) questionnaires in a sample of women with DIE.
Materials and methods
With the focus on surgical patient care, this cross-sectional pre-planned observational study (Canadian Task Force Classification II-2) preoperatively enrolled 77 consecutive Brazilian women with DIE who were admitted to the Crispi Institute for Minimally Invasive Surgery (Rio de Janeiro, RJ, Brazil) for surgical treatment. The consecutive cases were documented successively from June 2011 to August 2013 as the patients were being prepared to undergo laparoscopy for DIE due to pain and/or infertility.
We included only patients with DIE during menacme who were capable of completing the full SF36 and EHP30 HRQoL questionnaires (validated Brazilian versions). According to our service routine, the preoperative DIE diagnosis was based on three steps: medical history; physical examination; and magnetic resonance imaging (MRI), which has been a powerful and highly accurate tool for predicting the diagnosis of multiple sites of DIE [16]. This series included no women in whom the assessed pain symptoms were attributed to conditions other than endometriosis since the gold-standard diagnostic method (visual inspection by laparoscopy with histological confirmation) subsequently confirmed DIE in all patients. Endometriosis was considered histologically confirmed when endometrial glands and stroma were present upon microscopic examination.
As part of the basic medical history for evaluating women with endometriosis, we quantified the intensities of dysmenorrhea, deep dyspareunia, chronic pelvic pain, and menstrual and non-menstrual dyschezia using a 11-point visual analog scale (VAS: 0-10) according to Gerlinger et al. [17]. On this scale, a score of 0 represented no pain, whereas 10 represented the worst pain ever felt. This is the scale used most often to assess endometriosis-related pain [18]. Although several patients in the study may already have received some treatment for endometriosis (including ongoing hormonal treatment), this variable was not assessed.
Concerning HRQoL, the full validated Brazilian versions of two self-reported HRQoL questionnaires were applied: the SF36 (more general) and the EHP30 (focusing on endometriosis-related complaints). The SF36 and EHP30 questionnaires have been the most often used and most specific tools for assessing HRQoL in women with endometriosis [19,20].
Objectively, the SF36 includes 8 different domains that are quantified by a different number of specific questions: Vitality (VIT; 5 questions), Physical Functioning (PF; 10 questions), Bodily Pain (BPain; 2 questions), General Health Perception (GHP; 5 questions), Physical-Role Functioning (PRF; 4 questions), Emotional-Role Functioning (ERF; 3 questions), Social-Role Functioning (SRF; 2 questions), and Mental Health (MH; 5 questions) [19].
The EHP30 questionnaire is a user-friendly self-reporting tool suitable for use in endometriosis-related clinical research. It consists of a core instrument that indicates the extent of self-reported ill health in each measured HRQoL domain. The core instrument is available as a long form that has 5 scales of scores covering the domains: Pain (11 questions), Control and Powerlessness (CP; 6 questions), Social Support (SS; 4 questions), Emotional Well-Being (EWB; 6 questions), and Self-Image (SI; 3 questions). In addition to the core instrument, 6 optional supplementary modules (a total of 23 extra questions) cover areas of health status that may not affect every endometriosis sufferer but may be particularly relevant. Their domains (not applied for all women) include Work (5 questions), Relationship with Child/Children (2 questions), Sexual Relationship (SR; 5 questions), Feelings About Medical Profession (FAMP; 4 questions), Feelings About Treatment (FAT; 3 questions), and Feelings About Infertility (FAI; 4 questions) [20].
Both questionnaires were originally standardized on a scale of 0–100. An SF36 scale score of 100 indicates the best health status, while 0 indicates the worst health status. Inversely, an EHP30 score of 0 indicates the best health status, while 100 indicates the worst health status.
1. Statistics
The pain scores were preliminarily considered in a hierarchical agglomerative clustering method using the average linkage between the groups to gather variables into homogeneous and distinct clusters (rather than observations) based on the squared Euclidean distance. Since the measures of distance depend on the units in which variables are measured and influenced by the variable that takes numerically higher values, the variables were standardized as z-scores and the result was summarized in a dendrogram. This combined exploratory multivariate strategy has been used to check for trends and confounding factors by considering multiple variables simultaneously [21]. The correlations among the scores were tested by bivariate non-parametric (Spearman) analysis.
The database was managed using Microsoft Office Access® (version 2010; Microsoft Corp., Redmond, WA, USA). The charts and statistics were developed using IBM® SPSS® Statistics Standard Grad Pack 20 (IBM Corp., Armonk, NY, USA). The statistical results were considered significant when P<0.05 (2-sided).
2. Ethical approval
This study was previously approved by the Research Ethics Committee (CEP IFF-FIOCRUZ: 0035.0.008.000-11), an entity of the National Research Ethics Commission of the Brazilian Ministry of Health, in accordance with the Guidelines and Regulatory Standards for Research Involving Human Beings (CNS196/96). All patients gave informed consent prior to joining the study.
Results
1. Women with deep infiltrating endometriosis
The characteristics of the DIE sample including obstetrical data are summarized in Table 1. Postoperatively, DIE was histologically confirmed in all cases through microscopic examination (as expected). A median of 5 different sites (min, 1; max, 15) were affected by endometriosis. In summary, 49.2% (95% confidence interval [CI], 37.3–60.9) of women had endometriosis in the ovary, 50.8% (95% CI, 38.2–62.5) in the anterior compartment, and 92.3% (95% CI, 84.9–98.4) in the posterior compartment.
Table 1. Patient characteristics including obstetrical history (n=77).
| Characteristics | Min | P10 | Median | P90 | Max | No. (%) | 95% CI |
|---|---|---|---|---|---|---|---|
| Age (yr) | 21 | 29 | 35 | 43 | 46 | ||
| Gesta (No.) | 0 | 0 | 0 | 1 | 2 | ||
| Vaginal delivery (No.) | 0 | 0 | 0 | 0 | 1 | ||
| C-section (No.) | 0 | 0 | 0 | 1 | 2 | ||
| Abortion (No.) | 0 | 0 | 0 | 1 | 2 | ||
| Nulliparous | 48 (62.3) | 52.2–76.1 | |||||
| Caucasiana) | 52 (67.5) | 56.3–78.8 | |||||
| Stable relationship with a male sexual partner | 65 (84.4) | 76.1–93.0 | |||||
| Never smoked | 59 (77.6) | 67.6–87.3 | |||||
| College graduates | 53 (68.8) | 57.7–80.3 |
All women had completed high school.
P10 and P90, 10th and 90th percentiles.
a)The other 25 women were mixed (African descent).
Regarding asymptomatic women (VAS=0), approximately 26.8% of women reported no dysmenorrhea (95% CI, 16.9–36.6); 19.7%, no dyspareunia (95% CI, 9.9–29.6); 35.2%, no chronic pelvic pain (95% CI, 24.0–46.5); 46.5%, no menstrual dyschezia (95% CI, 35.2–57.7); and 60.0%, no non-menstrual dyschezia (95% CI, 47.2–71.4). On the other hand, considering the prevalence of unbearable pain (VAS ≥ 9), approximately 16.9% (95% CI, 8.5–26.8) reported experiencing unbearable cramps during menstruation; 38.0% (CI 95%: 26.8–49.3), unbearable pain during intercourse; 14.1% (95% CI, 7.0–22.5), unbearable chronic pelvic pain; 9.9% (95% CI, 4.2–16.9), unbearable menstrual dyschezia; and 4.3% (95% CI, 0–8.6), unbearable non-menstrual dyschezia. About 7.0% of the women were completely asymptomatic, that is, showed VAS=0 on all assessed pain scales (95% CI, 1.4–14.1).
2. Interrelationships among endometriosis-related pain symptoms
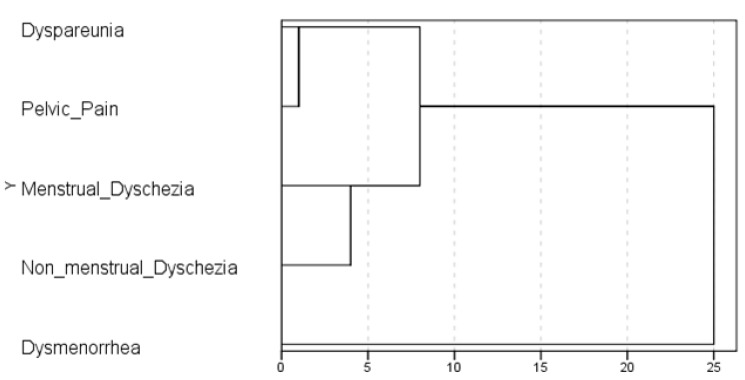
When the intensity of the 5 main endometriosis-related symptoms (dysmenorrhea, deep dyspareunia, chronic pelvic pain, and menstrual and non-menstrual dyschezia) were combined in a multivariate analysis capable of identifying groups of correlated variables, dysmenorrhea showed the lowest similarity, that is, the most independent behavior. On the other hand, chronic pelvic pain and dyspareunia were the most similar. After considering all pain scores simultaneously, this exploratory approach suggests a quite complex relationship among them. A visual representation of the distance at which clusters are combined (dendrogram) is shown in Fig. 1, and it suggests that dysmenorrhea is an independent symptom.
Fig. 1. Dendrogram of hierarchical agglomerative clustering analysis using average linkage to group variables based on squared Euclidean distance (n=77). The ratio of the rescaled distances (z-score) is the same as the ratio of the original distances.
The same understanding was reached by bivariate analysis. In the assessment of the correlation among the main endometriosis-related pain symptoms, dysmenorrhea showed no statistically significant correlation with the other symptoms (Table 2).
Table 2. Correlation among different endometriosis-related pain symptoms of 77 women with histologically confirmed deep endometriosis.
| Symptoms | Dyspareunia | Chronic pelvic pain | M-dyschezia | NM-dyschezia |
|---|---|---|---|---|
| Dysmenorrhea | 0.141 (0.244) | 0.194 (0.108) | 0.143 (0.238) | 0.107 (0.380) |
| Dyspareunia | 0.441 (<0.001) | 0.375 (0.001) | 0.336 (0.004) | |
| Chronic pelvic pain | 0.388 (0.001) | 0.472 (<0.001) | ||
| M-dyschezia | 0.488 (<0.001) |
Chronic pelvic pain defined as recurrent or constant pain in the lower abdominal region that has lasted for at least 6 months [22]. M: menstrual. Pain was quantified through a visual analogue scale (0–10). Non-parametric bivariate Spearman correlations expressed by coefficient of correlation and P values (between parentheses). Significant correlations (P<0.05) in bold.
NM, non-menstrual.
3. Assessment of the health-related quality of life
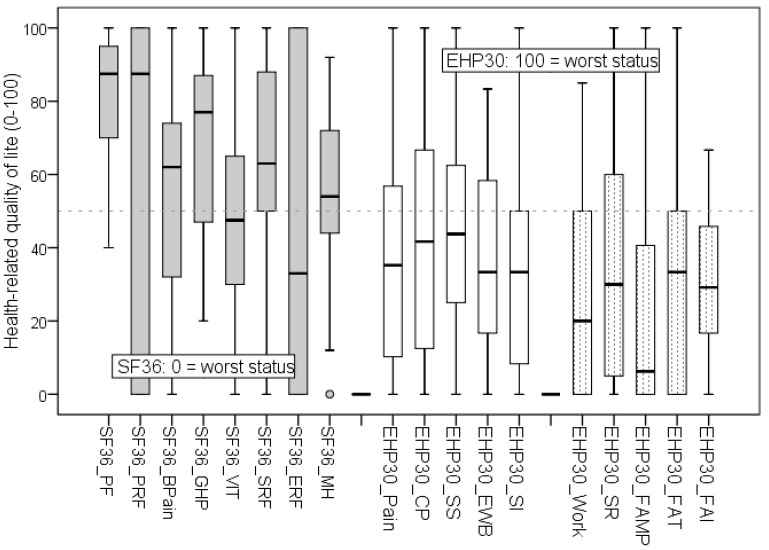
An overview of the HRQoL domains of the SF36 and EHP30 full questionnaires is shown in Fig. 2. The EHP30 optional supplementary module Relationship with Child/Children was not included in the study because it showed several missing values, mainly due to the prevalence of nulliparous status in the sample (62.2%; 95% CI, 52.2–76.1).
Fig. 2. Boxplot showing scores (quartiles) of the different health-related quality of life scales: Short Form 36 (SF36; 0 represents the worst health status) and Endometriosis Health Profile (EHP30; 0 represents the best health status).
SF36 domains: Physical Functioning (PF), Physical-Role Functioning (PRF), Bodily-Pain (BPain), General Health Perception (GHP), Vitality (VIT), Social-Role Functioning (SRF), Emotional-Role Functioning (ERF), and Mental Health (MH). EHP30 core instrument: Pain, Control and Powerlessness (CP), Social Support (SS), Emotional Well-Being (EWB), and Self-Image (SI). EHP30 supplementary optional modules: Work, Sexual Relationship (SR), Feelings About Medical Profession (FAMP), Feelings About Treatment (FAT), and Feelings About Infertility (FAI). EHP-30 Relationship with Child/Children domain was not assessed due to the major prevalence of nulliparous women (62.2%; 95% confidence interval, 52.2–76.1).
Dysmenorrhea and chronic pelvic pain were the main symptoms that correlated with all domains of the SF36 and EHP30 (core instrument) questionnaires. All symptoms (not only the significant ones) showed a negative correlation coefficient with the SF36 scores and a positive correlation with the EHP30 scores, which led to the same biological interpretation: the more intense the pain, the worse the quality of life (Table 3).
Table 3. Bivariate Spearman correlation coefficients of different pain scores and domains of health-related quality of life in women with histologically confirmed deep endometriosis (P values shown in parentheses; n=77).
| Domain | Age | Dysmenorrhea | Dyspareunia | Chronic pelvic pain | M-Dyschezia | NM-Dyschezia | |
|---|---|---|---|---|---|---|---|
| SF36 | |||||||
| PF | 0.076 (0.579) | −0.473 (<0.001) | −0.216 (0.110) | −0.292 (0.029) | −0.316 (0.018) | −0.388 (0.003) | |
| PRF | −0.037 (0.789) | −0.463 (<0.001) | −0.204 (0.132) | −0.324 (0.015) | −0.460 (<0.001) | −0.272 (0.043) | |
| BPain | 0.131 (0.334) | −0.324 (0.015) | −0.290 (0.030) | −0.541 (<0.001) | −0.395 (0.003) | −0.381 (0.004) | |
| GHP | −0.046 (0.736) | −0.348 (0.009) | −0.202 (0.135) | −0.329 (0.013) | −0.161 (0.237) | −0.255 (0.058) | |
| VIT | 0.264 (0.049) | −0.544 (<0.001) | −0.169 (0.213) | −0.447 (0.001) | −0.374 (0.005) | −0.364 (0.006) | |
| SRF | 0.034 (0.803) | −0.470 (<0.001) | −0.093 (0.497) | −0.498 (<0.001) | −0.246 (0.068) | −0.194 (0.153) | |
| ERF | 0.026 (0.847) | −0.523 (<0.001) | −0.187 (0.168) | −0.286 (0.033) | −0.324 (0.015) | −0.210 (0.120) | |
| MH | 0.049 (0.721) | −0.495 (<0.001) | −0.112 (0.411) | −0.482 (<0.001) | −0.261 (0.052) | −0.215 (0.112) | |
| EHP30 | |||||||
| Pain | −0.237 (0.078) | 0.301 (0.024) | 0.332 (0.012) | 0.375 (0.004) | 0.187 (0.167) | 0.056 (0.681) | |
| CP | −0.217 (0.108) | 0.440 (0.001) | 0.385 (0.003) | 0.523 (<0.001) | 0.231 (0.087) | 0.173 (0.202) | |
| SS | 0.006 (0.967) | 0.435 (0.001) | 0.186 (0.169) | 0.491 (<0.001) | 0.180 (0.185) | 0.264 (0.049) | |
| EWB | −0.061 (0.654) | 0.358 (0.007) | 0.117 (0.389) | 0.408 (0.002) | 0.130 (0.338) | 0.144 (0.289) | |
| SI | −0.169 (0.212) | 0.490 (<0.001) | 0.227 (0.093) | 0.457 (<0.001) | 0.132 (0.332) | 0.244 (0.070) | |
| EHP30 Suppl. | |||||||
| Work (n=51) | −0.112 (0.445) | 0.327 (0.019) | 0.391 (0.005) | 0.348 (0.012) | 0.330 (0.018) | 0.359 (0.010) | |
| SR (n=56) | 0.080 (0.569) | 0.518 (<0.001) | 0.260 (0.053) | 0.472 (<0.001) | 0.244 (0.071) | 0.297 (0.026) | |
| FAMP (n=56) | 0.066 (0.641) | 0.267 (0.046) | 0.022 (0.872) | 0.293 (0.028) | 0.119 (0.382) | 0.122 (0.371) | |
| FAT (n=43) | 0.091 (0.563) | 0.080 (0.596) | 0.229 (0.126) | 0.157 (0.297) | 0.198 (0.187) | 0.311 (0.036) | |
| FAI (n=49) | −0.452 (0.002) | 0.018 (0.904) | 0.046 (0.751) | 0.269 (0.062) | 0.258 (0.074) | 0.237 (0.102) | |
Chronic pelvic pain defined as recurrent or constant pain in the lower abdominal region that has lasted for at least 6 months [22]. Pain was quantified through visual analogue scale (0–10). Non-parametric bivariate Spearman correlations expressed by coefficient of correlation and P values (between parentheses). Significant correlations (P<0.05) in bold. SF36 domains: Physical Functioning (PF), Physical-Role Functioning (PRF), Bodily Pain (BPain), General Health Perception (GHP), Vitality (VIT), Social-Role Functioning (SRF), Emotional-Role Functioning (ERF), and Mental Health (MH). EHP30 domains: Pain, Control and Powerlessness (CP), Social Support (SS), Emotional Well-Being (EWB), and Self-Image (SI). EHP30 supplementary optional modules: Work, Sexual Relationship (SR), Feelings About Medical Profession (FAMP), Feelings About Treatment (FAT), and Feelings About Infertility (FAI). EHP30 supplementary optional modules: Work, Sexual Relationship (SR), Feelings About Medical Profession (FAMP), Feelings About Treatment (FAT), and Feelings About Infertility (FAI). EHP-30 Relationship with Child/Children domain was not assessed due to the major prevalence of nulliparous women (62.2%; 95% CI, 52.2–76.1).
SF36, Short Form 36 (100 is the best and 0 is the worst health status); EHP30, Endometriosis Health Profile (0 is the best and 100 is the worst health status).
Although the SF36 and EHP30 instruments have a specific domain that focuses on pain symptoms (Bodily Pain and Pain, respectively), only the EHP30 questionnaire includes specific questions about dyspareunia. However, despite their different approaches, there was a significant negative (although moderate) correlation between the SF36 Bodily Pain and EHP30 Pain scores (ρ=−0.310; P=0.013), which supports the notion that both questionnaires evaluate pain, although differently.
Discussion
This cross-sectional pre-planned observational interdisciplinary study assessed the associations between the main endometriosis-related pain symptoms and HRQoL impairment. Although recognizing the existence of variables other than pain, we identified dysmenorrhea and chronic pelvic pain as the most important pain symptoms affecting the women's quality of life. Furthermore, considering the simultaneous presence of different pain symptoms in women with DIE, our results suggest that dysmenorrhea occurs independently.
The presence of dyspareunia has been an independent and significant negative predictor of sexual function, and women with dyspareunia have lower coital frequency, desire, and arousal. Women with dyspareunia experience orgasm less often, which has been correlated with a decrease in overall well-being [23]. However, despite this, dyspareunia was only one of many determinants of sexual function in women with endometriosis [24]. Indeed, concerning the correlations between deep dyspareunia and the HRQoL scores assessed in the present study, the conclusions were quite similar in both questionnaires: Deep dyspareunia was the less important symptom, even when the focus was placed on the sexual relationship, about which there are specific questions in the EHP30 (Table 3).
Different investigators have emphasized that, although complete excision of the endometriosis seems to offer significant improvement in sexual function, quality of life, and pelvic pain in symptomatic patients with DIE, surgery may be associated with complications and adverse new-onset symptoms and should be performed only after thorough consultation with the patient [25]. The current options for treatment may themselves have undesirable consequences, such as major urinary and bowel complications after surgery [26,27]. The present findings can help physicians discuss the possible interactions among the main endometriosis-associated symptoms and the differences in their impacts on HRQoL. Physicians and researchers must consider the differences between physicians' and patients' perceptions of endometriosis pain and that deep symptoms (i.e., dyspareunia) might be underreported by patients due to social and cultural constraints [12]. Moreover, one must be very cautious when interpreting/comparing HRQoL median (or mean) scores because endometriosis is an individual condition, as are treatment consequences.
Regarding limitations, this study may show a mathematical point of concern that is the nature of self-rating scores (particularly at one or both extremes of the scales); that is, whether it is a ratio or ordinal and is linear or nonlinear. This discussion (although not usual during medical meetings) has been reported by researchers with different points of view [28]. Therefore, in the present study, we used both ordinal (non-parametric correlation) and ratio (cluster analysis) methods to assess the variables to avoid this issue. Moreover, we did not develop predictive models; rather, we merely demonstrated different exploratory ways to confirm and compare the negative effects of pain on HRQoL, which pointed in the same direction regardless of the statistical approach.
Regarding the external validity of this study, we admit the possibility of some selection bias due to the characteristics of the sample, which includes specific racial aspects (75% white), prevalence of smoking habit (<16%), level of education (75% college graduates), and level of information about the disease (access to a specialized health unit with a multidisciplinary team of surgeons). Nevertheless, the study has strong positive points that must be considered since certain sources of measurement bias were minimized, such as: the sample included only accurately diagnosed cases of DIE; the HRQoL assessments were made through 2 different major questionnaires (SF36 and EHP30); and the data were collected before the patients had undergone surgery, that is, when the symptoms existed but the consequences (good or bad) of the treatment did not to avoid memory bias.
Some investigators have emphasized that the medical treatment of endometriosis with pain may be insufficient and psychological intervention may be required since depressive symptoms were independent and significant negative predictors of quality of life in patients with endometriosis [23]. Moreover, improving global sexual function, not just reducing pain during intercourse, should be considered a major clinical goal of endometriosis treatment [29].
Several studies have discussed the benefits of both medical and surgical treatments based on HRQoL questionnaire scores [30,31]. The surgical excision of lesions is feasible and significantly improves the main symptoms and HRQoL, an effect that may persist for >4 years [2,32]. Besides, pain symptoms have a significant impact on the physical and mental components of quality of life [33]. However, studies to date have not considered the very different domains of HRQoL assessed simultaneously by different questionnaires when evaluating women with endometriosis.
Our findings support the concept that the global sexual impact of endometriosis requires further investigation that focuses not only on pain during intercourse but also on psychological and relational dimensions, including the partner's sexual function [34].
This study involved different specialists with the purpose of gaining a better understanding of the disease by strongly focusing on how different kinds of endometriosis-related pain may affect quality of life. This focus accords with those of recent recommendations about Research Priorities in Endometriosis, which have emphasized that the main goal should be to treat endometriosis-related pain and improve the HRQoL [35].
Acknowledgements
This study was supported by the Brazilian Federal Government Programs PIBIC/PIBITI/PIP of the Fundação Instituto Oswaldo Cruz (Fiocruz) of the Ministry of Health and the National Council for Scientific and Technological Development – (CNPq) of the Ministry of Science and Technology (PIP IFF-008-FIO-13-3-4). The authors are grateful to Roberta Pacheco da Luz Fonseca (nurse and patient) and Dr. Claudio Moura Andrade Jr. (surgeon experienced in the field of endometriosis) for contributing to the discussion and reviewing the manuscript.
Footnotes
Conflict of interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Eriksen HL, Gunnersen KF, Sørensen JA, Munk T, Nielsen T, Knudsen UB. Psychological aspects of endometriosis: differences between patients with or without pain on four psychological variables. Eur J Obstet Gynecol Reprod Biol. 2008;139:100–105. doi: 10.1016/j.ejogrb.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Facchin F, Barbara G, Saita E, Mosconi P, Roberto A, Fedele L, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol. 2015;36:135–141. doi: 10.3109/0167482X.2015.1074173. [DOI] [PubMed] [Google Scholar]
- 3.Laganà AS, Condemi I, Retto G, Muscatello MR, Bruno A, Zoccali RA, et al. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur J Obstet Gynecol Reprod Biol. 2015;194:30–33. doi: 10.1016/j.ejogrb.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 4.Warren JW, Clauw DJ, Wesselmann U, Howard FM, Gallicchio L, Morozov V. Functional somatic syndromes as risk factors for hysterectomy in early bladder pain syndrome/interstitial cystitis. J Psychosom Res. 2014;77:363–367. doi: 10.1016/j.jpsychores.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Cornillie FJ, Oosterlynck D, Lauweryns JM, Koninckx PR. Deeply infiltrating pelvic endometriosis: histology and clinical significance. Fertil Steril. 1990;53:978–983. doi: 10.1016/s0015-0282(16)53570-5. [DOI] [PubMed] [Google Scholar]
- 6.Vercellini P. Introduction: Management of endometriosis: moving toward a problem-oriented and patient-centered approach. Fertil Steril. 2015;104:761–763. doi: 10.1016/j.fertnstert.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Ferrero S, Alessandri F, Racca A, Leone Roberti Maggiore U. Treatment of pain associated with deep endometriosis: alternatives and evidence. Fertil Steril. 2015;104:771–792. doi: 10.1016/j.fertnstert.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 8.Porto BT, Ribeiro HS, Galvão MA, Sekula VG, Aldrigui JM, Ribeiro PA. Histological classification and quality of life in women with endometriosis. Rev Bras Ginecol Obstet. 2015;37:87–93. doi: 10.1590/SO100-720320140004650. [DOI] [PubMed] [Google Scholar]
- 9.de Freitas Fonseca M, Sessa FV, de Carvalho Aragao L, de Resende JA. Junior, Crispi CP. The association between dyspareunia and dysmenorrhea in women with deep endometriosis: a pre-planned observational study. Ann Public Health Res. 2015;2:1018. [Google Scholar]
- 10.Evangelista A, Dantas T, Zendron C, Soares T, Vaz G, Oliveira MA. Sexual function in patients with deep infiltrating endometriosis. J Sex Med. 2014;11:140–145. doi: 10.1111/jsm.12349. [DOI] [PubMed] [Google Scholar]
- 11.Fauconnier A, Chapron C, Dubuisson JB, Vieira M, Dousset B, Bréart G. Relation between pain symptoms and the anatomic location of deep infiltrating endometriosis. Fertil Steril. 2002;78:719–726. doi: 10.1016/s0015-0282(02)03331-9. [DOI] [PubMed] [Google Scholar]
- 12.Fauconnier A, Staraci S, Huchon C, Roman H, Panel P, Descamps P. Comparison of patient- and physician-based descriptions of symptoms of endometriosis: a qualitative study. Hum Reprod. 2013;28:2686–2694. doi: 10.1093/humrep/det310. [DOI] [PubMed] [Google Scholar]
- 13.Lukic A, Di Properzio M, De Carlo S, Nobili F, Schimberni M, Bianchi P, et al. Quality of sex life in endometriosis patients with deep dyspareunia before and after laparoscopic treatment. Arch Gynecol Obstet. 2016;293:583–590. doi: 10.1007/s00404-015-3832-9. [DOI] [PubMed] [Google Scholar]
- 14.Nnoaham KE, Hummelshoj L, Webster P, d'Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366–373.e8. doi: 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minson FP, Abrão MS, Sardá Júnior J, Kraychete DC, Podgaec S, Assis FD. Importance of quality of life assessment in patients with endometriosis. Rev Bras Ginecol Obstet. 2012;34:11–15. [PubMed] [Google Scholar]
- 16.Medeiros LR, Rosa MI, Silva BR, Reis ME, Simon CS, Dondossola ER, et al. Accuracy of magnetic resonance in deeply infiltrating endometriosis: a systematic review and meta-analysis. Arch Gynecol Obstet. 2015;291:611–621. doi: 10.1007/s00404-014-3470-7. [DOI] [PubMed] [Google Scholar]
- 17.Gerlinger C, Schumacher U, Faustmann T, Colligs A, Schmitz H, Seitz C. Defining a minimal clinically important difference for endometriosis-associated pelvic pain measured on a visual analog scale: analyses of two placebo-controlled, randomized trials. Health Qual Life Outcomes. 2010;8:138. doi: 10.1186/1477-7525-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourdel N, Alves J, Pickering G, Ramilo I, Roman H, Canis M. Systematic review of endometriosis pain assessment: how to choose a scale? Hum Reprod Update. 2015;21:136–152. doi: 10.1093/humupd/dmu046. [DOI] [PubMed] [Google Scholar]
- 19.Stull DE, Wasiak R, Kreif N, Raluy M, Colligs A, Seitz C, et al. Validation of the SF-36 in patients with endometriosis. Qual Life Res. 2014;23:103–117. doi: 10.1007/s11136-013-0442-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mengarda CV, Passos EP, Picon P, Costa AF, Picon PD. Validation of Brazilian Portuguese version of quality of life questionnaire for women with endometriosis (Endometriosis Health Profile Questionnaire--EHP-30) Rev Bras Ginecol Obstet. 2008;30:384–392. doi: 10.1590/s0100-72032008000800003. [DOI] [PubMed] [Google Scholar]
- 21.Fonseca MF, De Souza Hacon S, Grandjean P, Choi AL, Bastos WR. Iron status as a covariate in methylmercury-associated neurotoxicity risk. Chemosphere. 2014;100:89–96. doi: 10.1016/j.chemosphere.2013.12.053. [DOI] [PubMed] [Google Scholar]
- 22.Campbell F, Collett BJ. Chronic pelvic pain. Br J Anaesth. 1994;73:571–573. doi: 10.1093/bja/73.5.571. [DOI] [PubMed] [Google Scholar]
- 23.De Graaff AA, Van Lankveld J, Smits LJ, Van Beek JJ, Dunselman GA. Dyspareunia and depressive symptoms are associated with impaired sexual functioning in women with endometriosis, whereas sexual functioning in their male partners is not affected. Hum Reprod. 2016;31:2577–2586. doi: 10.1093/humrep/dew215. [DOI] [PubMed] [Google Scholar]
- 24.Vercellini P, Somigliana E, Buggio L, Barbara G, Frattaruolo MP, Fedele L. “I can't get no satisfaction”: deep dyspareunia and sexual functioning in women with rectovaginal endometriosis. Fertil Steril. 2012;98:1503–1511.e1. doi: 10.1016/j.fertnstert.2012.07.1129. [DOI] [PubMed] [Google Scholar]
- 25.Setälä M, Härkki P, Matomäki J, Mäkinen J, Kössi J. Sexual functioning, quality of life and pelvic pain 12 months after endometriosis surgery including vaginal resection. Acta Obstet Gynecol Scand. 2012;91:692–698. doi: 10.1111/j.1600-0412.2012.01394.x. [DOI] [PubMed] [Google Scholar]
- 26.de Resende JA, Júnior, Cavalini LT, Crispi CP, de Freitas Fonseca M. Risk of urinary retention after nerve-sparing surgery for deep infiltrating endometriosis: a systematic review and meta-analysis. Neurourol Urodyn. 2017;36:57–61. doi: 10.1002/nau.22915. [DOI] [PubMed] [Google Scholar]
- 27.Oliveira MA, Pereira TR, Gilbert A, Tulandi T, de Oliveira HC, De Wilde RL. Bowel complications in endometriosis surgery. Best Pract Res Clin Obstet Gynaecol. 2016;35:51–62. doi: 10.1016/j.bpobgyn.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 28.Myles PS, Troedel S, Boquest M, Reeves M. The pain visual analog scale: is it linear or nonlinear? Anesth Analg. 1999;89:1517–1520. doi: 10.1097/00000539-199912000-00038. [DOI] [PubMed] [Google Scholar]
- 29.Barbara G, Facchin F, Meschia M, Berlanda N, Frattaruolo MP, Vercellin IP. When love hurts. A systematic review on the effects of surgical and pharmacological treatments for endometriosis on female sexual functioning. Acta Obstet Gynecol Scand. 2017;96:668–687. doi: 10.1111/aogs.13031. [DOI] [PubMed] [Google Scholar]
- 30.Dubernard G, Rouzier R, David-Montefiore E, Bazot M, Darai E. Use of the SF-36 questionnaire to predict quality-of-life improvement after laparoscopic colorectal resection for endometriosis. Hum Reprod. 2008;23:846–851. doi: 10.1093/humrep/den026. [DOI] [PubMed] [Google Scholar]
- 31.Vercellini P, Frattaruolo MP, Somigliana E, Jones GL, Consonni D, Alberico D, et al. Surgical versus low-dose progestin treatment for endometriosis-associated severe deep dyspareunia II: effect on sexual functioning, psychological status and health-related quality of life. Hum Reprod. 2013;28:1221–1230. doi: 10.1093/humrep/det041. [DOI] [PubMed] [Google Scholar]
- 32.Touboul C, Ballester M, Dubernard G, Zilberman S, Thomin A, Daraï E. Long-term symptoms, quality of life, and fertility after colorectal resection for endometriosis: extended analysis of a randomized controlled trial comparing laparoscopically assisted to open surgery. Surg Endosc. 2015;29:1879–1887. doi: 10.1007/s00464-014-3880-4. [DOI] [PubMed] [Google Scholar]
- 33.De Graaff AA, D'Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod. 2013;28:2677–2685. doi: 10.1093/humrep/det284. [DOI] [PubMed] [Google Scholar]
- 34.Barbara G, Facchin F, Buggio L, Somigliana E, Berlanda N, Kustermann A, et al. What is known and unknown about the association between endometriosis and sexual functioning: a systematic review of the literature. Reprod Sci. 2017;24:1566–1576. doi: 10.1177/1933719117707054. [DOI] [PubMed] [Google Scholar]
- 35.Rogers PA, Adamson GD, Al-Jefout M, Becker CM, D'Hooghe TM, Dunselman GA, et al. Research priorities for endometriosis. Reprod Sci. 2017;24:202–226. doi: 10.1177/1933719116654991. [DOI] [PMC free article] [PubMed] [Google Scholar]