Abstract
Based on the current understanding of a preventive effect of bilateral salpingectomy on ovarian/fallopian/peritoneal cancers, the Korean Society of Obstetrics and Gynecology, Korean Society of Gynecologic Endocrinology, Korean Society of Gynecologic Oncology, Korean Society of Maternal Fetal Medicine, and Korean Society for Reproductive Medicine support the following recommendations:
• Women scheduled for hysterectomy for benign gynecologic disease should be informed that bilateral salpingectomy reduces the risk of ovarian/fallopian/peritoneal cancer, and they should be counseled regarding this procedure at the time of hysterectomy.
• Although salpingectomy is generally considered as a safe procedure in terms of preserving ovarian reserve, there is a lack of evidences representing its long-term outcomes. Therefore, patients should be informed about the minimal potential of this procedure for decreasing ovarian reserve.
• Prophylactic salpingectomy during vaginal hysterectomy is favorable in terms of prevention of ovarian/fallopian/peritoneal cancer, although operation-related complications minimally increase with this procedure, compared to the complications associated with vaginal hysterectomy alone. Conversion to open or laparoscopic approach from vaginal approach to perform prophylactic salpingectomy is not recommended.
• Women who desire permanent sterilization at the time of cesarean delivery could be counseled for prophylactic salpingectomy before surgery on an individual basis.
Keywords: Fallopian tubes, Salpingectomy, Ovarian neoplasms, Hysterectomy, Prophylactic surgical procedures
Introduction
In Korea, ovarian cancer is the tenth most prevalent cancer and the eighth leading cause of cancer-related deaths in women [1]. In 2015, 9.6 new cases of ovarian cancer and 7.2 cancer-related deaths per 100,000 women were reported. Epithelial ovarian carcinoma (EOC), which accounts for more than 85% of all ovarian cancers, is more aggressive than non-EOC, and it is responsible for 90% of ovarian cancer-related deaths. The four most common subtypes of EOC are serous (80‒85%), clear cell (5%), endometrioid (10%), and mucinous (3%) ovarian neoplasm, as categorized by their traditional histomorphologic features [2,3]. A 2-tier grading system for serous ovarian carcinoma subdivided these tumors into low-grade serous carcinoma (LGSC, type I) and high-grade serous carcinoma (HGSC, type II) [4], and HGSC is 20 times more likely than LGSC to cause death [5]. As effective screening methods for early detection of EOC are still unknown, more than 70% of ovarian cancers are diagnosed in advanced stages, with poor survival outcomes [6].
The risk factors for ovarian cancer include age, menopausal status, parity, and family history. Germline mutation in BRCA1 or BRCA2 is associated with autosomal-dominant hereditary breast and ovarian cancer (HBOC) and hereditary non-polyposis colorectal cancer (HNPCC), and it carries an approximately 40% lifetime risk of ovarian cancer [7,8]. Germline mutation in BRCA1 or BRCA2 is found in 12‒15% of cases of EOC, and the carcinomas that develop in patients with these mutations are commonly high-grade serous in type [9,10,11]. The lifetime risks of EOC (mainly endometrioid and clear cell types) owing to mutations in MSH2, MLH1, and MSH6, which are known as mismatch repair genes associated with HNPCC, are approximately 10%, 8%, and 7%, respectively [12]. Although HBOC and HNPCC are the most well-known conditions that predispose to EOC, mutations in BRIP1, RAD51D, and RAD51C are associated with a 10‒15% lifetime risk of cancer [13,14,15].
To prevent EOC in women at a high risk for the disease, removal of the ovaries and fallopian tubes, known as risk-reducing salpingo-oophorectomy (RRSO), is commonly recommended. The recommended age range for performing RRSO in women with BRCA1 and BRCA2 germline mutations is 35–40 years and 40–45 years, respectively, as per the National Comprehensive Cancer Network (NCCN) guideline [16]. In 2016, the Korean Society of Obstetrics and Gynecology (KSGO) recommended that women with a BRCA germline mutation should be counseled about RRSO at 35–40 years of age on an individual basis [17]. Meanwhile, as RRSO results in surgical menopause, which can affect the cardiovascular system, osteoporotic health, and quality of life [18,19], prophylactic salpingectomy and delayed oophorectomy (PSDO) is now under clinical trials as an alternative of RRSO in premenopausal women at a high risk for ovarian cancer (NCT02321228, NCT01907789). Contrary to RRSO in high-risk women, the surgical strategy to prevent EOC in women at average risk* is controversial.
*Women at an average risk for EOC (those who meet all criteria)
Age 18 years and older
No known family history of ovarian, breast, or colon cancer
No known germline mutation in BRCA1, BRCA2, mismatch repair genes (MLH1, MSH2, MSH6, PMS2), EPCAM, BRIP1, RAD51C, and RAD51D, which increases the risk of ovarian/fallopian/peritoneal cancer
Carcinogenesis in the ovarian epithelium — a tubal paradigm
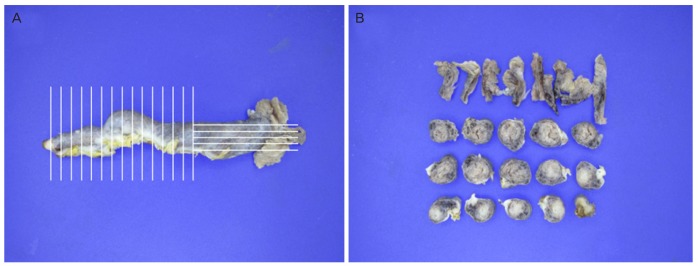
LGSC has been hypothesized to arise from a serous cystadenoma or adenofibroma, and is characterized by mutations in the KRAS, BRAF, or ERBB2 genes, in which approximately two-thirds of the tumors harbor a mutation [20,21,22]. Unlike LGSC, mutations in KRAS, BRAF, or ERBB2 rarely occur in HGSC. In contrast, TP53 mutation frequently occurs in more than 80% cases of HGSC [23,24]. TP53 mutations result in an increased nonfunctional p53; this is referred to as a “p53 signature,” which is commonly identified in HGSC [22]. The increasing RRSO in the high-risk women and improved pathologic assessments would have enabled pathologists to detect more p53 signatures derived from TP53 mutation adjacent to serous tubal intraepithelial carcinoma (STIC) lesions [25]. Sectioning and Extensively Examining the Fimbriated End (SEE-FIM) protocol involves a meticulous examination of the fallopian tubes of high-risk women who undergo RRSO; it entails amputation of each fimbria at the infundibulum, longitudinal sectioning of the fimbria, and extensive cross-sectioning of the remainder of the tube at 2–3-mm intervals [26] (Fig. 1). STIC is now generally accepted as the earliest morphologically recognizable form of HGSC.
Fig. 1. Sectioning and extensive examination the fimbriated end protocol. (A) A fallopian tube demonstrating longitudinal sectioning of the fimbria and extensive cross-sectioning of the remainder of the tube at 2–3-mm intervals and (B) preparing cross-sections of the fallopian tube.
STIC and invasive tubal carcinoma were detected more frequently in patients who had a genetic predisposition to EOC; however, they were also detected in non-mutation carriers with HGSC, with a prevalence of 48‒75% [27,28,29,30]. According to the results of a recently published Gynecologic Oncology Group-0199 trial, occult, invasive, or serous tubal intraepithelial lesions were identified in 25 out of 966 high-risk women who underwent RRSO: 4.6%, 3.5%, and 0.5% of occult lesions were detected in women who had BRCA1 germline mutation, BRCA2 germline mutation, and were high-risk non-carriers, respectively [31]. More than 90% of these precancerous lesions were localized to the fimbrial end in both women who had BRCA germline mutations and in those who did not have the mutations [26,28,29]. The average time from STIC to HGSC was estimated as 6.5 (range, 1.4–10.7) years, and the time between the initiation of the HGSC and the development of peritoneal carcinomatosis appears to be very short in women with metastatic STIC lesions [32].
Although increasing evidences for the pathological association between STIC and HGSC are still observed, it is noteworthy that all HGSCs do not have tubal precancerous lesions. The possibility of non-tubal origin and an alternative carcinogenetic process persists, and further studies on ovarian tissue factors including hormonal milieu or ovulation are warranted.
Preventive effect of salpingectomy on ovarian/fallopian/peritoneal cancers
It is known that not only sterilization but also tubal ligation confers a decreased risk of EOC. Several meta-analyses have demonstrated an approximately 26–34% decrease in the risk of EOC in women undergoing tubal ligation [33,34,35,36,37]. This effect is more pronounced in endometrioid and clear cell tumors than in serous tumors. It has been understood that the location of the ligation near the utero-tubal junction prevents the retrograde transport and ovarian seeding of endometriotic cells, which has been consistently linked to endometrioid and clear cell tumors. On the contrary, the risk of serous tumors is considerably reduced by excisional tubal sterilization rather than non-excisional tubal sterilization. Several nationwide studies have reported that salpingectomy decreases the risk of ovarian cancer by 42–77% [38,39,40]. Lessard-Anderson et al. [38] suggested that compared to other sterilization methods, excisional sterilization may more effectively reduce the risk of ovarian cancer. They compared the number of tubal sterilization cases between 194 women with serous ovarian/peritoneal cancer and matched them with 388 controls. The rate of excisional tubal sterilization was lower in the cancer group than in the controls (2.6% vs. 6.4%), and the adjusted risk of serous ovarian/peritoneal cancer was decreased by 64% after excisional tubal sterilization compared to the risk after non-excisional tubal sterilization or no sterilization (odds ratios [ORs], 0.36; 95% confidence interval [CI], 0.13–1.02). Similarly, Madsen et al. conducted a case-control study based on a nationwide registry (case group of ovarian cancer, n=13,241) [39]. They reported that tubal sterilization and bilateral salpingectomy reduce the risk of EOC by 13% and 42%, respectively (OR, 0.87; 95% CI, 0.78–0.98 and OR, 0.58; 95% CI, 0.36–0.95, respectively). Falconer et al. also revealed that salpingectomy for benign indications is associated with a reduced risk of ovarian cancer, in a national population-based cohort study [40]. Women who had previously undergone a salpingectomy (n=251,465) showed a significantly lower risk for ovarian cancer than that of women who had not undergone a salpingectomy (OR, 0.65; 95% CI, 0.52–0.81). In particular, bilateral salpingectomy was associated with a 50% decreased risk of ovarian cancer, compared to unilateral salpingectomy (OR, 0.35; 95% CI, 0.17–0.73 and OR, 0.71; 95% CI, 0.56–0.91, respectively). Although the retrospective studies cannot confirm the causal relationship for prevention of ovarian cancer, these nationwide population-based results are quite noteworthy. In 2016, Yoon et al. [41] reported the results of a meta-analysis based on these nationwide population-based studies. The risks of ovarian cancer were compared between 3,509 women who underwent bilateral salpingectomy and 5,655,702 women who did not undergo salpingectomy. Bilateral salpingectomy was revealed to reduce the risk of ovarian cancer by 49% (OR, 0.51; 95% CI, 0.35–0.75; I2=0%).
Other position statements on prophylactic salpingectomy and its current state
Several professional gynecologic boards have carefully stated a favorable position of prophylactic salpingectomy at the time of benign gynecologic surgery or other intraperitoneal surgery [42,43,44,45,46,47] (Table 1). In 2013, the Society of Gynecologic Oncology (SGO) stated the following: “For women at average risk of ovarian cancer, risk-reducing salpingectomy should also be discussed and considered with patients at the time of abdominal or pelvic surgery, hysterectomy or in lieu of tubal ligation” [43,44]. Subsequently, the Royal College of Obstetricians and Gynecologists (RCOG) and the American College of Obstetricians and Gynecologists also stated, in 2014 and 2015, respectively, that bilateral salpingectomy should be considered at the time of benign gynecologic surgery or other intraperitoneal surgery in women who have completed their families [45,46]. These societies supported that bilateral salpingectomy has a potential benefit to prevent ovarian cancer, with minimal procedure-related complications.
Table 1. Other position statements on prophylactic salpingectomy by professional gynecologic boards.
| Year | Associations or experts | Recommendations |
|---|---|---|
| 2011 | Royal Australian and New Zealand college of obstetricians and gynecologists [42] | Doctors should discuss the risks and benefits of bilateral salpingectomy with patients undergoing hysterectomy for benign disease. |
| 2013 | Society of Gynecologic Oncology [43,44] | For women at average risk of ovarian cancer, risk-reducing salpingectomy should also be discussed and considered at the time of abdominal or pelvic surgery, hysterectomy or in lieu of tubal ligation. |
| 2014 | Royal College of Obstetricians and Gynecologists [45] | Women who are not at high risk for BRCA mutation and those who have completed their families should be carefully considered for prophylactic removal of the fallopian tubes with conservation of ovaries at the time of gynecological or other intraperitoneal surgery. |
| 2015 | American college of obstetricians and gynecologists [46] | Women at population-level risk of ovarian cancer who are undergoing ovary-sparing hysterectomy for benign indications should be offered bilateral salpingectomy to reduce their risk of ovarian cancer. |
| 2015 | Commission Ovary of the Arbeitsgemeinschaft Gynäkologische Onkologie [47] | During preoperative counseling prior to hysterectomy, all patients should be informed about the potential beneficial impact of opportunistic salpingectomy and the associated risks. |
Prophylactic salpingectomy is increasingly performed in many countries. In the United States, the rate of performing prophylactic salpingectomy with hysterectomy increased by 371% in 5 years (2008–2013) [48]. However, it was revealed that 45.5% of American physicians do not routinely perform prophylactic salpingectomy in real practice, and 69.4% of them said that they actually do not believe that salpingectomy exerts a preventive effect on ovarian cancer, in a survey study [49]. Although there is a wide discrepancy in the responses of physicians from European countries, 32.7–80% of physicians routinely perform prophylactic salpingectomy during benign gynecologic surgery [50,51,52]. In Austria, 70% (269/382) of the respondents stated that they would offer or discuss prophylactic salpingectomy in average-risk women undergoing gynecological surgery for benign indications, and that salpingectomy was the preferred method for surgical sterilization at the time of cesarean delivery [51,53]. Prophylactic salpingectomy at the time of hysterectomy for benign disease was applied to women in 70–80% of institutions accredited by an academic society and in 50–65% of institutions not accredited by an academic society in Japan [54]. In China, the rate of bilateral salpingectomy increased threefold (from 22% to 60%) from 2007 to 2017 [55]. Corresponding data from Korea are unavailable.
Concerns regarding potential disadvantages of prophylactic salpingectomy
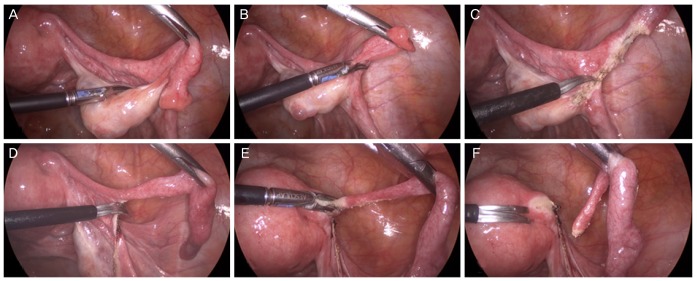
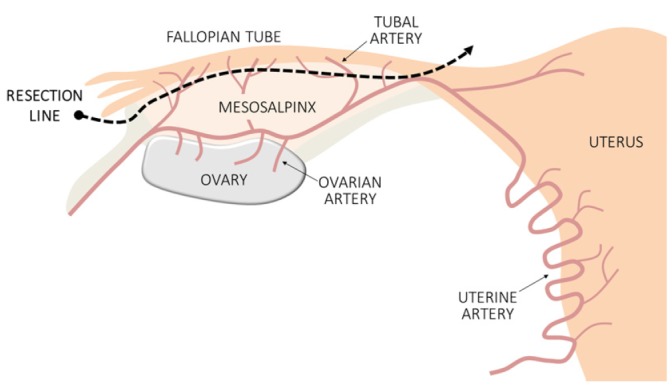
During salpingectomy, the fallopian tubes are typically resected from the fimbrial end to the uterine cornu. In particular, the fimbrial end should be completely removed, as most STICs originate from the fimbrial end. Some authors suggested that salpingectomy does not cause functional damage to the ovary, because the ovarian blood supply is guaranteed both by infundibulopelvic vessels and the ovarian branch of the uterine artery [56]. However, the potential disadvantages to the ovarian reserve owing to diminished ovarian arterial blood flow after salpingectomy are still debatable. Therefore, resection is performed at the posterior margin of the fallopian tubes, while conserving the mesosalpinx (Figs. 2 and 3).
Fig. 2. Laparoscopic salpingectomy. Fallopian tubes are resected from the fimbrial end to the uterine cornu. Careful resection is performed at the posterior margin of the fallopian tubes, while conserving the mesosalpinx.
Fig. 3. Schematic representation of salpingectomy.
1. Prophylactic salpingectomy during open and laparoscopic hysterectomy
It was reported that compared to hysterectomy alone, salpingectomy along with hysterectomy did adversely affect ovarian reserve. Morelli et al. [57] retrospectively compared the data of 79 women who underwent laparoscopic hysterectomy along with bilateral salpingectomy with those of 79 women who underwent laparoscopic hysterectomy alone. They reported that there was no significant difference between the 2 groups in the postoperative changes of anti-Müllerian hormone (AMH, P=0.35), follicle-stimulating hormone (FSH, P=0.15), antral follicle count (AFC, P=0.09), mean ovarian diameters (P=0.57), and peak systolic velocity (PSV, P=0.61). Similarly, the ovarian reserve-related factors including AMH were compared between women who underwent hysterectomy alone and those who underwent hysterectomy along with salpingectomy in almost all previous studies, and results consistent to those observed in the study by Morelli et al. [57] were reported [58,59,60,61]. However, limited evidence in terms of the short-term outcomes after salpingectomy exists, and no long-term outcome (such as an effect on actual menopausal timing) of this procedure has been reported. Many authors also showed that compared to hysterectomy alone, salpingectomy along with hysterectomy did not impair surgical outcomes including operation time, hemoglobin decrement, return to normal activity, and operation-related complications. Only Van Lieshout et al. [61] reported a significantly longer operation time in the group of women who underwent salpingectomy plus hysterectomy than in the group of women who underwent hysterectomy alone (2.0 [1.3–2.4] vs. 1.5 [1.3–2.1] hours, P=0.02).
Using a Markov Monte Carlo simulation model, Kwon et al. [62] proved that although salpingectomy along with hysterectomy was socioeconomically less expensive than hysterectomy alone ($11,044.32±1.56 vs. $11,206.52±29.81), it was more effective in terms of increased life expectancy (21.12±0.02 vs. 21.10±0.03 years). These results indicate that salpingectomy could be a life-saving procedure, in that it could decrease the risk of ovarian cancer and unplanned pregnancies in high-risk women. A recently published study with cost-effectiveness analysis showed that prophylactic salpingectomy along with laparoscopic hysterectomy would save nearly $24 million per year and could confer an additional 500 quality-adjusted life years (QALYs) [63].
2. Prophylactic salpingectomy during vaginal hysterectomy
When technically feasible, vaginal hysterectomy remains the minimally invasive surgical option of choice for benign disease. However, salpingectomy during vaginal hysterectomy requires a different technical skill, and it is associated with a modest increase in operation-related complications. Therefore, a recent study demonstrated that in 16.5% of cases, salpingectomy is performed with vaginal hysterectomy, while in 55.8% of cases, salpingectomy is performed with hysterectomy via other routes [64]. Unfortunately, there is a lack of evidence for prophylactic salpingectomy in clinical procedures other than open and laparoscopic hysterectomy. Cadish et al. [65] suggested that salpingectomy with vaginal hysterectomy is a cost-effective strategy ($7,350.62 vs. $8,113.45) considering its cancer- preventive effect, although operation-related complications minimally increase with this procedure, compared to those observed with vaginal hysterectomy alone (7.95% vs. 7.68%).
3. Prophylactic salpingectomy during cesarean delivery
Tubal ligation has been performed in women who desire permanent sterilization at the time of cesarean delivery. Although there are limited data on prophylactic salpingectomy performed in pregnant women, evaluation of the feasibility and safety of this procedure in this setting is important in terms of prevention of ovarian/fallopian/peritoneal cancer. However, a careful surgical approach is required, because the increased vascular supply to the broad ligament and fallopian tubes during pregnancy can easily lead to vascular injury and blood loss (Fig. 4). Several randomized controlled trials showed that bilateral salpingectomy during cesarean delivery seems as safe as tubal ligation in terms of operation-related complications. Subramaniam et al. [66] enrolled women at 35 weeks of gestation or greater who desired permanent sterilization at the time of cesarean delivery, and randomized a total of 80 women after skin incision to bilateral salpingectomy or bilateral tubal ligation (n=40 per group). Bilateral salpingectomy required 15 more minutes than cesarean delivery alone did (75.4±29.1 vs. 60.0±23.3 minutes, P=0.004). However, no sterilization-related complications were observed in any of the groups. Ganer Herman et al. [67] also showed longer operation time in women who underwent bilateral salpingectomy than in those who did not undergo salpingectomy (66.0±20.5 vs. 52.0±15.8 minutes, P=0.01); however, the rates of operation-related complications and hemoglobin decrement were similar in the 2 groups. The authors stated that salpingectomy seems to be a safer alternative to tubal ligation in women who desire permanent sterilization, during a planned cesarean delivery.
Fig. 4. Salpingectomy during cesarean delivery. (A, B) The engorged vessels in the mesosalpinx and the broad ligament during pregnancy; (C) resection of the tubo-ovarian ligament and mesosalpinx as close as possible to the fallopian tube, with ligation of engorged tubal vessels; and (D) resection of the fallopian tube at the uterine cornu.
Conclusion
Current evidences support that the fallopian tube plays a major role in the carcinogenesis of ovarian/fallopian/peritoneal cancer, and that bilateral salpingectomy seems to be an effective risk-reducing option. With the increasing adoption of prophylactic salpingectomy, the rate of ovarian/fallopian/peritoneal cancer in the general population is expected to gradually decrease over time. Women not at a high risk for EOC who have completed their families should be carefully considered for prophylactic salpingectomy at the time of their scheduled gynecologic surgery. Further prospective trials for prophylactic salpingectomy in various clinical settings, including non-gynecologic surgery, are warranted.
Acknowledgements
For the creation and development of this position statement, the “Prophylactic Salpingectomy―Position Statement Committee” including Miseon Kim (committee's secretary), Young-Han Kim (KSMFM representative), Yong Beom Kim (affiliate of scientific committee in KSOG), Jayeon Kim (KSRM representative), Jae-Weon Kim (committee's chair), Mi Hye Park (affiliate of scientific committee in KSOG), Joo Hyun Park (KSGE representative), Jeong Ho Rhee (affiliate of scientific committee in KSOG), Myong Cheol Lim (KSGO representative), and Joon-Seok Hong (secretary of scientific committee in KSOG) was formed. The position statement was reviewed and approved by all committee members.
Footnotes
Conflict of interest: The authors, as members of the “Prophylactic Salpingectomy―Position Statement Committee,” are listed in the acknowledgements, and they declare no potential conflicts of interest. None of the authors received funding for the creation of this position statement.
This statement asserts the official position of the Korean Society of Obstetrics and Gynecology (KSOG), and is not a document with the aim of guiding decisions.
References
- 1.Jung KW, Won YJ, Kong HJ, Lee ES. Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50:303–316. doi: 10.4143/crt.2018.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soslow RA. Histologic subtypes of ovarian carcinoma: an overview. Int J Gynecol Pathol. 2008;27:161–174. doi: 10.1097/PGP.0b013e31815ea812. [DOI] [PubMed] [Google Scholar]
- 3.McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. 2011;43:420–432. doi: 10.1097/PAT.0b013e328348a6e7. [DOI] [PubMed] [Google Scholar]
- 4.Vang R, Shih IM, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16:267–282. doi: 10.1097/PAP.0b013e3181b4fffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delair D, Soslow RA. Key features of extrauterine pelvic serous tumours (fallopian tube, ovary, and peritoneum) Histopathology. 2012;61:329–339. doi: 10.1111/j.1365-2559.2011.04167.x. [DOI] [PubMed] [Google Scholar]
- 6.Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schrader KA, Hurlburt J, Kalloger SE, Hansford S, Young S, Huntsman DG, et al. Germline BRCA1 and BRCA2 mutations in ovarian cancer: utility of a histology-based referral strategy. Obstet Gynecol. 2012;120:235–240. doi: 10.1097/AOG.0b013e31825f3576. [DOI] [PubMed] [Google Scholar]
- 8.Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD, 2nd, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28:3570–3576. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanda R, Schumm LP, Cummings S, Fackenthal JD, Sveen L, Ademuyiwa F, et al. Genetic testing in an ethnically diverse cohort of high-risk women: a comparative analysis of BRCA1 and BRCA2 mutations in American families of European and African ancestry. JAMA. 2005;294:1925–1933. doi: 10.1001/jama.294.15.1925. [DOI] [PubMed] [Google Scholar]
- 10.Anglian Breast Cancer Study Group. Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer. 2000;83:1301–1308. doi: 10.1054/bjoc.2000.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancaster JM, Powell CB, Kauff ND, Cass I, Chen LM, Lu KH, et al. Society of Gynecologic Oncologists Education Committee statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol. 2007;107:159–162. doi: 10.1016/j.ygyno.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Ryan NA, Morris J, Green K, Lalloo F, Woodward ER, Hill J, et al. Association of mismatch repair mutation with age at cancer onset in lynch syndrome: implications for stratified surveillance strategies. JAMA Oncol. 2017;3:1702–1706. doi: 10.1001/jamaoncol.2017.0619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loveday C, Turnbull C, Ramsay E, Hughes D, Ruark E, Frankum JR, et al. Germline mutations in RAD51D confer susceptibility to ovarian cancer. Nat Genet. 2011;43:879–882. doi: 10.1038/ng.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loveday C, Turnbull C, Ruark E, Hughes D, Ruark E, Frankum JR, et al. Germline RAD51C mutations confer susceptibility to ovarian cancer. Nat Genet. 2012;44:475–476. doi: 10.1038/ng.2224. [DOI] [PubMed] [Google Scholar]
- 15.Rafnar T, Gudbjartsson DF, Sulem P, Jonasdottir A, Sigurdsson A, Jonasdottir A, et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nat Genet. 2011;43:1104–1107. doi: 10.1038/ng.955. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network. Genetic/familial high-risk assessment: breast and ovarian (version 1.2019) [Internet] Fort Washington (PA): National Comprehensive Cancer Network; [cited 2018 Jul 19]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/genetics_screening.pdf. [Google Scholar]
- 17.Choi MC, Lim MC, Suh DH, Song YJ, Kim TJ, Chang SJ, et al. Position statements on genetic test for peritoneal, ovarian, and fallopian tubal cancers: Korean Society of Gynecologic Oncology (KSGO) J Gynecol Oncol. 2016;27:e36. doi: 10.3802/jgo.2016.27.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker WH, Broder MS, Chang E, Feskanich D, Farquhar C, Liu Z, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the nurses' health study. Obstet Gynecol. 2009;113:1027–1037. doi: 10.1097/AOG.0b013e3181a11c64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 20.Singer G, Shih IM, Truskinovsky A, Umudum H, Kurman RJ. Mutational analysis of K-ras segregates ovarian serous carcinomas into two types: invasive MPSC (low-grade tumor) and conventional serous carcinoma (high-grade tumor) Int J Gynecol Pathol. 2003;22:37–41. doi: 10.1097/00004347-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 22.Long Roche KC, Abu-Rustum NR, Nourmoussavi M, Zivanovic O. Risk-reducing salpingectomy: let us be opportunistic. Cancer. 2017;123:1714–1720. doi: 10.1002/cncr.30528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonome T, Lee JY, Park DC, Radonovich M, Pise-Masison C, Brady J, et al. Expression profiling of serous low malignant potential, low-grade, and high-grade tumors of the ovary. Cancer Res. 2005;65:10602–10612. doi: 10.1158/0008-5472.CAN-05-2240. [DOI] [PubMed] [Google Scholar]
- 24.Davidson B, Zhang Z, Kleinberg L, Li M, Flørenes VA, Wang TL, et al. Gene expression signatures differentiate ovarian/peritoneal serous carcinoma from diffuse malignant peritoneal mesothelioma. Clin Cancer Res. 2006;12:5944–5950. doi: 10.1158/1078-0432.CCR-06-1059. [DOI] [PubMed] [Google Scholar]
- 25.Kurman RJ, Shih IM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am J Surg Pathol. 2006;30:230–236. doi: 10.1097/01.pas.0000180854.28831.77. [DOI] [PubMed] [Google Scholar]
- 27.Przybycin CG, Kurman RJ, Ronnett BM, Shih IM, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34:1407–1416. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 28.Morrison JC, Blanco LZ, Jr, Vang R, Ronnett BM. Incidental serous tubal intraepithelial carcinoma and early invasive serous carcinoma in the nonprophylactic setting: analysis of a case series. Am J Surg Pathol. 2015;39:442–453. doi: 10.1097/PAS.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 29.Kindelberger DW, Lee Y, Miron A, Hirsch MS, Feltmate C, Medeiros F, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 30.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, et al. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J Clin Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman ME, Piedmonte M, Mai PL, Ioffe OB, Ronnett BM, Van Le L, et al. Pathologic findings at risk-reducing salpingo-oophorectomy: primary results from Gynecologic Oncology Group Trial GOG-0199. J Clin Oncol. 2014;32:3275–3283. doi: 10.1200/JCO.2013.54.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labidi-Galy SI, Papp E, Hallberg D, Niknafs N, Adleff V, Noe M, et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat Commun. 2017;8:1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaitskell K, Coffey K, Green J, Pirie K, Reeves GK, Ahmed AA, et al. Tubal ligation and incidence of 26 site-specific cancers in the Million Women Study. Br J Cancer. 2016;114:1033–1037. doi: 10.1038/bjc.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narod SA, Sun P, Ghadirian P, Lynch H, Isaacs C, Garber J, et al. Tubal ligation and risk of ovarian cancer in carriers of BRCA1 or BRCA2 mutations: a case-control study. Lancet. 2001;357:1467–1470. doi: 10.1016/s0140-6736(00)04642-0. [DOI] [PubMed] [Google Scholar]
- 35.Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol. 2013;42:579–589. doi: 10.1093/ije/dyt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rice MS, Murphy MA, Vitonis AF, Cramer DW, Titus LJ, Tworoger SS, et al. Tubal ligation, hysterectomy and epithelial ovarian cancer in the New England case-control study. Int J Cancer. 2013;133:2415–2421. doi: 10.1002/ijc.28249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rice MS, Murphy MA, Tworoger SS. Tubal ligation, hysterectomy and ovarian cancer: a meta-analysis. J Ovarian Res. 2012;5:13. doi: 10.1186/1757-2215-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lessard-Anderson CR, Handlogten KS, Molitor RJ, Dowdy SC, Cliby WA, Weaver AL, et al. Effect of tubal sterilization technique on risk of serous epithelial ovarian and primary peritoneal carcinoma. Gynecol Oncol. 2014;135:423–427. doi: 10.1016/j.ygyno.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madsen C, Baandrup L, Dehlendorff C, Kjaer SK. Tubal ligation and salpingectomy and the risk of epithelial ovarian cancer and borderline ovarian tumors: a nationwide case-control study. Acta Obstet Gynecol Scand. 2015;94:86–94. doi: 10.1111/aogs.12516. [DOI] [PubMed] [Google Scholar]
- 40.Falconer H, Yin L, Grönberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107:dju410. doi: 10.1093/jnci/dju410. [DOI] [PubMed] [Google Scholar]
- 41.Yoon SH, Kim SN, Shim SH, Kang SB, Lee SJ. Bilateral salpingectomy can reduce the risk of ovarian cancer in the general population: a meta-analysis. Eur J Cancer. 2016;55:38–46. doi: 10.1016/j.ejca.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Brand AH. The RANZCOG College Statement on prophylactic oophorectomy in older women undergoing hysterectomy for benign disease: is the evidence sufficient to change practice? Aust N Z J Obstet Gynaecol. 2011;51:296–300. doi: 10.1111/j.1479-828X.2011.01308.x. [DOI] [PubMed] [Google Scholar]
- 43.Society of Gynecologic Oncology. SGO clinical practice statement: salpingectomy for ovarian cancer prevention [Internet] Chicago (IL): Society of Gynecologic Oncology; [cited 2018 Jul 19]. Available from: https://www.sgo.org/clinical-practice/guidelines/sgo-clinical-practice-statement-salpingectomy-for-ovarian-cancer-prevention/ [Google Scholar]
- 44.Walker JL, Powell CB, Chen LM, Carter J, Bae Jump VL, Parker LP, et al. Society of Gynecologic Oncology recommendations for the prevention of ovarian cancer. Cancer. 2015;121:2108–2120. doi: 10.1002/cncr.29321. [DOI] [PubMed] [Google Scholar]
- 45.Royal College of Obstetricians and Gynecologists. High-grade serous carcinomas, the distal fallopian tube as the origin of non-uterine pelvic (scientific impact paper No. 44) London: Royal College of Obstetricians and Gynecologists; 2014. [Google Scholar]
- 46.American College of Obstetricians and Gynecologists. ACOG Committee on Practice Bulletins--Gynecology; ACOG Committee on Genetics; Society of Gynecologic Oncologists. ACOG Practice Bulletin No. 103: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2009;113:957–966. doi: 10.1097/AOG.0b013e3181a106d4. [DOI] [PubMed] [Google Scholar]
- 47.Pölcher M, Hauptmann S, Fotopoulou C, Schmalfeldt B, Meinhold-Heerlein I, Mustea A, et al. Opportunistic salpingectomies for the prevention of a high-grade serous carcinoma: a statement by the Kommission Ovar of the AGO. Arch Gynecol Obstet. 2015;292:231–234. doi: 10.1007/s00404-015-3697-y. [DOI] [PubMed] [Google Scholar]
- 48.Hanley GE, McAlpine JN, Pearce CL, Miller D. The performance and safety of bilateral salpingectomy for ovarian cancer prevention in the United States. Am J Obstet Gynecol. 2017;216:270.e1–270.e9. doi: 10.1016/j.ajog.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 49.Gill SE, Mills BB. Physician opinions regarding elective bilateral salpingectomy with hysterectomy and for sterilization. J Minim Invasive Gynecol. 2013;20:517–521. doi: 10.1016/j.jmig.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Chene G, de Rochambeau B, Le Bail-Carval K, Beaufils E, Chabert P, Mellier G, et al. Current surgical practice of prophylactic and opportunistic salpingectomy in France. Gynecol Obstet Fertil. 2016;44:377–384. doi: 10.1016/j.gyobfe.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Venturella R, Rocca M, Lico D, Trapasso S, Di Cello A, Gizzo S, et al. Prophylactic bilateral salpingectomy for the prevention of ovarian cancers: what is happening in Italy? Eur J Cancer Prev. 2016;25:410–415. doi: 10.1097/CEJ.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 52.Guldberg R, Wehberg S, Skovlund CW, Mogensen O, Lidegaard O. Salpingectomy as standard at hysterectomy? A Danish cohort study, 1977–2010. BMJ Open. 2013;3:e002845. doi: 10.1136/bmjopen-2013-002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kapurubandara S, Qin V, Gurram D, Anpalagan A, Merkur H, Hogg R, et al. Opportunistic bilateral salpingectomy during gynaecological surgery for benign disease: a survey of current Australian practice. Aust N Z J Obstet Gynaecol. 2015;55:606–611. doi: 10.1111/ajo.12402. [DOI] [PubMed] [Google Scholar]
- 54.Mikami M, Nagase S, Yamagami W, Ushijma K, Tashiro H, Katabuchi H, et al. Opportunistic bilateral salpingectomy during benign gynecological surgery for ovarian cancer prevention: a survey of Gynecologic Oncology Committee of Japan Society of Obstetrics and Gynecology. J Gynecol Oncol. 2017;28:e52. doi: 10.3802/jgo.2017.28.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Du H, Bao L, Liu W. Opportunistic salpingectomy at benign gynecological surgery for reducing ovarian cancer risk: a 10-year single centre experience from China and a literature review. J Cancer. 2018;9:141–147. doi: 10.7150/jca.21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venturella R, Morelli M, Lico D, Di Cello A, Rocca M, Sacchinelli A, et al. Wide excision of soft tissues adjacent to the ovary and fallopian tube does not impair the ovarian reserve in women undergoing prophylactic bilateral salpingectomy: results from a randomized, controlled trial. Fertil Steril. 2015;104:1332–1339. doi: 10.1016/j.fertnstert.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Morelli M, Venturella R, Mocciaro R, Di Cello A, Rania E, Lico D, et al. Prophylactic salpingectomy in premenopausal low-risk women for ovarian cancer: primum non nocere. Gynecol Oncol. 2013;129:448–451. doi: 10.1016/j.ygyno.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 58.Findley AD, Siedhoff MT, Hobbs KA, Steege JF, Carey ET, McCall CA, et al. Short-term effects of salpingectomy during laparoscopic hysterectomy on ovarian reserve: a pilot randomized controlled trial. Fertil Steril. 2013;100:1704–1708. doi: 10.1016/j.fertnstert.2013.07.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sezik M, Ozkaya O, Demir F, Sezik HT, Kaya H. Total salpingectomy during abdominal hysterectomy: effects on ovarian reserve and ovarian stromal blood flow. J Obstet Gynaecol Res. 2007;33:863–869. doi: 10.1111/j.1447-0756.2007.00669.x. [DOI] [PubMed] [Google Scholar]
- 60.Song T, Kim MK, Kim ML, Jung YW, Yun BS, Seong SJ, et al. Impact of opportunistic salpingectomy on anti-Müllerian hormone in patients undergoing laparoscopic hysterectomy: a multicentre randomised controlled trial. BJOG. 2017;124:314–320. doi: 10.1111/1471-0528.14182. [DOI] [PubMed] [Google Scholar]
- 61.Van Lieshout LA, Pijlman B, Vos MC, de Groot MJ, Houterman S, Coppus SF, et al. Opportunistic salpingectomy in women undergoing hysterectomy: Results from the HYSTUB randomised controlled trial. Maturitas. 2018;107:1–6. doi: 10.1016/j.maturitas.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 62.Kwon JS, McAlpine JN, Hanley GE, Finlayson SJ, Cohen T, Miller DM, et al. Costs and benefits of opportunistic salpingectomy as an ovarian cancer prevention strategy. Obstet Gynecol. 2015;125:338–345. doi: 10.1097/AOG.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 63.Dilley SE, Havrilesky LJ, Bakkum-Gamez J, Cohn DE, Michael Straughn J, Jr, Caughey AB, et al. Cost-effectiveness of opportunistic salpingectomy for ovarian cancer prevention. Gynecol Oncol. 2017;146:373–379. doi: 10.1016/j.ygyno.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 64.Garcia C, Martin M, Tucker LY, Lyon L, Armstrong MA, McBride-Allen S, et al. Experience with opportunistic salpingectomy in a large, community-based health system in the United States. Obstet Gynecol. 2016;128:277–283. doi: 10.1097/AOG.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 65.Cadish LA, Shepherd JP, Barber EL, Ridgeway B. Risks and benefits of opportunistic salpingectomy during vaginal hysterectomy: a decision analysis. Am J Obstet Gynecol. 2017;217:603.e1–603.e6. doi: 10.1016/j.ajog.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Subramaniam A, Blanchard CT, Erickson BK, Szychowski J, Leath CA, Biggio JR, et al. Feasibility of complete salpingectomy compared with standard postpartum tubal ligation at cesarean delivery: a randomized controlled trial. Obstet Gynecol. 2018;132:20–27. doi: 10.1097/AOG.0000000000002646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ganer Herman H, Gluck O, Keidar R, Kerner R, Kovo M, Levran D, et al. Ovarian reserve following cesarean section with salpingectomy vs tubal ligation: a randomized trial. Am J Obstet Gynecol. 2017;217:472.e1–472.e6. doi: 10.1016/j.ajog.2017.04.028. [DOI] [PubMed] [Google Scholar]