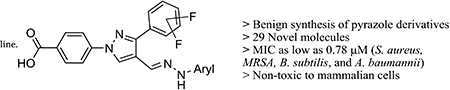
Abstract
Microbial resistance to antibiotics is an unresolved global concern, which needs urgent and coordinated action. One of the guidelines of the Centers for Disease Control and Preventions (CDC) to combat antibiotic resistance is the development of new antibiotics to treat drug-resistant bacteria. In our effort to find new antibiotics, we report the synthesis and antimicrobial studies of 30 new pyrazole derivatives. These novel molecules have been synthesized by using readily available starting materials and benign reaction conditions. Some of these molecules have shown activity with MIC values as low as 0.78 μg/mL against four bacterial strains; Staphylococcus aureus, methicillin-resistant S. aureus, Bacillus subtilis, and Acinetobacter baumannii. Furthermore, active molecules are non-toxic to mammalian cell
Keywords: Antimicrobial, Pyrazole, Hydrazone, Acinetobacter baumannii, MRSA
TOC Figure
Microbial resistance to antibiotics is an unsolved American and global concern. 1 Without urgent and coordinated action, we are moving toward an era in which normal infections or minor injuries may become fatal. Guidelines from the Centers for Disease Control and Prevention (CDC) recommend combatting antibiotic resistance2 by promoting the development of new antibiotics and new diagnostic tests for drug-resistant bacteria. The ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) bacteria cause two-thirds of nosocomial infections in the United States, and these bacteria are effectively escaping the existing antibiotics. The need for combating bacterial resistance to existing drugs is greater than ever.3
A. baumannii, a Gram-negative bacterium, is normally found in soil and water. It is responsible for different diseases in humans. A. baumannii in particular causes 80% of the diseases associated with the Acinetobacter genus. Acinetobacter infections threaten the lives of people with weakened immune systems, chronic lung disease, and diabetes. Acinetobacter infection outbreaks commonly happen in healthcare settings, particularly intensive care units, and this bacterium can live in tracheostomy sites or open wounds for several days without causing infection.4 Since 2003, the beginning of the Iraq war, several US service members have been infected by A. baumannii which is a huge problem in veterans.5,6 It is challenging for the medical community to find new antibiotics that can treat the infected people with multi-drug resistant (MDR) A. baumannii because almost all the approved antibiotics have failed. In late February 2017, the World Health Organization (WHO) has released a list of 12 drug resistant bacteria that pose the greatest threat to human health and for which new antibiotics are desperately needed. Carbapenem-resistant A. baumannii is on the top of the list and Enterobacter occupies the third position.7,8 It is very important to find novel molecules that can inhibit the growth of non-fermenting bacteria. These types of bacteria are opportunistic and nosocomial pathogens and several non-fermenting bacteria are multi-drug resistant such as A. baumannii and P. aeruginosa.9 A. baumannii has a highly impermeable outer membrane that prevents the uptake of most molecules including several antibiotics.10 That’s why finding new antibiotics to prevent the growth of A. baumannii infection is very challenging.
S. aureus, a Gram-positive bacterium, is found in the nares of about 30% of the population. This bacterium may cause sepsis, pneumonia, endocarditis, and osteomyelitis. S. aureus resistant to different antibiotics has evolved into different strains such as methicillin-resistant S. aureus (MRSA), vancomycin-intermediate S. aureus (VISA), and vancomycin-resistant S. aureus (VRSA).11 Two percent of the world population are nasal carriers of MRSA. Invasive infection caused by S. aureus and its drug resistant strains has decreased over the years but MRSA and other drug resistant strains are still a major threat in healthcare settings.12
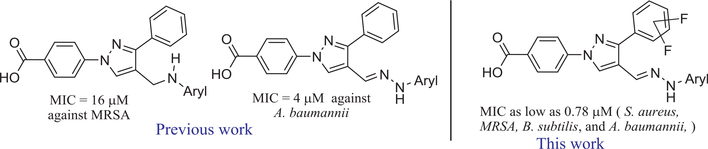
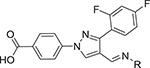
Recently, we have reported the synthesis of novel pyrazole derivatives as potent antimicrobial agents. Pyrazole-derived arylamines and hydrazones were found to be anti-MRSA and anti-A. baumannii agents, respectively (Fig. 1).13,14
Fig. 1.
Pyrazole derived hydrazones as potent antimicrobial agents.
Pyrazole derivatives are known to show a wide range of pharmaceutical properties including antimicrobial activities.15,16 In drug design, the fluorine atom is a common substitution in various synthesized compounds. The unique properties of fluorine play an important role in medicinal chemistry. Fluorine has small size (van der Waals radius 1.47 Å), high electronegativity, and high lipophilicity. Incorporating fluorine in a compound is beneficial as it changes the physicochemical and pharmacokinetic properties of the resultant molecule.17 Fluorine increases the metabolic stability and membrane permeation ability of a molecule. Another property of fluorinated compounds is improved binding affinity of the drug to the target protein. It has been found that even a single substitution of fluorine in a drug results in an intense pharmacological effect. Since 2011, around 51 fluorinated molecules have been approved, or are in the last stages of clinical studies (phase II/III). In addition, between 2001 and 2011, ~40 fluorinated drugs have been approved by the FDA.18
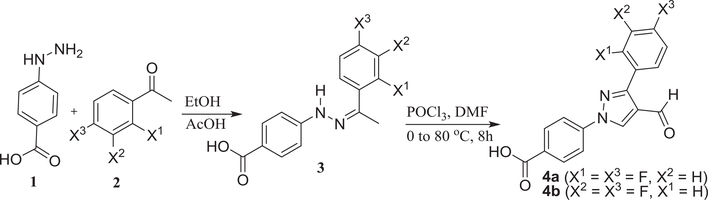
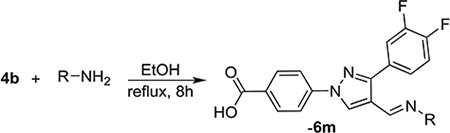
Based on the above facts of fluorine substituted molecules such as improved stability and membrane permeability, we designed fluorinated molecules built on our lead compounds reported previously.13,19 To our delight, we found several molecules with broader and several fold improved activities against both Gram-positive and Gram-negative bacteria. Reaction of 4-hydrazinobenzoic acid (1) with bisfluoro acetophenyl derivatives (2) led to the formation of hydrazones (3). Evaporation of the solvent followed by reaction with POCl3/DMF (Vilsmeier-Haack reaction) formed the formyl pyrazole derivative (4). Reaction of these two aldehydes (4a & 4b) with different hydrazine derivatives formed hydrazones. All the synthesized molecules were tested against the ESKAPE pathogens, Bacillus subtilis, and Escherichia coli. Several compounds have shown good activity against Gram-positive bacteria: B. subtilis, S. aureus, and MRSA, and a Gram-negative bacterium, A. baumannii (Scheme 1).
Scheme 1.
Synthesis of bisfluorophenyl pyrazole-derived aldehyde.
Reaction of 4-[3-(2,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl] benzoic acid (4a) with different hydrazine derivatives formed new hydrazone derivatives (Table 1). Phenyl hydrazone derivative (5a) showed activity against S. aureus, MRSA, B. subtäis, and A. baumanni with an MIC value of 25 μg/mL. Fluoro substituted compounds (5b, 5c, and 5d) showed similar activity against S. aureus and MRSA but increased activity against B. subtäis and A. baumannii with an MIC as low as 6.25 μg/mL. Fluoro substitution increased the activity but its position (ortho: 5b, meta: 5c, and para: 5d) did not alter the activities of resultant molecules. Bisfluoro substitution on the phenyl ring (5e) showed good activity against S. aureus, MRSA, and A. baumannii with an MIC value of 12.5 μg/mL. This compound showed very good activity against B. subtilis with an MIC value as low as 3.12 μg/mL. Polyfluorinated (tetra and penta) compounds (5f and 5g) did not show any significant activity against all the tested bacteria. The bischloro derivative (5h) showed potent activity against all three tested Grampositive bacteria with MIC value as low as 0.78 μg/mL. This compound (5h) is the most potent in the whole series. This compound also showed good activity against A. baumanni with an MIC value of 12.5 μg/mL. Bromo substituted hydrazone (5i) showed good activity against Grampositive bacteria and the activity against A. baumannii is very potent with an MIC value of 1.56 μg/mL. Strong electron withdrawing groups such as nitro and carboxylic acid on the phenyl ring of the hydrazones (5j, 5k, and 5l) ceased the activity of the hydrazone derivatives against all the tested bacteria. Hydrazone derivatives of aliphatic compounds such as piperidine (5m) and N-methyl piperazine (5n) did not show any activity.
Table 1.
Synthesis and antimicrobial studies of 2, 4 difluoro substituted hydrazone derivatives, Gram-positive bacteria: S. aureus (Sa) and B. subtilis (Bs), Gram-negative bacteria: A. baumannii (Ab), and E. aerogenes (Ea), and NA = no activity. Values are the average of two closely related experimental values.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comp. No. | R | Microorganism (MIC in μg/mL |
Product yield (%) | |||||
| Sa | MRSA | Bs | Ab | PA | Ea | |||
| 5a | 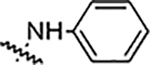 |
25 | 25 | 25 | 25 | NA | NA | 86% |
| 5b | 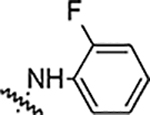 |
25 | 25 | 12.5 | 12.5 | NA | NA | 90% |
| 5c | 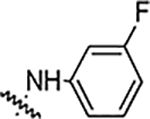 |
25 | 25 | 12.5 | 6.25 | NA | NA | 88% |
| 5d | 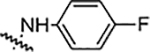 |
25 | 25 | 6.25 | 6.25 | NA | NA | 82% |
| 5e | 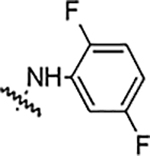 |
12.5 | 12.5 | 3.12 | 12.5 | NA | NA | 89% |
| 5f | 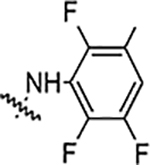 |
NA | NA | NA | NA | NA | NA | 92% |
| 5g | 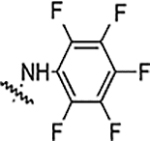 |
NA | NA | NA | NA | NA | NA | 81% |
| 5h | 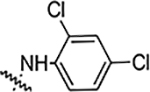 |
3.125 | 0.78 | 0.78 | 12.5 | NA | NA | 80% |
| 5i | 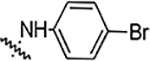 |
12.5 | 12.5 | 12.5 | 1.56 | NA | NA | 83% |
| 5j | NA | NA | NA | NA | NA | NA | 95% | |
| 5k | 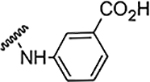 |
NA | NA | NA | NA | NA | NA | 78% |
| 5l | NA | NA | NA | NA | Na | NA | 89% | |
| 5m | 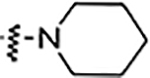 |
NA | NA | NA | NA | NA | NA | 76% |
| 5n | 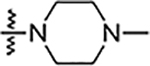 |
NA | NA | NA | NA | NA | NA | 90% |
| DMSO | NA | NA | NA | NA | NA | NA | ||
| Ciprofloxacin | 1.56 | |||||||
| Colistin | 12.5 | 0.78 | 0.39 | |||||
| Vancomycin | 6.25 | 12.5 | ||||||
Reaction of 3,4-bisfluophenyl derived aldehyde (4b) with hydrazine derivatives resulted the formation of hydrazones (Table 2) in good yield. Phenyl hydrazone derivative (6a) inhibited the growth of S. aureus, MRSA and B. subtilis (6a) with an MIC value of 25 μg/mL. This compound also inhibited the growth of A. baumannii with a lower MIC value, 6.25 μg/mL. Ortho (6b) and meta (6c) substituted fluorine derivatives showed the inhibition of Gram-positive bacteria (S. aureus, MRSA, and B. subtilis) with an MIC value of 12.5 μg/mL. Para-fluoro substituted derivative (6d) showed weaker activity (25 μg/mL) than its ortho and meta regioisomers (6b and 6c). These monofluoro phenyl hydrazones inhibited the growth of A. baumanni effectively with an MIC value of 6.25 μg/mL. Bisfluoro (6e) did not show any activity against S. aureus or MRSA but inhibited the growth of B. subtilis and A. baumannii at 3.1 μg/mL and 6.2 μg/mL respectively. 2,3,5,6-Tetrafluorophenyl derivative (6f) inhibited S. aureus and MRSA with an MIC value of 12.5 μg/mL. This polyfluorinated compound (6f) showed better activity against B. subtilis (6.25 μg/mL) and this compound (6f) did not show any activity against the tested Gram-negative bacteria. 4-Tri-fluoromethyl phenyl derivative (6g) showed the potent activity against Gram-positive bacteria with an MIC value as low as 0.78 μg/mL. This compound showed the highest activity among all the compounds of this series. 4-Chloro substituted phenyl hydrazone (6h) inhibited the growth of Gram-positive bacteria (S. aureus, MRSA and B. subtilis) with an MIC value of 6.25 μg/mL. This compound (6h) showed enhanced activity against A. baumannii with an MIC value of 1.56 μg/mL. Substitution of a hydrogen with one more chlorine (6i) enhanced the activity against S. aureus and MRSA with an MIC value of 1.56 μg/mL and this bischloro derivative (6i) showed better activity against B. subtilis and A. baumannii with MIC values as low as 0.78 and 3.125 μg/mL respectively. 4-Bromophenyl derivative (6j) inhibited the growth of tested bacteria: S. aureus with an MIC value of 3.125 μg/mL, MRSA and B. subtilis with an MIC value of 1.56 μg/mL. This compound (6j) showed very good activity against A. baumannii with an MIC value as low as 0.78 μg/mL. As discussed in the previous series (5a-n), strong electron withdrawing groups such as nitro (6k) and carboxylic acid (6l) did not show any activity against the tested bacteria. Methyl piperazine (6m) and methyl hydrazine (6n) derivatives also failed to inhibit the growth of any bacteria.
Table 2.
Synthesis and antimicrobial data of 3, 4 difluoro substituted hydrazone derivatives.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Comd. No. | R | Microorganism (MIC μg/mL) |
Product yield (%) | |||||
| Sa | MRSA | B s | Ab | P a | E a | |||
| 6a | 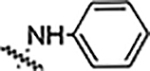 |
25 | 25 | 25 | 6.25 | NA | NA | 88% |
| 6b | 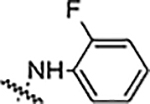 |
12.5 | 12.5 | 12.5 | 6.25 | NA | NA | 85% |
| 6c | 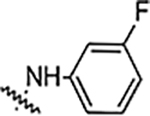 |
12.5 | 12.5 | 12.5 | 6.25 | NA | NA | 90% |
| 6d | 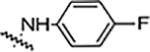 |
25 | 25 | 25 | 6.25 | NA | NA | 88% |
| 6e | 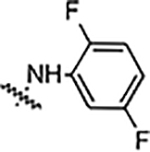 |
NA | NA | 3.125 | 6.25 | NA | NA | 80% |
| 6f | 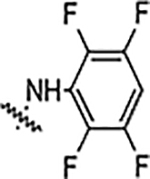 |
12.5 | 12.5 | 6.25 | NA | NA | NA | 78% |
| 6g | 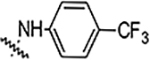 |
0.78 | 0.78 | 0.78 | 3.12 | NA | NA | 81% |
| 6h | 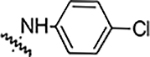 |
6.25 | 6.25 | 6.25 | 1.56 | NA | NA | 91% |
| 6i | 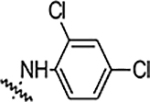 |
1.56 | 1.56 | 0.78 | 3.125 | NA | NA | 75% |
| 6j | 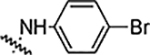 |
3.12 | 1.56 | 1.56 | 0.78 | NA | NA | 89% |
| 6k | NA | NA | NA | NA | NA | NA | 90% | |
| 6l | NA | NA | NA | NA | NA | NA | 81% | |
| 6m | 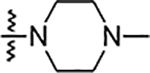 |
NA | NA | NA | NA | NA | NA | 72% |
Furthermore, five of the most active anti-MRSA compounds were tested against two additional strains of S. aureus, LAC (MRSA) and UAMS-1 (methicillin-sensitive S. aureus [MSSA]).20 2,4-Bisfluoroderivatives (5h and 5i) inhibited the growth of LAC (MRSA) and UAMS-1 (MSSA) at higher concentration compared to the tested S. aureus and MRSA strains. 3,4-Bisfluoro derivatives inhibited the growth of LAC (MRSA) and UAMS-1 (MSSA) with an MIC value as low as 3.12 and 6.25 μg/mL, respectively (Table 3).
Table 3.
Growth inhibition data of active molecules against other Staphylococcus aureus strains.
| Comp. No. | Microorganism (MIC in μg/mL) |
|
|---|---|---|
| LAC (MRSA) | UAMS-1 (MSSA) | |
| 5h | 12.5 | 25.0 |
| 5i | 25.0 | 25.0 |
| 6g | 3.12 | 6.25 |
| 6i | 6.25 | 6.25 |
| 6j | 12.5 | 12.5 |
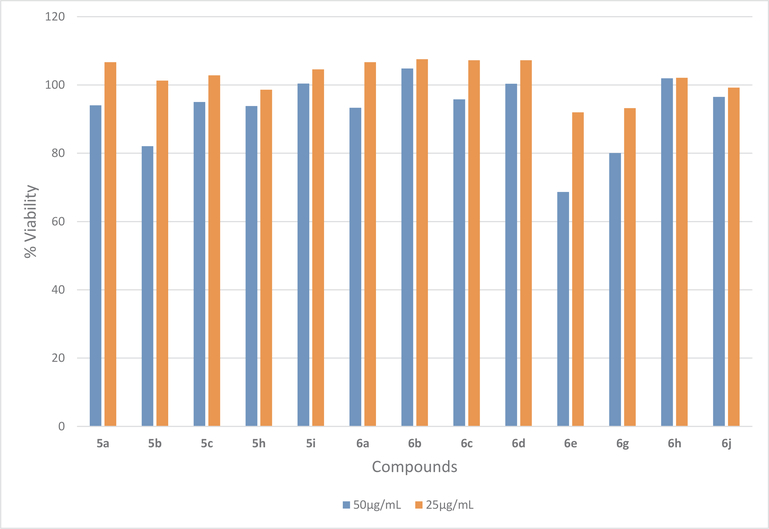
Synthesized compounds were tested against 60 cancer cell lines of National Cancer Institute under the Developmental Therapeutic Program (DTP)21 and these compounds did not show any significant cytotoxicity against human cancer cell lines at 10 μM concentration. Compounds showing activity against tested bacteria were also evaluated for growth inhibition of Human Embryonic Kidney (HEK-293) cell line at 25 and 50 μg/mL concentration via resazurin assay.13 To our delight, none of these active compounds showed any significant growth inhibition at 25 μg/mL concentration. HEK293 cells also tolerated the active compounds at 50 μg/mL concentration after incubating for 24 h. Cell viability was more than 80% for all the compounds except 6e, which showed 69% viability at 50 μg/mL concentration (Fig. 2).
Fig. 2.
Cytotoxic assay of active compounds on HEK293 cell line by using resazurin assay.
Based on the antimicrobial properties of the synthesized molecules, it can be concluded that these compounds are more active against Gram-positives bacteria than Gram-negative bacteria. Halogen substitution on the phenyl ring of the hydrazone have shown better activity than that of strongly electron withdrawing groups. Highly polar moieties such as nitro (5j and 6k) and carboxylic acids (5k, 5l, and 6l) and non-aromatic hydrazones (5m, 5n, and 6m) failed to show any activities against any bacteria. Hydrophobic and electron withdrawing groups (5h, 6g, 6i, and 6j) produced the most active bacterial growth inhibitors.
We have reported an efficient synthesis of new hydrazone derivatives of 4-[3-(2,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(3,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid. Thirty compounds have been synthesized and characterized by 1H NMR and 13C NMR spectroscopy and mass spectrometry. These new molecules have been tested against several Gram-positive and Gramnegative bacterial strains. The MIC values of some of the molecules showed promising activities against different bacteria. We found several molecules that inhibited A. baumannii growth with an MIC value as low as 0.73 μg/mL. Three compounds showed activities against Grampositive bacteria, S. aureus and B. subtilis at therapeutic concentration. Thus, these potent antimicrobial agents are good starting point for further antibacterial drug development. Further docking and enzyme inhibition studies, growth inhibition studies against pathogenic bacterial strains will be reported soon.
ARTICLE INFO InChIKeys:
NIPISOWJKCIIHK-LGJNPRDNSA-N
MVLUBVHJPZOZLV-KKMKTNMSSA-N
RXERGSIGGJWJQK-KKMKTNMSSA-N
HDYVUMZUWCJPKC-KKMKTNMSSA-N
RXESTGQXLDYIKG-IPBVOBEMSA-N
PFEMWRAYTCTHIG-OOEWDAAOSA-N
WIKWAYNIPJPLTA-OWNKRRAQSA-N
TWFYOLDKHQKSCA-IPBVOBEMSA-N
FETAFRLLXZXYCT-KKMKTNMSSA-N
HTHJFNCAJAARGB-RPPGKUMJSA-N
UWBWHTJUAPCNHF-KKMKTNMSSA-N
UWGGQMGEFMBVLR-KKMKTNMSSA-N
FPMNUHFIZZDCKX-DHRITJCHSA-N
AVLRRMXQQJNKGP-DHRITJCHSA-N
JIXROJKYLGSGTF-LGJNPRDNSA-N
UUHAWRQSWYCALO-KKMKTNMSSA-N
IQLGHNZLARMWNN-KKMKTNMSSA-N
AQVBDLJEOUZANE-KKMKTNMSSA-N
VAXHWIHXKIPNRD-IPBVOBEMSA-N
ZUGRLNIFEHJKOS-OOEWDAAOSA-N
QEWMZDUKJDLAQH-PNQUVVCRSA-N
ADNDOVPYDVEIIC-KKMKTNMSSA-N
SUWFPEZITOEUQR-IPBVOBEMSA-N
LFXQJGXFFMMSCD-KKMKTNMSSA-N
JORSTRRMGOZNNU-RPPGKUMJSA-N
SBLBFRUAAPEBKY-KKMKTNMSSA-N
LOERTZFIRUROPJ-DHRITJCHSA-N
JYMHEGQCQAJSTQ-GLCIPLLTSA-N
Acknowledgements
This publication was made possible by the Research Technology Core of the Arkansas INBRE program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429 from the National Institutes of Health to record the Mass Spectrometry data. This publication was made possible by the Arkansas INBRE program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429 from the National Institutes of Health. ABI mini-grant 200, 136 also helped to complete this project.
Footnotes
Declarations of interest
None.
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bmcl.2018.07.016. These data include MOL files and InChiKeys of the most important compounds described in this article.
References
- 1.Friedrich MJ. Who survey reveals misconceptions about antibiotic resistance. JAMA. 2016;315:242. [Google Scholar]
- 2.CDC Antibiotic/Antimicrobial Resistance. https://www.cdc.gov/drugresistance/federal-engagement-in-ar/national-strategy/index.html (accessed 10/19/2016).
- 3.Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. [DOI] [PubMed] [Google Scholar]
- 4.CDC Acinetobacter in Healthcare Settings. Acinetobacter in Healthcare Settings (accessed 9/22/2016).
- 5.Huang XZ, Chahine MA, Frye JG, et al. Molecular analysis of imipenem-resistant Acinetobacter baumannii isolated from US service members wounded in Iraq, 2003–2008. Epidemiol Infect. 2012;140:2302–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott P, Deye G, Srinivasan A, et al. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. http://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed 2/28/2017).
- 8.Willyard C The drug-resistant bacteria that pose the greatest health threats. Nature. 2017;543:15. [DOI] [PubMed] [Google Scholar]
- 9.Su SC, Vaneechoutte M, Dijkshoorn L, Wei YF, Chen Yl, Chang TC. Identification of non-fermenting Gram-negative bacteria of clinical importance by an oligonucleotide array. J Med Microbiol. 2009;58:596–605. [DOI] [PubMed] [Google Scholar]
- 10.Tommasi R, Brown DG, Walkup GK, Manchester JI, Miller AA. ESKAPEing the labyrinth of antibacterial discovery. Nat Rev Drug Discov. 2015;14:529–542. [DOI] [PubMed] [Google Scholar]
- 11.CDC Staphylococcus aureus in Healthcare Settings. https://www.cdc.gov/hai/organisms/staph.html (accessed 10/28/2017).
- 12.CDC Methicillin-resistant Staphylococcus aureus (MRSA). https://www.cdc.gov/mrsa/tracking/index.html (accessed 10/28/2017).
- 13.Allison D, Delancey E, Ramey H, et al. Synthesis and antimicrobial studies of novel derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-Acinetobacter baumannii agents. Bioorg Med Chem Lett. 2017;27:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alam MA. Preparation of 1,3-diphenyl azomethines and N-arylamines as antimicrobial agents and a method of synthesizing the antimicrobial agents. WO2017205814A1, 2017.
- 15.Faria JV, Vegi PF, Miguita AGC, dos Santos MS, Boechat N, Bernardino AMR. Recently reported biological activities of pyrazole compounds. Bioorg Med Chem. 2017;25:5891–5903. [DOI] [PubMed] [Google Scholar]
- 16.Khan KA, Faidallah HM. 1-Substituted carbamoyl and thiocarbamoyl-4,5-dihydro-1H-pyrazoles as possible cytotoxic and antimicrobial agents. J Enzyme Inhib Med Chem. 2016;31:619–627. [DOI] [PubMed] [Google Scholar]
- 17.Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. Applications of fluorine in medicinal chemistry. J Med Chem. 2015;58:8315–8359. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Wang J, Gu Z, et al. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II-III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem Rev. 2016;116:422–518. [DOI] [PubMed] [Google Scholar]
- 19.Brider J, Rowe T, Gibler DJ, et al. Synthesis and antimicrobial studies of azomethine and N-arylamine derivatives of4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-methicillin-resistant Staphylococcus aureus agents. Med Chem Res. 2016;25:2691–2697. [Google Scholar]
- 20.Meeker DG, Beenken KE, Mills WB, et al. Evaluation of antibiotics active against methicillin-resistant Staphylococcus aureus based on activity in an established biofilm. Antimicrob Agents Chemother. 2016;60:5688–5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Developmental Therapeutics Program. https://dtp.cancer.gov/default.htm (accessed 1/16/2018).