Abstract
Research on the developmental origins of health and disease (DOHaD) has traditionally focused on how maternal exposures around the time of pregnancy might influence offspring health and risk of disease. We acknowledge that for some exposures this is likely to be correct, but argue that the focus on maternal pregnancy effects also reflects implicit and deeply-held assumptions that 1) causal early life exposures are primarily transmitted via maternal traits or exposures, 2) maternal exposures around the time of pregnancy and early infancy are particularly important, and 3) other factors, such as paternal factors and postnatal exposures in later life, have relatively little impact in comparison. These implicit assumptions about the “causal primacy” of maternal pregnancy effects set the agenda for DOHaD research and, through a looping effect, are reinforced rather than tested. We propose practical strategies to redress this imbalance through maintaining a critical perspective about these assumptions.
Keywords: DOHaD, Developmental origins, Maternal effects, Research translation, Paternal effects, Causal inference, Prenatal, Epidemiology
Highlights
-
•
Many maternal pregnancy exposures are assumed to affect offspring health.
-
•
Other factors like paternal and postnatal exposures are also likely to be important.
-
•
Nevertheless, maternal pregnancy exposures are assumed to be most important.
-
•
We need to retain a critical perspective regarding this assumption.
-
•
Improving the causal evidence base and contextualising findings will help.
1. Introduction
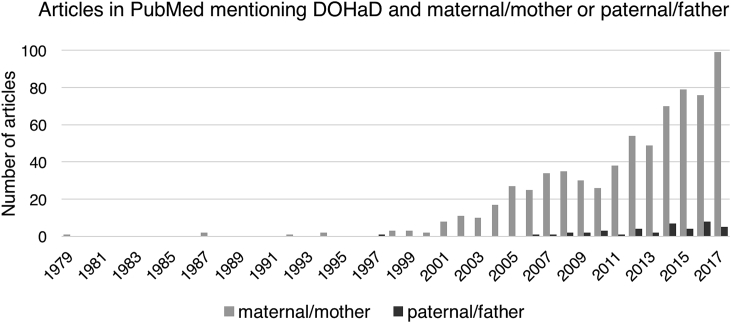
The developmental origins of health and disease (DOHaD) hypothesis asserts that our early life experiences, including those occurring in utero, can affect our longterm health. Research in DOHaD has traditionally focused on the health and lifestyle of mothers around the time of pregnancy in relation to the health of their children. For example, a PubMed search for terms relating to DOHaD in addition to “maternal” or “mother” reveals 702 papers, compared to just 41 for the same terms in addition to “paternal” or “father” (Fig. 1).
Fig. 1.
Number of articles in PubMed mentioning DOHaD ("developmental origins" OR ″DOHaD″ OR ″Barker hypothesis" OR ″fetal origins") and either (“maternal” OR “mother) or (“paternal” OR “father”). Search conducted December 2017.
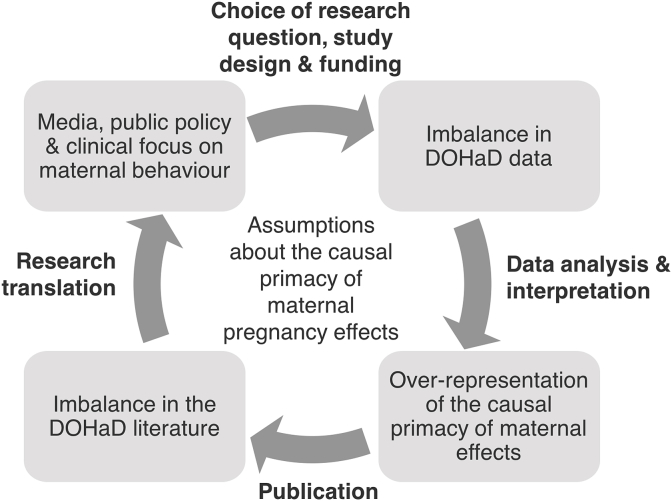
We argue that this imbalance in the amount of research published on potential “maternal pregnancy effects” reflects implicit and deeply-held assumptions regarding which early-life exposures are likely to cause later disease, and when these exposures are likely to occur. Specifically, these assumptions are that 1) causal early life exposures are primarily transmitted via maternal traits or exposures, 2) maternal factors around the time of pregnancy and early infancy are particularly important, and 3) other factors, including paternal and postnatal factors, have relatively little impact in comparison. Assumptions about the “causal primacy” of maternal pregnancy effects set the agenda for DOHaD research and are reinforced by a looping effect at all stages – from study design, to the results obtained, to the ways findings are interpreted and translated to guide public health policy and clinical practice (Fig. 2). This looping effect produces an iterative, self-reinforcing dynamic in which initial assumptions about relevant causal relations, driven by material pragmatics as well as human goals and values, become naturalized as more real and/or cognitively salient than other possible alternatives (Barad, 2007; Hacking, 1995; Lombrozo and Vasilyeva, 2017).
Fig. 2.
Assumptions that the health, lifestyle and behaviours of mothers around the time of pregnancy have the largest causal influence on their children's health and risk of disease drives DOHaD research at all stages, from study design to research translation, and is also reinforced by DOHaD research itself.
1.1. Origins of assumptions about the causal primacy of maternal pregnancy effects
Cultural assumptions that mothers play the most important role in shaping their children's health have a long history (Sommerfeld, 1989), stemming from the clearly proximal and intimate relationship between a mother and child during pregnancy and breastfeeding and from historical tendencies for mothers to be the primary caregiver. These ideas are also found in scientific theories, including in interpretations of the earliest DOHaD findings.
DOHaD began life as the “Barker hypothesis”, arising from initial ecological studies by David Barker et al. suggesting population level associations between low birthweight and neonatal death and an increased risk of ischemic heart disease in adult survivors (Barker et al., 1993, 1989; Barker and Osmond, 1986). The group hypothesised that prenatal environmental factors that result in low birthweight and neonatal death would also influence adult heart disease. The principal factor influencing low birthweight was assumed to be fetal undernutrition, directly influenced by the nutritional, hormonal and metabolic environment afforded by the mother (Barker, 2007). Thus, assumptions that early-life exposures cause later disease, and that these exposures are primarily transmitted by the mother during pregnancy, were foundational in the history of the field. As further studies emerged exploring more maternal pregnancy exposures and offspring outcomes, the Barker hypothesis became the Fetal Origins of Adult Disease (FOAD) hypothesis, and was extended to DOHaD in 2003 to recognise the role of exposures occurring beyond pregnancy in the postnatal developmental period (Wadhwa et al., 2009). However, despite this extended remit, the majority of DOHaD research still focuses on exposures occurring in the intrauterine period: a recent review found only limited overlap between publications in DOHaD and lifecourse epidemiology (Ben-Shlomo et al., 2016).
Today, there is a large and increasing body of published literature linking maternal health, lifestyle and behaviour around the time of pregnancy to offspring health at birth and in later life. Well-known examples include the increased and decreased risk of congenital anomalies associated with taking Thalidomide (Vargesson, 2015) or folic acid supplements (MRC Vitamin Study Research Group, 1991), respectively, and the robust and likely causal association between maternal smoking and low birthweight (Pereira et al., 2017; Rice et al., 2009; Tyrrell et al., 2012). These sit alongside a much larger proportion of the literature offering correlative, rather than causal, claims relating a wide range of maternal pregnancy exposures to an equally wide range of offspring health outcomes. This type of correlative research, with little attempt to use methods that might improve causal understanding, has remained the overwhelming focus over recent decades. This both reflects and fuels assumptions about the causal primacy of maternal effects, while ignoring or down-playing the role of the myriad of other factors that can shape long-term health, including the systems and social inequalities that influence health and behaviours of all family members, and hence the impact of wider family, postnatal and potential “paternal” effects (Braun et al., 2017; Romanus et al., 2016). Without establishing a more critical perspective, DOHaD research risks reinforcing rather than testing implicit assumptions regarding the causal primacy of maternal pregnancy effects.
2. How DOHaD research reinforces assumptions about the causal primacy of maternal pregnancy effects throughout the research process
2.1. Choosing a research question, designing a study and getting funding
In picking a research question, researchers consider several factors, including their a priori hypotheses, which will be partly influenced by underlying assumptions about causality, clinical importance and what is already known according to the relevant literature. As Fig. 1 suggests, there is a wealth of previous evidence to support funding applications to study maternal pregnancy effects. Comparatively little is known about other influences, including potential paternal, partner and postnatal effects. The rationale for looking beyond maternal pregnancy effects is less easy to support with references from the literature and such studies might be considered riskier, by both researchers and funders.
Study design and developing a research question are involved in a looping effect in which the research question is often limited by the study design and available data, which themselves are often shaped by initial proposed research questions. If there is little or poor-quality data on a factor, there is limited ability to study it well. An important example is birth cohort studies, which are fundamental to DOHaD research, but often have a higher quantity and quality of data available for mothers than fathers/partners (Table 1). This imbalance might be influenced by an underlying assumption that mothers exert a stronger influence on offspring health outcomes and their data should therefore be the priority. While some birth cohort studies have recognised the importance of studying fathers and have worked hard to include them, recruitment rates have suffered from difficulties recruiting and retaining fathers throughout the study period (Kiernan, 2014). Many prospective studies recruit mothers during antenatal clinics, which is an effective strategy to study maternal exposures; in countries with universal health care, this practice maximises the chance of recruiting a large sample representative of the population. Conversely, fathers attend antenatal clinics less frequently, so there is less opportunity for direct recruitment (Bryson, 2014; Kiernan, 2014; Redshaw and Heikkila, 2010). More broadly, men are less likely to engage in research (Dunn, 2004; Mindell et al., 2015; Tolonen et al., 2015) (despite being more likely to be invited (Holdcroft, 2007)), which might be explained by gender roles, gender identities and gendered stratification of labour.
Table 1.
Recruitment rates and data collection for partners in a small selection of the major population-based birth cohort studies that inform DOHaD research. We selected cohorts for inclusion in this table based on our familiarity with the recruitment procedure and availability of data, and consulted with studies to ensure accuracy.
| Cohort | Recruitment context | Number recruited | Data collected for mothers | Data collected for partners |
|---|---|---|---|---|
| Born in Bradford (BIB) (Wright et al., 2013) | 2007-2010; Bradford, UK |
|
|
|
| Avon Longitudinal Study of Parents and Children (ALSPAC) (Boyd et al., 2013; Fraser et al., 2013) | 1991-1992; Bristol and surrounding area, UK |
|
|
|
| Generation R (Kooijman et al., 2016) | 2002-2006; Rotterdam, the Netherlands |
|
|
|
| The Norwegian Mother and Child Cohort Study (MoBa) (Magnus et al., 2016) | 1999-2008; Norway |
|
|
|
| Project Viva (Oken et al., 2015) | 1999-2002; Eastern Massachusetts, USA |
|
|
|
Together, the higher intensity of existing research on maternal pregnancy effects (providing a foundation for ‘safe’ research funding requests) and the better availability of data to study maternal compared to paternal effects (and other factors) contribute to a structural imbalance in DOHaD data that limits the range of research questions that can be addressed well and therefore the range of studies likely to be funded.
2.2. Data analysis and interpretation
The structural imbalance in DOHaD research data has the potential to influence how these data are analysed and interpreted. Limited or poor quality data on other exposures might restrict researchers to analyse the effect of maternal pregnancy exposures in isolation, thus inflating their importance and ignoring other factors, including paternal factors. Where information on paternal exposures is available, differences in the quality of data in comparison to maternal data could mean any paternal effect is underestimated. For example, greater measurement error of a trait such as parental body mass index (BMI) in partners could contribute to a weaker association with offspring BMI in partners as compared with mothers (Lawlor et al., 2007). This problem is likely to arise if maternal weight and height are measured at a clinic or self-reported, while paternal weight and height data rely on less accurate maternal-report. In addition, if more data are missing for partners than mothers, analyses will have lower statistical power to detect partner/paternal effects. Similarly, missing partner data might introduce selection bias. For example, missing paternal information has been more likely for infants of mothers who are adolescents, unmarried, less educated and smokers in some studies (Gaudino et al., 1999; Tan et al., 2004), which could reduce estimates of the paternal effect, while not biasing estimates of the maternal effect. Bias can also be introduced by non-paternity, whereby the higher genetic similarity between mothers and offspring will overestimate the relative importance of the maternal pregnancy effect (Lawlor and Mishra, 2009).
Experimental designs, in which researchers manipulate the early life environment in some way and then follow children up to measure the outcome, are often impractical, prohibitively expensive and/or entirely unethical. By merely observing associations, researchers cannot be sure whether a studied maternal factor actually causes an offspring outcome or whether the association is better explained by other “confounding” factors that tend to correlate with the maternal factor. For example, the often-cited “natural experiment” famine studies, in which individuals prenatally exposed or unexposed to famine are followed-up in later life (reviewed in Lumey et al. (2011)), do not provide evidence that maternal undernutrition has the strongest causal effect on offspring adult health; maternal exposure to famine is likely to correlate with multiple other factors, including paternal undernutrition, parental stress, and postnatal factors in the aftermath. A classic example where assumed causality was shown to be wrong is the series of “crack baby” studies from the 1980s and 1990s that documented a wide range of abnormalities in children of mothers who took cocaine during pregnancy. A later review found no evidence of an independent effect of maternal prenatal cocaine exposure after adjustment for the confounding effects of tobacco, alcohol, cannabis or quality of the child's environment (Frank et al., 2001).
Through analysis of data that is observational and imbalanced, DOHaD research could produce biased estimates that overstate the role of maternal exposures as the most important causal determinants of offspring health.
2.3. Publication
A maternal-skewed imbalance in DOHaD data and research intensity increases the chances of a positive association between a maternal factor and offspring health being reported in the literature. In contrast, the relatively low intensity of research on paternal effects, coupled with problems such as an increased likelihood of finding a null association due to measurement error, may decrease the chances of publication of a positive association with a paternal factor.
Publication bias, which occurs when the outcome of a study influences the decision to publish, may further fuel the collection of published positive maternal associations. Assumptions about the causal primacy of maternal pregnancy effects could drive publication bias in two main ways: authors may be less likely to submit papers describing null results, and editors and reviewers may be more likely to accept papers reporting associations in line with their own assumptions (Lawlor, 2007). Studies that report an association are more likely to be published than those that report no association, particularly if the direction is in line with what would be considered ‘common sense’(Ioannidis, 2005; Stern and Simes, 1997). This problem is of course not limited to DOHaD research, but the field has been previously criticised for publication bias (Huxley et al., 2002).
Together, higher quality and quantity of research on maternal pregnancy effects and publication bias seem like plausible contributors to the disparate pattern of published maternal versus paternal DOHaD studies illustrated in Fig. 1.
2.4. Research translation, public engagement, and impact on policy and clinical practice
Systematic bias in data collection, analysis, and interpretation influences the translation of DOHaD research into policy, clinical practice and the popular media. Journalists often focus on maternal pregnancy effects, simplifying or ignoring methodological limitations, inconclusive results and the role of other contributing factors (Richardson et al., 2014; Winett et al., 2016), while presenting the evidence as causal. This gives the impression that prenatal maternal exposures and behaviors are conveyed to the fetus as direct, amplified, unbuffered effects, as in one headline “You are What Your Mum Ate” (ABC News, 2012). By implicating the maternal body as a central site for the introduction of health deficits, mothers are positioned as “vectors” (Richardson, 2015) for chronic diseases and intergenerational harms across a wide range of outcomes with varying levels of supporting evidence. One headline describing mothers as “smoking guns” in the obesity epidemic epitomizes this alarmist, inflammatory discourse around harms of maternal behaviour to helpless fetuses, to future generations, and ultimately to social welfare (Warin et al., 2012). In many narratives, pregnant women appear as individually responsible for specific harms to their offspring. A Science news item titled “The Nutritional Sins of the Mother” positioned the mother as a hostile assailant: “when subjected to a suboptimal prenatal environment, offspring feel the effects of the maternal assault” (Purnell, 2014). Framing the evidence in this way ignores the fact that much of the evidence for DOHaD describes population-level trends, rather than individual risk (Davey Smith, 2011), and examines complex exposures that involve not just individual factors but also the systems and social inequalities that influence health behaviours (Korenbrot and Moss, 2000).
Complex DOHaD findings have been rushed into simplified, direct advice to pregnant women and public health policy, including in the expanding area of preconceptional care (Waggoner, 2015). Recent policy guidance from the United States Centers for Disease Control (CDC) advised sexually active women of reproductive age not using birth control to abstain completely from alcohol use. Such advice is based on an extreme interpretation of the precautionary principle rather than good quality evidence for causal maternal pregnancy effects. A recent review showed there has been very little research assessing effects of low level drinking during pregnancy (Mamluk et al., 2017), and although there is probably no way to scientifically prove a “safe” level, current evidence is not sufficient to advise abstention in all women who are not using birth control. Without a sound evidence base, it is difficult for translators of DOHaD research to the public to know where to draw the line in developing precautionary health advice. For example, a review of DOHaD research published in the Atlantic Magazine raised questions about women working outside the home during pregnancy and considered the implications of introducing policies permitting, or even requiring, women to “reduce their hours, change duties, or take time off while pregnant” (Velasquez-Manoff, 2015). In contrast, aside from the context of low fertility, men are rarely prescribed advice on how to behave around the time their partner is pregnant (Almeling, n.d.; Daniels, 2011; Shawe et al., 2015).
Recent changes to antenatal care practice implicitly suggest that future offspring health is more important than maternal health, for example by recommending diagnostic criteria for gestational diabetes that minimises assumed future offspring adiposity risk over established postnatal maternal type 2 diabetes risk (Lawlor, 2013). A further example is recommendations for how much weight a woman should gain in pregnancy. In most Western countries, the practice of repeatedly weighing women throughout pregnancy stopped in the early-to-mid-1990s as it was recognised that gestational weight gain (GWG) was a poor predictor of fetal growth retardation or adverse pregnancy outcomes, and the practice was associated with maternal anxiety (Farrar and Duley, 2007). In the last decade, the emergence of the obesity epidemic has seen a return to weighing women throughout pregnancy, but now, rather than aiming to identify babies at risk of intrauterine growth restriction, the recommendations are designed to limit GWG to prevent future offspring overweight or obesity. This is despite the fact that GWG is a complex mix of maternal fat deposition, volume expansion, fetal growth, amniotic fluid and placental growth, each of which reflect very different processes; there is very limited evidence that total weight gain can be safely modified in pregnancy and no convincing evidence that it has a causal effect on future offspring adiposity (Lawlor, 2013; Lawlor et al., 2012).
These cases illustrate how DOHaD-informed clinical practice, advice and policy intended to reduce risks to the fetus not only fails to arm women with the evidence needed to assess risk for themselves, but can also have coercive and autonomy-limiting effects for women (Richardson and Almeling, 2016). Such harms include increasing guilt and obsessive self-vigilant behaviors in women of reproductive age, as well as the corrosive effects of everyday surveillance and social reproach and even the threat of legal detention and charges of child abuse. Following cases of women refused entry to concerts or bars, or denied orders of alcohol or other foods believed to carry risks during pregnancy, the New York Commission on Human Rights was forced to issue a statement that “using safety as a pretext for discrimination or as a way to reinforce traditional gender norms or stereotypes is unlawful” (McPhate, 2016). In 18 of the United States, the use of intoxicants by pregnant women is legally classed as child abuse (Miranda et al., 2015). Hundreds of women, largely poor, non-white women, have been subject to forced interventions in health care settings, removal of their children or social benefits, or prosecution for behaviour during pregnancy on the basis of claims about harms to the fetus (Paltrow and Flavin, 2013).
Concerns about DOHaD findings contributing to maternal blame do not stop at social implications, but also influence the agenda for DOHaD research itself. In a looping effect, increased focus on maternal behaviour in public health discourse and the media reinforces implicit starting assumptions in DOHaD research (Lombrozo, 2014).
3. Strategies to maintain a critical perspective
If DOHaD research is to provide high quality evidence to support implementation of effective policies and clinical practice to improve health, it will be necessary to maintain, throughout the research process, a critical perspective on the core assumption that maternal exposures around the time of pregnancy are of greatest causal importance in offspring health outcomes. This can be achieved practically in several ways.
3.1. Improve the quantity and quality of data collected on partners
The structural imbalance in DOHaD data can be lessened by addressing more research questions regarding potential postnatal, partner and paternal effects. It is encouraging to see increasing agreement on the importance of studying both direct (e.g. via biological effects in sperm) and indirect (e.g. via influence on the mother) paternal effects in DOHaD (Agricola et al., 2016; Braun et al., 2017; Day et al., 2016; Romanus et al., 2016; Soubry, 2017). This goes hand-in-hand with developing and implementing strategies to collect better-quality, more complete data on partners. For example, a review of birth cohort methodologies found that male partners were more likely to take part in a telephone interview than respond to a posted questionnaire (Kiernan, 2014). We recognise that even with special efforts, collecting data on partners can be extremely difficult. Strategies to improve the richness of data available for partners include linkage to national registries and taking advantage of the wealth of information that can be generated from biological samples. Aside from paternal genotype data providing insights about genetic background, epigenetic data is proving to be a valuable indicator of environmental exposures. For example, blood DNA methylation is such a powerful indicator of smoking that it outperforms self-reported smoking as a predictor of future lung cancer (Zhang et al., 2016).
3.2. Triangulate methods for assessing causality of pregnancy exposures in DOHaD
DOHaD studies can avoid over-stating a maternal pregnancy effect by ensuring it is not spurious and represents a true causal effect. Replication in an independent cohort provides confidence that the association is reproducible (Munafò et al., 2017). However, if the same factors are likely to play similarly confounding roles in independent cohorts, replication alone cannot provide strong evidence for a causal effect.
Increasingly, DOHaD studies by epidemiologists and economists (Almond and Currie, 2011) are using statistical techniques and study designs that can help reveal whether associations between early-life exposures and later-life outcomes are causal or more likely to be explained by confounding factors. Some of these “causal inference techniques” can also try to disentangle a causal effect of a specific exposure in pregnancy from postnatal and/or wider familial exposures. For example, evidence regarding detrimental effects of maternal pregnancy smoking on offspring fetal growth is supported by studies in which partner smoking is used as a “negative control”. In this design, we would expect the paternal exposure to have a weaker effect on the offspring outcome than the maternal exposure if the latter has a causal intrauterine effect, whereas similar magnitudes of association would support residual confounding for both the maternal and paternal association (Lawlor and Mishra, 2009). In the ALSPAC cohort, markedly lower offspring birthweight is seen in relation to maternal smoking, but not in relation to paternal smoking, at the time of their partner's pregnancy (Davey Smith, 2008). By contrast, despite evidence that maternal smoking during pregnancy is associated with attention deficit hyperactivity disorder (ADHD) in children when conventional multivariable regression approaches are used (Langley et al., 2005), a study that applied the paternal negative control design found similar effect estimates for maternal and paternal smoking around the time of pregnancy (Langley et al., 2012). This suggests that associations between maternal pregnancy smoking and subsequent ADHD are better explained by shared familial genetics or lifestyle rather than a causal intrauterine effect.
The application of causal inference techniques has also challenged the conclusion of several DOHaD studies of the effect of maternal obesity in pregnancy on offspring adiposity (Lawlor, 2013). Multivariable regression analyses have identified associations between higher maternal BMI at the start of pregnancy and higher offspring BMI and fat mass in childhood, adolescence and early adulthood (Lawlor, 2013; Yu et al., 2013), leading to the suggestion that more obese mothers might cause their children to be fatter through a direct mechanism occurring in utero. Two studies have now attempted to disentangle any maternal effect from confounding factors by applying a causal inference technique called Mendelian randomization (MR) (Davey Smith and Hemani, 2014). This approach, based on the instrumental variable approach developed in the field of economics, uses genetic variants robustly associated with an exposure (in this case, maternal BMI) as an instrumental variable for that exposure in statistical models. These MR studies suggest that the association between maternal BMI and offspring adiposity is more likely driven by confounding than a causal intrauterine effect (Lawlor et al., 2008; Richmond et al., 2017).
It is important to note that there is no single panacea to overcome issues with observational evidence; each causal inference technique has its own limitations. However, each relies on different assumptions, so triangulating evidence garnered using several techniques can be a powerful approach to distinguish causation from mere correlation (Lawlor et al., 2017b). For example, when MR studies are used to test DOHaD effects, in particular when the maternal exposure and offspring outcome are the same (as in the case of maternal-offspring BMI), there are additional assumptions and sources of bias that might explain these null findings (Lawlor et al., 2017a). However, the consistency of findings between MR studies, paternal BMI negative control studies (Corsi et al., 2015; Davey Smith et al., 2007; Fleten et al., 2012; Lawlor, 2013; Lawlor et al., 2008), and within-sibling analyses, which can control for measured and unmeasured familial level confounding (Lawlor, 2013; Lawlor et al., 2011), all strengthen the conclusion that maternal higher BMI at the start of pregnancy is unlikely to causally affect later offspring risk of greater adiposity.
3.3. Scrutinise paternal, social, postnatal and other likely effects on development and health
By considering maternal factors around the time of pregnancy in isolation, the maternal pregnancy effect is presented as substantial, without a clear understanding of its contribution relative to the other factors that shape child health. Instead, studies should provide a perspective on the relative effect sizes. For example, in a study of over 10,000 children, low maternal physical condition was associated with childhood intelligence, but so were several other factors, including birth outside of marriage and preterm birth. The most important predictor was father's social class, even after adjustment for maternal characteristics (Lawlor et al., 2005). Similarly, another study showed that exposure to long term poverty had a greater effect on socioemotional and cognitive development than maternal age, drinking or smoking in pregnancy (Korenman et al., 1995). These examples also highlight the need to consider the larger social and structural contexts that shape maternal and paternal health behaviours.
For outcomes that are likely to be strongly influenced by genetics and similarities between parents (e.g. due to assortative mating), overestimation of the maternal effect due to non-paternity can be mitigated by conducting sensitivity analyses adjusting the maternal and paternal effects for simulated levels of non-paternity (Lawlor and Mishra, 2009). Increased quality of data on partners and wider societal influences and minimising the structural bias in DOHaD data will help reduce issues around missing data, measurement error and selection bias, which will improve statistical power and the ability to compare relative maternal and non-maternal/postnatal effects.
Imbalance in the DOHaD literature can be redressed by increasing the intensity of research on non-maternal (including paternal) effects and encouraging authors to publish negative results on both maternal and paternal effects. There is a need for increased criticality amongst authors, reviewers, editors and funders of how underlying assumptions about maternal pregnancy effects can influence publication bias.
3.4. Place risk in context in translation of DOHaD research
To ameliorate concerns about increased surveillance and legal targeting of mothers, researchers and the media have a responsibility to accurately describe the strength of findings and to contextualize them within both the broader scientific literature and the social environment (Richardson et al., 2014). When paternal factors have not been explored, this should be conveyed as conditioning or limiting any conclusions about specific maternal responsibility. Where evidence for paternal effects is available, as in the case of parental age and risk of autism spectrum disorders (Croen et al., 2007), health advice should be aimed at both parents. There is good reason to believe that such a strategy might be more effective; for example, smoking cessation in expectant mothers is consistently associated with her partner's provision of support for her quitting, and by his quitting himself (McBride et al., 2004). Communicators must also scrutinise and re-frame language that implies direct harm of fetuses by individual mothers, given that epidemiological research in DOHaD provides evidence of trends in populations rather than accurate prediction of risk in individuals (Davey Smith, 2011; Winett et al., 2016), and maternal (and paternal and offspring) health behaviours are influenced by systems and social inequalities, not just individual factors.
Public health advice should convey the level of risk in a way that empowers individuals with the ability to assess the evidence and form their own opinion. As an example, the UK's Chief Medical Officer currently advises that pregnant women completely abstain from drinking alcohol during pregnancy, but although the crux of this guidance is largely based on the precautionary principle, some qualitative assessment of the level of risk is also provided: “The risk of harm to the baby is likely to be low if a woman has drunk only small amounts of alcohol before she knew she was pregnant or during pregnancy … It is unlikely in most cases that their baby has been affected” (Department of Health, 2016).
3.5. Promote multidisciplinary research involving social scientists
Finally, collaboration of population health scientists with social scientists can support ongoing examination of the social implications of DOHaD research, as well as the role of social assumptions within causal reasoning. Such collaborations are best built into research proposals and funding, so that social and ethical considerations can inform science throughout the research process (Müller et al., 2017).
4. Conclusion
Assumptions that maternal factors around the time of pregnancy exert a strong causal effect on offspring health influence DOHaD evidence and its interpretation at all stages of the research process. Strategies to maintain a critical perspective on these assumptions will help to redress the imbalance in DOHaD research. Ultimately this will maximise the field's potential to improve the lives of women, men and children through a better understanding of early-life factors that shape our life-long health.
Funding information
GCS and DAL work in a unit that receives funds from the University of Bristol and UK Medical Research Council [MC_UU_00011/5 and MC_UU_00011/6]. DAL's contribution to this work is supported by grants from the US NIH [R01 DK1034] and the European Union's Seventh Framework Programme [FP/2007-2013)/ERC Grant Agreement (Grant number 66945; DevelopObese)]. DAL is a National Institute of Health Research Senior Investigator [NF-SI-0611-10196]. The views expressed in this paper are those of the authors and not necessarily any funders. The funders had no influence on the content of the paper.
Declarations of interest
None.
Acknowledgements
We wish to thank George Davey Smith, Rebecca Richmond, Luisa Zuccolo and Caroline Relton for conversations about some of the themes in this article, and/or for commenting on previous versions. We also wish to thank Janine Felix, Christian Page and Emily Oken for checking/providing the information in Table 1 about Generation R, MoBa and Project Viva, respectively.
References
- ABC News . ABC news; 2012. You Are what Your Mum Ate.http://www.abc.net.au/news/2012-03-14/you-are-what-your-mum-ate3a-obesity-research/3887848?site=melbourne [WWW Document]. [Google Scholar]
- Agricola E., Gesualdo F., Carloni E., D'Ambrosio A., Russo L., Campagna I., Pandolfi E., Tozzi A.E. Investigating paternal preconception risk factors for adverse pregnancy outcomes in a population of internet users. Reprod. Health. 2016;13:37. doi: 10.1186/s12978-016-0156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeling, R., n.d. Guynecology: Men, Medical Knowledge and Reproduction [Forthcoming] [WWW Document]. URL http://www.renealmeling.com/research.html.
- Almond D., Currie J. Killing me softly: the fetal origins hypothesis. J. Econ. Perspect. 2011;25:153–172. doi: 10.1257/jep.25.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad K. 2007. Meeting the Universe Halfway: Quantum Physics and the Entanglement of Matter and Meaning, Quantum Physics and the Entanglement of Matter and Meaning. [DOI] [Google Scholar]
- Barker D.J., Gluckman P.D., Godfrey K.M., Harding J.E., Owens J.A., Robinson J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet (London, England) 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Barker D.J., Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barker D.J., Winter P.D., Osmond C., Margetts B., Simmonds S.J. Weight in infancy and death from ischaemic heart disease. Lancet (London, England) 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Barker D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y., Cooper R., Kuh D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int. J. Epidemiol. 2016;45:973–988. doi: 10.1093/ije/dyw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd A., Golding J., Macleod J., Lawlor D.A., Fraser A., Henderson J., Molloy L., Ness A., Ring S., Davey Smith G. Cohort profile: the “children of the 90s”—the index offspring of the avon longitudinal study of parents and children. Int. J. Epidemiol. 2013;42(1):111–127. doi: 10.1093/ije/dys064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J.M., Messerlian C., Hauser R. Fathers matter: why It's time to consider the impact of paternal environmental exposures on Children's health. Curr. Epidemiol. Reports. 2017;4:46–55. doi: 10.1007/s40471-017-0098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson C. 2014. Maximising the Involvement of Fathers And/or Partners in Life Study: what Can We Learn from Other Relevant UK Studies? [Google Scholar]
- Corsi D.J., Subramanian S.V., Ackerson L.K., Davey Smith G. Is there a greater maternal than paternal influence on offspring adiposity in India? Arch. Dis. Child. 2015;100:973–979. doi: 10.1136/archdischild-2014-307690. [DOI] [PubMed] [Google Scholar]
- Croen L.A., Najjar D.V., Fireman B., Grether J.K. Maternal and paternal age and risk of autism spectrum disorders. Arch. Pediatr. Adolesc. Med. 2007;161:334. doi: 10.1001/archpedi.161.4.334. [DOI] [PubMed] [Google Scholar]
- Daniels C.R. 2011. Exposing Men: the Science and Politics of Male Reproduction, Exposing Men: the Science and Politics of Male Reproduction. [DOI] [Google Scholar]
- Davey Smith G. Epidemiology, epigenetics and the “Gloomy Prospect”: embracing randomness in population health research and practice. Int. J. Epidemiol. 2011;40:537–562. doi: 10.1093/ije/dyr117. [DOI] [PubMed] [Google Scholar]
- Davey Smith G. Assessing intrauterine influences on offspring health outcomes: can epidemiological studies yield robust findings? Basic Clin. Pharmacol. Toxicol. 2008;102:245–256. doi: 10.1111/j.1742-7843.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- Davey Smith G., Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey Smith G., Steer C., Leary S., Ness A. Is there an intrauterine influence on obesity? Evidence from parent child associations in the Avon Longitudinal Study of Parents and Children (ALSPAC) Arch. Dis. Child. 2007;92:876–880. doi: 10.1136/adc.2006.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J., Savani S., Krempley B.D., Nguyen M., Kitlinska J.B. Influence of paternal preconception exposures on their offspring: through epigenetics to phenotype. Am. J. Stem Cells. 2016;5:11–18. [PMC free article] [PubMed] [Google Scholar]
- Department of Health . 2016. UK Chief Medical Officers' Alcohol Guidelines Review: Summary of the Proposed New Guidelines.https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/489795/summary.pdf [WWW Document] [Google Scholar]
- Dunn K.M. Patterns of consent in epidemiologic research: evidence from over 25,000 responders. Am. J. Epidemiol. 2004;159:1087–1094. doi: 10.1093/aje/kwh141. [DOI] [PubMed] [Google Scholar]
- Farrar D., Duley L. Commentary: but why should women be weighed routinely during pregnancy? Int. J. Epidemiol. 2007;36:1283–1284. doi: 10.1093/ije/dym210. [DOI] [PubMed] [Google Scholar]
- Fleten C., Nystad W., Stigum H., Skjaerven R., Lawlor D.A., Davey Smith G., Naess O. Parent-offspring body mass index associations in the Norwegian mother and child cohort study: a family-based approach to studying the role of the intrauterine environment in childhood adiposity. Am. J. Epidemiol. 2012;176:83–92. doi: 10.1093/aje/kws134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D.A., Augustyn M., Knight W.G., Pell T., Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: a systematic review. J. Am. Med. Assoc. 2001;285:1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A., Macdonald-Wallis C., Tilling K., Boyd A., Golding J., Davey Smith G., Henderson J., Macleod J., Molloy L., Ness A., Ring S., Nelson S.M., Lawlor D.A. Cohort profile: the avon longitudinal study of parents and children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013;42:97–110. doi: 10.1093/ije/dys066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudino J.A., Jenkins B., Rochat R.W. No fathers' names: a risk factor for infant mortality in the State of Georgia, USA. Soc. Sci. Med. 1999;48:253–265. doi: 10.1016/s0277-9536(98)00342-6. [DOI] [PubMed] [Google Scholar]
- Hacking I. 1995. The Looping Effects of Human Kinds. Causal Cogn. An Interdiscip. Approach. [DOI] [Google Scholar]
- Holdcroft A. Gender bias in research: how does it affect evidence based medicine? J. R. Soc. Med. 2007;100:2–3. doi: 10.1177/014107680710000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley R., Neil A., Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- Ioannidis J.P.A. Why most published research findings are false. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan K. 2014. Fathers and Partners in National and International Birth Cohort Studies. [Google Scholar]
- Kooijman M.N., Kruithof C.J., van Duijn C.M., Duijts L., Franco O.H., van IJzendoorn M.H., de Jongste J.C., Klaver C.C.W., van der Lugt A., Mackenbach J.P., Moll H.A., Peeters R.P., Raat H., Rings E.H.H.M., Rivadeneira F., van der Schroeff M.P., Steegers E.A.P., Tiemeier H., Uitterlinden A.G., Verhulst F.C., Wolvius E., Felix J.F., Jaddoe V.W.V. The Generation R Study: design and cohort update 2017. Eur. J. Epidemiol. 2016;31:1243–1264. doi: 10.1007/s10654-016-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenbrot C.C., Moss N.E. Preconception, prenatal, perinatal, and postnatal influences on health. In: Smedley B., Syme S., editors. Institute of Medicine (US) Committee on Capitalizing on Social Science and Behavioral Research to Improve the Public's Health. Promoting Health: Intervention Strategies from Social and Behavioral Research. National Academies Press (US); Washington DC: 2000. [PubMed] [Google Scholar]
- Korenman S., Miller J.E., Sjaastad J.E. Long-term poverty and child development in the United States: results from the NLSY. Child. Youth Serv. Rev. 1995;17:127–155. doi: 10.1016/0190-7409(95)00006-X. [DOI] [Google Scholar]
- Langley K., Heron J., Davey Smith G., Thapar A. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. Am. J. Epidemiol. 2012;176:261–268. doi: 10.1093/aje/kwr510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K., Rice F., van den Bree M.B.M., Thapar A. Maternal smoking during pregnancy as an environmental risk factor for attention deficit hyperactivity disorder behaviour. A review. Minerva Pediatr. 2005;57:359–371. [PubMed] [Google Scholar]
- Lawlor D.A. The society for social medicine john pemberton lecture 2011. Developmental overnutrition--an old hypothesis with new importance? Int. J. Epidemiol. 2013;42:7–29. doi: 10.1093/ije/dys209. [DOI] [PubMed] [Google Scholar]
- Lawlor D.A. Quality in epidemiological research: should we be submitting papers before we have the results and submitting more hypothesis-generating research? Int. J. Epidemiol. 2007;36:940–943. doi: 10.1093/ije/dym168. [DOI] [PubMed] [Google Scholar]
- Lawlor D.A., Batty G.D., Morton S.M.B., Deary I.J., Macintyre S., Ronalds G., Leon D.A. Early life predictors of childhood intelligence: evidence from the Aberdeen children of the 1950s study. J. Epidemiol. Community Health. 2005;59:656–663. doi: 10.1136/jech.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.A., Davey Smith G., O'Callaghan M., Alati R., Mamun A.A., Williams G.M., Najman J.M. Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the mater-university study of pregnancy and its outcomes. Am. J. Epidemiol. 2007;165:418–424. doi: 10.1093/aje/kwk030. [DOI] [PubMed] [Google Scholar]
- Lawlor D.A., Lichtenstein P., Långström N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation. 2011;123:258–265. doi: 10.1161/CIRCULATIONAHA.110.980169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.A., Mishra G.D. 2009. Family Matters: Designing, Analysing and Understanding Family Based Studies in Life Course Epidemiology, Family Matters: Designing, Analysing and Understanding Family Based Studies in Life Course Epidemiology. [DOI] [Google Scholar]
- Lawlor D.A., Relton C., Sattar N., Nelson S.M. Maternal adiposity--a determinant of perinatal and offspring outcomes? Nat. Rev. Endocrinol. 2012;8:679–688. doi: 10.1038/nrendo.2012.176. [DOI] [PubMed] [Google Scholar]
- Lawlor D.A., Richmond R., Warrington N., McMahon G., Davey Smith G., Bowden J., Evans D.M. Using Mendelian randomization to determine causal effects of maternal pregnancy (intrauterine) exposures on offspring outcomes: sources of bias and methods for assessing them. Wellcome open Res. 2017;2:11. doi: 10.12688/wellcomeopenres.10567.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.A., Tilling K., Davey Smith G. Triangulation in aetiological epidemiology. Int. J. Epidemiol. 2017;45 doi: 10.1093/ije/dyw314. dyw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D.A., Timpson N.J., Harbord R.M., Leary S., Ness A., McCarthy M.I., Frayling T.M., Hattersley A.T., Davey Smith G. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombrozo T. 2014. Using Science to Blame Mothers.https://www.npr.org/sections/13.7/2014/08/25/343121679/using-science-to-blame-mothers-check-your-values [WWW Document]. 13.7 Cosm. Cult. NPR. [Google Scholar]
- Lombrozo T., Vasilyeva N. The Oxford Handbook of Causal Reasoning. 2017. Causal explanation; pp. 415–432. [Google Scholar]
- Lumey L.H., Stein A.D., Susser E. Prenatal famine and adult health. Annu. Rev. Publ. Health. 2011;32:237–262. doi: 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P., Birke C., Vejrup K., Haugan A., Alsaker E., Daltveit A.K., Handal M., Haugen M., Høiseth G., Knudsen G.P., Paltiel L., Schreuder P., Tambs K., Vold L., Stoltenberg C. Cohort profile update: the Norwegian mother and child cohort study (MoBa) Int. J. Epidemiol. 2016;45:382–388. doi: 10.1093/ije/dyw029. [DOI] [PubMed] [Google Scholar]
- Mamluk L., Edwards H.B., Savović J., Leach V., Jones T., Moore T.H.M., Ijaz S., Lewis S.J., Donovan J.L., Lawlor D., Fraser A., Zuccolo L. Low alcohol consumption and pregnancy and childhood outcomes: time to change guidelines indicating apparently “safe” levels of alcohol during pregnancy? A systematic review and meta-analyses. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-015410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride C.M., Baucom D.H., Peterson B.L., Pollak K.I., Palmer C., Westman E., Lyna P. Prenatal and postpartum smoking abstinence. Am. J. Prev. Med. 2004;27:232–238. doi: 10.1016/j.amepre.2004.06.005. [DOI] [PubMed] [Google Scholar]
- McPhate M. 2016. Bartenders Can't Refuse Pregnant Women Alcohol, New York City Says.https://www.nytimes.com/2016/05/10/nyregion/bartenders-cant-refuse-pregnant-women-alcohol-new-york-city-says.html [WWW Document]. New York Times. [Google Scholar]
- Mindell J.S., Giampaoli S., Goesswald A., Kamtsiuris P., Mann C., Männistö S., Morgan K., Shelton N.J., Verschuren W.M., Tolonen H., HES Response Rate Group Sample selection, recruitment and participation rates in health examination surveys in Europe – experience from seven national surveys. BMC Med. Res. Meth. 2015;15:78. doi: 10.1186/s12874-015-0072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda L., Dixon V., Reyes C. 2015. How States Handle Drug Use during Pregnancy.https://projects.propublica.org/graphics/maternity-drug-policies-by-state [WWW Document]. Pro Publica. [Google Scholar]
- MRC Vitamin Study Research Group Prevention of neural tube defects: results of the medical research Council Vitamin study. Lancet (London, England) 1991;338:131–137. [PubMed] [Google Scholar]
- Müller R., Hanson C., Hanson M., Penkler M., Samaras G., Chiapperino L., Dupré J., Kenney M., Kuzawa C., Latimer J., Lloyd S., Lunkes A., Macdonald M., Meloni M., Nerlich B., Panese F., Pickersgill M., Richardson S., Rüegg J., Schmitz S., Stelmach A., Villa P. The biosocial genome? EMBO Rep. 2017;18:1677–1682. doi: 10.15252/embr.201744953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò M.R., Nosek B.A., Bishop D.V.M., Button K.S., Chambers C.D., Percie du Sert N., Simonsohn U., Wagenmakers E.-J., Ware J.J., Ioannidis J.P.A. A manifesto for reproducible science. Nat. Hum. Behav. 2017;1:21. doi: 10.1038/s41562-016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken E., Baccarelli A.A., Gold D.R., Kleinman K.P., Litonjua A.A., De Meo D., Rich-Edwards J.W., Rifas-Shiman S.L., Sagiv S., Taveras E.M., Weiss S.T., Belfort M.B., Burris H.H., Camargo C.A., Huh S.Y., Mantzoros C., Parker M.G., Gillman M.W. Cohort profile: project viva. Int. J. Epidemiol. 2015;44:37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltrow L.M., Flavin J. Arrests of and forced interventions on pregnant women in the United States, 1973-2005: implications for women's legal status and public health. J. Health Polit. Policy Law. 2013;38:299–343. doi: 10.1215/03616878-1966324. [DOI] [PubMed] [Google Scholar]
- Pereira P.P. da S., Da Mata F.A.F., Figueiredo A.C.G., de Andrade K.R.C., Pereira M.G. Maternal active smoking during pregnancy and low birth weight in the americas: a systematic review and meta-analysis. Nicotine Tob. Res. 2017;19:497–505. doi: 10.1093/ntr/ntw228. [DOI] [PubMed] [Google Scholar]
- Purnell B.A. The nutritional sins of the mother. Science. 2014;345 doi: 10.1126/science.345.6198.782-j. (80-. ) 782–782. [DOI] [Google Scholar]
- Redshaw M., Heikkila K. vol. 63. Natl. Perinat. Epidemiol. Unit, Univ. Oxford; 2010. (Delivered with Care: a National Survey of Women's Experience of Maternity Care 2010). [Google Scholar]
- Rice F., Harold G.T., Boivin J., Hay D.F., van den Bree M., Thapar A. Disentangling prenatal and inherited influences in humans with an experimental design. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2464–2467. doi: 10.1073/pnas.0808798106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson S.S. Maternal bodies in the postgenomic order. Postgenomics. 2015:210–231. doi: 10.1215/9780822375449. [DOI] [Google Scholar]
- Richardson S.S., Almeling R. 2016. The CDC Risks its Credibility with New Pregnancy Guidelines.https://www.bostonglobe.com/opinion/2016/02/08/the-cdc-risks-its-credibility-with-new-pregnancy-guidelines/2SCHzNCqcWNDRguol7kzwK/story.html [WWW Document]. Boston Globe. [Google Scholar]
- Richardson S.S., Daniels C.R., Gillman M.W., Golden J., Kukla R., Kuzawa C., Rich-Edwards J. Society: don't blame the mothers. Nature. 2014;512:131–132. doi: 10.1038/512131a. [DOI] [PubMed] [Google Scholar]
- Richmond R.C., Timpson N.J., Felix J.F., Palmer T., Gaillard R., McMahon G., Davey Smith G., Jaddoe V.W., Lawlor D.A. Using genetic variation to explore the causal effect of maternal pregnancy adiposity on future offspring adiposity: a mendelian randomisation study. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanus S., Neven P., Soubry A. Extending the Developmental Origins of Health and Disease theory: does paternal diet contribute to breast cancer risk in daughters? Breast Cancer Res. 2016;18:103. doi: 10.1186/s13058-016-0760-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawe J., Delbaere I., Ekstrand M., Hegaard H.K., Larsson M., Mastroiacovo P., Stern J., Steegers E., Stephenson J., Tydén T. Preconception care policy, guidelines, recommendations and services across six European countries: Belgium (Flanders), Denmark, Italy, The Netherlands, Sweden and the United Kingdom. Eur. J. Contracept. Reprod. Health Care. 2015;20:77–87. doi: 10.3109/13625187.2014.990088. [DOI] [PubMed] [Google Scholar]
- Sommerfeld D.P. The origins of mother blaming: historical perspectives on childhood and motherhood. Infant Ment. Health J. 1989;10:14–24. https://doi.org/10.1002/1097-0355(198921)10:1<14::AID-IMHJ2280100103>3.0.CO;2-Y. [Google Scholar]
- Soubry A. Epigenetics as a driver of developmental origins of health and disease: did we forget the fathers? Bioessays. 2017:1700113. doi: 10.1002/bies.201700113. [DOI] [PubMed] [Google Scholar]
- Stern J.M., Simes R.J. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ. 1997;315 doi: 10.1136/bmj.315.7109.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H., Wen S.W., Walker M., Demissie K. Missing paternal demographics: a novel indicator for identifying high risk population of adverse pregnancy outcomes. BMC Pregnancy Childbirth. 2004;4:21. doi: 10.1186/1471-2393-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolonen H., Ahonen S., Jentoft S., Kuulasmaa K., Heldal J., European Health Examination Pilot Project Differences in participation rates and lessons learned about recruitment of participants – the european health examination survey pilot project. Scand. J. Publ. Health. 2015;43:212–219. doi: 10.1177/1403494814565692. [DOI] [PubMed] [Google Scholar]
- Tyrrell J., Huikari V., Christie J.T., Cavadino A., Bakker R., Brion M.-J.A., Geller F., Paternoster L., Myhre R., Potter C., Johnson P.C.D., Ebrahim S., Feenstra B., Hartikainen A.-L., Hattersley A.T., Hofman A., Kaakinen M., Lowe L.P., Magnus P., McConnachie A., Melbye M., Ng J.W.Y., Nohr E.A., Power C., Ring S.M., Sebert S.P., Sengpiel V., Taal H.R., Watt G.C.M., Sattar N., Relton C.L., Jacobsson B., Frayling T.M., Sørensen T.I.A., Murray J.C., Lawlor D.A., Pennell C.E., Jaddoe V.W.V., Hypponen E., Lowe W.L., Jarvelin M.-R., Davey Smith G., Freathy R.M. Genetic variation in the 15q25 nicotinic acetylcholine receptor gene cluster (CHRNA5–CHRNA3–CHRNB4) interacts with maternal self-reported smoking status during pregnancy to influence birth weight. Hum. Mol. Genet. 2012;21:5344–5358. doi: 10.1093/hmg/dds372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargesson N. Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res. Part C Embryo Today - Rev. 2015;105:140–156. doi: 10.1002/bdrc.21096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez-Manoff M. 2015. Should You Bring Your Unborn Baby to Work?https://www.theatlantic.com/magazine/archive/2015/03/should-you-bring-your-unborn-baby-to-work/384977/ [WWW Document]. Atl. [Google Scholar]
- Wadhwa P.D., Buss C., Entringer S., Swanson J.M. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner M.R. Cultivating the maternal future: public health and the prepregnant self. Signs J. Women Cult. Soc. 2015;40:939–962. doi: 10.1086/680404. [DOI] [Google Scholar]
- Warin M., Zivkovic T., Moore V., Davies M. Mothers as smoking guns: fetal overnutrition and the reproduction of obesity. Fem. Psychol. 2012;22:360–375. doi: 10.1177/0959353512445359. [DOI] [Google Scholar]
- Winett L.B., Wulf A.B., Wallack L. Framing strategies to avoid mother-blame in communicating the origins of chronic disease. Am. J. Publ. Health. 2016;106:1369–1373. doi: 10.2105/AJPH.2016.303239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J., Small N., Raynor P., Tuffnell D., Bhopal R., Cameron N., Fairley L., Lawlor D.A., Parslow R., Petherick E.S., Pickett K.E., Waiblinger D., West J. Cohort Profile: the Born in Bradford multi-ethnic family cohort study. Int. J. Epidemiol. 2013;42:978–991. doi: 10.1093/ije/dys112. [DOI] [PubMed] [Google Scholar]
- Yu Z., Han S., Zhu J., Sun X., Ji C., Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Elgizouli M., Schöttker B., Holleczek B., Nieters A., Brenner H. Smoking-associated DNA methylation markers predict lung cancer incidence. Clin. Epigenet. 2016;8:127. doi: 10.1186/s13148-016-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]