The protective efficacy of IPTp with dihydroartemisinin-piperaquine was strongly associated with higher piperaquine exposure. Modeling suggests that daily or weekly administration of lower dosages of dihydroartemisinin-piperaquine, compared to standard dosing, will maintain piperaquine levels above target concentrations, potentially limiting toxicity.
Keywords: intermittent preventive treatment for malaria in pregnancy, dihydroartemisinin-piperaquine, Plasmodium falciparum, pharmacokinetic/pharmacodynamic modeling
Abstract
Background
Dihydroartemisinin-piperaquine (DHA-PQ) is highly efficacious as intermittent preventive therapy for malaria during pregnancy (IPTp). Determining associations between piperaquine (PQ) exposure, malaria risk, and adverse birth outcomes informs optimal dosing strategies.
Methods
Human immunodeficiency virus–uninfected pregnant women (n = 300) were enrolled in a placebo-controlled trial of IPTp at 12–20 weeks’ gestation and randomized to sulfadoxine-pyrimethamine every 8 weeks, DHA-PQ every 8 weeks, or DHA-PQ every 4 weeks during pregnancy. Pharmacokinetic sampling for PQ was performed every 4 weeks, and an intensive pharmacokinetic substudy was performed in 30 women at 28 weeks’ gestation. Concentration-effect relationships were assessed between exposure to PQ; the prevalence of Plasmodium falciparum infection during pregnancy; outcomes at delivery including placental malaria, low birth weight, and preterm birth; and risks for toxicity. Simulations of new dosing scenarios were performed.
Results
Model-defined PQ target venous plasma concentrations of 13.9 ng/mL provided 99% protection from P. falciparum infection during pregnancy. Each 10-day increase in time above target PQ concentrations was associated with reduced odds of placental parasitemia, preterm birth, and low birth weight, though increases in PQ concentrations were associated with QT interval prolongation. Modeling suggests that daily or weekly administration of lower dosages of PQ, compared to standard dosing, will maintain PQ trough levels above target concentrations with reduced PQ peak levels, potentially limiting toxicity.
Conclusions
The protective efficacy of IPTp with DHA-PQ was strongly associated with higher drug exposure. Studies of the efficacy and safety of alternative DHA-PQ IPTp dosing strategies are warranted.
Clinical Trials Registration
In sub-Saharan Africa, >25 million pregnancies occur each year in areas where malaria is endemic, and malaria in pregnancy is estimated to cause nearly 1 million low-birth-weight deliveries [1–3]. The World Health Organization recommends malaria preventive measures in African countries where Plasmodium falciparum remains endemic, including use of long-lasting insecticide-treated bed nets (LLIN) and intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine (IPTp-SP) [4]. Despite these measures, rates of placental malaria and poor birth outcomes are high in many parts of Africa. In Tororo District in eastern Uganda, the prevalence of histopathologically confirmed placental malaria was 50% among women receiving both LLINs and IPTp-SP [5]. This and other studies have raised concern for waning efficacy of both LLINs due to resistance to pyrethroid insecticides [6–8], and SP due to P. falciparum resistance [9].
IPTp with dihydroartemisinin-piperaquine (DHA-PQ) [5, 10] offers a promising alternative to IPT-SP, as it provides rapid killing of circulating parasites by the short-acting artemisinin, DHA, and prolonged posttreatment prophylaxis due to the long PQ half-life [11]. Pregnant women living in Tororo randomized to IPTp with DHA-PQ had a significantly reduced burden of malaria and lower prevalence of histopathologically confirmed placental malaria compared to women randomized to IPTp-SP [5]. DHA-PQ dosed every 4 weeks was superior to DHA-PQ every 8 weeks, but did not eliminate malaria risks. Similarly, pregnant women living in western Kenya randomized to IPTp with DHA-PQ had significantly lower prevalence of malaria infection during pregnancy and at delivery compared to those receiving IPTp with SP or undergoing intermittent screening and treatment with DHA-PQ [10].
Associations during pregnancy between DHA-PQ pharmacokinetic (PK) exposure, risks of malaria, adverse birth outcomes, and potential toxicity have not been previously defined, and are necessary to optimize DHA-PQ regimens for IPTp. To address this goal, we performed population PK sampling every 4 weeks during pregnancy, and integrated data from an intensive PK substudy [12], to develop a detailed, population pharmacokinetics/pharmacodynamics (PK/PD) model.
METHODS
Study Design and Clinical Procedures
This study was part of a double-blinded randomized controlled trial comparing SP every 8 weeks vs DHA-PQ every 8 weeks or every 4 weeks among 300 HIV-uninfected women [5] in Tororo, Uganda, an area of high malaria transmission intensity [13]. Eligible participants were pregnant women of all gravidities with an estimated gestational age between 12 and 20 weeks confirmed by ultrasound. Complete entry criteria have been summarized previously [5]. Study participants provided written informed consent. Women received an LLIN at enrollment and all medical care at a dedicated study clinic. Routine visits occurred every 4 weeks, including dried blood spot collections to determine the presence or absence of P. falciparum DNA using loop-mediated isothermal amplification (LAMP; Eiken Chemical, Japan) [14]. Women presenting with fever (temperature ≥38.0°C) or history of fever in the previous 24 hours and with Plasmodium parasites by thick smear were diagnosed with malaria and treated with artemether-lumefantrine. Adverse events (AEs) were assessed and graded according to standardized criteria at every visit [15]. At delivery, an evaluation for adverse birth outcomes including birth weight and preterm birth was completed, and biological specimens including placental blood and tissue were collected.
Procedures were in accordance with the ethical standards of the responsible committees on human experimentation of Makerere University, the Uganda National Council of Science and Technology, and the University of California, San Francisco.
Study Drug Administration
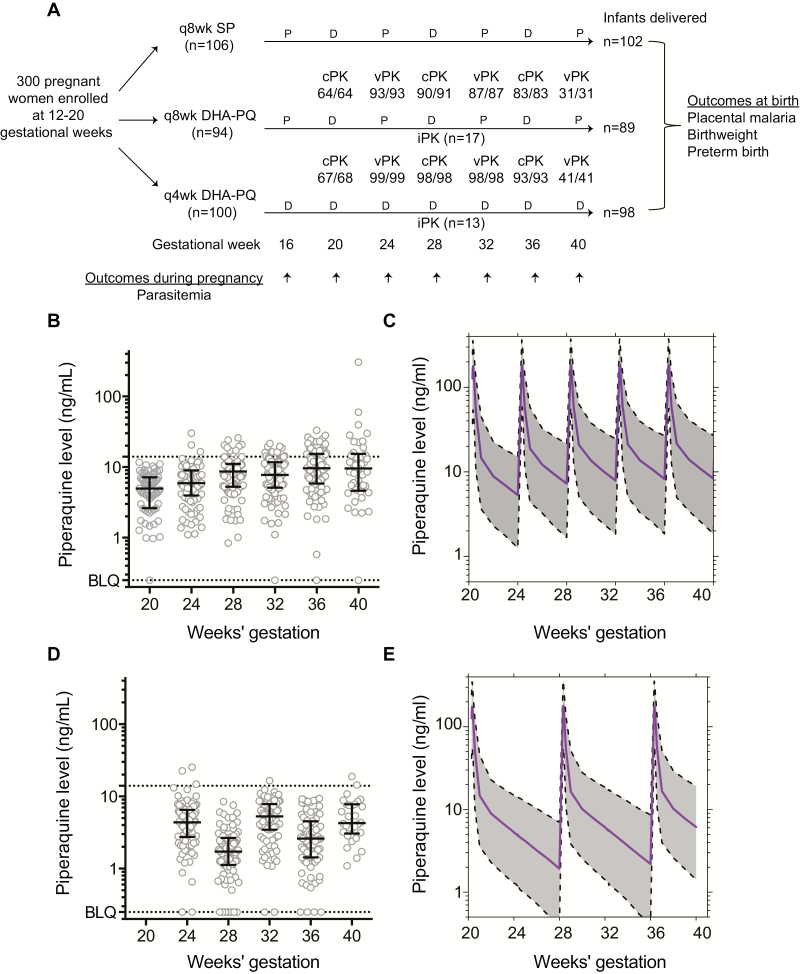
Study pharmacists allocated treatment and prepared study drugs [5]. Each treatment (dose) with DHA-PQ (Duo-Cotexin, Holley-Cotec, Beijing, China, 40 mg/320 mg tablets, Ugandan national drug authority registration number NDA/MAL/HDP/2743) consisted of 3 tablets given once daily for 3 consecutive days. Participants allocated to every-8-week DHA-PQ received study drugs at 20, 28, and 36 weeks of gestational age. Women allocated to every-4-week DHA-PQ received study drugs starting at either 16 or 20 weeks of gestational age (Figure 1A). Matching placebos were used so all women received the same number of pills.
Figure 1.
Trial profile and derivation of population pharmacokinetics (PK) model. A, Trial profile showing women enrolled, randomized, and sampled for piperaquine during pregnancy; infants delivered; and outcomes assessed. Numbers below population PK sampling show samples analyzed/collected at each timepoint. B–E, Raw and modeled PK data between dihydroartemisinin-piperaquine dosing every 4 weeks (B and C) and every 8 weeks (D and E). Abbreviations: BLQ, below the limit of quantitation; cPK, capillary sample; D, active drug; DHA-PQ, dihydroartemisinin-piperaquine; iPK, intensive PK study; P, placebo; q4wk, every 4 weeks; q8wk, every 8 weeks; SP, sulfadoxine-pyrimethamine; vPK, venous sample.
PK Sample Collection
Women provided blood samples for population PK 28 or 56 days after DHA-PQ administration. Sparse venous samples were collected at 24, 32, and 40 weeks’ gestation, and capillary samples at 20, 28, and 36 weeks’ gestation. A subset of women randomized to DHA-PQ every 8 weeks (n = 17) or every 4 weeks (n = 13) were enrolled in an intensive PK substudy completed after DHA-PQ administration around the 28-week gestational visit [12]. Sampling for PQ quantitation occurred before and after DHA-PQ administration on the third day of the 3-day treatment dose; specifically, 13 venous samples were collected from 0 to 456 hours (19 days) postdose (Supplementary Methods). PQ 24-hour capillary and venous results were used to establish a correlation that was incorporated into the final PK/PD model. Twelve-lead electrocardiograms (ECGs) were carried out prior to DHA-PQ administration on the first day and 3–4 hours following administration on the third day. QT and RR intervals were measured manually using calipers, and the corrected QT interval (QTc) was calculated using the Fridericia formula (QTcF, ), which provided better heart rate correction than the Bazett formula in this study (Supplementary Figure 1).
PQ Quantitation
PK samples were centrifuged within 60 minutes at 2000g for 10 minutes, and plasma was stored at –80°C. PQ concentrations were determined using high-performance liquid chromatography–tandem mass spectrometry, as described previously [16], with modifications to lower the calibration range to 0.5–50 ng/mL and a new calibration range of 10–1000 ng/mL. The lower limit of quantification was 0.5 ng/mL and the coefficient of variance was <10% for all quality control concentrations.
Population PK Analysis of PQ
Data were analyzed using the nonlinear mixed-effects approach in NONMEM VI and the first order conditional estimation method. An initial PK model using venous data was developed and, in a stepwise manner, capillary samples were added to a joint model (Supplementary Methods).
Malaria in Pregnancy, Adverse Events, and Delivery Outcomes
Malaria infection during pregnancy was assessed by the presence or absence of parasites at each routine visit using LAMP [5]. Grade 3–4 AEs and serious adverse events (SAEs) assessed at clinic visits were included as outcomes. Delivery outcomes were placental malaria (by histopathology or LAMP of placental blood), low birth weight (<2500 g), and preterm birth (<37 weeks).
Statistical Analysis
Statistical analyses were performed using NONMEM, R version 3.2.2, and Stata version 14 (StataCorp). Relationships between population PQ concentrations, gestational weeks of pregnancy, and parasite prevalence during pregnancy were assessed using mixed-effects logistic regression.
Derivation of Target PQ Concentrations for Protection Against Malaria Infection During Pregnancy
A simultaneous continuous-categorical PK/PD modeling approach was applied to link PQ PK data to longitudinal LAMP measurements by mixed-effects logistic regression modeling using NONMEM and the LAPLACE method. Target concentrations were defined as venous plasma PQ concentrations using the upper bounds of uncertainty for 95% and 99% protection. Once the target concentration was estimated by integration, secondary PK parameters, such as cumulative area under the concentration–time curve (AUC), time above target concentration, and cumulative AUC above target concentration were derived.
PK/PD Analysis of Delivery and Safety Outcomes
Parameters derived from the population PK model (cumulative AUC, time above target concentration, and cumulative AUC above target concentration), were used to establish exposure–response models for delivery outcomes using log-binomial regression models. Similarly, the relationship between PQ exposure parameters and any grade 3–4 AEs and SAEs identified was evaluated using logistic regression modeling and/or proportional odds models. Relationship between PK and QTc was modeled using simultaneous PK/PD modeling (Supplementary Methods).
PK/PD Analysis: Simulations
Simulations of new dosing scenarios were performed using final PK/PD models.
RESULTS
Study Cohort and Clinical Outcomes
Between June and October 2014, 300 women were enrolled and randomized; 106 to SP every 8 weeks, 94 to DHA-PQ every 8 weeks, and 100 to DHA-PQ every 4 weeks (Figure 1A). At enrollment, mean age was 22 years, 69% were ≤16 weeks’ gestation, 37% were primigravida, and 57% had malaria parasites detected by LAMP in peripheral blood. PQ analysis was completed in >99% of samples collected every 4 weeks; 289 women (96.3%) were followed through delivery and 282 (94.0%) had placental tissue collected for histopathology.
Derivation of Population PK Model
There was a significant increase in measured PQ trough concentrations over the course of pregnancy among women randomized to every-4-week DHA-PQ (mean, 5.0 ng/mL at 24 weeks’ gestation vs 19.5 ng/mL at 40 weeks’ gestation, P < .001; Figure 1B), and to every-8-week DHA-PQ (mean, 4.97 ng/mL at 24 weeks’ gestation vs 5.95 ng/mL at 40 weeks’ gestation, P = .05; Figure 1D). A linear model with a venous/capillary ratio of 0.91 best fit the correlation of venous and capillary concentrations (Supplementary Figure 2).
A 2-compartment model using intensive (n = 419) and sparse (n = 874) PK data adequately predicted plasma concentrations and provided improved stability compared to a 3-compartment model, which has previously been reported for pregnant women receiving PQ [17, 18] (Supplementary Methods). PQ parameter estimates were consistent with previous estimates (Supplementary Table 1) [12]. Simulation-based diagnostics (visual predictive checks; Supplementary Figure 3) demonstrated good agreement between observations and model simulations.
PQ Target Concentrations for Malaria Prevention
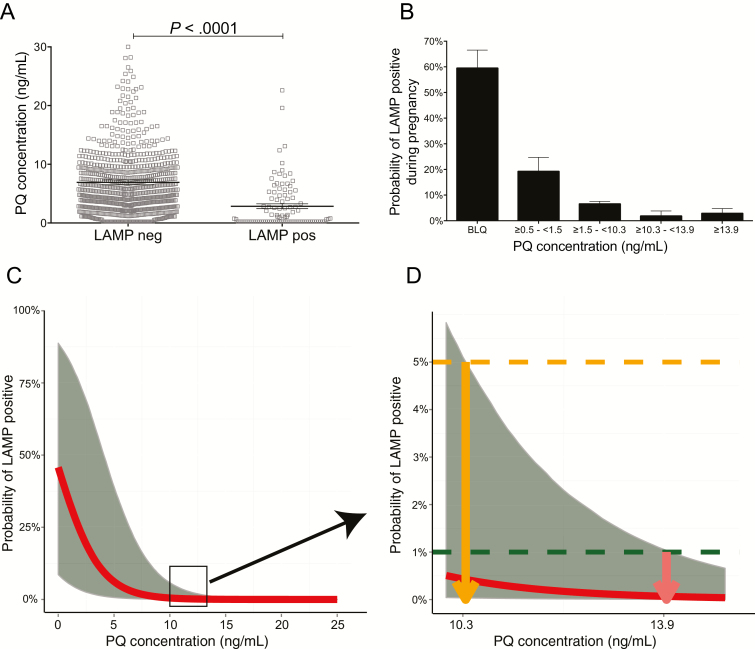
We used a concentration-effect approach to relate PQ concentrations to parasite prevalence at routine monthly visits. Samples with parasitemia had significantly lower PQ levels than those free of parasites (mean, 2.85 ng/mL vs 6.92 ng/mL, P < .001; Figure 2A). There was a strong association between PQ concentration and the probability of parasitemia. When PQ concentrations fell below the level of quantification, the probability of parasitemia was 59.7% (95% confidence interval [CI], 45.8%–72.2%); in contrast, for PQ levels >10.3 ng/mL the probability of parasitemia was 2.4% (95% CI, 0%–4.7%; Figure 2B).
Figure 2.
Derivation of target concentration to prevent parasitemia during pregnancy. A, Raw population piperaquine (PQ) levels at loop-mediated isothermal amplification (LAMP)–positive or LAMP-negative visits. B, Increasing raw population PQ levels (stratified into 5 categories) associated with lower probability of LAMP positivity during pregnancy. Marginal estimates were derived using mixed-effects logistic regression, accounting for repeated observations within individuals (below the limit of quantitation [BLQ] of 0.5 ng/mL). C and D, Modeled trough PQ levels of 10.3 and 13.9 define upper bounds of uncertainty for 95% and 99% protection from malaria infection during pregnancy, respectively. Abbreviations: LAMP, loop-mediated isothermal amplification; PQ, piperaquine.
We then performed simultaneous continuous-categorical PK/PD modeling to define target PQ concentrations protective against P. falciparum parasitemia (LAMP positivity) during pregnancy. Using this approach, we estimated that venous PQ levels of 10.3 ng/mL and 13.9 ng/mL define the upper bounds of uncertainty for 95% and 99% protection from parasitemia during pregnancy, respectively (Figure 2C and 2D).
Increasing PQ Exposure During Pregnancy Was Associated With Improved Delivery Outcomes
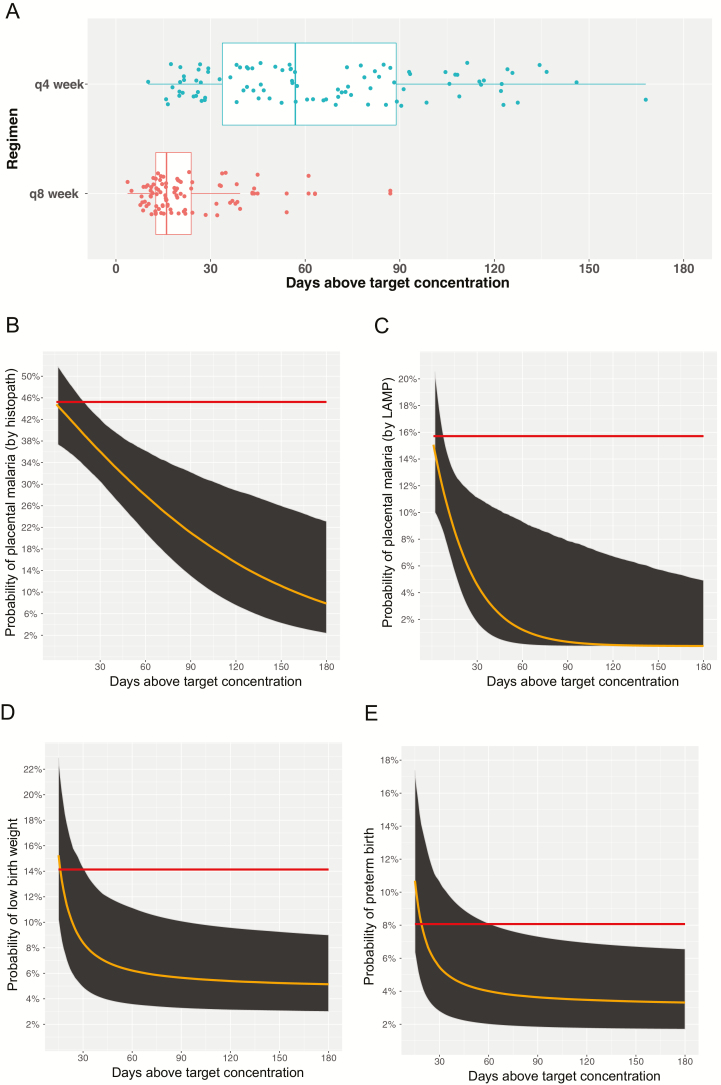
Using the PQ target concentration of 13.9 ng/mL, we derived secondary PK parameters, including cumulative AUC, time above target concentration, and cumulative AUC above target concentration, and evaluated associations between these PQ exposure parameters and delivery outcomes. The probability of placental malaria (by histopathology or placental parasitemia), preterm birth, and low birth weight were significantly reduced with increasing PQ exposure (defined as days during pregnancy concentrations remained >13.9 ng/mL; Figure 3). For every 10-day increase in time >13.9 ng/mL, there was a 7% reduction in the odds of placental malaria by histopathology, 33% reduction in the odds of placental parasitemia, and 26% reduction in the odds of either preterm birth and low birth weight (Table 1). Similar associations were found between the cumulative PQ AUC and the cumulative PQ AUC above the 13.9 ng/mL target threshold and delivery outcomes (Table 1).
Figure 3.
Increasing piperaquine (PQ) exposure associated with improved outcomes at birth. A, Days above target PQ concentration among women randomized to dihydroartemisinin-piperaquine every 4 weeks vs every 8 weeks. Probability of placental malaria (B and C), preterm birth (D), and low birth weight (E) modeled over time above target PQ concentration. Red line represents probability of outcome at delivery among women randomized to intermittent preventive treatment during pregnancy with sulfadoxine-pyrimethamine. Similar results obtained with cumulative area under the curve (AUC) and cumulative AUC above target. Abbreviations: LAMP, loop-mediated isothermal amplification; q4week, every 4 weeks; q8week, every 8 weeks.
Table 1.
Piperaquine Exposure-Response Models for Delivery Outcomes
| PK Predictor | Delivery Outcome | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Placental Malaria (Histopathology) | Any Placental Malaria (LAMP) | Preterm Birth | Low Birth Weight | |||||||||
| OR | RSE, % | P Value | OR | RSE, % | P Value | OR | RSE, % | P Value | OR | RSE, % | P Value | |
| DHA-PQ every 8 wk | 0.68 | 14 | <.01 | 0.17 | 18 | <.0001 | 1.53 | 16 | .2 | 1.11 | 17 | .23 |
| DHA-PQ every 4 wk | 0.54 | 12 | <.05 | 0.10 | 19 | <.001 | 0.63 | 16 | .3 | 0.57 | 15 | .76 |
| Cumulative PQ AUC | 0.83 | 30 | <.01 | 0.52 | 35 | <.00001 | 0.67 | 16 | <.01 | 0.67a | 16 | <.01 |
| Time above PQ target of 13.9 ng/mLb | 0.93 | 32 | <.01 | 0.67 | 54 | <.0001 | 0.74 | 19 | <.01 | 0.74c | 19 | <.05 |
| Cumulative PQ AUC above target of 13.9 ng/mLd | 0.86 | 31 | <.01 | 0.46 | 39 | <.00001 | 0.64 | 16 | <.01 | 0.64e | 16 | <.05 |
Abbreviations: AUC, area under the concentration–time curve; DHA-PQ, dihydroartemisinin-piperaquine; LAMP, loop-mediated isothermal amplification; OR, odds ratio; PQ, piperaquine; RSE, relative standard error.
aFor 1000 mg × day/L AUC increase postthreshold of AUC approximately 2000.
bFor every 10 days’ increase in time above target.
cFor 10 days’ time increase postthreshold of minimum approximately 20 days.
dFor every 1000 ng × day/L increase in AUC above target.
eFor 1000 mg × day/L AUC increase postthreshold of AUC approximately 5000.
Increasing PQ Exposure and Adverse Effects
Grade 3–4 AEs and SAEs were uncommon, and of similar incidence among treatment groups [5]. The probability (with relative standard error) of experiencing grade 3–4 AEs (14.1% [15%], 11.7% [15%], and 7% [15%] for cumulative AUC, time above target, and AUC above target, respectively) or SAEs (5.6% [15%], 9.6% [16%], and 3% [17%] for cumulative AUC, time above target, and AUC above target, respectively) were not associated with PQ PK parameters.
We modeled PQ concentrations expected at the time of ECG and found a positive correlation between PQ concentration and QTc measurement (an increase of 5 msec per 100 ng/mL increase in PQ concentration, P < .001; Supplementary Figure 4B), consistent with published reports.
PK/PD Modeling Suggests Improved Efficacy if DHA-PQ Is Administered as a Single Daily or Weekly Dosage
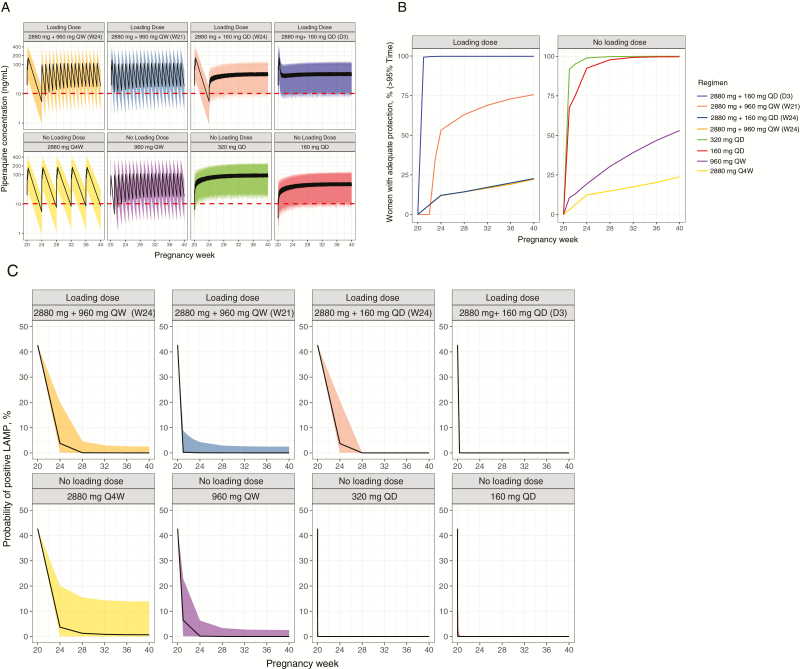
We hypothesize that DHA-PQ dose modifications, offering lower doses more frequently, will be associated with improved exposure and efficacy against malaria, and lower peak concentrations, limiting AEs [19]. Using our PK/PD model, we evaluated several potential dosing strategies (Figure 4A). Our model predicted that more frequent (daily or weekly) PQ administration, with or without a loading dose of a standard 3-day treatment course at the start of chemoprevention, would be associated with a greater proportion of women exceeding target concentrations for >95% of the time during pregnancy (Figure 4B). Accordingly, these models suggest that daily or weekly PQ administration, with or without a treatment loading dose, would be associated with a lower probability of parasitemia during pregnancy (Figure 4C).
Figure 4.
Simulated piperaquine (PQ) concentrations for dihydroartemisinin-piperaquine dosing with or without an initial loading dose for chemoprevention during pregnancy. A, Modeled PQ concentrations over weeks of pregnancy for 8 different dosing strategies. All simulations used 5000 virtual subjects, with chemoprevention initiation at 20 weeks, and continuing through 40 weeks of gestation. Regimens indicate PQ dosing in mg. For regimens with loading dose, daily regimens begin either 3 days (D3) or 4 weeks (W24) following loading dose, and weekly regimens begin either 1 week (W21) or 4 weeks (W24) following loading dose. The horizontal dotted line represents a target PQ concentration of 10.3 ng/mL. B, Percentage of women predicted to have >95% of duration of pregnancy above target concentration for each dosing regimen. C, Model-predicted probabilities of parasitemia during pregnancy with different dosing regimens. Abbreviations: LAMP, loop-mediated isothermal amplification; Q4W, every 4 weeks; QD, once daily; QW, once weekly.
Regarding outcomes at delivery, for women with median PQ clearance, our model predicted that single daily or weekly PQ administration would be associated with a lower probability of placental malaria by histopathology in comparison to standard monthly dosing (Table 2; Supplementary Figure 5A), with minimal additional reduction in placental malaria if a loading dose was provided. Furthermore, our model predicted that, for high-risk women in the ≥95th percentile of PQ clearance (ie, low PQ exposure during pregnancy), protection against placental malaria and adverse birth outcomes would be greater with single daily and/or weekly administration of PQ than with standard monthly dosing (Table 2). Importantly, single daily or weekly administration is predicted to yield lower PQ peak concentrations (Supplementary Figure 5B), suggesting decreased risk of dose-dependent QTc prolongation (Supplementary Figure 5C).
Table 2.
Impact of Different Regimens on Probability of Delivery Outcomes
| Regimen | Delivery Outcome | |||||
|---|---|---|---|---|---|---|
| PQ Dosing Regimen | Loading Dose (Standard Treatment Dose) |
Frequency | Probability of Positive LAMP | Probability of Positive Histopathology | Probability of Low Birth Weight | Probability of Preterm Birth |
| Woman with typical (median) PQ clearance | ||||||
| 2880 mg + 160 mg QD (day 3) | Yes | Daily | <0.01 (<.01–.15) | 0.12 (.05–.27) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 2880 mg + 160 mg QD (week 24) | Yes | Daily | <0.01 (<.01–.14) | 0.15 (.08–.29) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 2880 mg + 960 mg QW (week 21) | Yes | Weekly | <0.01 (<.01–.15) | 0.13 (.06–.28) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 2880 mg + 960 mg QW (week 24) | Yes | Weekly | <0.01 (<.01–.14) | 0.17 (.09–.30) | 0.05 (.03–.09) | 0.04 (.02–.07) |
| 2880 mg Q4W | No | Monthly | <0.01 (<.01–.13) | 0.26 (.19–.35) | 0.06 (.03–.11) | 0.04 (.02–.08) |
| 160 mg QD | No | Daily | <0.01 (<.01–.15) | 0.12 (.05–.27) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 320 mg QD | No | Daily | <0.01 (<.01–.15) | 0.12 (.05–.27) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 960 mg QW | No | Weekly | <0.01 (<.01–.14) | 0.14 (.07–.28) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| High-risk woman with high clearance (95% percentile of clearance distribution) | ||||||
| 2880 mg + 160 mg QD (day 3) | Yes | Daily | <0.01 (<.01–.15) | 0.12 (.05–.27) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 2880 mg + 160 mg QD (week 24) | Yes | Daily | <0.01 (<.01–.14) | 0.16 (.08–.29) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 2880 mg + 960 mg QW (week 21) | Yes | Weekly | 0.03 (<.01–.13) | 0.33 (.28–.40) | 0.07 (.04–.13) | 0.05 (.02–.09) |
| 2880 mg + 960 mg QW (week 24) | Yes | Weekly | 0.04 (<.01–.13) | 0.35 (.29–.41) | 0.08 (.04–.13) | 0.05 (.03–.10) |
| 2880 mg Q4W | No | Monthly | 0.07 (.02–.13) | 0.39 (.33–.45) | 0.11 (.07–.18) | 0.08 (.04–.14) |
| 160 mg QD | No | Daily | <0.01 (<.01–.15) | 0.13 (.06–.27) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 320 mg QD | No | Daily | <0.01 (<.01–.15) | 0.13 (.05–.27) | 0.05 (.03–.09) | 0.03 (.02–.07) |
| 960 mg QW | No | Weekly | 0.03 (<.01–.13) | 0.34 (.28–.40) | 0.07 (.04–.13) | 0.05 (.02–.10) |
Data are presented as probability (95% confidence intervals).
Abbreviations: LAMP, loop-mediated isothermal amplification; PQ, piperaquine; Q4W, every 4 weeks; QD, once daily; QW, once weekly.
DISCUSSION
Control of malaria in pregnancy requires not only eliminating circulating parasites, but also preventing placental malaria and improving birth outcomes. Although we previously reported that IPTp with DHA-PQ conferred superior protection against malaria in pregnancy compared to IPTp-SP, IPTp with DHA-PQ every 4 weeks did not eliminate risks [5]. This PK/PD analysis defined target PQ concentrations associated with protection against malaria infection during pregnancy. Importantly, increasing time above target PQ concentrations during pregnancy was associated with improved outcomes at delivery, including placental malaria, preterm birth, and low birth weight. Furthermore, modeling suggests that alternative regimens for DHA-PQ as IPTp, including single daily or weekly administration, may improve efficacy and safety.
One other published study has evaluated PQ exposure and outcomes in the context of DHA-PQ as IPT, in nonpregnant Thai adults. In this study of adults living in an area of low malaria transmission intensity in Thailand, venous PQ levels >31 ng/mL at the end of a dosing interval were necessary to confer protection [20]. In our study of pregnant Ugandan women, lower concentrations achieved high levels of protection (13.9 ng/mL yielded 99% protection). Differences in these putative protective thresholds may be due to multiple factors, including pregnancy status, transmission intensity, underlying immunity, and/or presence of artemisinin or partner drug resistance. Nonetheless, it is encouraging that the identified target concentrations are largely achievable with current dosing strategies. Furthermore, alterations in dosing may result in a greater proportion of women protected during pregnancy.
For any preventive therapy, limiting drug-related toxicity is of primary importance. With DHA-PQ, of concern is QTc prolongation associated with peak PQ concentrations [19]. Administration of a compressed, 2-day regimen of DHA-PQ (with the same total dose as the standard 3-day regimen) for malaria prevention in Cambodian male adults resulted in QTc prolongation of >500 msec in 4 of 47 individuals, leading to premature study termination [19], with QTc changes correlating with PQ peak concentrations. Considering both pregnant and nonpregnant women, we observed a modest QTc increase of 17 msec following treatment [12], consistent with recent reports [21]. In the present study, modeling PQ concentrations at the time that ECGs were obtained revealed a positive correlation between PQ concentration and QTc interval, consistent with published reports [19]. However, no clinically worrisome QTc prolongations (ie, >480 msec) and no clinically significant arrhythmias were observed.
Although monthly DHA-PQ as IPTp was effective and safe, our modeling suggests that this regimen can be improved. Simulations suggest that daily or weekly administration at a lower dosage of PQ will improve efficacy, especially for women exhibiting high PQ clearance. Furthermore, peak PQ concentrations are predicted to be significantly lower with these alternative dosing strategies compared to standard dosing, mitigating peak concentration-dependent QT prolongation. Importantly, single weekly administration of DHA-PQ for IPT has also been advocated recently in simulation studies of pediatric populations [22] and nonpregnant adults [23]. It is important to note that a single weekly administration may offer a less complicated regimen than 3 consecutive daily administrations once monthly, and so one would predict improved adherence with the weekly regimen [23].
A limitation of the current study is our focus on the PK/PD of PQ, and not of the shorter-acting artemisinin DHA. In the setting of monthly dosing, where efficacy is presumably dependent primarily on preventing new malarial infections through extended exposure to PQ, PK/PD of DHA is unlikely to be of significant importance. However, as parasitemia may be present at the time DHA-PQ is first initiated, providing the standard 3 days of DHA-PQ administration as a “load” would eliminate any circulating parasites and ensure that PQ target concentrations are rapidly achieved. For daily or weekly dosing of DHA-PQ, usage of the standard DHA-PQ ratio may be appropriate, assuming that toxicity will not increase appreciably with more frequent dosing (at lower dosages) of either PQ or DHA. This will require additional investigation and should initially be tested in nonpregnant adults. In addition, although there is mixed evidence in the literature regarding the impact of food on PQ disposition [24–26], we did not control for meals in this study. We focused instead primarily on the association of PQ exposure in the setting of “real-world” practice and the most relevant clinical and laboratory outcomes. Our models also did not account for a potential time delay from when an infection emerges from the liver to the time it is detectable by LAMP, and therefore target PQ levels proposed may be a slight underestimation. Furthermore, the LAMP assay used to detect infection with P. falciparum is unable to distinguish between asexual parasites from gametocytes which do not contribute to adverse pregnancy outcomes. We also did not present data modeling PK/PD associations with selection of potential PQ drug resistance markers; these investigations are ongoing.
In conclusion, increasing PQ exposure during pregnancy was associated with reduced placental malaria and improved birth outcomes. Modeling suggests that a single daily or weekly administration of lower dosages of PQ may limit toxicity and improve birth outcomes. Trials assessing modified dosing strategies for DHA-PQ will be needed, beginning in nonpregnant adults, before definitive conclusions can be made regarding optimal IPTp dosing strategies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all the staff in the Infectious Diseases Research Collaboration Tororo study clinic.
Financial support. This work was supported by the National Institutes of Health (grant numbers P01HD059454 and R01AI117001).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dellicour S, Tatem AJ, Guerra CA, Snow RW, ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 2010; 7:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Desai M, ter Kuile FO, Nosten F et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis 2007; 7:93–104. [DOI] [PubMed] [Google Scholar]
- 3. Walker PG, ter Kuile FO, Garske T, Menendez C, Ghani AC. Estimated risk of placental infection and low birthweight attributable to Plasmodium falciparum malaria in Africa in 2010: a modelling study. Lancet Glob Health 2014; 2:e460–7. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. World malaria report, 2015. Geneva, Switzerland: WHO, 2015. [Google Scholar]
- 5. Kakuru A, Jagannathan P, Muhindo MK et al. Dihydroartemisinin-piperaquine for the prevention of malaria in pregnancy. N Engl J Med 2016; 374:928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mawejje HD, Wilding CS, Rippon EJ, Hughes A, Weetman D, Donnelly MJ. Insecticide resistance monitoring of field-collected Anopheles gambiae s.l. populations from Jinja, eastern Uganda, identifies high levels of pyrethroid resistance. Med Vet Entomol 2013; 27:276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ramphul U, Boase T, Bass C, Okedi LM, Donnelly MJ, Müller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Trans R Soc Trop Med Hyg 2009; 103:1121–6. [DOI] [PubMed] [Google Scholar]
- 8. Verhaeghen K, Van Bortel W, Trung HD, Sochantha T, Keokenchanh K, Coosemans M. Knockdown resistance in Anopheles vagus, An. sinensis, An. paraliae and An. peditaeniatus populations of the Mekong region. Parasit Vectors 2010; 3:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA 2007; 297:2603–16. [DOI] [PubMed] [Google Scholar]
- 10. Desai M, Gutman J, L’lanziva A et al. Intermittent screening and treatment or intermittent preventive treatment with dihydroartemisinin-piperaquine versus intermittent preventive treatment with sulfadoxine-pyrimethamine for the control of malaria during pregnancy in western Kenya: an open-label, three-group, randomised controlled superiority trial. Lancet 2015; 386:2507–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tarning J, Ashley EA, Lindegardh N et al. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob Agents Chemother 2008; 52:1052–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kajubi R, Huang L, Jagannathan P et al. Antiretroviral therapy with efavirenz accentuates pregnancy-associated reduction of dihydroartemisinin-piperaquine exposure during malaria chemoprevention. Clin Pharmacol Ther 2017; 102:520–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kamya MR, Arinaitwe E, Wanzira H et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg 2015; 92:903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hopkins H, González IJ, Polley SD et al. Highly sensitive detection of malaria parasitemia in a malaria-endemic setting: performance of a new loop-mediated isothermal amplification kit in a remote clinic in Uganda. J Infect Dis 2013; 208:645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. National Institute of Allergy and Infectious Diseases. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events 2014. Available at: https://rsc.tech-res.com/docs/default-source/safety/daids_ae_grading_table_v2_nov2014.pdf?sfvrsn=8. Accessed 1 January 2018.
- 16. Kjellin LL, Dorsey G, Rosenthal PJ, Aweeka F, Huang L. Determination of the antimalarial drug piperaquine in small volume pediatric plasma samples by LC-MS/MS. Bioanalysis 2014; 6:3081–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoglund RM, Adam I, Hanpithakpong W et al. A population pharmacokinetic model of piperaquine in pregnant and non-pregnant women with uncomplicated Plasmodium falciparum malaria in Sudan. Malar J 2012; 11:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tarning J, Rijken MJ, McGready R et al. Population pharmacokinetics of dihydroartemisinin and piperaquine in pregnant and nonpregnant women with uncomplicated malaria. Antimicrob Agents Chemother 2012; 56:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manning J, Vanachayangkul P, Lon C et al. Randomized, double-blind, placebo-controlled clinical trial of a two-day regimen of dihydroartemisinin-piperaquine for malaria prevention halted for concern over prolonged corrected QT interval. Antimicrob Agents Chemother 2014; 58:6056–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lwin KM, Phyo AP, Tarning J et al. Randomized, double-blind, placebo-controlled trial of monthly versus bimonthly dihydroartemisinin-piperaquine chemoprevention in adults at high risk of malaria. Antimicrob Agents Chemother 2012; 56:1571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chotsiri P, Wattanakul T, Hoglund RM et al. Population pharmacokinetics and electrocardiographic effects of dihydroartemisinin-piperaquine in healthy volunteers. Br J Clin Pharmacol 2017; 83:2752–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sambol NC, Tappero JW, Arinaitwe E, Parikh S. Rethinking dosing regimen selection of piperaquine for malaria chemoprevention: a simulation study. PLoS One 2016; 11:e0154623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Permala J, Tarning J, Nosten F, White NJ, Karlsson MO, Bergstrand M. Prediction of improved antimalarial chemoprevention with weekly dosing of dihydroartemisinin-piperaquine. Antimicrob Agents Chemother 2017; 61. doi:10.1128/AAC.02491-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reuter SE, Evans AM, Shakib S et al. Effect of food on the pharmacokinetics of piperaquine and dihydroartemisinin. Clin Drug Investig 2015; 35:559–67. [DOI] [PubMed] [Google Scholar]
- 25. Annerberg A, Lwin KM, Lindegardh N et al. A small amount of fat does not affect piperaquine exposure in patients with malaria. Antimicrob Agents Chemother 2011; 55:3971–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hai TN, Hietala SF, Van Huong N, Ashton M. The influence of food on the pharmacokinetics of piperaquine in healthy Vietnamese volunteers. Acta Trop 2008; 107:145–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.