Glecaprevir/pibrentasvir is approved to treat hepatitis C virus genotype 1–6 infection. Patients coinfected with human immunodeficiency virus type 1 achieved high cure rates after 8- (without cirrhosis) and 12- (compensated cirrhosis) week treatment, without adjustments to antiretroviral therapy regimens.
Keywords: hepatitis C, HIV coinfection, EXPEDITION-2, direct-acting antiviral, cirrhosis
Abstract
Background
Once-daily glecaprevir coformulated with pibrentasvir (glecaprevir/pibrentasvir) demonstrated high rates of sustained virologic response 12 weeks after treatment (SVR12) in patients with hepatitis C virus (HCV) genotype 1–6 infection. This phase 3 study evaluated the efficacy and safety of glecaprevir/pibrentasvir in patients with chronic HCV genotype 1–6 and human immunodeficiency virus type 1 (HIV-1) coinfection, including patients with compensated cirrhosis.
Methods
EXPEDITION-2 was a phase 3, multicenter, open-label study evaluating glecaprevir/pibrentasvir (300 mg/120 mg) in HCV genotype 1–6/HIV-1–coinfected adults without and with compensated cirrhosis for 8 and 12 weeks, respectively. Patients were either HCV treatment-naive or experienced with sofosbuvir, ribavirin, or interferon, and antiretroviral therapy (ART) naive or on a stable ART regimen. Treatment-experienced genotype 3–infected patients were excluded. The primary endpoint was the SVR12 rate.
Results
In total, 153 patients were enrolled, including 16 (10%) with cirrhosis. The SVR12 rate was 98% (n = 150/153; 95% confidence interval, 95.8–100), with no virologic failures in 137 patients treated for 8 weeks. One genotype 3–infected patient with cirrhosis had on-treatment virologic failure. Most adverse events were mild in severity; 4 patients (2.6%) had serious adverse events, all deemed unrelated to glecaprevir/pibrentasvir. Treatment discontinuation was rare (<1%). All patients treated with ART maintained HIV-1 suppression (<200 copies/mL) during treatment.
Conclusions
Glecaprevir/pibrentasvir for 8 weeks in noncirrhotic and 12 weeks in cirrhotic patients is a highly efficacious and well-tolerated treatment for HCV/HIV-1 coinfection, regardless of baseline HCV load or prior treatment with interferon or sofosbuvir.
Clinical trial registration
Among the estimated 80 million individuals infected with hepatitis C virus (HCV) worldwide, approximately 2–3 million have human immunodeficiency virus type 1 (HIV-1) coinfection [1–3]. The era of combination antiretroviral therapy (cART) has greatly improved life expectancy for HIV-infected patients, such that liver disease has become a leading cause of morbidity and mortality [4]. Compared with infection with HCV alone, HCV/HIV-1 coinfection is associated with accelerated liver disease progression, increased risk for hepatic decompensation, and liver failure, highlighting the importance of treating HCV infection in this population [5, 6]. Curative treatment of HCV infection is possible with direct-acting antiviral (DAA) therapy, with most recent guidelines recommending that HCV/HIV-1–coinfected and HCV-monoinfected patients be treated alike and cautioning primarily for drug–drug interactions (DDIs) with ART [7–9]. Because patient adherence is likely to improve with shorter treatment durations, an approved pangenotypic, 8-week regimen is desirable and would require fewer medical resources, allowing for the treatment of more patients [10].
Glecaprevir is a nonstructural (NS) protein 3/4A protease inhibitor identified by AbbVie and Enanta that has been coformulated with pibrentasvir, an NS5A inhibitor. Together, these pangenotypic DAAs demonstrate a high barrier to resistance and potent anti-HCV activity in vitro, with half maximal effective concentration (EC50) of glecaprevir and pibrentasvir ranging 0.85–4.6 nanomolar and 1–5 picomolar across HCV genotypes 1–6, respectively [11, 12]. Glecaprevir/pibrentasvir is minimally metabolized in the liver, has negligible renal excretion, is primarily excreted in the bile, and has a favorable DDI profile with most commonly administered concomitant medications, including proton pump inhibitors (PPIs) and opiate substitution therapies [13, 14]. In healthy participants, no clinically significant drug–drug interactions were demonstrated between glecaprevir/pibrentasvir and commonly used antiretroviral agents, including raltegravir, rilpivirine, elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, and abacavir/dolutegravir/lamivudine [15, 16].
In recent phase 2 and 3 studies, 8-week treatment with once-daily, ribavirin-free glecaprevir/pibrentasvir yielded sustained virologic response 12 weeks after treatment (SVR12; HCV RNA less than lower limit of quantification) rates of 93%–100% in patients with HCV genotype 1–6 infection without cirrhosis, including a 100% SVR12 rate in 15 HCV genotype 1–infected patients coinfected with HIV-1 [17, 18]; 12-week treatment in HCV genotype 1–6-infected patients with cirrhosis yielded an SVR12 rate of 99% [19]. EXPEDITION-2 was the first study dedicated to evaluate the safety and efficacy of an 8-week treatment duration of glecaprevir/pibrentasvir in an entirely HIV-1–coinfected population of patients with HCV genotype 1–6 infection without cirrhosis; 12-week treatment was also evaluated in coinfected patients with compensated cirrhosis. The results from this study support the recent approval of glecaprevir/pibrentasvir for patients with chronic HCV genotype 1–6 infection, including those with HIV-1 coinfection [14].
METHODS
Study Design
EXPEDITION-2 was a nonrandomized, open-label, phase 3, multicenter study conducted at 36 sites in Australia, Belarus, France, Germany, Poland, Puerto Rico, Russia, the United Kingdom, and the United States. The efficacy and safety of 8-week or 12-week treatment with glecaprevir/pibrentasvir in HCV/HIV-1–coinfected patients without or with compensated cirrhosis, respectively, were evaluated. The study was conducted according to Good Clinical Practice guidelines, the Declaration of Helsinki, and all applicable regulations, with independent ethics committee or institutional review board approval for all study sites.
Patients
Patients aged ≥18 years and with a body mass index ≥18 kg/m2 were eligible if they had chronic infection with HIV-1 (positive for anti-HIV antibody) and HCV (any genotype 1–6, with HCV RNA at least 1000 IU/mL). Patients with genotype 3 infection could not have received previous treatment for HCV; all other patients could be either HCV treatment-naive or experienced with interferon, pegylated interferon with or without ribavirin, or sofosbuvir plus ribavirin with or without pegylated interferon. Compensated cirrhosis status was defined by liver biopsy, transient elastography (≥12.5 kPa), or serum biomarkers (FibroTest ≥0.75 and aspartate aminotransferase to platelet ratio index >2). Absence of cirrhosis was defined as a transient elastography score <12.5 kPa or FibroTest and APRI values of ≤0.48 and <1, respectively. More details on cutoffs and determination of cirrhosis are located in the Supplementary Appendix and Supplementary Table 1. All patients were either antiretroviral therapy (ART)–naive with a CD4+ count ≥500 cells/mm3 or ≥29% of total T cells, or on a qualifying ART regimen at least 8 weeks prior to screening, with CD4+ count ≥200 cells/mm3 or ≥14% of total T cells and plasma HIV-1 RNA below the lower limit of quantification (20 copies/mL). Qualified ART regimens for all patients were raltegravir, dolutegravir, rilpivirine, elvitegravir/cobicistat and, for patients without cirrhosis only, darunavir (DRV) plus ritonavir (r), DRV/cobicistat, and lopinavir/r. Allowed nucleoside/nucleotide reverse transcriptase inhibitor backbone agents for all patients were tenofovir disoproxil fumarate, tenofovir alafenamide, abacavir, emtricitabine, and lamivudine. Patients with a positive test result at screening for hepatitis B surface antigen or coinfection with >1 HCV genotype were excluded, as were patients with compensated cirrhosis with current or past evidence of Child-Pugh B or C classification. Alanine aminotransferase (ALT) or aspartate aminotransferase (AST) values >10 times the upper limit of normal, albumin <3.0 g/dL, creatinine clearance <50 mL/min, and platelet count <60000 cells/mm3 or 90000 cells/mm3 for patients with or without cirrhosis, respectively, were also exclusionary. All patients provided written informed consent.
Procedures
Coformulated glecaprevir/pibrentasvir (300 mg/120 mg) was orally dosed as three 100-mg/40-mg tablets taken once daily with food for either 8 (patients without cirrhosis) or 12 (patients with cirrhosis) weeks. Plasma samples for HCV RNA quantification were collected at screening, day 1, and weeks 1, 2, 4, 8, and, for patients in the 12-week arm, week 12. Next-generation sequencing was conducted on plasma samples collected from all patients at baseline, and presence of baseline polymorphisms relative to subtype-specific reference sequences at amino-acid positions 155, 156, and 168 in NS3 and 24, 28, 30, 31, 58, 92, and 93 in NS5A was evaluated using a 15% detection threshold (Illumina MiSeq, San Diego, CA). For patients with virologic failure, treatment-emergent substitutions in NS3 and NS5A relative to the patient’s baseline sequence were analyzed. Compliance was measured by pill count. Adverse event monitoring, vital sign measurements, physical examination, and laboratory tests were performed throughout the study to assess safety and tolerability; analyses were performed on the intent-to-treat population.
Statistical Analysis and Outcomes
The primary efficacy endpoint was the proportion of total patients with sustained virologic response (HCV RNA <15 IU/mL) 12 weeks after last day of treatment (SVR12) and was assessed using the COBAS AmpliPrep/COBAS TaqMan HCV Quantitative Test, v2.0 (Roche Molecular Diagnostics, Pleasanton, CA). The secondary endpoints were the proportion of patients with on-treatment virologic failure and post-treatment relapse. Additional efficacy analyses were conducted to determine whether HCV genotype, fibrosis stage, a history of injection drug use, or concomitant opiate substitution therapy affected SVR12 rates in noncirrhotic patients receiving 8 weeks of treatment. All analyses were prespecified and conducted in the intent-to-treat population, which included all patients who received at least 1 dose of study drug.
The study had a planned enrollment of 160 patients, with no more than 110 with genotype 1 infection. All statistical tests and confidence intervals (CIs) were 2-sided with a significance level of .05. Confidence intervals for the primary and secondary endpoints were calculated using the normal approximation to the binomial distribution; Wilson’s method was used to calculate confidence intervals for the subgroup analyses and whenever a rate of 100% occurred. Noninferiority to a historical 96% SVR12 rate (sofosbuvir plus ledipasvir or grazoprevir plus elbasvir) [20, 21] was calculated using a 6% noninferiority margin and achieved if the lower confidence bound of the 95% confidence interval for the SVR12 rate was greater than 90%. Statistical summaries were performed using SAS software, version 9.3. This study is registered with www.ClinicalTrials.gov (NCT02738138).
RESULTS
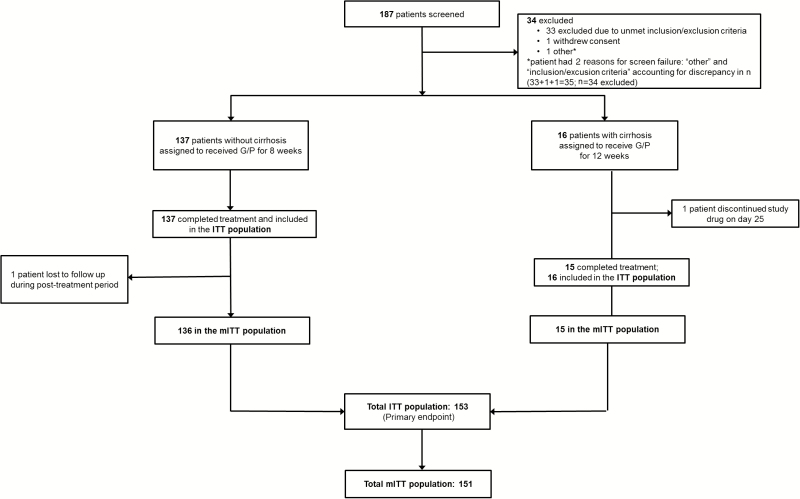
Patients were screened between 17 May 2016 and 12 September 2016, and 153 were enrolled (Figure 1). The majority of patients were male, white, and HCV treatment-naive. Although patients with genotype 5 infection were eligible, none were enrolled. The most common ART anchor agent was dolutegravir. Of the 2 HCV targets, polymorphisms in NS5A (29% in the 8-week arm and 38% in the 12-week arm) were more frequent than those in NS3 (2% in the 8-week arm and 6% in the 12-week arm). One patient had polymorphisms in both targets (Table 1).
Figure 1.
Trial profile. Patient disposition from screening through study completion is shown. Abbreviations: G/P, glecaprevir/pibrentasvir; ITT, intent-to-treat (includes all patients who received at least 1 dose of the study drug); mITT, modified intent-to-treat (excludes nonvirologic failures.)
Table 1.
Baseline Demographics and Disease Characteristics
| Characteristic | Without Cirrhosis | With Cirrhosis |
|---|---|---|
| 8 wk | 12 wk | |
| (n = 137) | (n = 16) | |
| Male, no. (%) | 113 (82) | 15 (94) |
| Age, median (range), y | 45 (23–74) | 50 (35–62) |
| BMI, median (range), kg/m2 | 25.0 (18.1–40.6) | 27.6 (21.6–38.2) |
| Race, no. (%)a | ||
| White | 106 (77) | 15 (94) |
| Black | 24(18) | 1 (6) |
| Genotype, no. (%)b | ||
| 1 | 87 (64) | 10 (63) |
| Subtype 1a | 66 (48) | 5 (31) |
| Subtype 1b | 21 (15) | 5 (31) |
| 2c | 9 (7) | 1 (6) |
| 3c | 22 (16) | 4 (25) |
| 4c | 16 (12) | 1 (6) |
| 5c | 0 | 0 |
| 6c | 3 (2) | 0 |
| HCV RNA, median (range), log10 IU/mLd | 6.2 (4.0–7.4) | 6.1 (4.4–7.0) |
| HCV treatment-naive, no. (%) | 111 (81) | 14 (88) |
| HCV treatment-experienced, no. (%) | 26 (19) | 2 (13) |
| IFN-based | 23 (17) | 2 (13) |
| SOF-based | 3 (2) | 0 |
| Fibrosis stage, no. (%) | ||
| F0–F1 | 120 (88) | 0 |
| F2 | 2 (1) | 0 |
| F3 | 15 (11) | 0 |
| F4 | 0 | 16 (100) |
| Baseline polymorphisms, no. (%) | ||
| NS3 only | 1 (1)e | 1 (6) |
| NS5A only | 36 (28)e | 6 (38) |
| NS3 and NS5A | 1 (1)e | 0 |
| None | 92 (71)e | 9 (56) |
| CD4+ cell count ≥500 cells/mm3, no. (%) | 92 (67) | 9 (56) |
| No antiretroviral therapy, no. (%) | 9 (7) | 0 |
| HIV RNA, median (range), copies/mL | 13900 (<20–279000) | NA |
| Anchor ARV Agent, no. (%)f,g | ||
| Raltegravir (BID) | 39 (28) | 6 (38) |
| Dolutegravir (QD or BID) | 62 (45) | 5 (31) |
| Rilpivirine (QD) | 27 (20) | 5 (31) |
| Elvitegravir/cobicistat (QD) | 1 (1) | 0 |
| N(t)RTI backbone agent, no. (%) | ||
| Tenofovir disoproxil fumarate | 74 (54) | 13 (81) |
| Tenofovir alafenamide | 6 (4) | 0 |
| Abacavir | 49 (36) | 3 (19) |
| Emtricitabine | 74 (54) | 12 (75) |
| Lamivudine | 53 (39) | 4 (25) |
| Concomitant PPI use, no. (%) | 11 (8) | 1 (6) |
| IDU within 12 months, no. (%) | 12 (9) | 1 (6) |
| IDU >12 months prior to screening, no. (%) | 62 (45) | 10 (63) |
| On opiate substitution therapy, no. (%) | 11 (8) | 2 (13) |
Abbreviations: ARV, antiretroviral; BID, twice-daily; BMI, body mass index; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IDU, injection drug use; IFN, interferon; NA, not applicable; N(t)RTI, nucleoside/nucleotide reverse transcriptase inhibitor; PPI, proton pump inhibitor; QD, once-daily; SOF, sofosbuvir.
aRace was self-reported.
bGenotype was determined by the Versant HCV Genotype INNO-LiPA Assay v2.0 and confirmed by phylogenetic analysis of available sequences.
cThe following subtypes were reported: GT2a, GT2b, GT2c, GT3a, GT4a, GT4d, GT6e, GT6n, GT6xa.
dHCV RNA was quantified by Roche COBAS AmpliPrep/TaqMan v2.0.
en = 130 and is representative of the number of samples with sequences available for both targets.
fDarunavir (DRV) + ritonavir (r) QD, DRV/cobicistat (COBI) QD, and lopinavir/r BID were also allowed for patients without cirrhosis.
gNo patients were enrolled who were taking DRV/r, DRV/COBI, or lopinavir/r as anchor ARV.
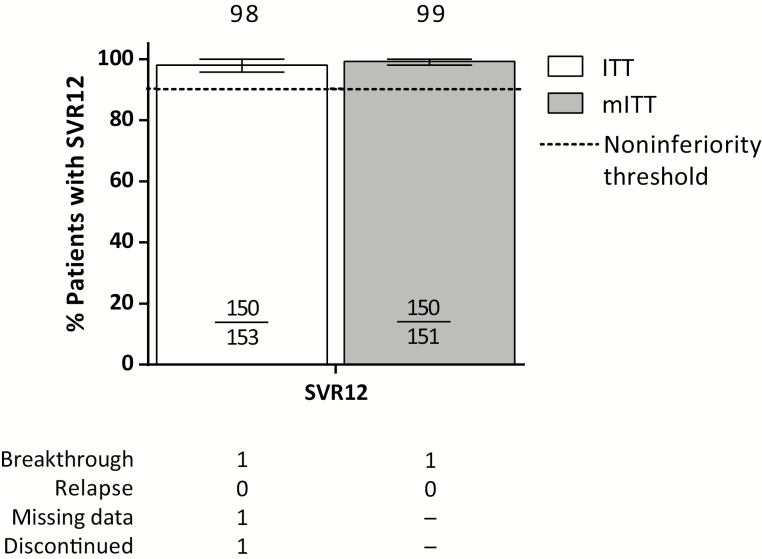
Of the 153 patients treated with glecaprevir/pibrentasvir, 150 achieved SVR12 (98%; 95% CI, 95.8%–100%) (Figure 2). Noninferiority to the historical standard-of-care was achieved. Ninety-five percent of patients had HCV RNA <lower limit of quantification (LLOQ) by week 4 of treatment (Supplementary Table 2.) One HCV treatment-naive patient with genotype 3 infection and compensated cirrhosis (12-week treatment arm) had on-treatment virologic breakthrough at treatment week 8. At the time of failure, this patient had treatment-emergent substitutions Y56H and Q168R in NS3 and S24F and M28K in NS5A; the patient was 85% compliant with treatment. One patient was missing SVR12 data and thus did not achieve SVR12; however, this patient had achieved SVR4 and still had HCV RNA <LLOQ upon return 24 weeks after treatment (SVR24). The third patient who did not achieve SVR12 discontinued treatment on day 25 due to a serious adverse event; this patient is discussed in more detail below. Excluding the patient missing SVR12 data (who achieved SVR24) and the patient who prematurely discontinued treatment, the SVR12 rate was 100% (n = 136/136; 95% CI, 97.3%–100%) in patients without cirrhosis treated for 8 weeks and 93% (n = 14/15; 95% CI, 70.2%–98.8%) in patients with compensated cirrhosis treated for 12 weeks. There were no relapses. In all patients, baseline characteristics such as baseline HCV viral load, presence of baseline NS3 or NS5A polymorphisms, fibrosis score, and ART regimen had no impact on achievement of SVR12 (Supplementary Table 3).
Figure 2.
Efficacy of glecaprevir/pibrentasvir. Rates of sustained virologic response 12 weeks after treatment (SVR12) are shown in the intent-to-treat (white) and modified intent-to-treat (gray) populations. Dotted line represents the noninferiority threshold applied to the primary endpoint (SVR12) analysis. Reasons for nonresponse are tabled. Abbreviations: ITT, intent-to-treat (includes all patients who received at least 1 dose of the study drug); mITT, modified intent-to-treat (excludes nonvirologic failures); SVR12, sustained virologic response 12 weeks after treatment.
The safety profile of glecaprevir/pibrentasvir was generally similar in all patients regardless of treatment duration (Table 2). The majority of adverse events were mild in severity. The most common adverse events were fatigue and nausea. Four patients experienced serious adverse events, none of which were related to the study drug. One genotype 2–infected patient with cirrhosis experienced serious adverse events unrelated to glecaprevir/pibrentasvir of cerebrovascular accident and cerebral hemorrhage on day 23 that led to discontinuation 2 days later; the patient did not achieve SVR12. The remaining 3 serious adverse events occurred in 1 patient each, all without cirrhosis, and were all unrelated to glecaprevir/pibrentasvir: upper gastrointestinal hemorrhage on treatment day 25, obliterating arteriopathy on post-treatment day 28, and urolithiasis on post-treatment day 15. All 3 patients achieved SVR12. One patient in the 8-week treatment arm had a grade 3 total bilirubin elevation on day 10 that continued through day 31; both direct and indirect bilirubin values were elevated, but levels normalized by day 59 without treatment interruption. There were no treatment-emergent grade 3 or higher transaminase elevations. There were no clinically meaningful decreases in renal function (estimated GFR) during the course of treatment, regardless of ARV regimen (Supplementary Table 4). All patients taking ART maintained HIV-1 suppression while on glecaprevir/pibrentasvir treatment.
Table 2.
Safety and Tolerability
| Event | Without Cirrhosis | With Cirrhosis |
|---|---|---|
| 8 wk | 12 wk |
|
| (n = 137) | (n = 16) | |
| Any AE | 86 (63) | 8 (50) |
| Grade 1 (mild) | 52 (60) | 3 (38) |
| Serious AE | 3 (2)a | 1 (6)b |
| DAA-related serious AE | 0 | 0 |
| AE leading to discontinuation | 0 | 1 (6)b |
| AEs occurring in ≥5% of overall patients | ||
| Fatigue | 18 (13) | 0 |
| Nausea | 12 (9) | 1 (6) |
| Headache | 12 (9) | 0 |
| Nasopharyngitis | 12 (9) | 0 |
| Laboratory abnormalities | ||
| ALT, grade ≥3 (>5 × ULN) | 0 | 0 |
| AST, grade ≥3 (>5 × ULN) | 0 | 0 |
| Total bilirubin, grade ≥3 (>3 × ULN) | 1 (0.7) | 0 |
Data are given as no. (%).
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; DAA, direct-acting antiviral; ULN, upper limit of normal.
aUpper gastrointestinal hemorrhage, obliterating arteriopathy, and urolithiasis in 1 patient each, all unrelated to glecaprevir/pibrentasvir.
bCerebrovascular accident and cerebral hemorrhage, both unrelated to glecaprevir/pibrentasvir.
DISCUSSION
Coformulated, once-daily glecaprevir/pibrentasvir achieved an overall SVR12 rate of 98% in HCV/HIV-1–coinfected patients with or without cirrhosis, with no relapses. In coinfected patients without cirrhosis, 8-week glecaprevir/pibrentasvir achieved an SVR12 rate of 99%, with no virologic failures. The safety profile of glecaprevir/pibrentasvir was similarly favorable for patients without cirrhosis treated for 8 weeks and patients with compensated cirrhosis treated for 12 weeks; serious adverse events, clinically significant laboratory abnormalities, and treatment discontinuations were rare in both treatment arms. The low rate of virologic failure suggests that achievement of SVR12 was not impacted by high baseline viral load, presence of baseline polymorphisms, cirrhosis status, or any other baseline factor. In the ION-4 study conducted exclusively in patients with HCV/HIV-1 coinfection, a 12-week treatment duration with ledipasvir/sofosbuvir resulted in an SVR12 rate of 96% with 2 virologic breakthroughs and 10 relapses; all 10 patients who relapsed were black, and black race (n = 115; 34% enrolled) was the only significant predictor of relapse [22]. Although a comparatively small number of black coinfected patients enrolled in our study (n = 25; 16%), it is encouraging that all achieved SVR12 with no incidence of relapse, even with a shorter duration of treatment.
A short-duration, pangenotypic HCV treatment may be an important part of the World Health Organization goal of global HCV elimination by 2030 [23]. Until recently, 8-week treatment durations for other regimens were limited. The ION-3 study, conducted in noncirrhotic, HCV genotype 1–monoinfected, treatment-naive patients with a baseline viral load <6 million IU/mL, yielded an SVR12 rate of 94% following 8 weeks of sofosbuvir/ledipasvir [24]. Although recent real-world data have shown that 27 of 28 (96%) coinfected patients achieved SVR12 when treated for 8 weeks with sofosbuvir/ledipasvir [25], the 8-week duration is not recommended by US guidelines in coinfected patients [9]. More recently, the 3-DAA regimen sofosbuvir/velpatasvir/voxilaprevir was approved in Europe for 8 weeks in treatment-naive, noncirrhotic patients with HCV genotype 1–6 infection, although this regimen did not meet the primary endpoint (noninferiority to 12 wk of sofosbuvir/velpatasvir) [26, 27]. Indeed, US label guidelines indicate that sofosbuvir/velpatasvir/voxilaprevir be administered for 12 weeks in patients with HCV genotype 1–6 infection and only in those who failed prior therapy with an NS5A DAA [28]. Here, 8-week glecaprevir/pibrentasvir yielded an SVR12 rate of 99% with no virologic failures in 137 HIV-1–infected patients with HCV genotype 1, 2, 3, 4, or 6 coinfection, regardless of baseline factors, including HCV load or prior treatment experience with sofosbuvir and/or interferon.
Twelve weeks of glecaprevir/pibrentasvir treatment is the only pangenotypic HCV regimen recommended by both the American Association for the Study of Liver Diseases and the European Association of the Liver treatment guidelines for HCV treatment-naive coinfected patients with compensated cirrhosis [8, 9]. In the C-EDGE study, 12-week elbasvir plus grazoprevir yielded an SVR12 rate of 96% in 218 coinfected patients, including 100% SVR12 in 35 patients with cirrhosis [20]. In the US study ASTRAL-5, conducted in 106 patients coinfected with HCV/HIV-1, including 19 patients with cirrhosis, 12-week sofosbuvir/velpatasvir yielded an SVR12 rate of 95%, with 2 relapses [29]. Here, there were no relapses in 16 coinfected patients with cirrhosis following 12-week treatment with glecaprevir/pibrentasvir; 1 HCV treatment-naive patient with genotype 3 infection and cirrhosis experienced breakthrough.
The favorable safety and DDI profile of glecaprevir/pibrentasvir observed in this study is consistent with that observed in >2000 patients treated with glecaprevir/pibrentasvir [18, 30], suggesting that the regimen can be administered with minimal on-treatment monitoring. Although some regimens containing HCV protease inhibitors have been associated with gastrointestinal adverse events [31–33], glecaprevir/pibrentasvir was well-tolerated: <10% of patients experienced nausea, and <5% of patients experienced diarrhea. Glecaprevir/pibrentasvir had a favorable DDI profile with ARVs that included integrase inhibitors, nucleoside reverse transcriptase inhibitors, and the nonnucleoside reserve transcriptase inhibitor rilpivirine. Use of tenofovir disoproxil fumarate has been associated with onset or worsening of renal impairment and may be exacerbated by coadministration with certain concomitant medications such as HIV-1 protease inhibitors [34, 35]. Here, 87 (57%) patients were taking tenofovir disoproxil fumarate as their nucleoside/nucleotide reverse transcriptase inhibitor, and no patient experienced worsening of renal function. Exposures of glecaprevir/pibrentasvir may be increased by coadministration with ritonavir- or cobicistat-containing regimens; however, exposure-safety analysis of phase 1–3 available data at the time of study initiation indicated that the regimens allowed in this study were not expected to impact the safety profile for glecaprevir/pibrentasvir [15, 16]. Due to the timing of a protocol amendment allowing these additional anchor agents, no patients on darunavir- or lopinavir-containing ART regimens and only 1 patient on elvitegravir/cobicistat enrolled. Similar to other DAA-containing HCV therapies, coadministration of glecaprevir/pibrentasvir with ARV regimens that induce P-glycoprotein and cytochrome P450 (such as efavirenz) was not permitted in this study and should be avoided [8, 36].
One limitation of this study is that a small number of patients with genotype 6 infection and no patients with genotype 5 infection were enrolled, despite eligibility criteria allowing for enrollment of all 6 major HCV genotypes. Patients with HCV genotype 5 and 6 are generally difficult to recruit due to their low global prevalence rates of 0.8% and 5%, respectively [37, 38]. A second limitation is the low number of patients with compensated cirrhosis that were enrolled; however, recent reports suggest these proportions are consistent with current HCV/HIV epidemiology, as HCV treatment among coinfected individuals has become more widely adopted [39]. Lastly, the study design did not include randomization by treatment duration. However, HCV genotype 1 patients coinfected with HIV-1 were randomized to 8- versus 12-week treatment durations in the ENDURANCE-1 study [17].
Treatment with all-oral, once-daily glecaprevir/pibrentasvir yielded high SVR12 rates in HCV/HIV-1–coinfected patients that were similarly high to those reported in patients with HCV monoinfection; treatment discontinuations and grade 3 laboratory abnormalities occurred in <1% of all patients. The low rate of virologic failure (n = 1/153; <1%) indicates that treatment outcome was not impacted by baseline patient or viral characteristics, and there were no virologic failures in the 137 noncirrhotic patients treated for 8 weeks. These results support the indication of glecaprevir/pibrentasvir as the first 8-week pangenotypic treatment option for HCV/HIV-1–coinfected patients without cirrhosis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors would like to thank the patients and their families who participated in this study and the study investigators and their staff. AbbVie would like to thank Karmin Robinson-Morgan of AbbVie for her contributions to the study. Medical writing support was provided by Zoë Hunter, PhD, of AbbVie.
Financial support. This study was supported by AbbVie, Inc.
Potential conflicts of interest. The design, study conduct, analysis, and financial support of the study (NCT02738138) were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the content. All authors had access to all relevant data and participated in writing, review, and approval of this presentation. J. K. R. has received grant/research support from Gilead; has served as a consultant/advisor to Abbott, Abbvie, Bionor, Gilead, Hexal, Janssen, Merck, and ViiV; and has been a speaker at educational events for AbbVie, Gilead, Janssen, and Merck. K. L. has served as an advisor/consultant/speaker board member to Abbvie, BMS, Gilead, Janssen, and Merck. C. O. has received grant/research support from AbbVie, Gilead, GSK, Janssen, and ViiV. D. W. has received grant/research support from AbbVie, Gilead, Merck, and Tacere Therapeutics; and has served as a consultant/advisor to AbbVie, Gilead, and Merck. A. F. L. has received grant/research support from AbbVie, Gilead, and Merck. R. S.-M. has received grant/research support from AbbVie; and is a consultant/advisor for Janssen and Merck. R. F. has served as a consultant/advisory board member/speaker to AbbVie, Alfa Wasserman, BMS, Gilead, Janssen, Merck, and Roche. S. B. has served as an advisor/consultant/speaker board member to Abbvie, BMS, Gilead, Janssen, Merck, and ViiV. K. E. S. has received grant/research support from AbbVie, Merck, Gilead, BMS, Innovio, and Intercept; and has served on the advisory board of Gilead, Merck, and MedImmune. T. S. has served as an investigator for AbbVie. P. R. has received grant/research support from AbbVie, Bristol-Meyers Squibb, Gilead, Merck, Idenix, ViiV, and Janssen; has served as a consultant/advisor to AbbVie, Merck, and Gilead; has served as a speaker for Gilead, ViiV, and Merck; and has been a stockholder of Gilead. J. Sa. has served on the advisory boards of AbbVie, Gilead, Merck, and BMS; has received research support from Gilead and AbbVie; and has been a speaker for Gilead and BMS. J. Sl. has served as a speaker for AbbVie, BMS, Gilead, Merck, Janssen, and Genentech. M. S. has served as a consultant/advisor to AbbVie, Gilead, Janssen, and Trek; has served on the data safety monitoring board of Gilead (funds paid to Johns Hopkins University); and has received grant/research support from AbbVie, Gilead, Merck, and Janssen (paid to Johns Hopkins University). Z. Z., S. S., T. I. N., M. P. K., N. S. S., and R. T. are employees of AbbVie, Inc, and may hold stock or options. R. M. V. and A. G. were employees of AbbVie during the time the work was performed and may hold stock or options. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44:S6–9. [DOI] [PubMed] [Google Scholar]
- 2. Peters L, Klein MB. Epidemiology of hepatitis C virus in HIV-infected patients. Curr Opin HIV AIDS 2015; 10:297–302. [DOI] [PubMed] [Google Scholar]
- 3. Platt L, Easterbrook P, Gower E, et al. . Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis. Lancet Infect Dis 2016; 16:797–808. [DOI] [PubMed] [Google Scholar]
- 4. Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol 2008; 48:353–67. [DOI] [PubMed] [Google Scholar]
- 5. Soriano V, Vispo E, Fernandez-Montero JV, Labarga P, Barreiro P. Update on HIV/HCV coinfection. Curr HIV/AIDS Rep 2013; 10:226–34. [DOI] [PubMed] [Google Scholar]
- 6. Lacombe K, Rockstroh J. HIV and viral hepatitis coinfections: advances and challenges. Gut 2012; 61(suppl 1):i47–58. [DOI] [PubMed] [Google Scholar]
- 7. Operskalski EA, Kovacs A. HIV/HCV co-infection: pathogenesis, clinical complications, treatment, and new therapeutic technologies. Curr HIV/AIDS Rep 2011; 8:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol 2018. In press. doi: 10.1016/j.jhep.2018.03.026 [DOI] [PubMed] [Google Scholar]
- 9. American Association for the Study of Liver Disease, Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C virus 2017. Available at: http://www.hcvguidelines.org. Accessed 14 June 2017.
- 10. Townsend K, Petersen T, Gordon LA, et al. . Effect of HIV co-infection on adherence to a 12-week regimen of hepatitis C virus therapy with ledipasvir and sofosbuvir. AIDS 2016; 30:261–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ng T, Krishnan P, Pilot-Matias T, et al. . In vitro antiviral activity and resistance profile of the next generation hepatitis C virus NS5A inhibitor pibrentasvir. Antimicrob Agents Chemother 2017; 61:pii: e02558-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ng TI, Tripathi R, Reisch T, et al. . In vitro antiviral activity and resistance profile of the next-generation hepatitis C virus NS3/4A protease inhibitor glecaprevir. Antimicrob Agents Chemother 2018; 62:pii: e01620-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kosloski M, Zhao W, Asatryan A, Kort J, Geoffroy P, Liu W. No clinically relevant drug-drug interactions between methadone or buprenorphine/naloxone and anti-viral combination glecaprevir and pibrentasvir. Antimicrob Agents Chemother 2017; 61(10):pii: e00958-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. AbbVie, Inc. Mavyret (glecaprevir/pibrentasvir). Prescribing Information. North Chicago, IL: AbbVie, Inc, 2017. [Google Scholar]
- 15. Kosloski MP, Dutta S, Viani RM, et al. . Glecaprevir and pibrentasvir interactions with combination antiretroviral regimens (abstract 413). In: Program and abstracts of the Conference on Retroviruses and Opportunistic Infections (Seattle, WA) 2017. [Google Scholar]
- 16. Oberoi RK, Kosloski MP, Ding B, et al. . Interactions between ABT‐493 plus ABT‐530 combination and rilpivirine or raltegravir (abstract 453). In: Program and abstracts of the Conference on Retroviruses and Opportunistic Infections (Boston, MA) 2016. [Google Scholar]
- 17. Zeuzem S, Foster GR, Wang S, et al. . Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med 2018; 378:354–69. [DOI] [PubMed] [Google Scholar]
- 18. Puoti M, Foster G, Wang S, et al. . High SVR rates with eight and twelve weeks of pangenotypic glecaprevir/pibrentasvir: integrated efficacy analysis of genotype 1–6 patients without cirrhosis. J Hepatol 2017; 66:S721. [Google Scholar]
- 19. Forns X, Lee SS, Valdes J, et al. . Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis 2017; 17:1062–8. [DOI] [PubMed] [Google Scholar]
- 20. Rockstroh JK, Nelson M, Katlama C, et al. . Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV 2015; 2:e319–27. [DOI] [PubMed] [Google Scholar]
- 21. Gilead Sciences. HARVONI (ledipasvir and sofosbuvir) tablets [package insert] 2015. Available at: https://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf. Accessed October 2017.
- 22. Naggie S, Cooper C, Saag M, et al. ; ION-4 Investigators Ledipasvir and sofosbuvir for HCV in patients coinfected with HIV-1. N Engl J Med 2015; 373:705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization. Combating hepatitis B and C to reach elimination by 2030 2016. Available at: http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.04_eng.pdf?ua=1. Accessed 10 August 2017.
- 24. Kowdley KV, Gordon SC, Reddy KR, et al. ; ION-3 Investigators Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014; 370:1879–88. [DOI] [PubMed] [Google Scholar]
- 25. Ingiliz P, Christensen S, Kimhofer T, et al. . Sofosbuvir and ledipasvir for 8 weeks for the treatment of chronic hepatitis C virus (HCV) infection in HCV-monoinfected and HIV-HCV-coinfected individuals: results from the german hepatitis C cohort (GECCO-01). Clin Infect Dis 2016; 63:1320–4. [DOI] [PubMed] [Google Scholar]
- 26. Jacobson IM, Lawitz E, Gane EJ, et al. . Efficacy of 8 weeks of sofosbuvir, velpatasvir, and voxilaprevir in patients with chronic HCV infection: 2 phase 3 randomized trials. Gastroenterology 2017; 153:113–22. [DOI] [PubMed] [Google Scholar]
- 27. Gilead Sciences International Ltd. VOSEVI (sofosbuvir/velpatasvir/voxilaprevir). Summary of Product Characteristics 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004350/WC500235373.pdf. Accessed 1 October 2017.
- 28. Gilead Sciences. VOSEVI (sofosbuvir/velpatasvir/voxilaprevir). Prescribing Information. Foster City, CA: Gilead Sciences, 2017. [Google Scholar]
- 29. Wyles D, Bräu N, Kottilil S, et al. ; ASTRAL-5 Investigators Sofosbuvir and velpatasvir for the treatment of hepatitis C virus in patients coinfected with human immunodeficiency virus type 1: an open-label, phase 3 study. Clin Infect Dis 2017; 65:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gane EJ, Poordad F, Zadeikis N, et al. . Efficacy, safety, and pharmacokinetics of glecaprevir/pibrentasvir in adults with chronic genotype 1–6 hepatitis C virus infection and compensated cirrhosis: an integrated analysis. Hepatology 2017; 66:1–148.28965360 [Google Scholar]
- 31. Kwo PY, Vinayek R. The therapeutic approaches for hepatitis C virus: protease inhibitors and polymerase inhibitors. Gut Liver 2011; 5:406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chae HB, Park SM, Youn SJ. Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives. ScientificWorldJournal 2013; 2013:704912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banerjee D, Reddy KR. Review article: safety and tolerability of direct-acting anti-viral agents in the new era of hepatitis C therapy. Aliment Pharmacol Ther 2016; 43:674–96. [DOI] [PubMed] [Google Scholar]
- 34. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis 2010; 51:496–505. [DOI] [PubMed] [Google Scholar]
- 35. Goicoechea M, Liu S, Best B, et al. ; California Collaborative Treatment Group 578 Team Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis 2008; 197:102–8. [DOI] [PubMed] [Google Scholar]
- 36. Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015; 62:932–54. [DOI] [PubMed] [Google Scholar]
- 37. Messina JP, Humphreys I, Flaxman A, et al. . Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 2015; 61:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gower E, Estes C, Blach S, Razavi-Shearer K, Razavi H. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014; 61:S45–57. [DOI] [PubMed] [Google Scholar]
- 39. Collins LF, Chan A, Zheng J, et al. . Direct-acting antivirals improve access to care and cure for patients with HIV and chronic HCV infection. Open Forum Infect Dis 2018; 5:ofx264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.