To the Editor—Zika virus emerged in French Guiana in December 2015 shortly after its first occurrence on the American continent [1]. In this context of emergence, molecular diagnostic approach is essential to confirm first human cases and to monitor the outbreak. Nevertheless, its use must be based on a robust diagnostic algorithm built from data of Zika virus RNA persistence in body fluids with minimal bias.
Here, we prospectively assessed Zika virus RNA persistence by real-time reverse transcription polymerase chain reaction in the serum of 38 infected- patients at restricted time intervals from onset of symptoms until virus clearance [2]. Detection threshold was 1.8 log10 copies/mL. No censored data and no intermittent virus detection were observed. Patients were included based on Zika virus RNA detection in their serum or urine no longer after 5 days after symptom onset. The cohort comprised 13 females and 25 males with an age of 39 ± 8.8 (mean ± standard deviation) years, recruited between December 2015 and November 2016 in French Guiana, and all provided written informed consent. This study was approved by the independent ethics committee “Comité de Protection des Personnes Sud-Méditerranée I” in Marseille, France. Time to event analyses were performed using the packages survival (v 2.41.3), flexsurv (v 1.1) and survminer (v 0.4.1) in the R statistical environment [3]. An accelerated failure-time regression model (AFT) with a log-logistic error distribution was chosen over other distributions to be fitted to the data based on the lowest Akaike information criterion.
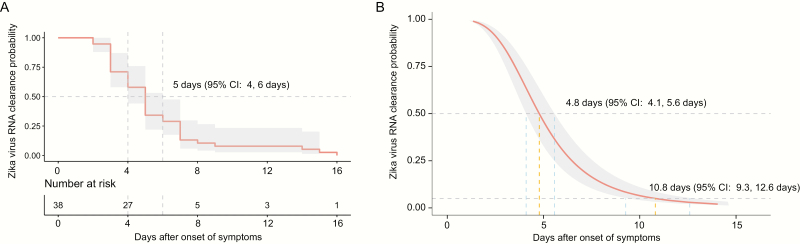
The minimum and maximum Zika virus RNA clearance time observed in our cohort were 3 and 16 days after symptoms onset, respectively. Median time from symptoms onset to Zika virus RNA clearance in serum was similar with both models: 5 days (95% confidence interval [CI] 4 days, 6 days) using the nonparametric Kaplan-Meier method and 4.8 days (95% CI 4.1 days, 5.6 days) using parametric AFT regression (Figure 1). According to the AFT regression, 95% of symptomatic patients would experience a Zika RNA clearance after 10.8 days (95% CI 9.3 days, 12.6 days) post symptoms onset. No viral load nor sex effect were observed on time to Zika RNA clearance (P = .68 and P = .45, log-rank test).
Figure 1.
Probabilities of Zika virus RNA clearance according to time after onset of symptoms. Kaplan-Meier estimates (A) and parametric estimates (B) of the Zika virus RNA clearance probability. A table displaying number of individuals at risk of loss of Zika virus RNA detection is provided in panel A. An accelerated failure-time regression model (AFT) with a log-logistic error distribution was used to provide parametric estimates. The medians are indicated for both analyses (A, B) and the 95th percentile is provided for the parametric analysis only (B). Light gray shading denotes 95% confidence intervals. Abbreviation: CI, confidence interval.
Our results are in accordance with the median estimates assessed by Eui Jeong and colleagues analyzed only from 9 imported Zika cases in South Korea [4]. However, several other studies established a median time to Zika virus clearance after symptom onset that equal or extend 10 days in plasma or whole blood [5–7]. In these works, several biases could have contributed to overestimate the duration of Zika viremia: (i) consideration of asymptomatic patients, (ii) very low sample size, (iii) weekly interval sampling, or (iv) intermittent detection in the follow-up assessment of Zika virus RNA [5–7]. Here we provide estimates of time-dependent probabilities of loss of RNA Zika virus detection after onset of symptoms by analyzing a cohort of an unprecedent sample size. Our results can provide guidance concerning the optimum time window after symptom onset for the implementation of molecular diagnosis tests on serum.
Notes
Funding. This work was supported by the Direction du Service de Santé des Armées [grant number 2016RC10] and by the European Virus Archive goes Global project through a grant from Horizon 2020 of the European Union [grant number 653316].
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. de Laval F, Matheus S, Maquart M et al. Prospective Zika virus disease cohort: systematic screening. Lancet 2016; 388:868. [DOI] [PubMed] [Google Scholar]
- 2. de Laval F, Matheus S, Labrousse T, Enfissi A, Rousset D, Briolant S. Kinetics of Zika viral load in semen. N Engl J Med 2017; 377:697–9. [DOI] [PubMed] [Google Scholar]
- 3. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: 2017. Available at https://www.R-project.org/ [Google Scholar]
- 4. Jeong YE, Cha GW, Cho JE, Lee EJ, Jee Y, Lee WJ. Viral and serological kinetics in Zika virus-infected patients in South Korea. Virol J 2017; 14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barzon L, Percivalle E, Pacenti M et al. Virus and antibody dynamics in travelers with acute Zika virus infection. Clin Infect Dis 2017; 66:1173–80. [DOI] [PubMed] [Google Scholar]
- 6. Mansuy JM, Mengelle C, Pasquier C et al. Zika virus infection and prolonged viremia in whole-blood specimens. Emerg Infect Dis 2017; 23:863–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paz-Bailey G, Rosenberg ES, Doyle K et al. Persistence of Zika virus in body fluids—preliminary report. N Engl J Med 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]