Abstract
Calprotectin (CPT) is released during inflammation, also in the context of atherosclerosis. The link between CPT and the atherosclerotic process was evaluated in several diseases. However, studies in axial spondyloarthritis (axSpA), associated with a high incidence of subclinical atherosclerosis, are scarce. Therefore, we assessed the association of CPT with subclinical atherosclerosis and metabolic risk factors in axSpA. CPT serum levels were measured by enzyme-linked immunosorbent assay in 163 axSpA patients and 63 controls. Subclinical atherosclerosis was determined in patients by carotid ultrasonography (assessing the presence/absence of carotid plaques and carotid intima-media thickness [cIMT]). Data on inflammation, disease activity, lipid profile and treatment were collected to evaluate its relationship with CPT. axSpA patients evidenced lower CPT levels than controls. CPT showed no association with plaques or cIMT in axSpA. CPT and HDL-cholesterol negatively correlated, while a positive association of CPT with the atherogenic index was disclosed. Additionally, axSpA patients with C-reactive protein values at diagnosis higher than 3 mg/L displayed higher CPT levels. Our study shows no relationship between CPT and markers of subclinical atherosclerosis in axSpA. Nevertheless, it demonstrates an association of CPT with adverse lipid profiles and inflammatory biomarkers, which could further influence on the development of atherosclerosis.
Introduction
Several metabolic abnormalities such as hyperglycemia, dyslipidemia, obesity and hypertension are considered risk factors for the development of cardiovascular (CV) disease. Most of them are clustered under the term ‘Metabolic syndrome’ (MeS), a pathologic state with growing prevalence that has been associated with the development not only of vascular and cardiac diseases, but also with other pathologies1–5.
CV disease is generally the result of an accelerated atherosclerotic process, initiated by damage to the vascular endothelium. This can lead to structural damage, manifested by thickening of the vascular wall (carotid intima-media thickness [cIMT]) as well as atheromatous plaque formation6. These morphological findings are considered surrogate markers of CV disease. In this sense, both in the general population and in patients with chronic inflammatory arthritis, an abnormal cIMT value or the presence of plaques predict the development of CV events such as ischemic heart disease or stroke7–11. As previously shown by our group, the presence of these two surrogate markers of CV disease can be determined by carotid ultrasound, a non-invasive imaging technique10–12.
Patients diagnosed with chronic inflammatory diseases, such as axial spondyloarthritis (axSpA), show higher morbidity and mortality rates due to CV disease, particularly atherosclerosis, when compared to the general population6,13,14. This is not only the result of a higher incidence of traditional CV risk factors and MeS features15–20, but also due to the inflammatory burden present in these patients, that acts as an additional independent CV risk factor21,22. In this regard, it is well known that inflammation triggers the expression of endothelial adhesion molecules, promoting thus endothelial damage and the formation of atherosclerotic plaques, an indicator of advanced atherosclerosis22.
Among the cells implicated in the development of atherosclerotic disease, neutrophils, monocytes and macrophages play a key role by secreting a large number of molecules which are further involved in the inflammatory process of atherosclerosis23. In this context, calprotectin (CPT), also known as myeloid-related protein 8/14 (MRP8/14) or S100A8/A9, is a heterodimeric complex of proteins released by these cells during inflammation24. In fact, it was reported that the secretion of CPT is stimulated by the interaction between phagocytes and the endothelium25. Accordingly, the link between CPT and the pathophysiology of atherosclerosis has been studied in several diseases26–29. However, previous studies on this issue that included axSpA patients are limited to only one performed in a small cohort of patients with different inflammatory arthropaties30.
Regarding the functional role of CPT, this protein exerts diverse intra- and extra-cellular functions, acting on different target tissues/organs, such as muscle, cartilage, bone, synovial tissue, vasculature and epithelium, among others31. Modulation of the inflammatory response by binding to different cell-surface proteins such as toll-like receptor 4, as well as oxidant-scavenging, antimicrobial and apoptosis-inducing activities are among the extracellular functions attributed to CPT31. Interestingly, both pro- and anti-inflammatory roles have been reported for CPT32. Additionally, previous studies showed that CPT is a sensitive and specific biomarker of systemic and local inflammation, mirroring in the latter case intestinal or synovial inflammation (when its levels are assessed in stool samples or synovial fluid, respectively)33–35. Fecal CPT levels have also been associated with disease activity in ankylosing spondylitis (AS)24,36. Furthermore, serum CPT was shown to be an independent marker of radiographic spinal progression in axSpA37. Also in this line, it was reported that CPT expression is highly upregulated in inflamed axial entheses in SKG mice, an experimental model of SpA38.
Taking all these considerations into account, in the present study we aimed to evaluate the potential association of CPT with the development of subclinical atherosclerotic disease and also with metabolic risk factors, including lipid profile and markers of inflammation, in a large cohort of axSpA patients.
Results
Differences in CPT levels between axSpA patients and controls
axSpA patients displayed statistically significantly lower CPT levels than controls (91.4 ± 26.1 vs. 102.3 ± 31.2 ng/mL, respectively, p = 0.006) after adjustment for sex, age at the time of the study and classic CV risk factors (Fig. 1, Table 1). No statistically significant differences were observed in CPT levels between AS and nr-axSpA when adjusting for sex, age at the time of the study, classic CV risk factors and HLA-B27 status (p > 0.05).
Figure 1.
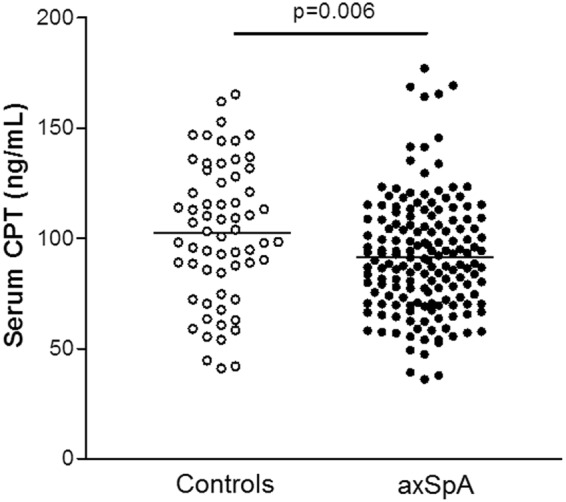
Differences in CPT serum levels between healthy controls and axSpA patients, after adjustment for sex, age at the time of the study and traditional CV risk factors (smoking, obesity, dyslipidemia and hypertension). Healthy controls (n = 63) are represented by empty circles (○), while axSpA patients (n = 163) are represented by filled circles (●). Horizontal bars indicate mean value of each group.
Table 1.
Demographical, laboratory and cardiovascular disease-related data in healthy controls and patients with axSpA (both AS and nr-axSpA patients).
| Variable | Controls (n = 63) | axSpA (n = 163) | AS (n = 119) | nr-axSpA (n = 44) |
|---|---|---|---|---|
| Men/Women, n | 28/35 | 90/73 | 73/46 | 17/27 |
| Age at study (years), mean ± SD | 50.9 ± 15.3 | 43.7 ± 11.6 | 44.9 ± 11.9 | 40.3 ± 10.3 |
| Age at axSpA diagnosis (years), mean ± SD | — | 37.2 ± 10.4 | 36.7 ± 10.7 | 38.7 ± 9.2 |
| History of classic cardiovascular risk factors, n (%) | ||||
| Current smokers | 12 (19.0) | 47 (28.8) | 39 (32.8) | 8 (18.2) |
| Obesity | 12 (19.0) | 32 (19.6) | 25 (21.0) | 7 (15.9) |
| Dyslipidemia | 13 (20.6) | 33 (20.2) | 25 (21.0) | 8 (18.2) |
| Hypertension | 12 (19.0) | 25 (15.3) | 19 (16.0) | 6 (13.6) |
| Body mass index (kg/m2) at study, mean ± SD | 26.8 ± 4.9 | 25.9 ± 4.5 | 26.1 ± 4.6 | 25.2 ± 4.2 |
| Systolic blood pressure (mm Hg) at study, mean ± SD | 127.4 ± 15.4 | 128.1 ± 15.2 | 129.2 ± 15.0 | 125.2 ± 15.5 |
| Diastolic blood pressure (mm Hg) at study, mean ± SD | 78.2 ± 8.8 | 77.9 ± 10.0 | 78.0 ± 10.3 | 77.8 ± 9.2 |
| Total cholesterol (mg/dL) at study, mean ± SD | 202.2 ± 33.9 | 193.1 ± 35.6 | 194.9 ± 36.1 | 188.0 ± 34.1 |
| HDL cholesterol (mg/dL) at study, mean ± SD | 59.1 ± 16.0 | 55.4 ± 16.4 | 53.8 ± 14.8 | 59.5 ± 19.6 |
| LDL cholesterol (mg/dL) at study, mean ± SD | 122.0 ± 31.8 | 118.2 ± 30.0 | 120.7 ± 31.1 | 111.4 ± 25.8 |
| Triglycerides (mg/dL) at study, mean ± SD | 94.7 ± 47.7 | 97.5 ± 54.5 | 98.7 ± 54.4 | 94.3 ± 55.3 |
| Atherogenic index (total cholesterol/HDL), mean ± SD | 3.6 ± 1.1 | 3.7 ± 1.0 | 3.8 ± 1.0 | 3.4 ± 0.9 |
| CRP (mg/L) at study, mean ± SD | 2.9 ± 4.4 | 5.7 ± 9.9 | 6.3 ± 10.9 | 4.0 ± 5.8 |
| CRP (mg/L) at axSpA diagnosis, mean ± SD | — | 11.5 ± 23.4 | 14.0 ± 26.6 | 4.9 ± 7.4 |
| ESR (mm/1st hour) at study, mean ± SD | 11.5 ± 4.4 | 11.3 ± 12.4 | 12.3 ± 13.3 | 8.4 ± 8.9 |
| CRP at axSpA diagnosis ≥ 3 mg/L, n (%) | — | 86 (52.8) | 71 (59.7) | 15 (34.1) |
| ESR (mm/1st hour) at axSpA diagnosis, mean ± SD | — | 15.1 ± 17.9 | 17.2 ± 20.2 | 10.3 ± 10.2 |
| BASDAI at study, median [IQ range] | — | 3.80 [1.75–5.50] | 3.70 [1.65–4.95] | 4.40 [2.60–6.10] |
| BASDAI at study, mean ± SD | — | 3.80 ± 2.22 | 3.60 ± 2.17 | 4.35 ± 2.29 |
| Therapy at study | ||||
| Anti-TNF-α treatment, n (%) | — | 49 (30.1) | 43 (36.1) | 6 (13.6) |
| DMARDs, n (%)* | — | 79 (48.5) | 62 (52.1) | 17 (38.6) |
| NSAIDs, n (%)** | — | 158 (96.9) | 114 (95.8) | 44 (100) |
| Statins, n (%) | — | 13/163 (8.0) | 10/119 (8.4) | 3/44 (6.8) |
| Carotid IMT (mm), mean ± SD | — | 0.608 ± 0.131 | 0.622 ± 0.14 | 0.568 ± 0.11 |
| Carotid plaques, n (%) | — | 51 (31.3) | 43 (36.1) | 8 (18.2) |
| CPT (ng/mL) | 102.3 ± 31.2 | 91.4 ± 26.1 | 91.4 ± 26.7 | 91.1 ± 24.9 |
AS: Ankylosing spondylitis; axSpA: Axial spondyloarthritis; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; CPT: Calprotectin; CRP: C-Reactive Protein; DMARDs: Disease Modifying Anti-Rheumatic Drugs; ESR: Erythrocyte Sedimentation Rate; HDL: High Density Lipoprotein; IMT: Intima-Media Thickness; IQ: Interquartile; LDL: Low Density Lipoprotein; nr-axSpA: non-radiographic axial spondyloarthritis; NSAIDs: Nonsteroidal anti-inflammatory drugs; SD: Standard Deviation; TNF: Tumour necrosis factor. *Mainly sulphasalazine, median dose: 2 grams/day. **Mainly naproxen, median dose: 1000 milligrams/day.
Association of CPT levels and markers of subclinical atherosclerosis
When we assessed the potential association of CPT levels with presence of plaques and cIMT values in axSpA, no statistically significant results were obtained after adjusting for sex, age at the time of the study, classic CV risk factors and HLA-B27 status (p > 0.05).
Relationship of CPT levels with routine laboratory markers of inflammation, lipid profile, disease activity and treatment
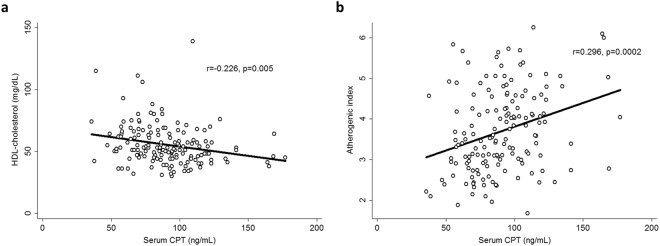
We observed a negative correlation of CPT with high density lipoprotein (HDL)-cholesterol (r = −0.226, p = 0.005, Fig. 2a) and a positive correlation with the atherogenic index (AI) (r = 0.296, p = 0.0002, Fig. 2b) in axSpA. This association between CPT and parameters of lipid profile was further confirmed in the AS subgroup (r = −0.285, p = 0.003 for HDL-cholesterol, and r = 0.285, p = 0.003 for AI) and in the non-radiographic axSpA (nr-axSpA) subgroup (r = 0.371, p = 0.02 for AI). In addition, we found an association with C-reactive protein (CRP) levels at diagnosis in axSpA patients (r = 0.169, p = 0.03). When patients were stratified according to the subtype of axSpA, it was observed that this association was due to the AS subgroup (r = 0.188, p = 0.05). Accordingly, the mean CPT concentration in axSpA patients with CRP levels at diagnosis higher than 3 mg/L was more elevated than that found in patients with lower CRP levels at diagnosis (95.5 ± 26.4 vs. 86.8 ± 25.2 ng/mL, respectively, p = 0.01). This significant difference in CPT levels among patients with high/low CRP levels at diagnosis was also observed in AS (p = 0.001). No statistically significant association was disclosed between CPT levels and disease activity (assessed by Bath Ankylosing Spondylitis Disease Activity Index [BASDAI]) or markers of inflammation (CRP and erythrocyte sedimentation rate [ESR]) at the time of the study, or with ESR at the time of axSpA diagnosis (p > 0.05). All these results were adjusted for sex, age at the time of the study, classic CV risk factors and HLA-B27 status as potential confounding factors.
Figure 2.
Association of serum levels of CPT with HDL-cholesterol (a) and atherogenic index (b) in axSpA patients after adjustment for sex, age at the time of the study, classic CV risk factors and HLA-B27 status.
The different treatments that our axSpA patients were receiving at the time of the study (anti- tumour necrosis factor-α [anti-TNF-α] therapy, disease modifying anti-rheumatic drugs [DMARDs], nonsteroidal anti-inflammatory drugs [NSAIDs] and statins) were not found to exert any influence on their CPT serum levels after adjustment for sex, age at the time of the study, classic CV risk factors and HLA-B27 status (p > 0.05).
When we evaluated the association of CPT and lipid profile parameters in healthy controls, we observed a statistically significant correlation with HDL-cholesterol and AI after adjustment for sex, age at the time of the study and classic CV risk factors (r = −0.376; p = 0.004 for HLD-cholesterol, and r = 0.393; p = 0.003 for AI). No statistically significant association of CPT was noted regarding CRP and ESR levels at study in the controls group after adjustment for those potential confounding factors (p > 0.05).
Discussion
CV disease is one of the leading causes of death in axSpA patients39. In this regard, the chronic inflammatory state as well as the higher incidence of traditional CV risk factors and MeS features in these patients play a key role in the development of CV disease in axSpA15–20. The elucidation of the molecular mechanisms that are implicated in the onset and progression of CV disease in axSpA could help to a better understanding of this comorbidity. Thereby, serum biomarkers could be used as an alternative, non-invasive approach to assess CV risk in axSpA patients, combined with imaging techniques such as carotid ultrasound. Thereupon, during the last years, much effort has been made to identify circulating biomarkers of CV risk in different chronic inflammatory diseases such as AS. Among them, adipokines, MeS-related molecules, as well as biomarkers of endothelial cell activation and inflammation have been reported to exert relevant roles in the development of CV disease40. However, given the complex physiological effects of these molecules, the identification of the ‘perfect’ biomarker of CV risk in chronic inflammatory diseases for its use in the daily clinical practice has not been achieved yet. Consequently, data on this issue are still inconclusive and require further studies.
This prompted us to assess whether CPT, a protein released during inflammation and potentially linked to CV disease in inflammatory pathologies such as type 2 diabetes mellitus or Sjögren’s syndrome26–29, could be associated with subclinical atherosclerosis and metabolic risk factors in axSpA.
Current data on CPT levels in patients with axSpA are very heterogeneous. Even if most of the studies report higher circulating levels of this protein in these patients41–44, others do not find differences as compared to controls24,36. In this study, that included a large cohort of patients with axSpA, we disclosed significantly lower CPT serum concentrations in our patients when compared to controls. In this regard, a recent study revealed that CPT levels decrease after the end of a 6-month intensive exercise programme in AS and nr-axSpA patients, accompanied by clinical improvement45. This may be particularly relevant, given that regular exercise and physical therapy is recommended to complement medical therapy in axSpA, aimed to ameliorate physical disability as well as cardiorespiratory complications of the disease46. Thus, it is possible that physical exercise could have an influence on circulating CPT levels, lowering them, and hence possibly explaining our results. Unfortunately, we could not get enough information on the physical activity/therapy performed by our patients. Another potential explanation for the decreased levels of circulating CPT observed in our patients could be that they are reflecting an accumulation of CPT in the synovial fluid, based on the small size of CPT (36.5 kDa), which enables it to diffuse between inflamed tissues and circulation47. In this regard, previous reports have shown that CPT may diffuse from the inflamed joints into circulation47,48. Similarly, it has been suggested that inflammatory cells expressing CPT are activated and transmigrate from peripheral circulation, through the endothelium, to the inflamed tissues34,43. Thereby, as suggested by Levitova et al., decreased serum levels of CPT may mirror local inflammation45 (e.g. in synovial tissue). Even though axSpA is a predominantly axial disease, rarely presenting peripheral involvement, around 30% of our axSpA patients did exhibit peripheral arthritis/synovitis. Accordingly, it is possible that serum levels of CPT may be reflecting a certain degree of local inflammation in sacroiliac, costovertebral or facet joints, which are often affected in axSpA. In addition, it was reported that the levels of circulating CPT decrease after effective treatment in rheumatic diseases31,49. In the present study we assessed the influence of the different therapies that patients were receiving at the time of the study. At this time, the different modalities of treatment did not show any statistically significant effect on CPT serum levels. It is important to note that, in spite of the low activity of the disease displayed by our patients (based on the BASDAI value), they probably still have a certain degree of inflammation. Thereupon, another potential hypothesis to explain our results could be that this might be triggering compensatory mechanisms to reduce the detrimental effect of this remaining inflammation (by reducing, for example, the levels of pro-inflammatory molecules such as CPT, either in a direct or indirect fashion).
Regarding the role of CPT in CV disease, as above mentioned, in previous studies this molecule has been linked to atherosclerosis in different chronic inflammatory diseases26–30. Among them, the only study that included axSpA patients was one in which 12 AS patients were studied along with 15 rheumatoid arthritis and 9 psoriatic arthritis patients (as a single group). In such a study, it was reported that CPT associated with aortic stiffness, but not with cIMT values30. In our present study we did not disclose an association between CPT levels and cIMT values or presence of plaques, as surrogate markers of CV disease. However, when we assessed the potential link between CPT levels and metabolic risk factors, an association between CPT and lipid profile parameters emerged in our axSpA patients. In this regard, we found an inverse association between HDL-cholesterol and CPT in AS, and a positive correlation between this protein and the AI in both types of axSpA. These results on lipid association with CPT were further confirmed in our control cohort. Our results are in line with those obtained in previous studies, in which CPT negatively correlated with HDL-cholesterol in individuals with no previous history of CV disease and in obese patients27,50,51. This further supports the importance of CPT in the atherosclerotic process, regardless of the pathogenic context. Hence, CPT adds to a list of inflammatory markers that are associated with decreased HDL-cholesterol levels52. Low HDL-cholesterol levels and high AI are markers of inflammation and dyslipidemia, factors that further enhance the risk of developing atherosclerosis. However, whether this is a direct effect of CPT on lipid metabolism or an indirect effect mediated by the inflammatory status should be further evaluated.
In accordance with the pro-inflammatory role of CPT, in the whole group of axSpA we disclosed a positive association with the levels of CRP, a widely known biomarker of systemic inflammation, at the time of diagnosis of the disease. This was also confirmed in the subgroup of AS patients, supporting the data previously reported by others27,36,44. In a further analysis, we observed that axSpA patients with CRP levels at disease diagnosis higher than 3 mg/L, a CRP level considered representative of high vascular risk53, also showed higher serum CPT levels. However, in line with previous reports41,42, no association was observed between CPT and BASDAI at the time of the study. Similarly, no association was noted between CPT and ESR at the time of disease diagnosis, or ESR and CRP at the time of the study. Even if some authors have previously reported a correlation between CPT and/or ESR36,41, others did not find such association42. It is plausible to think that CRP and ESR levels at the time of the study could be under the influence of the medical treatment received, physical therapy and changes in lifestyle aimed to improve the quality of life of axSpA patients.
It is worth mentioning that, as occurs with other rheumatic diseases, the current tools used to predict the individual’s absolute risk for CV disease (such as lipid profile, CRP or other inflammatory markers) were found to underestimate the actual CV risk of patients with axSpA12. Consequently, the search for additional tools that may help to identify axSpA patients at high risk of CV events is of main importance. Thereby, we believe a combination of a series of biomarkers (among them CPT), rather than the assessment of a single one, along with the use of non-invasive surrogate markers, such as the carotid ultrasonography, may be needed to reach an adequate stratification of the actual CV risk in axSpA patients.
In summary, our study shows no relationship between CPT levels and markers of subclinical atherosclerosis in axSpA patients undergoing therapy who had low disease activity. Nevertheless, it demonstrates that CPT is associated with adverse lipid profiles and inflammatory markers in a large cohort of axSpA patients. This may be suggesting an indirect action of this molecule on the development of atherosclerosis. The fact that lower CPT serum levels were observed in our patients when compared to controls warrants further investigation to determine whether axSpA-intrinsic factors, specific characteristics of our patients or even the therapy used for the management of the disease could be implicated in the modulation of their circulating serum CPT levels, but not affecting its association with CRP or parameters linked to adverse lipid profile. Moreover, further molecular studies are warranted to elucidate the exact mechanisms by which CPT exerts its action in the development and progression of atherosclerotic disease in our patients. Additionally, it will be interesting to perform comparative studies on the association of CPT with subclinical atherosclerosis and metabolic risk factors in other inflammatory arthritides, such as psoriatic arthritis and rheumatoid arthritis.
Methods
Patients and controls
All the experiments involving humans and human blood samples were carried out in accordance with the approved guidelines and regulations, according to the Declaration of Helsinki. Furthermore, all experimental protocols were approved by the Ethics Committee of Clinical Research of Cantabria (CEIC-C, Number of reference 7/2016). Informed written consent was obtained from all subjects.
163 patients diagnosed with axSpA seen over a 3-year period at Hospital Universitario Marqués de Valdecilla and Hospital de Laredo (Cantabria, Spain) that fulfilled the ASAS classification criteria54 were recruited for this study. None of them had experienced CV events or had diabetes mellitus, chronic kidney disease, inflammatory bowel disease or psoriasis. 44 out of 163 patients fulfilled the definitions for nr-axSpA54, while the remaining 119 patients also fulfilled definitions for AS according to the 1984 modified New York criteria55. 63 healthy controls (who did not have history of CV events or chronic inflammatory diseases) were also recruited for the comparative analysis.
Data on sex, age, body mass index (BMI), blood pressure, total cholesterol, HDL- and low density lipoprotein (LDL)-cholesterol, and triglycerides at the time of study, and history of traditional CV risk factors (smoking, obesity, dyslipidemia and hypertension) were collected. Obesity was defined if BMI (calculated as weight in kilograms divided by height in squared meters) was >30. Patients were considered to have dyslipidemia if they had hypercholesterolemia and/or hypertriglyceridemia (defined as diagnosis of hypercholesterolemia or hypertriglyceridemia by the patients’ family physician, or total cholesterol and/or triglyceride levels in fasting plasma being >220 and >150 mg/dL, respectively). In those patients with total cholesterol between 200 and 220 mg/dL, a diagnosis of dyslipidemia was considered if the AI (total cholesterol/HDL-cholesterol) was ≥4.1. Patients were diagnosed as having hypertension if blood pressure was >140/90 mmHg or if they were taking antihypertensive agents. Information on CRP and ESR at the time of the study and at disease diagnosis was assessed, as well as information on disease activity at the time of the study (by calculating the BASDAI value) and therapy, including treatment with anti-TNF-α agents, DMARDs, NSAIDs and statins. The main demographic, laboratory and CV disease-related data of controls and patients are displayed in Table 1.
Carotid ultrasonography
Carotid ultrasonography was performed in all the patients to assess the presence of abnormal cIMT values in the common carotid artery and the presence of focal plaques in the extracranial carotid tree (as surrogate markers of CV disease), as previously reported10,11,13.
Study protocol
Determinations were made in the fasting state. Blood samples were taken for measurement of ESR (Westergren), CRP (latex immunoturbidimetry) and lipids (enzymatic colorimetry). Commercial enzyme-linked immunosorbent assay (ELISA) kits were used to measure serum CPT (HK325, Hycult Biotech, the Netherlands) according to the manufacturer’s instructions. All samples were analysed in duplicate.
Statistical analysis
The analyses were first performed in the whole cohort of axSpA patients, and later patients were stratified into AS and nr-axSpA, since there is still concern on whether AS and nr-axSpA are distinct but overlapping disorders or two phases of the same disease56. Data were expressed as mean ± standard deviation (SD) and/or median [interquartile (IQ) range] for continuous variables, and number of individuals (n) and percentage (%) for categorical variables. Differences in CPT levels among the study groups were assessed by ANCOVA adjusting for potential confounding factors: sex, age at the time of the study and classic CV risk factors (smoking, obesity, dyslipidemia and hypertension). When comparing CPT levels between the two subtypes of axSpA we also performed an adjustment for ANCOVA using the above mentioned confounding factors along with HLA-B27 status. Correlation between CPT and continuous variables was performed via estimation of the Pearson partial correlation coefficient (r) adjusting for sex, age at the time of the study, classic CV risk factors and HLA-B27 status. Associations between categorical features and CPT concentrations were assessed by ANCOVA adjusting for sex, age at the time of the study, classic CV risk factors and HLA-B27 status. The correlation between CPT levels and continuous variables in healthy controls was performed via estimation of the Pearson partial correlation coefficient (r) adjusting for sex, age at the time of the study and classic CV risk factors.
Pearson partial correlation coefficients (r) were categorized as follows: 0.1 < |r| < 0.3 = small correlation; 0.3 < |r| < 0.5 = medium/moderate correlation; |r| > 0.5 = large/strong correlation. Two-sided p values ≤ 0.05 were considered to indicate statistical significance. Statistical analysis was performed using STATA® v. 11.1 (StataCorp, College Station, TX, USA).
Acknowledgements
We wish to thank all the patients and controls that participated in this study and Begoña Ubilla for technical assistance. FG is a recipient of a Sara Borrell post-doctoral fellowship from the Instituto de Salud Carlos III (ISCIII) (Spain), co-funded by the European Social Fund (ESF, “Investing in your future”) (grant CD15/00095). SR-M is supported by funds of the RETICS Program (RIER) RD16/0012/0009 (ISCIII, co-funded by the European Regional Development Fund, ERDF). VM is supported by funds of a Miguel Servet type I programme (grant CP16/00033) (ISCIII, co-funded by ERDF). RL-M is a recipient of a Miguel Servet type I programme fellowship from the ISCIII, co-funded by the ESF (grant CP16/00033). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
F.G., J.R.-G. and S.R.-M. carried out the conception and design of the study, were involved in the statistical analysis and interpretation of data, and in the elaboration of the manuscript. In addition, J.R.-G. recruited patients for the study and performed the carotid ultrasound study. A.C., V.M., R.E., C.M., V.P., R.B., J.L.H., J.L. and O.G. helped in the acquisition and interpretation of data, and contributed to the elaboration of the manuscript. R.L.-M. and M.A.G.-G. contributed to the elaboration of the protocol of study, helped in the interpretation of data and were responsible of the final drafting and elaboration of the manuscript. All authors have approved the final article.
Data Availability
All data generated or analysed during this study are included in this published article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fernanda Genre, Javier Rueda-Gotor and Sara Remuzgo-Martínez contributed equally.
Raquel López-Mejías and Miguel A. González-Gay jointly supervised this work.
Contributor Information
Raquel López-Mejías, Email: rlopezmejias78@gmail.com.
Miguel A. González-Gay, Email: miguelaggay@hotmail.com
References
- 1.Martínez MC, Andriantsitohaina R. Extracellular Vesicles in Metabolic Syndrome. Circ Res. 2017;120:1674–1686. doi: 10.1161/CIRCRESAHA.117.309419. [DOI] [PubMed] [Google Scholar]
- 2.Pucci G, et al. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: A review of the literature. Pharmacol Res. 2017;120:34–42. doi: 10.1016/j.phrs.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Tune JD, Goodwill AG, Sassoon DJ, Mather KJ. Cardiovascular consequences of metabolic syndrome. Transl Res. 2017;183:57–70. doi: 10.1016/j.trsl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brede S, Serfling G, Klement J, Schmid SM, Lehnert H. Clinical Scenario of the Metabolic Syndrome. Visc Med. 2016;32:336–341. doi: 10.1159/000449028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerekes G, et al. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol. 2014;10:691–696. doi: 10.1038/nrrheum.2014.121. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Juanatey C, et al. The high prevalence of subclinical atherosclerosis in patients with ankylosing spondylitis without clinically evident cardiovascular disease. Medicine (Baltimore). 2009;88:358–365. doi: 10.1097/MD.0b013e3181c10773. [DOI] [PubMed] [Google Scholar]
- 7.Nambi V, et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez-Juanatey C, Llorca J, Martin J, Gonzalez-Gay MA. Carotid intima-media thickness predicts the development of cardiovascular events in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2009;38:366–371. doi: 10.1016/j.semarthrit.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Evans MR, et al. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211–1220. doi: 10.1002/art.30265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrales A, et al. Cardiovascular risk stratification in rheumatic diseases: carotid ultrasound is more sensitive than Coronary Artery Calcification Score to detect subclinical atherosclerosis in patients with rheumatoid arthritis. Ann Rheum Dis. 2013;72:1764–1770. doi: 10.1136/annrheumdis-2013-203688. [DOI] [PubMed] [Google Scholar]
- 11.Corrales A, et al. Carotid ultrasound is useful for the cardiovascular risk stratification of patients with rheumatoid arthritis: results of a population-based study. Ann Rheum Dis. 2014;73:722–727. doi: 10.1136/annrheumdis-2012-203101. [DOI] [PubMed] [Google Scholar]
- 12.Rueda-Gotor J, et al. Carotid ultrasound in the cardiovascular risk stratification of patients with ankylosing spondylitis: results of a population-based study. Clin Exp Rheumatol. 2016;34:885–892. [PubMed] [Google Scholar]
- 13.Rueda-Gotor J, et al. Atherosclerotic disease in axial spondyloarthritis: increased frequency of carotid plaques. Clin Exp Rheumatol. 2015;33:315–320. [PubMed] [Google Scholar]
- 14.Papagoras C, et al. Cardiovascular risk profile in patients with spondyloarthritis. Joint Bone Spine. 2014;81:57–63. doi: 10.1016/j.jbspin.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Han C, et al. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33:2167–2172. [PubMed] [Google Scholar]
- 16.Mathieu S, Gossec L, Dougados M, Soubrier M. Cardiovascular profile in ankylosing spondylitis: a systematic review and meta-analysis. Arthritis Care Res (Hoboken). 2011;63:557–563. doi: 10.1002/acr.20364. [DOI] [PubMed] [Google Scholar]
- 17.Szabo SM, et al. Increased risk of cardiovascular and cerebrovascular diseases in individuals with ankylosing spondylitis: a population-based study. Arthritis Rheum. 2011;63:3294–3304. doi: 10.1002/art.30581. [DOI] [PubMed] [Google Scholar]
- 18.Bremander A, Petersson IF, Bergman S, Englund M. Population-based estimates of common comorbidities and cardiovascular disease in ankylosing spondylitis. Arthritis Care Res (Hoboken). 2011;63:550–556. doi: 10.1002/acr.20408. [DOI] [PubMed] [Google Scholar]
- 19.Malesci D, et al. High prevalence of metabolic syndrome in patients with ankylosing spondylitis. Clin Rheumatol. 2007;26:710–714. doi: 10.1007/s10067-006-0380-5. [DOI] [PubMed] [Google Scholar]
- 20.Papadakis JA, et al. High prevalence of metabolic syndrome and cardiovascular risk factors in men with ankylosing spondylitis on anti-TNFalpha treatment: correlation with disease activity. Clin Exp Rheumatol. 2009;27:292–298. [PubMed] [Google Scholar]
- 21.Castañeda S, Nurmohamed MT, González-Gay MA. Cardiovascular disease in inflammatory rheumatic diseases. Best Pract Res Clin Rheumatol. 2016;30:851–869. doi: 10.1016/j.berh.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 22.Shen J, Shang Q, Tam LS. Targeting inflammation in the prevention of cardiovascular disease in patients with inflammatory arthritis. Transl Res. 2016;167:138–151. doi: 10.1016/j.trsl.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Chávez-Sánchez L, et al. Innate immune system cells in atherosclerosis. Arch Med Res. 2014;45:1–14. doi: 10.1016/j.arcmed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Duran A, et al. Fecal calprotectin is associated with disease activity in patients with ankylosing spondylitis. Bosn J Basic Med Sci. 2016;16:71–74. doi: 10.17305/bjbms.2016.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frosch M, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43:628–637. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 26.Larsson PT, Hallerstam S, Rosfors S, Wallén NH. Circulating markers of inflammation are related to carotid artery atherosclerosis. Int Angiol. 2005;24:43–51. [PubMed] [Google Scholar]
- 27.Pedersen L, et al. Plasma calprotectin and its association with cardiovascular disease manifestations, obesity and the metabolic syndrome in type 2 diabetes mellitus patients. BMC Cardiovasc Disord. 2014;14:196. doi: 10.1186/1471-2261-14-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balarini GM, et al. Serum calprotectin is a biomarker of carotid atherosclerosis in patients with primary Sjögren’s syndrome. Clin Exp Rheumatol. 2016;34:1006–1012. [PubMed] [Google Scholar]
- 29.Langley SR, et al. Extracellular matrix proteomics identifies molecular signature of symptomatic carotid plaques. J Clin Invest. 2017;127:1546–1560. doi: 10.1172/JCI86924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angel K, et al. Effect of 1-year anti-TNF-α therapy on aortic stiffness, carotid atherosclerosis, and calprotectin in inflammatory arthropathies: a controlled study. Am J Hypertens. 2012;25:644–650. doi: 10.1038/ajh.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ometto F, et al. Calprotectin in rheumatic diseases. Exp Biol Med (Maywood). 2017;242:859–873. doi: 10.1177/1535370216681551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiopu A, Cotoi OS. S100A8 and S100A9: DAMPs at the crossroads between innate immunity, traditional risk factors, and cardiovascular disease. Mediators Inflamm. 2013;2013:828354. doi: 10.1155/2013/828354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Rycke L, et al. Differential expression and response to anti-TNFalpha treatment of infiltrating versus resident tissue macrophage subsets in autoimmune arthritis. J Pathol. 2005;206:17–27. doi: 10.1002/path.1758. [DOI] [PubMed] [Google Scholar]
- 35.Kruithof E, et al. Identification of synovial biomarkers of response to experimental treatment in early-phase clinical trials in spondylarthritis. Arthritis Rheum. 2006;54:1795–1804. doi: 10.1002/art.21914. [DOI] [PubMed] [Google Scholar]
- 36.Klingberg E, Carlsten H, Hilme E, Hedberg M, Forsblad-d’Elia H. Calprotectin in ankylosing spondylitis–frequently elevated in feces, but normal in serum. Scand J Gastroenterol. 2012;47:435–444. doi: 10.3109/00365521.2011.648953. [DOI] [PubMed] [Google Scholar]
- 37.Turina MC, et al. Calprotectin serum level is an independent marker for radiographic spinal progression in axial spondyloarthritis. Ann Rheum Dis. 2014;73:1746–1748. doi: 10.1136/annrheumdis-2014-205506. [DOI] [PubMed] [Google Scholar]
- 38.Stavre Z, Maeda Y, Gravallese EM. Calprotectin Is Highly Upregulated in Inflamed Axial Entheses in SKG Mice [abstract] Arthritis Rheumatol. 2016;68:4057–4058. [Google Scholar]
- 39.Prati C, Claudepierre P, Pham T, Wendling D. Mortality in spondylarthritis. Joint Bone Spine. 2011;78:466–470. doi: 10.1016/j.jbspin.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Genre F, et al. Adipokines, biomarkers of endothelial activation, and metabolic syndrome in patients with ankylosing spondylitis. Biomed Res Int. 2014;2014:860651. doi: 10.1155/2014/860651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cypers H, et al. Elevated calprotectin levels reveal bowel inflammation in spondyloarthritis. Ann Rheum Dis. 2016;75:1357–1362. doi: 10.1136/annrheumdis-2015-208025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oktayoglu P, et al. Elevated serum levels of calprotectin (myeloid-related protein 8/14) in patients with ankylosing spondylitis and its association with disease activity and quality of life. J Investig Med. 2014;62:880–884. doi: 10.1097/JIM.0000000000000095. [DOI] [PubMed] [Google Scholar]
- 43.Turina MC, Yeremenko N, Paramarta JE, De Rycke L, Baeten D. Calprotectin (S100A8/9) as serum biomarker for clinical response in proof-of-concept trials in axial and peripheral spondyloarthritis. Arthritis Res Ther. 2014;16:413. doi: 10.1186/s13075-014-0413-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta L, Bhattacharya S, Agarwal V, Aggarwal A. Elevated levels of serum MRP8/14 in ankylosing spondylitis: associated with peripheral arthritis and active disease. Clin Rheumatol. 2016;35:3075–3079. doi: 10.1007/s10067-016-3448-x. [DOI] [PubMed] [Google Scholar]
- 45.Levitova A, et al. Clinical improvement and reduction in serum calprotectin levels after an intensive exercise programme for patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Res Ther. 2016;18:275. doi: 10.1186/s13075-016-1180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Heijde D, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76:978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- 47.Kopeć-Mędrek M, Widuchowska M, Kucharz EJ. Calprotectin in rheumatic diseases: a review. Reumatologia. 2016;54:306–309. doi: 10.5114/reum.2016.64907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kane D, et al. Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum. 2003;48:1676–1685. doi: 10.1002/art.10988. [DOI] [PubMed] [Google Scholar]
- 49.Abildtrup M, Kingsley GH, Scott DL. Calprotectin as a biomarker for rheumatoid arthritis: a systematic review. J Rheumatol. 2015;42:760–770. doi: 10.3899/jrheum.140628. [DOI] [PubMed] [Google Scholar]
- 50.Cotoi OS, et al. Plasma S100A8/A9 correlates with blood neutrophil counts, traditional risk factors, and cardiovascular disease in middle-aged healthy individuals. Arterioscler Thromb Vasc Biol. 2014;34:202–210. doi: 10.1161/ATVBAHA.113.302432. [DOI] [PubMed] [Google Scholar]
- 51.Mortensen OH, et al. Calprotectin–a novel marker of obesity. PLoS One. 2009;4:e7419. doi: 10.1371/journal.pone.0007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khovidhunkit W, et al. Infection and inflammation-induced proatherogenic changes of lipoproteins. J Infect Dis. 2000;181:S462–S472. doi: 10.1086/315611. [DOI] [PubMed] [Google Scholar]
- 53.Ridker PM, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham Risk Scores. Circulation. 2004;109:1955–1959. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- 54.Rudwaleit M, et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. [DOI] [PubMed] [Google Scholar]
- 55.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27:361–368. doi: 10.1002/art.1780270401. [DOI] [PubMed] [Google Scholar]
- 56.Genre F, et al. Implication of osteoprotegerin and sclerostin in axial spondyloarthritis cardiovascular disease: study of 163 Spanish patients. Clin Exp Rheumatol. 2018;36:302–309. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.