Abstract
Established fear memory becomes vulnerable to disruption after memory retrieval and extinction; this labile state is critical for inhibiting the return of fear memory. However, the labile state has a very narrow time window after retrieval, and underlying molecular mechanisms are not well known. To that end, we isolated the hippocampus immediately after fear memory retrieval and performed proteomics. We identified Neurobeachin (NBEA), an autism-related regulator of synaptic protein trafficking, to be upregulated after contextual fear memory retrieval. NBEA protein expression was rapid and transient after fear memory retrieval at the synapse. Nbea mRNA was enriched at the synapses, and the rapid induction of NBEA expression was blocked by inhibition of the mammalian target of rapamycin (mTOR)-dependent signaling pathway. Mice with cornu ammonis 1 (CA1)-specific Nbea shRNA knockdown showed normal fear acquisition and contextual fear memory but impaired extinction, suggesting an important role of Nbea in fear memory extinction processes. Consistently, Nbea heterozygotes showed normal fear acquisition and fear memory recall but showed impairment in extinction. Our data suggest that NBEA is necessary either for induction of memory lability or for the physiological process of memory extinction.
Introduction
Post-traumatic stress disorder (PTSD) is a neuropsychiatric disorder developed from exposure to severe traumatic events1. Contextual fear conditioning is a well-known protocol used to understand context-dependent fear memory consolidation, reconsolidation and extinction processes in which the hippocampus is the core brain region involved2. Extinction therapy has been widely used for the treatment of PTSD; however, not all populations of patients experience the beneficial effects of these therapies, and effective pharmacological interventions for PTSD patients are limited3. Fear memory retrieval renders the original memory labile, allowing a short time window for amnesic intervention. Then, reconsolidation restores the stability of the memory4. However, the labile state has a very narrow time window after retrieval, and the underlying molecular mechanisms are not well known.
De novo protein synthesis has been shown to be involved in extinction5 and memory lability to trigger instability of the original memory6,7. A memory-labile state is rapidly established after retrieval and has a very narrow time window8. Recently, the mammalian target of rapamycin (mTOR) has been widely accepted to be involved in local de novo protein synthesis, and its target proteins have been shown to play diverse roles in synaptic plasticity and activity-dependent synaptic transmission. The mTOR signaling pathway has been suggested to be involved in rapid modulation of memory susceptibility9,10. However, which molecules are rapidly expressed after fear memory retrieval and the roles of these newly synthesized proteins in memory susceptibility and extinction have not been studied.
Neurobeachin (NBEA) is a large, cytosolic multidomain protein selectively expressed in neurons and endocrine cells11. NBEA has been suggested to regulate postsynaptic neurotransmitter and ionotropic receptor trafficking to the cell surface12,13 and act as a negative regulator of regulated secretion14. NBEA also regulates synaptic architecture through actin cytoskeleton remodeling15. In zebrafish, NBEA is essential for the maintenance of extensive dendritic branching in mature neurons16. Further, NBEA was recently reported to regulate Notch-mediated gene transcription17. NBEA also directly interacts with protein kinase A (PKA) and regulates PKA-mediated phosphorylation11. Because Nbea is a candidate gene for autism spectrum disorder (ASD), Nbea heterozygous mice were generated using the gene-trap method, and 24-month-old female mice were phenotyped18,19. However, there are insufficient studies to identify the link between the molecular role of NBEA and the resulting behavioral phenotypes, especially fear memory processes.
Here, we performed unbiased proteomics to isolate the molecules involved in fear memory retrieval and found that NBEA was upregulated in the dorsal hippocampus. Nbea mRNA was enriched at the synapse, in which its expression was rapidly and transiently expressed immediately after retrieval. Suppression of NBEA expression by knockdown and knockout impaired extinction, suggesting that NBEA is necessary for either the induction of memory lability or the physiological process of memory extinction.
Results
Isolation of NBEA from the proteomic analysis after contextual fear memory retrieval
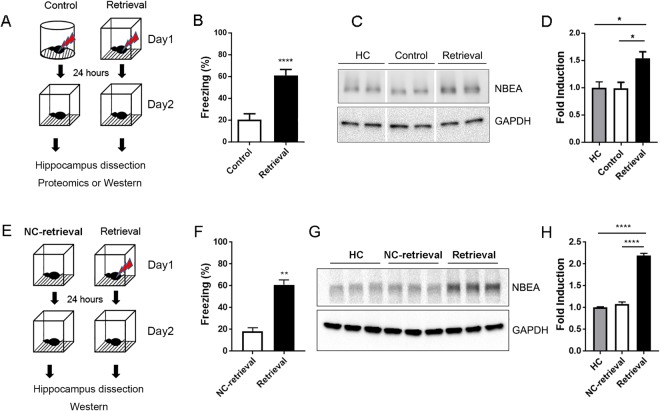
Memory retrieval renders the consolidated memory labile and that extinction training during the labile state can alter or erase fear memory (Fukushima et al.7). However, the molecular mechanisms underlying the memory retrieval-mediated process are not well known. To identify candidate molecules involved in fear memory retrieval, we utilized unbiased proteomics analysis. To isolate candidate molecules specific to the retrieval process, both a control and retrieval group were exposed to a foot shock (0.5 mA, 1 s) for the contextual fear conditioning. The next day, mice in the control group were placed in a different context from the conditioned context, and the retrieval group was placed in the same context (Fig. 1A). Importantly, there was a significant difference in the freezing level between groups on the retrieval test (Fig. 1B; t(18) = 5.398, ***p < 0.0001, unpaired t-test, two-tailed). Then, proteins were isolated from the hippocampus 5 min after fear memory retrieval and further processed for proteomic analysis. The peptide mixture from 5 mice of each group (control and retrieval) was labeled with TMT reagents. These labeled peptides were analyzed with LC-MS/MS analysis using an LTQ Orbitrap XL. A summary of the differentially expressed proteins (DEPs) is presented in Table 1. Among the 14 regulated proteins identified in the proteomic analysis, we decided to focus on the role of NBEA in the fear memory process because NBEA is known to regulate synaptic transmission by modulating the trafficking of receptors, including AMPA receptors, at synapses12,13,20. Recently, several studies have reported that AMPA receptor trafficking is significant for memory stabilization and destabilization after retrieval21,22. We confirmed the increased expression of NBEA after contextual fear retrieval. Since we focused on the rapid induction of protein expression and given the major role of NBEA in trafficking neurotransmitter receptors, we isolated synaptosomes from the dorsal hippocampus to examine the specific role of NBEA at the synapse. Here, NBEA expression at the synapse was significantly increased 5 min after memory retrieval compared to expression in the control group (Fig. 1C,D, Supple Fig. 4; F(2, 6) = 7.916, *p = 0.0208, one-way ANOVA, Tukey’s multiple comparisons post hoc test, HC vs Retrieval, *p = 0.0327, Control vs Retrieval, *p = 0.0307). Home cage samples were used to observe the basal expression of NBEA. Next, we further examined whether the increase in NBEA expression is specific to fear retrieval. To that end, mice were exposed to the same context as the retrieval group without foot shock on day 1 and re-exposed to the same context on day 2, which we described as neutral context retrieval (NC-retrieval) (Fig. 1E). Interestingly, compared to the home-cage group (HC), the NC-retrieval group expressed a similar amount of NBEA, but the retrieval group showed a significant induction in NBEA expression (Fig. 1G,H; F(2, 6) = 267, ****p < 0.0001, one-way ANOVA, Tukey’s multiple comparisons post hoc test, HC vs Retrieval, ****p < 0.0001, NC-retrieval vs Retrieval, ****p < 0.0001), indicating that the rapid increase in NBEA expression was fear-specific. The freezing level confirmed the delivery of foot shocks (0.7 mA, 1 s, 2 shocks) to retrieval group (Fig. 1F; t(4) = 7.826, **p = 0.0014, unpaired t-test, two-tailed).
Figure 1.
Proteomic analysis identified NBEA as one of the candidate molecules regulated by contextual fear retrieval in the hippocampus. (A) Schematic representation of the experimental protocol. Mice in the control group were fear conditioned with a shock in context A and underwent a retrieval test in context B. Mice in the retrieval group were fear conditioned with a shock in context B and underwent a retrieval test in the same context (context B). (B) Freezing level (%) during 5-min retrieval test. Freezing level in the retrieval group (61.19 ± 5.283, n = 10) was significantly higher than that in the control group (20.48 ± 5.381, n = 10), ***p < 0.0001, t-test, two-tailed. (C) Western blot image of NBEA expression in the control and retrieval group. GAPDH was used for the loading control. (D) Intensity measurement of the Western blot. NBEA expression was significantly higher in the retrieval group (1.541 ± 0.1187, n = 3) than in the control group (0.9913 ± 0.1091, n = 3), *p = 0.0327. NBEA expression was significantly higher in the retrieval group (1.541 ± 0.1187, n = 3) than in the home cage (HC) group (1 ± 0.1077, n = 3), *p = 0.0307, one-way ANOVA, Tukey’s multiple comparisons post hoc test. (E) Schematic representation of the experimental protocol. Mice in the NC (neutral context) retrieval group were handled as the same as the retrieval group with no shock of Day 1. (F) Freezing level in the retrieval group (60.89 ± 4.415, n = 3) was significantly higher than that in the NC (neutral context)-retrieval group (18.31 ± 3.179, n = 3), **p = 0.0014, t-test, two-tailed. (G) Western blot image of NBEA expression in the home cage (HC), NC-retrieval and retrieval groups. GAPDH was used as the loading control. (H) Intensity measurements of the Western blots. NBEA expression was significantly higher in the retrieval group (2.193 ± 0.05042, n = 3) than in the HC group (1 ± 0.01841, n = 3), ****p = 0.0001. NBEA expression was significantly higher in the retrieval group (2.193 ± 0.05042, n = 3) than in the NC-retrieval group (1.084 ± 0.04583, n = 3), ****p = 0.0001. There was no difference between the NC-retrieval group (1.084 ± 0.04583, n = 3) and the HC group in NBEA expression, p = 0.3146, one-way ANOVA, Tukey’s multiple comparisons post hoc test.
Table 1.
Proteins significantly changed by contextual fear retrieval.
| IPI | Swiss-Prot | Protein | Description | # unique peptides | Fold change (retrieval/control) |
|---|---|---|---|---|---|
| IPI00313151 | O35098 | DPYSL4 | Dihydropyrimidinase-related protein 4 | 4 | 0.50 |
| IPI00387430 | Q9D6J5 | NDUFB8 | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 8 | 2 | 0.50 |
| IPI00135563 | P55066 | NCAN | Neurocan core protein precursor | 4 | 0.55 |
| IPI00132460 | P61255 | RPL26 | 60S ribosomal protein L26 | 2 | 0.59 |
| IPI00154057 | Q2TJH4 | PCDH1 | Protocadherin 1 | 5 | 0.61 |
| IPI00420651 | Q6R891 | PPP1R9B | Neurabin-2 | 3 | 0.64 |
| IPI00471441 | Q9D0J8 | PTMS | Ptms protein | 4 | 1.50 |
| IPI00115680 | O08795 | PRKCSH | Glucosidase 2 subunit beta precursor | 2 | 1.50 |
| IPI00460558 | Q6PH08 | ERC2 | ERC protein 2 | 2 | 1.50 |
| IPI00652358 | P50429 | ARSB | Isoform 1 of Arylsulfatase B precursor | 4 | 1.54 |
| IPI00659932 | Q3UVX5 | GRM5 | Metabotropic glutamate receptor 5 precursor | 2 | 1.56 |
| IPI00320831 | Q9EPN1 | NBEA | Neurobeachin | 3 | 1.63 |
| IPI00403890 | O88448 | KLC1 | kinesin light chain 1 isoform 1D | 2 | 1.67 |
| IPI00420136 | O70492 | SNX3 | Sorting nexin 3 | 4 | 1.69 |
Significantly changed proteins are listed. The control group was exposed to an unconditioned stimulus (US) but retrieved memories in a different context than the conditioning context. The retrieval group retrieved memories in the same context as the conditioning context. Downregulated proteins are indicated in regular typeface, and upregulated proteins are indicated in bold typeface.
Rapid and transient expression of NBEA after fear memory retrieval
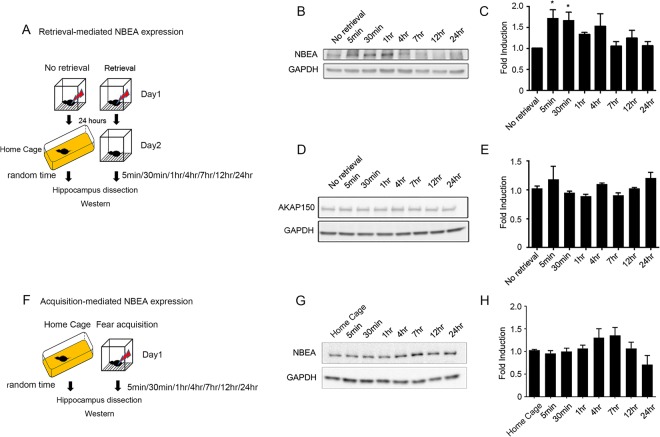
Next, we further examined the expression pattern of NBEA at the synapse at different time points after memory retrieval. Interestingly, the immediate induction of NBEA expression 5 min after retrieval lasted up to 4 h and dropped by 6 h after retrieval, correlating very well with the time course of the labile state of fear memory (Fig. 2A,B,C; F(7, 39) = 2.793, *p = 0.0187, one-way ANOVA). Since, NBEA is a well-known AKAP protein, which binds to PKA and regulates PKA signaling pathways, we also examined the expression of another well-studied AKAP protein, AKAP150, to determine whether the dynamic expression of NBEA after retrieval was unique to NBEA. Western blot analysis showed that there were no significant changes in AKAP150 expression after retrieval compared to the expression in the no-retrieval samples (Fig. 2D,E; F(7, 28) = 2.021, p = 0.0877, one-way ANOVA), suggesting that the dynamic expression after retrieval was not shown in one of the major AKAP isoforms present in the hippocampus23,24. In this experiment, the no-retrieval group, which was not exposed to any context after fear conditioning, was used as a control to examine NBEA expression 24 h after conditioning but immediately before retrieval. To determine whether this dynamic expression was specific to the retrieval process, we analyzed the time course of NBEA expression after fear conditioning. Interestingly, we did not find any significant changes in NBEA expression after fear conditioning, suggesting that the rapid induction of NBEA expression was specific to the fear retrieval process (Fig. 2F,G,H; F(7, 20) = 2.255, p = 0.0728, one-way ANOVA).
Figure 2.
The increase in NBEA expression was rapid and transient after contextual fear retrieval at the synapse in the dorsal hippocampus. (A) Schematic representation of the experimental protocol. Mice in the no-retrieval group were handled as the same as the retrieval group on Day 1 but were not exposed to the same context on Day 2. Random time means the no-retrieval group was sampled randomly, but most of the samples were taken between the 1 hr and 4 hr sampling intervals after fear retrieval. (B) Representative image of the time course of NBEA expression after retrieval. No-retrieval samples were obtained from mice that were fear conditioned and sacrificed 24 h after conditioning without retrieval. (C) Quantification of the time course of NBEA expression after retrieval. There was significant induction in NBEA expression at 5 min and 30 min after contextual fear retrieval. F(7, 39) = 2.793, *p = 0.0187, one-way ANOVA with post hoc Dunnett’s multiple comparisons test: No retrieval (1.005 ± 0.003658, n = 6) vs 5 min after retrieval (1.713 ± 0.2036, n = 5), *p = 0.0328; no retrieval vs 30 min after retrieval (1.661 ± 0.2003, n = 6), *p = 0.0400; no retrieval vs 1 h after retrieval (1.339 ± 0.04178, n = 6), p = 0.5568; no retrieval vs 4 h after retrieval (1.529 ± 0.2942, n = 6), p = 0.1411; no retrieval vs 7 h after retrieval (1.056 ± 0.1076, n = 6), p = 0.9997; no retrieval vs 12 h after retrieval (1.25 ± 0.1824, n = 6), p = 0.8216; no retrieval vs 24 h after retrieval (1.069 ± 0.09385, n = 6), p = 9996. (D) Representative image of the time course of AKAP150 expression after retrieval. (E) Quantification of the time course of AKAP150 expression after retrieval. There was no significant difference in AKAP150 expression after contextual fear retrieval. F(7, 28) = 2.021, p = 0.0877, one-way ANOVA. (F) Schematic representation of the experimental protocol. Random time means the home cage group was sampled randomly, but most of the samples were taken between the 1 hr and 4 hr sampling intervals after fear training. (G) Representative image of the time course of NBEA expression after conditioning. Home-cage samples were obtained from mice directly taken from the home cage and sacrificed. (H) Quantification of the time course of NBEA expression after fear conditioning. There was no significant difference in NBEA expression after fear conditioning. F (7, 20) = 2.255, p = 0.0728, one-way ANOVA.
De novo local protein synthesis of NBEA after retrieval
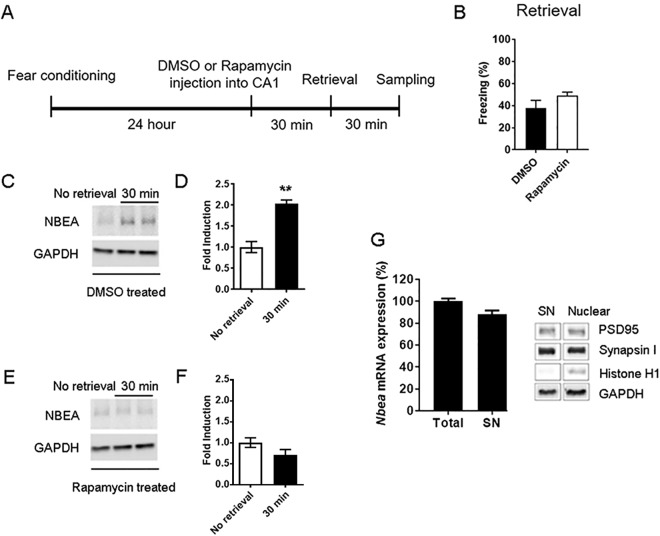
The rapid induction of NBEA expression allowed us to investigate the activation of local NBEA protein synthesis through the mTOR signaling pathway, which is known to be involved in rapid local protein synthesis25,26. Whether NBEA protein can be locally synthesized and expressed in an activity-dependent manner is unknown. To that end, rapamycin (1 μl of 50 μM in DMSO) or vehicle (DMSO) was administered into the CA1 of dorsal hippocampi 30 min before fear memory retrieval, followed by protein extraction 30 min after retrieval (Fig. 3A). Rapamycin infusion 30 min before retrieval did not change the level of freezing during retrieval (Fig. 3B; t(6) = 1.445, p = 0.1986, unpaired t-test, two-tailed) as shown in other reports27,28. However, the rapid induction of NBEA expression shown in the DMSO-infused group (Fig. 3C,D; t(4) = 6.625, *p = 0.0027, unpaired t-test, two-tailed) was completely blocked by rapamycin infusion (Fig. 3E,F; t(4) = 1.710, p = 0.1625, unpaired t-test, two-tailed), suggesting that NBEA is newly and rapidly synthesized at the synapse in response to fear memory retrieval through the mTOR signaling pathway. We also confirmed that NBEA mRNA was enriched in the purified synaptosome (Fig. 3G left), suggesting that NBEA was indeed actively synthesized at the synapse after contextual fear retrieval. The purity of the synaptosomes was validated with the histone H1 antibody to ensure that there was no nuclear contamination (Fig. 3G right and Supple Fig. 4).
Figure 3.
Increased NBEA expression after contextual fear retrieval at the synapse in the dorsal hippocampus was regulated by the mTOR signaling pathway. (A) Schematic representation of the experimental protocol. (B) Injection of rapamycin (49.03 ± 3.28, n = 4) into the dorsal CA1 hippocampus 30 min before contextual retrieval did not alter the freezing level during retrieval compared to injection of DMSO control (37.95 ± 6.934, n = 4). p = 0.1989, t-test, two-tailed. (C) Representative image of NBEA expression with DMSO treatment 30 min before contextual retrieval. (D) Quantification of NBEA expression with DMSO treatment. No retrieval (1.004 ± 0.1315, n = 3) vs 30 min after retrieval (2.038 ± 0.0843, n = 3), *p = 0.0027, t-test, two-tailed. (E) Representative image of NBEA expression with rapamycin treatment 30 min before contextual retrieval. (F) Quantification of NBEA expression with rapamycin treatment. No retrieval (1.005 ± 0.1142, n = 3) vs 30 min after retrieval (0.7149 ± 0.1254, n = 3), p = 0.1625, t-test, two-tailed. (G) Left, Nbea mRNA expression in total lysates (100.2 ± 2.341, n = 9) and the synaptosome fraction (88 ± 3.487, n = 9). Right, Confirmation of the purity of the synaptosome fraction, showing no nuclear contamination using the nuclear marker, histone H1.
Nbea knockdown in the CA1 hippocampus impaired extinction
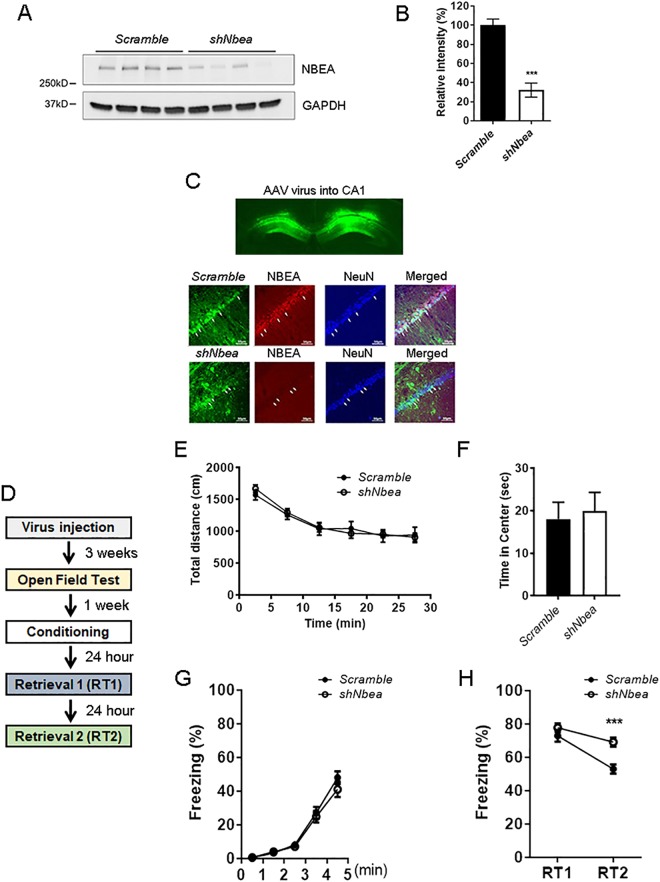
Since NBEA expression was increased in the hippocampus after contextual fear memory retrieval, we injected AAV encoding a sequence to knockdown NBEA (shNbea) or a scrambled sequence into the CA1 of the dorsal hippocampus, known to be involved in contextual fear retrieval and extinction, in WT mice. Before we tested the efficiency of the shRNA virus, we analyzed the baseline expression of NBEA in control mouse brains. In the hippocampus, NBEA was expressed in dentate gyrus (DG), CA3 and CA1 pyramidal cells (Supple Fig. S1A). Double staining with parvalbumin showed weak or no expression of NBEA in parvalbumin-positive cells in the thalamic reticular nucleus (TRN; Supple Fig. S1B), cortex (Supple Fig. S1C) and CA1 hippocampus (Supple Fig. S1D). To further confirm that NBEA is more weakly expressed in inhibitory neurons than in pyramidal cells, we examined the expression of NBEA in hippocampal tissue double-labeled with an antibody targeting GABA, an inhibitory neurotransmitter (Supple Fig. S1E), and in tissue from glutamate decarboxylase 67 (GAD67)-GFP transgenic mice to visualize GAD67-positive cells (Supple Fig. S1F). We did not see NBEA expression in those inhibitory neuronal populations; therefore, we concluded that baseline NBEA expression in inhibitory neurons is minimal. The efficiency of shNbea knockdown (KD) and the scrambled shRNA was confirmed with immunoblotting of lysates from each of the virus-treated in vitro primary hippocampal cultures (Fig. 4A,B; t(6) = 7.038, ***p = 0.0004, unpaired t-test, two-tailed). Furthermore, immunohistochemistry data clearly showed a suppression of NBEA expression with shNbea KD in the CA1 of the dorsal hippocampus but not in the group treated with the scrambled control (Fig. 4C). For the behavior analysis (Fig. 4D), we first examined general locomotor activity and anxiety-like behavior in the open field test. shNbea KD in the CA1 did not affect locomotion (Fig. 4E; F (5, 150) = 0.2602, p = 0.9341, two-way ANOVA) or anxiety (Fig. 4F; t(25) = 0.3197, p = 0.7519, unpaired t-test, two-tailed). Fear conditioning showed that shNbea KD in the CA1 did not affect fear acquisition (Fig. 4G; F (4, 100) = 0.8908, p = 0.4725, two-way ANOVA). Retrieval was tested with two sequential retrieval tests (RT1 and RT2) with a 24-h interval. To assess fear memory extinction within the group, we compared the freezing level between RT1 and RT2 of each group. As we observed from the Nbea+/− mutant data in Fig. 4D, th eshNbea KD group also showed attenuated extinction (Fig. 4H; shNbea, RT1-RT2: t(25) = 7.587, ****p < 0.0001, two-way ANOVA, Sidak’s multiple comparisons test) compared to the scrambled control (Fig. 4H; scramble, RT1-RT2: t(25) = 3.148, **p = 0.0084, two-way ANOVA, Sidak’s multiple comparisons test). Interestingly, we did not observe a significant effect of shNbea KD in RT1, the 24-h fear memory retrieval test performed 24 h after conditioning (Fig. 4H RT1; t(25) = 1.071, p = 0.2945, unpaired t-test, two-tailed), but we observed a significant difference in the behavior between the scrambled control and shNbea KD group in RT2 (Fig. 4H; F(1, 25) = 8.984, *p = 0.0061, two-way ANOVA, Sidak’s multiple comparisons test, RT1, p = 0.4475, RT2, ***p = 0.0007). These data indicate that the rapid induction of NBEA expression in pyramidal neurons in the CA1 hippocampus may play a role in either the inhibition of memory stability or the facilitation of memory instability after retrieval.
Figure 4.
Nbea knockdown in the dorsal hippocampal CA1 region resulted in impairments in extinction after contextual fear retrieval. (A) Validation of shNbea efficiency in cultured hippocampal neurons. Left, Western blot image of NBEA expression from AAV-scrambled or AAV-shNbea-treated cultured hippocampal neurons. Right, Quantification of NBEA expression. Scrambled (100 ± 6.321, n = 4) vs shNbea (32.14 ± 7.286, n = 4), ***p = 0.0004, t-test, two-tailed. (B) Upper, Representative image of AAV virus injection into the CA1 of the dorsal hippocampus. Middle, Immunohistochemistry for NBEA and NeuN in AAV-scrambled-injected CA1. Lower, Immunohistochemistry for NBEA and NeuN in AAV-shNbea-injected CA1. (C) Locomotor activity in the open field test. There was no significant difference in the locomotor activity between AAV-scrambled and AAV-shNbea-injected mice. F(5, 125) = 0.692, p = 0.6304, two-way ANOVA, post hoc Sidak’s multiple comparison test. Scale bars indicate 50 μm. (D) Anxiety-like behavior in the open field test. There was no significant difference in time spent in the center of the open field for the first 5 min of the test between the AAV-scrambled and AAV-shNbea-injected mice. Scrambled (18.05 ± 3.96, n = 14) and shNbea (19.94 ± 4.404, n = 13), p = 0.7519, t-test, two-tailed. (E) Schematic representation of the experimental protocol. (F) Fear conditioning. There was no significant difference in the freezing level during fear conditioning between the AAV-scrambled and AAV-shNbea-injected mice. F(4, 100) = 0.8908, p = 0.4725, two-way ANOVA, post hoc Sidak’s multiple comparison test. (G) Fear retrieval. F(1, 25) = 8.984, **p = 0.0061, two-way ANOVA with post hoc Sidak’s multiple comparisons test. There was no significant difference in the freezing level during retrieval 1 (RT1) between AAV-scrambled and AAV-shNbea-injected mice. Scrambled (72.93 ± 3.599, n = 14) and shNbea (69.17 ± 2.744, n = 13), p = 0.2945. However, there was a significant difference in the freezing level during retrieval 2 (RT2) between AAV-scrambled and AAV-shNbea-injected mice. Scrambled (53 ± 2.733, n = 14) and shNbea (77.75 ± 2.603, n = 13), ***p = 0. 0.0003.
Neurobeachin heterozygous mice showed impaired extinction
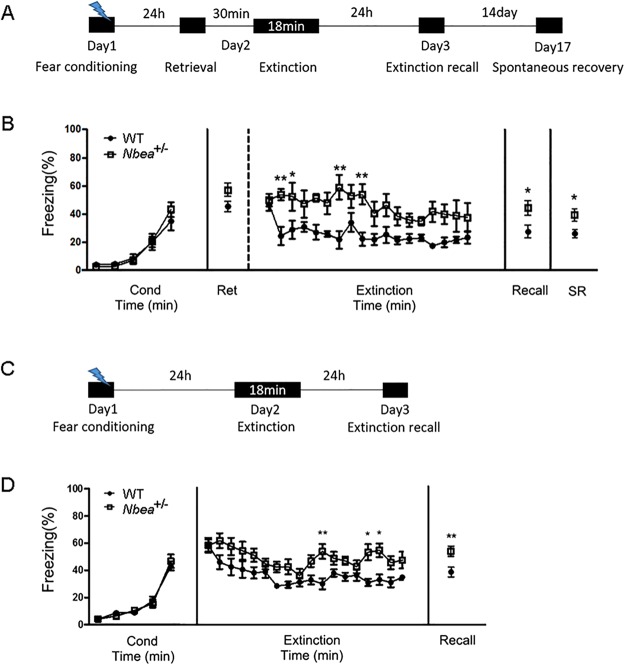
Due to the limitation of poor contextual extinction of surgerized mice and the variable infection rate and efficiency of the knockdown virus, we decided to generate Nbea knockout mice. This approach allowed us to perform extensive behavior analysis to further validate the effect of Nbea deficiency in fear reconsolidation or extinction including within-session extinction. We generated Nbea null mutant mice utilizing TALEN technology. By targeting exon 22, we obtained a 17-bp out-of-frame deletion mutant as shown in Supple Fig. S1A. The 17-bp deletion created a premature stop codon immediately after the truncation in exon 22. Genomic deletion was validated by the analysis of tail DNA with PCR. The deletion created a new BslI enzyme target sequence in the genomic DNA of the mutant allele. Mice with a homozygous mutation were embryonic lethal; therefore, only heterozygotes (indicated as Nbea+/− from here on) were used for assays and behavior tests. First, we confirmed the genomic deletion with the BslI restriction enzyme, which generates two bands (294 bp and 90 bp) in the mutant allele (Supple Fig. S1B). We also confirmed inhibition of the NBEA protein in Nbea+/− using Western blot analysis (Supple Fig. S1C; t(6) = 5.149, *p = 0.0021, unpaired t-test, two-tailed). For the behavior analysis, we first recorded the body weight of these mice since gene-trap-mediated null mutant mice (GH240) have previously shown dwarfism, but there were no significant differences in body weight at 10 weeks (Supple Fig. S1D; t(19) = 1.949, p = 0.0663, unpaired t-test, two-tailed) or 16 weeks (Supple Fig. S1D; t(12) = 1.468, p = 0.1678, unpaired t-test, two-tailed). To detect any phenotypic differences in general behaviors such as locomotor activity and anxiety-like behavior, the open field test was performed. Nbea+/− were hyperactive during the initial 5 min of the test, but their locomotor activity returned to normal levels for the rest of the exploration period (Supple Fig. S1E; F(5, 215) = 2.719, *p = 0.0210, two-way ANOVA). We also analyzed the time spent in the center zone of the arena as a measure of the anxiety level but found no difference between WT and Nbea+/− (Supple Fig. S1F; t(43) = 1.31, p = 0.1970, unpaired t-test, two-tailed). We examined fear conditioning and extinction in these mutant mice (Fig. 5A). Since the mutant was generated in the C57BL/6J background, we increased the intensity of shocks (0.7 mA, 1 s) to achieve a certain level of recall memory for further investigation of the extinction process. Using this protocol, we did not detect any differences in acquisition or 24-h context memory recall (Fig. 5B; F(4, 60) = 2.19, p = 0.0809, two-way ANOVA). Since Nbea+/− did not show any impairments in fear acquisition or contextual fear memory at the behavioral level, and NBEA expression was increased immediately after retrieval at the molecular level, we first tested the Nbea+/− on the retrieval-extinction protocol. Nbea+/− showed no difference in retrieval (Fig. 5B; t(15) = 1.824, p = 0.0881, unpaired t-test, two-tailed) and showed normal extinction learning (Fig. 5B; Time × Group interaction: F (17, 255) = 1.087, p = 0.3666, two-way ANOVA). However, there were differences in the freezing level between genotypes (Fig. 5B; Group: F(1, 15) = 13.64, *p = 0.0022, two-way ANOVA), suggesting that extinction was attenuated in Nbea+/−. The 24 h-extinction memory (t(15) = 2.944, *p = 0.0101, unpaired t-test, two-tailed) and spontaneous recall memory (t(15) = 2.255, *p = 0.0395, unpaired t-test, two-tailed) were significantly greater in Nbea+/− than in WT (Fig. 5B), suggesting that Nbea is critical for fear extinction. To validate the role of Nbea in fear extinction, we performed an extinction protocol without retrieval (Fig. 5C). As shown previously (Fig. 5B), we did not detect any differences in fear conditioning between the two genotypes (Fig. 5D; F(4, 68) = 0.6439, p = 0.6330). However, Nbea+/− showed normal extinction learning (Fig. 5D; Time × Group interaction: F(17, 289) = 0.9358, p = 0.5320) but attenuated extinction of the memory, indicated by higher freezing levels in Nbea+/− than in WT (Fig. 5D; Group: F(1, 17) = 12.69, **p = 0.0024). The impaired extinction in Nbea+/− was confirmed by significant differences in extinction recall between WT and Nbea+/− (Fig. 5D; t(17) = 3.094, **p = 0.0066, unpaired t-test, two-tailed). Altogether, Nbea+/− showed some tendency to increase the freezing level in retrieval, but the impairments in extinction processes were significant compared to WT, suggesting that NBEA may play a critical role extinction processes.
Figure 5.
Nbea+/− showed impairments in fear extinction. (A) Schematic representation of the experimental protocol. (B) Fear conditioning: There was no significant difference in freezing level during fear conditioning between WT (n = 7) and Nbea+/− (n = 10). Two-way ANOVA, F(4, 60) = 2.19, p = 0.0809. Retrieval: There was no difference in retrieval. WT (47.23 ± 3.587, n = 7) vs Nbea+/− (56.68 ± 3.524, n = 10), p = 0.0881, unpaired t-test, two-tailed. Extinction: There was a group difference during extinction. Interaction (Time × Group), F(17, 255) = 1.087, p = 0.3666; Group, F(1, 15) = 13.64, **p = 0.0022, Sidak’s multiple comparisons test. WT, n = 7; Nbea+/−, n = 10. Extinction recall: There was a significant difference in freezing level during extinction recall. WT (26.51 ± 3.072, n = 7) vs Nbea+/− (41.82 ± 3.762, n = 10), *p = 0.0101, unpaired t-test, two-tailed. Spontaneous recovery: There was a significant difference in freezing level during extinction recall. WT (25.86 ± 2.945, n = 7) vs Nbea+/− (39.07 ± 4.416, n = 10), *p = 0.0395, unpaired t-test, two-tailed. (C) Schematic representation of the experimental protocol. (D) Fear conditioning: There was no significant difference in freezing level during fear conditioning between WT (n = 10) and Nbea+/− (n = 9). Two-way ANOVA, F(4, 68) = 0.6439, p = 0.6330. Extinction: There was a group difference during extinction. Interaction (Time × Group), F(17, 289) = 0.9358, p = 0.5320; Group, F(1, 17) = 12.69, **p = 0.0024, Sidak’s multiple comparisons test. WT, n = 7; Nbea+/−, n = 10. Extinction recall: There was a significant difference in freezing level during extinction recall. WT (38.53 ± 3.568, n = 10) vs Nbea+/− (54.9 ± 3.924, n = 9), **p = 0.0066, unpaired t-test, two-tailed.
Discussion
Here, we performed an unbiased proteomics analysis to identify molecules involved in fear memory retrieval. We found that NBEA is rapidly and transiently upregulated in the dorsal hippocampus after fear memory retrieval. We also found that NBEA is enriched at the synapse, and its expression is regulated by the mTOR signaling pathway. Suppression of NBEA expression, either in the whole body or locally in the hippocampal CA1 region, impaired extinction, suggesting that NBEA may play a role in regulating fear extinction processes.
Exposure therapy is a behavior therapy used to treat anxiety disorders such as PTSD29. To overcome their distress, patients are exposed to a feared object or context without any danger present. Contextual information and the memory process is important for this form of therapy30; therefore, we utilized a contextual fear conditioning protocol (Fig. 1A). Since the dorsal hippocampus is an integration center for context-dependent fear memory processing31, we focused on identifying molecules within this structure in this study. The retrieval-mediated labile state of memories has been suggested to be an important factor in the permanent erasure of memory8,32, but the specific molecules involved in these processes are not known. Through proteomic analysis, we identified NBEA as one of the most prominently upregulated proteins in the hippocampus after fear memory retrieval. Given that NBEA is a trafficking molecule that targets neurotransmitter receptors to synapses, and protein sampling was performed 5 min after a 5-min retrieval session, we questioned whether an increase in the expression of NBEA after fear retrieval could be detected at the synapse. Local synaptic protein synthesis is known to be very rapid (within 5 min)25,33,34. Using synaptosomal fractionization, we clearly observed an increase in NBEA expression at 5 min after retrieval in the dorsal hippocampus (Fig. 1C,D). Thereafter, we only focused on NBEA expression at the synaptic level when examining the molecular dynamics of NBEA after retrieval. The time course of NBEA expression after retrieval (Fig. 2B,C) was similar to that of other proteins regulated by local translation25. The mTOR signaling pathway, also called the local protein synthesis pathway, is the main pathway that regulates the rapid induction of protein synthesis from existing mRNA at the synapse35. Rapamycin clearly inhibited the rapid induction of NBEA expression after retrieval, suggesting that retrieval indeed activates the new synthesis of NBEA at the synapse (Fig. 3). Therefore, the dynamic changes in NBEA expression appear to be necessary for the state-dependent modulation of fear memories, especially after fear memory retrieval. Interestingly, we did not observe any induction of NBEA after neutral-context retrieval, indicating that rapid NBEA protein induction at the synapse is closely related to fear retrieval (Fig. 1G,H). However, different sets of experiments may be necessary to test whether the relationship between NBEA expression and fear retrieval is generalizable to other types of retrieval learning that are not fear related.
Previous studies have shown that the mTOR signaling pathway is critical for the consolidation of fear memory reconsolidation27,28,36 and extinction37. In these studies, treatment with the mTOR inhibitor, rapamycin, immediately before or after retrieval impaired memory reconsolidation27, which is not consistent with our data. Although NBEA expression was regulated by mTOR, we found either increased fear memory or impaired extinction in both Nbea knockdown (Fig. 4) and Nbea+/− mice (Fig. 5). However, several studies support our data as well. For example, ketamine treatment, which is known to activate mTOR, accelerated the extinction process10. Another protein synthesis inhibitor, anisomycin, impaired extinction38. Therefore, specific mTOR downstream pathways may play different roles in memory stabilization and destabilization. In support of this idea, a study reported that two distinct molecular mechanisms are involved in the destabilization and restabilization of reactivated spatial memory39. In other words, inhibition of all proteins regulated by mTOR may mask the effects of a sub-group of proteins, including NBEA, involved in memory destabilization.
The mechanism by which the rapid induction of NBEA expression affects extinction is not clear since retrieval and extinction are known to be two distinct memory processes40. However, several studies have suggested a molecular link between retrieval and extinction4,41. NBEA is a regulator of ionotropic receptor trafficking to synapses13, and ionotropic glutamate receptor plasticity is required for memory consolidation and reconsolidation42. The learning, consolidation and reconsolidation of long-term memory require stable postsynaptic potentiation that is modulated by an increase in postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs)43. AMPARs are tetrameric glutamate receptors that have different properties depending on their subunit composition44. The majority of synaptic AMPARs are calcium-impermeable AMPARs (CI-AMPARs) that contain the GluA2 subunit and are stable at the synapse due to the interaction of the GluA2 subunits with synaptic molecules45. Moreover, most basal synaptic transmission is regulated through CI-AMPARs. On the other hand, GluA2-lacking AMPARs, calcium-permeable AMPARs (CP-AMPARs) are unstable at the synapse but involved in acute synaptic potentiation45. A recent study revealed the physiological roles of CI-AMPARs and CP-AMPARs in transforming a consolidated memory into an unstable memory and subsequently guiding extinction or reconsolidation4. In their results, memory retrieval caused a transient replacement of CI-AMPARs with CP-AMPARs. Furthermore, blocking CI-AMPAR endocytosis prevented the insertion of CP-AMPARs, thereby preventing the transformation of a consolidated memory to a labile state and leading to increased fear memory. Here, most intriguingly of all, the temporal profile of CP-AMPARs was very similar to that of NBEA expression after retrieval. Since we found that mice with Nbea KD showed enhanced fear memory (Fig. 4F), and the temporal patterns of NBEA and CP-AMPARs after retrieval are very similar (Fig. 2B,C), NBEA is speculated to regulate CP-AMPAR trafficking to the synapse. In support of this, NBEA interacts with protein kinase A (PKA)11, which phosphorylates GluA1-S845. GluA1-S845 phosphorylation enhances the mean channel open probability and promotes synaptic trafficking of GluA1-containing AMPARs (CP-AMPARs), especially to extrasynaptic sites. Therefore, in extinction, NBEA may alter the subunit composition of perisynaptic AMPARs by providing stability to GluA1 homomers, making NBEA necessary for the maintenance of CP-AMPARs on the surface, which is required for the extinction process46. In support of this idea, we found that NBEA interacts with PKA and GluA1 but not with GluA2 (Supple Fig. S3). Interestingly, AKAP150, which is one of major forms of PKA binding protein regulating synaptic transmission in the hippocampus, did not show dynamic changes in expression after retrieval, suggesting that NBEA might be an activity-dependent regulator that modulates PKA and AMPA receptor phosphorylation associated with contextual fear retrieval (Fig. 2D,E). Further analysis of the biochemical and electrophysiological role of NBEA in this process is required.
We generated Nbea null mutant mice, but the mutation was embryonic lethal. Therefore, we only used heterozygotes for our behavior assays. Nbea mutant mice have been generated in two different labs using gene-trap methods. Both Nbea knockout mouse lines showed perinatal lethality20,47. In the case of the GB140 line, several behavioral analyses have been performed. Nbea+/− showed enhanced conditioned fear memory and impaired spatial learning and memory as well as enhanced long-term potentiation in the CA1 area of the hippocampus19. However, we did not see enhanced conditioned fear memory compared to that in WT littermates. The group cited above used 24-month-old female mice for the tests, whereas we used 10–16-week-old males. In addition, dwarfism of Nbea+/− was reported18, but we did not see any differences in body weight between Nbea+/− and WT (Supple Fig. S2D). However, we did observe hyperactivity in the open field test, which was not shown in the previous GB140 line. Therefore, we believe that the differences in the phenotypes between GB140 and this newly generated Nbea+/− mouse line are due to several factors, such as the method used to generate the knockout mouse line (gene trapping for GB140 vs TALENs for our study), the dwarfism, the gender and age of the test groups.
Taken together, this study demonstrated that NBEA plays a role in either the induction of memory lability or the physiological process of memory extinction. Our research showed activity-dependent activation of NBEA at the synapse after retrieval, and impaired extinction by the dysregulation of NBEA in either the hippocampal CA1 or the whole body. Therefore, we suggest NBEA as a novel target for research on how it may contribute to PTSD extinction deficits or the efficacy of exposure therapy. Furthermore, regarding the possible role of Nbea as an autism molecule, this study may also facilitate research to identify potential pathways connecting PTSD and ASD48.
Materials and Methods
Animals
Adult male wild-type (WT) mice (10–16 weeks of age) of B6x129 F1 or B6 background were used for experiments. F1 mice were obtained by mating the parental strains C57BL/6J (N20-23) and 129s4/svJ (N20-23). B6x129 F1 mice were used because we used this strain in most of our previous fear and extinction studies49,50, and these mice were used for proteomics, western blot, immunoprecipitation and shNbea knockdown experiments. Nbea+/− mice were generated on the B6 background and were also included in the western blot analysis shown in Fig. 1E,F,G. All mice were housed with free access to food and water under controlled temperature and light conditions (23 °C, 12-h light: 12-h dark cycle). Experiments were performed during the light phase. All experimental procedures and animal care were performed in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of the Institute for Basic Science (IBS), Korea. All methods were performed in accordance with the relevant guidelines and regulations.
Preparation of protein extract for proteomics
Five minutes after contextual fear retrieval, the hippocampus was dissected and washed with ice-cold 1X phosphate-buffered saline (PBS) to reduce blood contamination, followed by lysis in 500 µl of lysis buffer (6 M urea, 50 mM Tris-Cl, pH 8.3, 5 mM EDTA, 0.05% SDS, 1x protease inhibitor cocktail, and 1x phosphatase inhibitor cocktail) by sonication at 4 °C for 1 min with an intermittent cooling period. After centrifugation at 12,000 rpm for 15 min at 4 °C, the supernatants were collected. The concentration of total protein was determined by Bradford assay. The tissue lysates were stored at −80 °C until use.
Protein digestion for mass spectrometry
A total of ten samples from two groups (5 mice per group, 40 µg lysates per sample) were adjusted to the same protein concentration, reduced with 10 mM dithiothreitol (DTT) for 30 min at 37 °C and then alkylated with 40 mM iodoacetamide for 1 h in the dark at 25 °C. After the samples were diluted 10-fold with 50 mM NH4HCO3, trypsin was added at a ratio of 1:40 (w:w), followed by incubation overnight at 37 °C. Two peptide mixtures were applied to a C18 cartridge. Purified peptide samples were dried and stored at 4 °C until liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
TMT-labeling proteomic analysis
The peptide mixture of the two groups (control and retrieval group) was used for tandem mass tag (TMT) reagent labeling according to the manufacturer’s protocols (Thermo Fisher Scientific, Waltham, MA, USA). Each sample was then labeled with one of two different TMT reagents (TMT126 for control and TMT129 for retrieval) according to the manufacturer’s protocols. After the labeling, the TMT-labeled peptide pool was cleaned with a C18 cartridge. Then, an OFFGEL electrophoresis was performed according to the manufacturer’s protocols (Agilent, Santa Clara, CA, USA). Desalted and dehydrated samples were reconstituted in OFFGEL solution. Isoelectric focusing was performed on an immobilized pH gradient (IPG) dry strip (13 cm, pH 3–10, linear; GE Healthcare, Chicago, IL, USA) using a 12-well frame for 20 kVh with a maximum current of 50 μA and power of 200 mW. The collected fractions were desalted using C18 spin columns (Thermo Fisher Scientific, Waltham, MA, USA), then evaporated by a speed vacuum and stored at −20 °C. LC-MS/MS analysis was performed using an LTQ Orbitrap XL (San Jose, CA, USA). The spray voltage was set to 1.9 kV, and the temperature of the heated capillary was set to 25 °C. The sample was subjected to reverse-phase chromatography using an Eksigent LC system (AB Sciex, Framingham, MA, USA). The mobile phases consisted of H2O (A) and acetonitrile (ACN) (B), where both phases contained 0.1% v/v formic acid. The flow rate was maintained at 300 nl/min. The peptides were eluted from the analytical column (C18) by a linear gradient running from 10% to 35% acetonitrile over 95 min to 80% B over the next 20 min and to 90% B over the final 15 min, followed by direct spray into the LTQ mass spectrometer. Mass spectra were acquired in the positive mode with an m/z window of 300–2,000, and a maximum of 3 precursors were selected for higher energy collisional dissociation (HCD) analysis in the Orbitrap (isolation width: 2; min. signal required: 1000; normalized collision energy: 45%) with dynamic exclusion. SEQUEST (XCorr Only) (ThermoFinnigan, San Jose, CA; version v.1.3.0.339) searches were performed against tryptic peptides, allowing for two missed cleavages with 25 ppm (monoisotopic) precursor mass tolerance and 25 ppm (monoisotopic) fragment mass tolerance detected in the Orbitrap. The searches were conducted against a mouse database (IPI ver. 3.87) with the decoy option enabled, and the iodoacetamide derivative of cysteine and TMT multiplexed quantitation chemistry of lysine and the N-terminus were specified in SEQUEST as fixed modifications. Methionine oxidation (+15.9949) was specified in SEQUEST as a variable modification. Scaffold (version Scaffold_4.0.5, Proteome Software Inc., Portland, OR) was used to validate MS/MS-based peptide and protein identifications. Peptide identifications were accepted if they could be established at a probability greater than 0.9 by the peptide prophet algorithm. Protein identifications were accepted if they could be established at a probability greater than 0.9 and contained at least 2 identified unique peptides. Protein probabilities were assigned by the protein prophet algorithm. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony. For the TMT quantification, the LIBRA (Trans-proteome pipeline, version 4.2.1) program was used to quantitate the label-based quantitation peptide and protein identifications. A fold change greater than 1.2 or less than 0.8 was considered statistically significant51,52.
Preparation of the synaptosome and immunoblotting analysis
Synaptosome purification has been described previously26. For the validation of NBEA expression after retrieval, whole hippocampal tissue was used. For the rest of the study, the dorsal hippocampus was dissected and homogenized in ice-cold lysis buffer using a Teflon-glass tube. For immunoblotting analysis, antibodies targeting the following proteins were used. Primary antibodies against the following antigens were used: synapsin I (1:1,000, Abcam, Cambridge, UK), postsynaptic density 95 (PSD95; 1:1,000, Abcam, Cambridge, UK), histone H1 (1:1,000, Abcam, Cambridge, UK), NBEA (K-20) (1:1,000, Santa Cruz Biotech., Dallas, TX, USA), A kinase anchor protein 150 (AKAP150; 1:1,000, Santa Cruz Biotech, Dallas, TX, USA) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:5,000, Cell signaling, Danvers, MA, USA). The secondary antibodies were horseradish peroxidase (HRP)-conjugated anti-mouse (1:5,000) or anti-rabbit (1:10,000) antibodies (PerkinElmer Life Sciences, Norwalk, CT, USA). Signal detection was performed using the LAS 2000 system (LAS-2000, Fuji, Tokyo, Japan) with enhanced chemiluminescence (Western-CDP star, PerkinElmer Life Sciences, Norwalk, CT, USA). Densitometric analysis of immunoreactivity for each protein was conducted using NIH ImageJ software.
Real-time PCR
Total RNA or synaptosomal RNA was isolated with GeneAll Hybrid-R, and cDNA was synthesized with a cDNA synthesis kit (Superscript Vilo, Invitrogen, Waltham, MA, USA). Then, PCR amplification was performed using 5X Qarta-probe qPCR mix (Qarta bio, Carson, CA, USA). Real-time quantitative RT-PCR was carried out using TaqMan Gene Expression assays and Applied Biosystems (ABI) Step One Plus (Applied Biosystems, Waltham, MA, USA). Primer sequences for Nbea were 5′-CCATCTATCCCTCATCCAAGTTT-3′ (sense), 5′-CCATGGACTCCTTGCCA AGCTGAT-3′ (probe), and 5′-CCTTCTCCTTTACAGTGTCTGG -3′ (antisense); the primer sequences for the internal control, beta-2 microglobulin, were 5′-GGTCTTTCTGGTGCTTGTCT-3′ (sense), 5′-CAAGTATACTCACGCCACCCACCG-3′ (probe), and 5′-TATGTTCGGCTTCCC ATTCTC-3′ (antisense). The PCR conditions were as follows: initial incubation at 50 °C for 2 min and denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min.
Drug delivery
For microinjection, cannulas (PlasticsOne, Roanoke, VA, USA) were bilaterally implanted with a 26-gauge guide cannula in the CA1 of the dorsal hippocampus (anterior-posterior (AP) −1.9 mm, lateral (L) +1.2 mm or −1.2 mm, and dorsal-ventral (DV) 1.5 mm from bregma) using a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA) under 2% Avertin anesthesia. A 33-gauge dummy cannula was inserted into each guide cannula to prevent clogging. The injection cannulas were cut to extend 0.2 mm beyond the guide cannulas. After 7 days of recovery, mice received bilateral 5-min infusions of DMSO (1 μl each) or rapamycin (1 μl each, 50 μM in DMSO) into the CA1 of the dorsal hippocampus, and the injection cannula remained in place for an additional 5 min to ensure diffusion away from the injector tip. Behavioral experiments were performed 30 min after the drug infusion. Thirty minutes after retrieval, the dorsal hippocampal tissue was isolated and western blot analysis was performed as described above. The control DMSO or rapamycin-only group was sacrificed 1 hour after drug infusion to match with the sampling time of the retrieval DMSO or rapamycin group.
Primary hippocampal neuron culture
Primary cultures of murine hippocampal neurons were done as previously described53, with minor modifications. A papain dissociation system (Worthington biochemical cooperation, Lakewood, NJ, USA) was used to dissociate murine hippocampal neurons. After mechanical trituration and centrifugation, the resulting cell pellet was resuspended in culture medium containing Neurobasal-A medium (Gibco, Waltham, MA, USA), 2% B27 serum (Gibco, Waltham, MA, USA), and 1x penicillin/streptomycin (Gibco, Waltham, MA, USA). Afterward, cells were plated at a high density of 5 × 105 cells/ml in 24-well plates (BD Bioscience, San Jose, CA, USA) coated with 1 mg/ml poly-D-lysine hydrobromide (Sigma-Aldrich, St. Louis, MO, USA). Hippocampal neurons were cultivated at 37 °C and 5% CO2 in a humidified incubator. After the cells were allowed to adhere for 3 h, more culture medium was added, and the cells were maintained for 10–14 days in culture before use. On day 5, 1 μl of high-titer adeno-associated virus (AAV; 1 × 1012 genome copy/ml) was added. The culture was maintained for an additional 5 days and then harvested for the protein assay.
AAV vector production
Separate hairpin RNA (shRNA) constructs were designed to target Nbea mRNA. Cloning was performed as previously described54. Briefly, DNA oligomers were synthesized and cloned into a modified pAAV-MCS vector, pAAV-shRNA (provided by Dr. Ralph J. DiLeone, Yale University School of Medicine). The loop sequence (CTTCCTGTCA) was inserted between the antisense (GAAGTCATGCAATTGACTGTGTGA) and sense (TCACACAGTCAATTGCATGACTTC) sequences. For control, the following scrambled shRNA sequence was used: GATTATACACCGACAATTCCGTGT (antisense) and ACACGGAATTGTCGGTGTATAATC (sense). The insertion was confirmed by sequencing. High-titer AAV virus (1 × 1012 genome copy/ml) was packaged with serotype DJ at KIST virus facility (http://virus.kist.re.kr).
Tissue preparation and immunohistochemistry
Mice were deeply anesthetized with Avertin (20 μl/g, intraperitoneally, i.p.) and transcardially perfused with PBS (pH 7.4) followed by a fixative, 4% paraformaldehyde in 0.01 M PBS (pH 7.4) at room temperature. Brains were removed from the skull and post-fixed overnight in the same solution at 4 °C. Using a vibrating microtome (Leica Microsystems, Wetzlar, Germany), we cut the brain containing hippocampal tissue into 50-μm-thick sections. Immunohistochemistry was done as previously described (Lee et al., 2009). Antibodies targeting the following proteins were used: NBEA (1:1000; Santa Cruz Biotech., Dallas, TX, USA) and NeuN (1:500; Millipore, Burlington, MA, USA). The Cy5-conjugated donkey anti-rabbit IgG antibody (1:300; Jackson ImmunoResearch, West Grove, PA, USA) and DyLight 405-conjugated donkey anti-mouse IgG antibody (1:300; Jackson ImmunoResearch, West Grove, PA, USA) were used as secondary antibodies. After the sections were washed with PBS, they were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and examined under a confocal laser-scanning microscope (Nikon, Shinagawa-Ku, Tokyo, Japan).
Open field test
The open field test was conducted as previously reported55. Briefly, each mouse was placed in a white acrylic chamber (40 × 40 × 40 cm) for 30 min to measure its locomotor activity. At the beginning of the test, the mouse was placed in the corner of the arena. To examine the anxiety level of the mouse, the exploration time in the center (20 × 20 cm) during the first 5 min was analyzed. Spontaneous movement was video recorded and automatically analyzed with EthoVision XT software, version 9 (Noldus, Wageningen, Netherlands).
Contextual fear conditioning, retrieval and extinction
Contextual fear conditioning was performed as previously described56, with minor modifications. All animals were handled for three days for 10 min each. As shown in Fig. 1, for the fear conditioning of mice used in the proteomic analysis and validation of the proteomic results, mice from both control and retrieval groups were placed in the conditioning chamber and received a foot shock at 180 s (0.5 mA, 1 s) through the rod floor. Then, mice were returned to their home cage 60 s after the end of the last shock. Twenty-four hours after training, the control group was placed in a novel context for 5 min. The retrieval group was placed in the same chamber in which conditioning occurred for 5 min. For time course studies and behavior analysis, two foot shocks were delivered at 180 s and 240 s (0.5 mA, 1 s for F1 mice for proteomic study, time course biochemistry and knockdown experiments, 0.7 mA, 1 s for B6 mice for knockout experiments). For the no-retrieval group, mice received foot shocks for conditioning, just as the retrieval group did, but were not placed in the same context as retrieval to examine baseline expression of NBEA 24 hrs after conditioning and before retrieval. For the retrieval-extinction experiment, 30 min after retrieval, mice were again placed in the same chamber for another 18 min, and 24 h later, the mice were placed in the same chamber for 5 min to measure extinction memory. For the extinction experiment, 24 h later, without a 5-min retrieval session, mice were directly exposed to the same context for 18 min, and extinction memory was observed for 5 min in the same context 24 h later. For the spontaneous recovery test, the memory was tested in the same chamber 2 weeks later for 5 min. Baseline activity, exploration and freezing were assessed automatically using EthoVision software (Noldus Information Technology, Wageningen, Nederland).
Statistical analysis
Data were analyzed using GraphPad Prism 7.03 (GraphPad Software Inc., La Jolla, CA, USA). Comparisons between two groups were determined by Student’s t-test. To analyze the time course expression of NBEA or AKAP150 after retrieval and conditioning, one-way ANOVA with post hoc Dunnett’s multiple comparisons test was used. To analyze the NBEA expression results shown in Fig. 1D,G, one-way ANOVA with post hoc Tukey’s multiple comparisons test was used. To analyze locomotor activity in the open field test and freezing in fear conditioning and extinction, two-way repeated measures ANOVA with post hoc Sidak’s multiple comparisons test was performed. All values indicated are means ± SEM, and the statistical significance is represented with asterisks at p values of <0.05 (*), <0.01 (**), <0.001 (***), and <0.0001 (****).
Electronic supplementary material
Acknowledgements
This study was supported by the Institute for Basic Science (IBS-R001-D1), Republic of Korea.
Author Contributions
B. Lee and H.-S. Shin designed the study. B. Lee and E. Bang performed major biochemical and behavioral studies. W.S. Yang and S. Lee performed proteomics. B. Lee, A. Paydar, S. Kim and S.E. Lee performed AAV purification and surgery. G. Ha and J.-H. Kim performed biochemical and histological studies for supplemental figures. T. Cho, M.-G. Kang, E. Cheong, K.-S. Kim, C. Lee, M.-H. Yu and H.-S. Shin provided reagents and contributed to the discussion. B. Lee, E. Bang and W.S. Yang performed the statistical analyses. B. Lee and H.-S. Shin wrote the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30589-1.
References
- 1.Pitman, R. K., Orr, S. P., Shalev, A. Y., Metzger, L. J. & Mellman, T. A. Psychophysiological alterations in post-traumatic stress disorder. Semin Clin Neuropsychiatry4, 234-241, 10.153/SCNP00400234 (1999). [DOI] [PubMed]
- 2.Miller MM, McEwen BS. Establishing an agenda for translational research on PTSD. Ann N Y Acad Sci. 2006;1071:294–312. doi: 10.1196/annals.1364.023. [DOI] [PubMed] [Google Scholar]
- 3.Smith NB, Doran JM, Sippel LM, Harpaz-Rotem I. Fear extinction and memory reconsolidation as critical components in behavioral treatment for posttraumatic stress disorder and potential augmentation of these processes. Neurosci Lett. 2017;649:170–175. doi: 10.1016/j.neulet.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Hong I, et al. Modulation of fear memory by retrieval and extinction: a clue for memory deconsolidation. Rev Neuroscience. 2011;22:205–229. doi: 10.1515/rns.2011.023. [DOI] [PubMed] [Google Scholar]
- 5.Sandusky LA, Flint RW, Jr., McNay EC. Effects of the protein synthesis inhibitor cycloheximide on anxiety-like extinction behavior in an animal model of post-traumatic stress. Behav Brain Res. 2012;231:208–212. doi: 10.1016/j.bbr.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Costanzi M, Cannas S, Saraulli D, Rossi-Arnaud C, Cestari V. Extinction after retrieval: effects on the associative and nonassociative components of remote contextual fear memory. Learn Mem. 2011;18:508–518. doi: 10.1101/lm.2175811. [DOI] [PubMed] [Google Scholar]
- 7.Fukushima H, et al. Enhancement of fear memory by retrieval through reconsolidation. Elife. 2014;3:e02736. doi: 10.7554/eLife.02736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoica L, et al. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci USA. 2011;108:3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girgenti MJ, Ghosal S, LoPresto D, Taylor JR, Duman RS. Ketamine accelerates fear extinction via mTORC1 signaling. Neurobiol Dis. 2017;100:1–8. doi: 10.1016/j.nbd.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X, et al. Neurobeachin: A protein kinase A-anchoring, beige/Chediak-higashi protein homolog implicated in neuronal membrane traffic. J Neurosci. 2000;20:8551–8565. doi: 10.1523/JNEUROSCI.20-23-08551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farzana F, et al. Neurobeachin Regulates Glutamate- and GABA-Receptor Targeting to Synapses via Distinct Pathways. Mol Neurobiol. 2016;53:2112–2123. doi: 10.1007/s12035-015-9164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nair R, et al. Neurobeachin regulates neurotransmitter receptor trafficking to synapses. J Cell Biol. 2013;200:61–80. doi: 10.1083/jcb.201207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Volders K, Nuytens K, Creemers JW. The autism candidate gene Neurobeachin encodes a scaffolding protein implicated in membrane trafficking and signaling. Curr Mol Med. 2011;11:204–217. doi: 10.2174/156652411795243432. [DOI] [PubMed] [Google Scholar]
- 15.Nuytens K, et al. Platelets of mice heterozygous for neurobeachin, a candidate gene for autism spectrum disorder, display protein changes related to aberrant protein kinase A activity. Mol Autism. 2013;4:43. doi: 10.1186/2040-2392-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller AC, Voelker LH, Shah AN, Moens CB. Neurobeachin is required postsynaptically for electrical and chemical synapse formation. Curr Biol. 2015;25:16–28. doi: 10.1016/j.cub.2014.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuand K, et al. Nuclear Localization of the Autism Candidate Gene Neurobeachin and Functional Interaction with the NOTCH1 Intracellular Domain Indicate a Role in Regulating Transcription. PLoS One. 2016;11:e0151954. doi: 10.1371/journal.pone.0151954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuytens K, et al. The dwarf phenotype in GH240B mice, haploinsufficient for the autism candidate gene Neurobeachin, is caused by ectopic expression of recombinant human growth hormone. Plos One. 2014;9:e109598. doi: 10.1371/journal.pone.0109598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuytens K, et al. Haploinsufficiency of the autism candidate gene Neurobeachin induces autism-like behaviors and affects cellular and molecular processes of synaptic plasticity in mice. Neurobiol Dis. 2013;51:144–151. doi: 10.1016/j.nbd.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Su Y, et al. Neurobeachin is essential for neuromuscular synaptic transmission. J Neurosci. 2004;24:3627–3636. doi: 10.1523/JNEUROSCI.4644-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong I, et al. AMPA receptor exchange underlies transient memory destabilization on retrieval. Proc Natl Acad Sci USA. 2013;110:8218–8223. doi: 10.1073/pnas.1305235110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rao-Ruiz P, et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14:1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- 23.Glantz SB, Amat JA, Rubin CS. cAMP signaling in neurons: patterns of neuronal expression and intracellular localization for a novel protein, AKAP 150, that anchors the regulatory subunit of cAMP-dependent protein kinase II beta. Mol Biol Cell. 1992;3:1215–1228. doi: 10.1091/mbc.3.11.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostroveanu A, et al. A-kinase anchoring protein 150 in the mouse brain is concentrated in areas involved in learning and memory. Brain Res. 2007;1145:97–107. doi: 10.1016/j.brainres.2007.01.117. [DOI] [PubMed] [Google Scholar]
- 25.Tsokas P, et al. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jobim PF, et al. Inhibition of mTOR by rapamycin in the amygdala or hippocampus impairs formation and reconsolidation of inhibitory avoidance memory. Neurobiol Learn Mem. 2012;97:105–112. doi: 10.1016/j.nlm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Gafford GM, Parsons RG, Helmstetter FJ. Consolidation and reconsolidation of contextual fear memory requires mammalian target of rapamycin-dependent translation in the dorsal hippocampus. Neuroscience. 2011;182:98–104. doi: 10.1016/j.neuroscience.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337–346. doi: 10.31887/DCNS.2015.17.3/akaczkurkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassab R, Alexandre F. Integration of exteroceptive and interoceptive information within the hippocampus: a computational study. Front Syst Neurosci. 2015;9:87. doi: 10.3389/fnsys.2015.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 33.Weiler IJ, Greenough WT. Metabotropic glutamate receptors trigger postsynaptic protein synthesis. Proc Natl Acad Sci USA. 1993;90:7168–7171. doi: 10.1073/pnas.90.15.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- 36.Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myskiw JD, Furini CRG, Schmidt B, Ferreira F, Izquierdo I. Extinction learning, which consists of the inhibition of retrieval, can be learned without retrieval. P Natl Acad Sci USA. 2015;112:E230–E233. doi: 10.1073/pnas.1423465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power AE, Berlau DJ, McGaugh JL, Steward O. Anisomycin infused into the hippocampus fails to block “reconsolidation” but impairs extinction: The role of re-exposure duration. Learn Memory. 2006;13:27–34. doi: 10.1101/lm.91206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim, R., Moki, R. & Kida, S. Molecular mechanisms for the destabilization and restabilization of reactivated spatial memory in the Morris water maze. Mol Brain4, 10.1186/1756-6606-4-9 (2011). [DOI] [PMC free article] [PubMed]
- 40.Pedreira, M. E. & Maldonado, H. Protein synthesis subserves reconsolidation or extinction depending on reminder duration. Neuron38, 863–869, 10.1016/S0896-6273(03)00352-0 (2003). [DOI] [PubMed]
- 41.Ouyang M, Thomas SA. A requirement for memory retrieval during and after long-term extinction learning. P Natl Acad Sci USA. 2005;102:9347–9352. doi: 10.1073/pnas.0502315102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rao-Ruiz, P., Schmitz, L. J. M., Smit, A. B. & Spijker, S. AMPA Receptor Plasticity in Retrieval, Reconsolidation and Post-retrieval Extinction of Memories. Novel Mechanisms of Memory, 21–41 (2016).
- 43.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 44.Gan Q, Salussolia CL, Wollmuth LP. Assembly of AMPA receptors: mechanisms and regulation. J Physiol. 2015;593:39–48. doi: 10.1113/jphysiol.2014.273755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huganir RL, Nicoll RA. AMPARs and synaptic plasticity: the last 25 years. Neuron. 2013;80:704–717. doi: 10.1016/j.neuron.2013.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medrihan L, et al. Neurobeachin, a protein implicated in membrane protein traffic and autism, is required for the formation and functioning of central synapses. J Physiol. 2009;587:5095–5106. doi: 10.1113/jphysiol.2009.178236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haruvi-Lamdan N, Horesh D, Golan O. PTSD and autism spectrum disorder: Co-morbidity, gaps in research, and potential shared mechanisms. Psychol Trauma. 2018;10:290–299. doi: 10.1037/tra0000298. [DOI] [PubMed] [Google Scholar]
- 49.Lee S, et al. Bidirectional modulation of fear extinction by mediodorsal thalamic firing in mice. Nat Neurosci. 2011;15:308–314. doi: 10.1038/nn.2999. [DOI] [PubMed] [Google Scholar]
- 50.Jeon D, et al. Enhanced learning and memory in mice lacking Na + /Ca2 + exchanger 2. Neuron. 2003;38:965–976. doi: 10.1016/S0896-6273(03)00334-9. [DOI] [PubMed] [Google Scholar]
- 51.Serang O, Cansizoglu AE, Kall L, Steen H, Steen JA. Nonparametric Bayesian evaluation of differential protein quantification. J Proteome Res. 2013;12:4556–4565. doi: 10.1021/pr400678m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D’Angelo G, et al. Statistical Models for the Analysis of Isobaric Tags Multiplexed Quantitative Proteomics. J Proteome Res. 2017;16:3124–3136. doi: 10.1021/acs.jproteome.6b01050. [DOI] [PubMed] [Google Scholar]
- 53.Lee B, Butcher GQ, Hoyt KR, Impey S, Obrietan K. Activity-dependent neuroprotection and cAMP response element-binding protein (CREB): kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J Neurosci. 2005;25:1137–1148. doi: 10.1523/JNEUROSCI.4288-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arendt DH, et al. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology. 2014;40:17–26. doi: 10.1016/j.psyneuen.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim S, et al. Deficiency of a brain-specific chemokine-like molecule, SAM3, induces cardinal phenotypes of autism spectrum disorders in mice. Sci Rep. 2017;7:16503. doi: 10.1038/s41598-017-16769-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keum S, et al. Variability in empathic fear response among 11 inbred strains of mice. Genes Brain Behav. 2016;15:231–242. doi: 10.1111/gbb.12278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.