Abstract
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease that leads to the loss of motor neurons. The molecular mechanisms of motor neuron degeneration are largely unknown and there are currently no effective therapies to treat this disease. In this work, we report whole transcriptome profiling of spinal cords of mutant transgenic hPFN1G118V mice and their wildtype transgenic hPFN1WT controls at a pre-symptomatic stage and at the end-stage of disease. Analyses revealed that end-stage hPFN1G118V mice had 890 differentially expressed genes (747 up-regulated, 143 down-regulated) when compared to pre-symptomatic hPFN1G118V mice, and they had 836 differentially expressed genes (742 up-regulated, 94 down-regulated) when compared to age-matched hPFN1WT controls. Pre-symptomatic hPFN1G118V mice were not significantly different from age-matched hPFN1WT controls. Ingenuity Pathway Analysis identified inflammatory pathways significantly activated in end-stage hPFN1G118V samples, suggesting an excess of glial activation at end-stage disease, possibly due to an increase in glial composition within the spinal cord during disease progression. In conclusion, our RNA-Seq data identified molecules and pathways involved in the mechanisms of neurodegeneration that could potentially serve as therapeutic targets for ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the loss of upper and lower motor neurons. Individuals affected by the disease develop progressive muscle weakness and atrophy, eventually leading to death due to respiratory failure1,2. While clinical studies and basic research have provided insight into mechanisms of ALS, no causative and treatable mechanism has been identified.
For more than 10 years, the complex pathogenesis of ALS has been evaluated with a variety of gene expression profiling methods, such as microarrays and RNA sequencing (RNA-Seq), coupled with whole-tissue or laser-capture microdissected tissue at several stages of disease in mutant SOD1 ALS mouse models3–8 and in postmortem patient tissues9–14. Most studies that analyzed gene expression in ALS mouse models (reviewed in15) were conducted before RNA-Seq had been developed or made widely accessible, so they relied heavily on microarray techniques. While useful and relatively inexpensive, microarray experiments often are limited in the number of genes that can be evaluated (sometimes less than 2,000 genes), which limits the detection scope to transcripts corresponding to genomic sequencing data that is available in the public domain at the time the experiments are conducted16. RNA-Seq, however, has the advantage of using virtually all RNAs and corresponding cDNA sequences in the tissue, which enables detection of virtually all known and novel transcripts in the cells or tissues. Additionally, background noise is lower with RNA-Seq than with microarrays, and RNA-Seq bypasses technical issues inherent to microarrays, such as cross-hybridization, nonspecific hybridization, and limited dynamic range16,17.
Recently, our lab developed transgenic mouse lines that overexpress human profilin1. One strain carries the gene with a mutation at position 118 (hPFN1G118V), and the other carries a wild-type copy (hPFN1WT)18. A second mouse model with mutation in PFN1 that over expresses PFN1C71G has been reported with robust ALS-like symptoms and pathologies19. hPFN1G118V is one of eight identified profilin1 mutations that have been reported in ALS patients20,21. The hPFN1G118V mouse model exhibits many key clinical and pathological signs consistent with human ALS, including loss of lower and upper motor neurons, aggregation of mutant profilin1, activation of glial cells, fragmented mitochondria, muscle atrophy, weight loss, abnormally ubiquitinated proteins, reduced expression of choline acetyltransferase, and reduced survival18. We examined transcriptomic changes in spinal cord tissue of hPFN1 mice to gain insights into the mechanism(s) of mutant hPFN1 neurotoxicity. Unlike human transcriptomic analyses, which are limited to tissues from patients with end-stage ALS, this mouse model provides the opportunity to examine changes that occur pre-symptomatically in the central nervous system, in addition to those that occur at the end-stage of disease.
The aim of this work was to identify molecular changes in spinal cords of the hPFN1G118V ALS mouse model at pre-symptomatic and end-stages. To our knowledge, this is the first study to use next-generation RNA-Seq to measure gene expression in hPFN1G118V mice at pre-symptomatic and end-stages. We report evidence that the overall transcriptome profiles of spinal cord tissues were highly similar, and that those of hPFN1G118V mice with end-stage disease clustered away from those of hPFN1WT mice. This study led to the discovery of 890 genes that were differentially expressed in mutant mice with end-stage disease, as compared to mutant mice that were pre-symptomatic (i.e., 50 days old); of the 890 genes, 747 were up-regulated and 143 were down-regulated.
Results
RNA-Seq data analysis
Sixteen spinal cord samples from male hPFN1G118V and age-matched hPFN1WT mice were used in this study. The experiments used male hPFN1G118V mice that were pre-symptomatic (50 days old; mutant young [MY]) or at the end-stage of disease (175–245 days old; mutant old [MO]), as well as age-matched hPFN1WT mice (wild-type young [WY], and wild-type old [WO], respectively) (Table 1). When hPFN1G118V mice could not right themselves within 20 seconds, they were considered at end-stage of disease18. Female mice were excluded to reduce effects on gene expression due to hormonal fluctuations of the estrus cycle. The numbers of sequenced fragments before and after the cleaning step, the lengths of cleaned sequences, and the GC content of cleaned sequences are presented in Table 1. After gene expression levels were calculated and unexpressed genes filtered out, 18,167 genes remained.
Table 1.
Sample identity and RNA-Seq statistics.
| Mouse ID | Genotype1 | Age (days) | Batch | Total sequenced fragments | Total cleaned fragments | Sequence length | %GC | qRT-PCR2 |
|---|---|---|---|---|---|---|---|---|
| MO1 | MUT | 198 | 1 | 31595682 | 30955215 | 36–76 | 48 | X |
| MO2 | MUT | 190 | 1 | 37449858 | 36869443 | 36–76 | 48 | X |
| MO3 | MUT | 245 | 1 | 51360499 | 50499947 | 36–76 | 48 | X |
| MO4 | MUT | 224 | 2 | 63553644 | 62373141 | 36–76 | 49 | |
| MO5 | MUT | 175 | 2 | 47682463 | 47120118 | 36–76 | 49 | |
| MY1 | MUT | 50 | 1 | 44323436 | 43616751 | 36–76 | 48 | X |
| MY2 | MUT | 50 | 1 | 42110571 | 41432780 | 36–76 | 48 | X |
| MY3 | MUT | 50 | 1 | 37757883 | 37093439 | 36–76 | 48 | X |
| WO1 | WT | 190 | 1 | 49828561 | 48951584 | 36–76 | 48 | X |
| WO2 | WT | 190 | 1 | 39015596 | 38404019 | 36–76 | 48 | X |
| WO3 | WT | 190 | 1 | 35855593 | 35207083 | 36–76 | 48 | X |
| WO4 | WT | 294 | 2 | 72735989 | 71927022 | 36–76 | 50 | |
| WO5 | WT | 294 | 2 | 65738082 | 64949263 | 36–76 | 49 | |
| WY1 | WT | 50 | 1 | 34559215 | 34048886 | 36–76 | 47 | X |
| WY2 | WT | 50 | 1 | 46519005 | 45747075 | 36–76 | 48 | X |
| WY3 | WT | 50 | 1 | 45006658 | 44309399 | 36–76 | 48 | X |
1MUT, hPFN1G118V; WT, hPFN1WT.
2“X” indicates samples assayed for qRT-PCR.
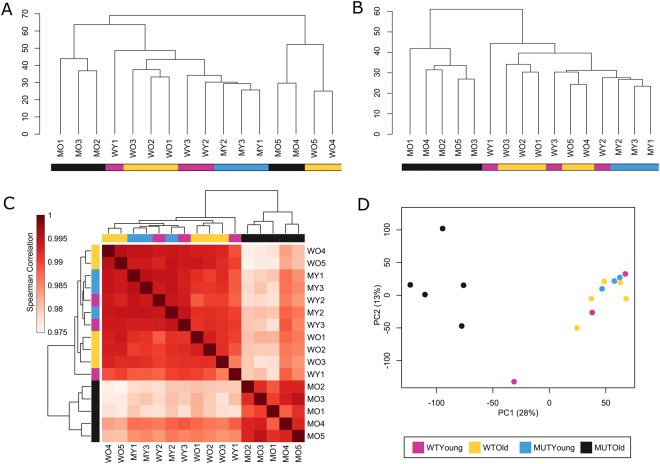
The sequence data analysis of spinal cord RNA samples in 2 batches showed significantly fewer gene sequences were detected in batch 1 than in batch 2, suggesting a potential batch effect between the two libraries. Unsupervised hierarchical clustering of the filtered log2(1 + FPKM) data before batch correction revealed that samples from batch 1 and 2 clustered into two separate clusters, independent of age and genotype (Fig. 1A). After batch-effect correction, unsupervised hierarchical clustering showed a clear separation of end-stage mutant mice from the other three groups (WO, WY, and MY) (Fig. 1B). The clustering of MO samples from different batches reveals biological reproducibility within the MO samples and illustrates the importance of the batch-correction step. Spearman’s rank correlation coefficient clustering (Fig. 1C) of the batch-effect-corrected data revealed similar separation of MO from all other samples. However, it should be noted that the lowest value of all Spearman’s rank correlation comparisons in this data set was 0.975, indicating that even the most different spinal cord transcriptomes were highly correlated. Principle component analysis (PCA) confirmed clustering of MO samples apart from all others, in agreement with Spearman’s and unsupervised hierarchical clustering analysis (Fig. 1D). No recognizable clustering pattern from WO, WY, and MY samples was detected in unsupervised hierarchical clustering, Spearman’s rank correlation, or PCA, suggesting a high degree of similarity among the three groups.
Figure 1.
Analysis of 16 spinal cord samples based on normalized expression values of 18,167 genes. (A,B) Unsupervised hierarchical clustering. (A) Separation of batch 1 and batch 2; no batch-effect correction. (B) Post-batch-effect correction; batch separation minimized, and clearly separate clustering of MO samples from the other samples. (C) Heatmap showing the pair-wise Spearman’s rank correlation between all samples and clustering of MO samples apart from the other three groups. (D) PCA plot of samples in which MO mice are clustered away from the other three groups.
Gene expression specificity by cell type
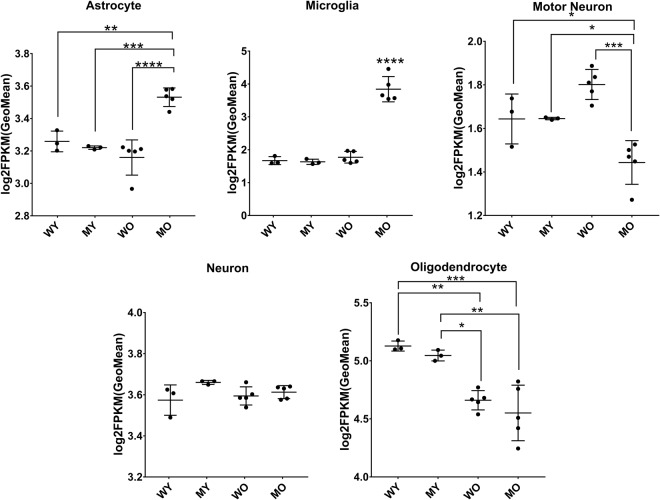
We verified the cell-type compositions of the spinal cord samples by analyzing expression of genes specifically expressed by neurons, motor neurons, astrocytes, oligodendrocytes, and microglia (Fig. 2, genes are listed in Supplementary Table S1)10,22. In MO samples, astrocyte and microglia compositions were significantly higher and motor neuron composition was significantly lower than in WO, WY, and MY. Significant changes in oligodendrocytes appeared to be dependent on the age of the mice in WO and MO samples, compositions of oligodendrocytes were significantly lower than in each of the younger samples but were not statistically different from each other. Neuron populations were not significantly different among the four groups.
Figure 2.
Cell-type composition of spinal cord samples. Changes in specific cell types indicated by geometric mean of the log2(1 + FPKM) values of cell-type marker genes for each cell type. Statistical differences in cell type composition are calculated by the Tukey Kramer test. Number of marker genes used per cell type: 60 for astrocyte, 5 for microglia, 5 for motor neuron, 72 for neuron, and 35 for oligodendrocyte. Error bars indicate SD; *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Differential expression
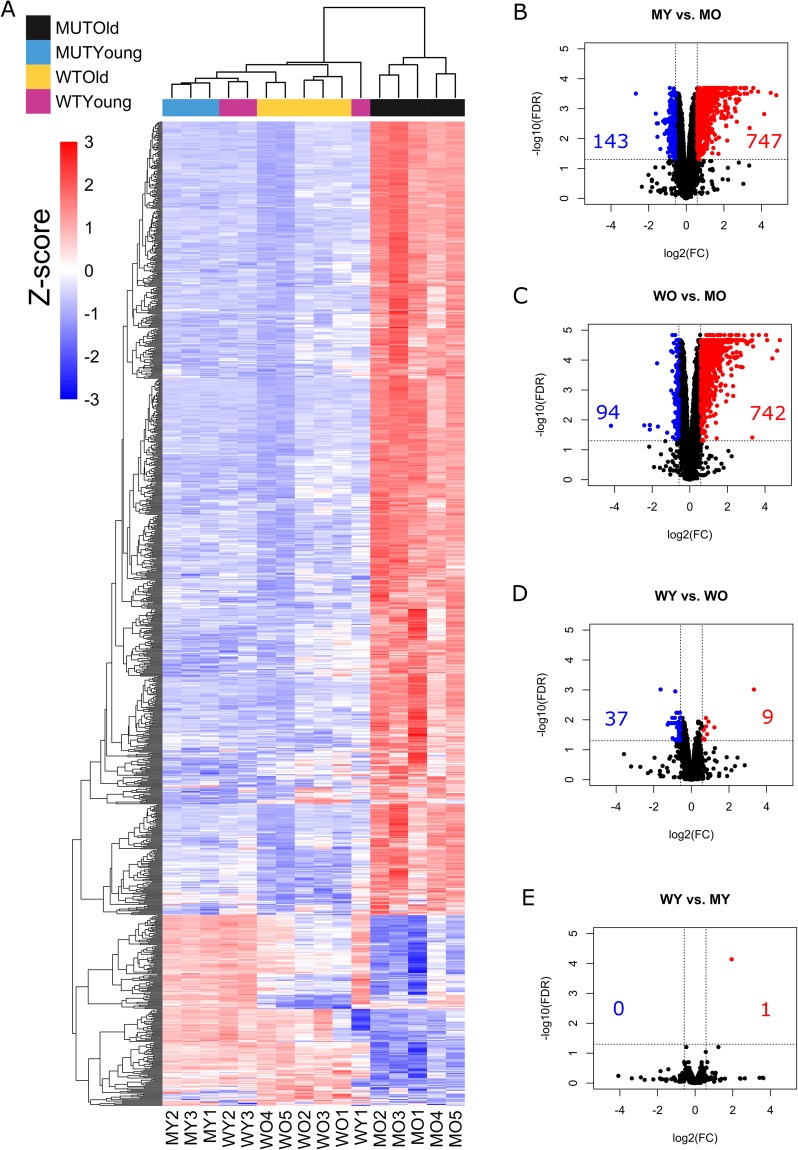
To determine the differential gene expression between presymtomatic and end-stage hPFN1G118V mice and age-matched hPFN1WT mice, we used limma, an open-source R-based software package that analyzes differential expression of log2-transformed FPKM values23. A total of 1,048 genes were significantly (FDR < 0.05) differentially expressed with an absolute fold change of 1.5 or greater for four comparisons: WO vs. MO, MY vs. MO, WY vs. WO, and WY vs. MY (Supplementary Table S2). A heatmap shows that most differentially expressed genes were up-regulated in spinal cord samples from the MO group (i.e., hPFN1G118V mice at end-stage; Fig. 3A).
Figure 3.
Heatmap and volcano plots of differential expression. (A) Heatmap shows 1,048 significantly (FDR ≤ 0.05) differentially expressed genes across four comparisons (MY vs. MO, WO vs. MO, MY vs. WY, WY vs. WO) from each spinal cord sample. Colors above the heatmap indicate mouse age and genotype. Each row of the heatmap represents the z-score transformed log2(1 + FPKM) values of one differentially expressed gene across all samples (blue, low expression; red, high expression). (B–E) Volcano plots of significantly differentially expressed genes, (FDR ≤ 0.05 and |FC| ≥ 1.5; red, up-regulated; blue, down-regulated). Numbers of genes up-regulated or down-regulated are denoted.
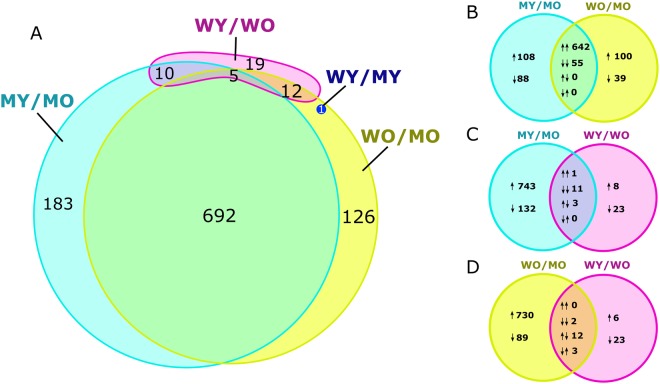
Expression varied most between hPFN1G118V mice that were at end-stage of disease and those that were pre-symptomatic (MY vs. MO), in which 890 genes were differentially expressed, with 747 up-regulated and 143 down-regulated, as shown in the volcano plot (Fig. 3B). When end-stage hPFN1G118V mice were compared to age-matched hPFN1WT mice (WO vs. MO), 836 differentially expressed genes were identified, with 742 up-regulated and 94 down-regulated (Fig. 3C). A subset of 697 genes were differentially expressed in both MO vs. MY and WO vs. MO comparisons as shown by Venn diagram (Fig. 4A); of these, 642 were up-regulated, 55 were down-regulated, and none were regulated in opposite directions in both comparisons (Fig. 4B). The WY vs. WO comparison revealed that only 46 genes are differentially expressed (9 up-regulated, 37 down-regulated) (Fig. 3D); 27 of the 46 were shared with the MY vs. MO comparison, the WO vs. MO comparison, or both, suggesting potential overlap of dys-regulated expression of specific genes involved in progression of motor neuron disease and aging (Fig. 4A). Analysis of WY vs. MY identified only one significantly dys-regulated gene, Prnp, which was up-regulated (Fig. 3E). This gene was significantly up-regulated also in the WO vs. MO comparison (Fig. 4A).
Figure 4.
Venn diagrams of differential expression. (A) Venn diagram indicating the number of significantly differentially expressed genes (FDR ≤ 0.05 and |FC| ≥ 1.5) across the four comparisons (MY vs MO, WO vs MO, WY vs WO, WY vs MY). (B–D) Detailed analysis between three comparisons illustrating overlap between up- and down-regulated genes. Pairs of arrows in intersection refer to direction of fold change in comparisons on the left and right sides, respectively.
Pathway analysis
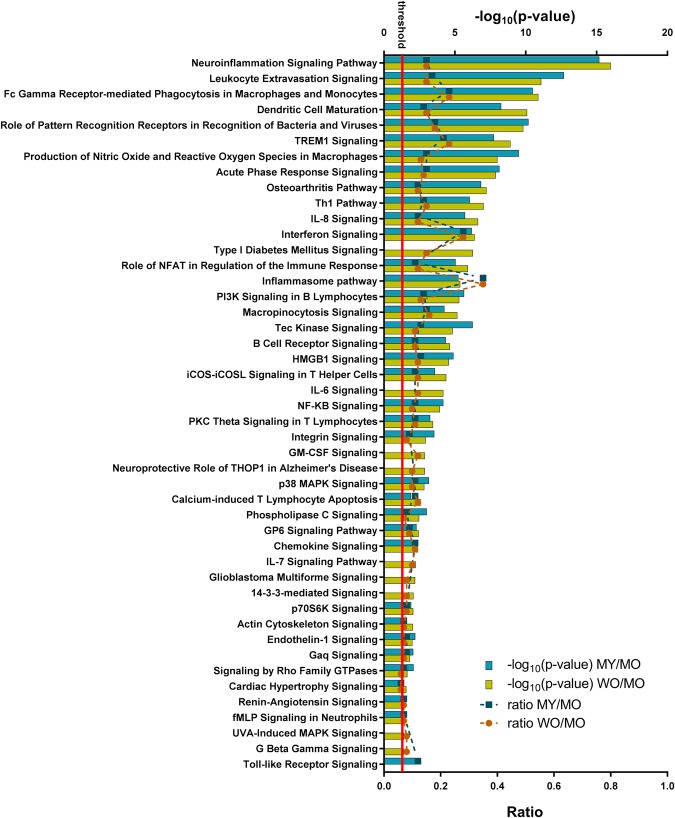
Ingenuity Pathway Analysis identified functionally relevant cellular pathways that were linked to differentially expressed genes in the MY vs. MO (890 genes) and WO vs. MO (836 genes) comparisons. Because the ideal set size for IPA from gene expression data is 200–3,000, we could not analyze the 46-gene set from the WY vs. WO comparison. Of the identified pathways that were considered significant (Fisher Exact P-value < 0.05; Supplementary Table S3), IPA predicted significant activation (z-score >2) of 37 pathways in the MY vs. MO comparison and 45 pathways in the WO vs. MO comparison (Fig. 5).
Figure 5.
Significantly activated pathways in hPFN1G118V spinal cord tissue. IPA identified pathways (P ≤ 0.05) that are significantly activated (z-score ≥2) in differentially expressed genes from MY vs. MO and/or WO vs. MO comparisons. The –log10(P-value) is reported on the upper axis, with the threshold P-value indicated by a red line at –log10(P-value) = 1.3. The ratio between the number of differentially expressed genes and the number of genes in the specific pathway is reported on the bottom axis. Lack of a bar from MY vs. MO or WO vs. MO for a specific pathway indicates that the comparison did not meet the criteria of P ≤ 0.05 and/or z-score ≥2.
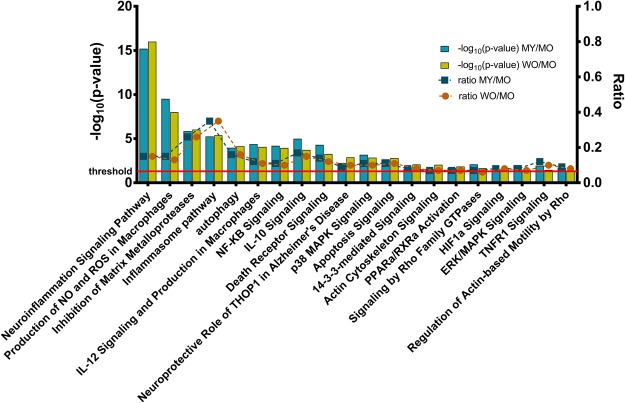
Pathways with biological relevance to ALS are listed in Fig. 6. Differentially expressed genes corresponded to, most notably, components of neuroinflammation signaling, radical-generating pathways, inhibition of matrix metalloproteases, inflammasome pathway, autophagy, interleukin signaling and production, NF-kB signaling, apoptosis, MAP kinase signaling, and actin cytoskeleton signaling. The neuroinflammation signaling pathway had one of the lowest P-values of the identified pathways in both comparisons and was predicted to be significantly up-regulated (z-score of ~5.6 for WO vs. MO and ~5.5 for MY vs. MO). Of the genes that were linked to selected ALS-relevant pathways, the highest degree of overlap occurred with those linked to the inflammasome. End-stage hPFN1G118V samples (i.e., MO) had significantly increased levels of transcripts encoding components of inflammasome complexes (Pycard, Aim2, Casp1, Naip2), as well as other molecules important to the inflammasome pathway (Casp8, Tlr4, Il1b, Ctsb).
Figure 6.
ALS-relevant pathways. IPA identified significantly activated pathways that are relevant to ALS. The –log10(P-value) is reported on the left axis, with the threshold P-value (P ≤ 0.05) indicated by a red line at –log10(P-value) = 1.3. The ratio between the number of differentially expressed genes and the number of genes in the specific pathway is reported on the right axis. (NO, nitric oxide; ROS reactive oxygen species).
Validation of RNA-Seq results
RNA-Seq results were validated with qRT-PCR analysis of RNA samples from spinal cords of mice from batch 1. From the differentially expressed genes identified in the four comparisons, we randomly selected 12 candidates with |log2FC| > 0.58 (or |FC| > 1.5) and log2(1 + FPKM) > 1 across all samples for the given gene (Table 2).The rationale for selecting genes at random for validation was to avoid scientific bias. Because Prnp was the only significantly differentially expressed gene in the WY vs. MY comparison, it was included among the 12 genes in the validation set. At least one up-regulated gene and one down-regulated gene was chosen from the genes identified in the other three comparisons for validation of RNA-Seq.
Table 2.
List of qRT-PCR validated genes*.
| Gene Symbol | Description | MY/MO | WO/MO | WY/MY | WY/WO |
|---|---|---|---|---|---|
| Abca8a | ATP-binding cassette, sub-family A (ABC1), member 8a | + | + | ||
| Ccl6 | chemokine (C-C motif) ligand 6 | + | + | ||
| Chodl | chondrolectin | − | − | ||
| Fmod | fibromodulin | + | + | ||
| Hmgcs1 | 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 | − | − | ||
| Opalin | oligodendrocytic myelin paranodal and inner loop protein | − | |||
| Prnp | prion protein | + | + | ||
| S100a4 | S100 calcium binding protein A4 | + | + | ||
| Serpina3n | serine (or cysteine) peptidase inhibitor, clade A, member 3 N | + | + | ||
| Tlr2 | toll-like receptor 2 | + | + | ||
| Trem2 | triggering receptor expressed on myeloid cells 2 | + | + | ||
| Vim | vimentin | + | + |
*Comparisons in which genes were found to have |log2FC | ≥ 0.58 (or |FC| ≥ 1.5) are denoted by + for up-regulation and – for down-regulation.
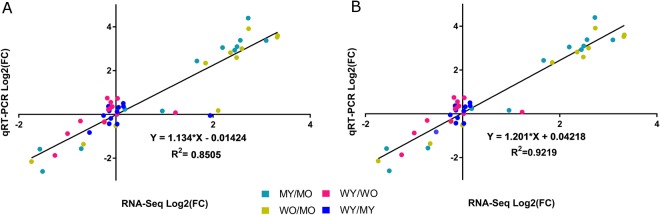
Results of the qRT-PCR analysis confirmed those of RNA-Seq for all genes across all four comparisons, with the exception of Prnp expression in WO vs. MO and in WY vs. MY. When comparing WO and MO, RNA-Seq analysis of Prnp resulted in log2(FC) of 2.10, but qRT-PCR resulted in log2(FC) of 0.182. When comparing WY and MY, RNA-Seq analysis of Prnp resulted in log2(FC) of 1.94, but qRT-PCR resulted in log2(FC) of −0.048. The RNA-Seq data showed that Prnp was up-regulated when hPFN1G118V mice were compared to hPFN1WT mice within the same age groups, but the qRT-PCR data showed only insignificant changes in log2(FC) of Prnp. When all RNA-Seq-derived log2(FC) values for the set of 12 genes in all four comparisons were correlated with the qRT-PCR log2(FC) values, the values were highly consistent (Fig. 7A). However, when Prnp results from WO vs. MO and from WY vs. MY were removed from the analysis, the Pearson correlation coefficient increased from R = 0.92 (P < 10−15) to R = 0.96 (P < 10−15), and the coefficient of determination increased from R2 = 0.85 to R2 = 0.92 (Fig. 7B).
Figure 7.
Results from qRT-PCR validation. Linear regression plots between log2(FC) values computed by limma from RNA-Seq data and log2(FC) values detected by qRT-PCR for 12 selected genes across four comparisons: MY vs. MO, WO vs. MO, WY vs. WO, and WY vs. MY. (A) Linear regression plot of all samples measured. (B) Linear regression plot of all samples except Prnp qRT-PCR outliers from WO vs. MO and WY vs. MY comparisons.
Discussion
ALS is a complex neurodegenerative disease with no viable treatment, and its causative mechanistic pathways have yet to be elucidated. In this study, we used RNA-Seq to evaluate transcriptomic changes in spinal cord tissues of hPFN1G118V mice as compared to those of age-matched hPFN1WT mice, at pre-symptomatic (50 days; the earliest time at which hPFN1 aggregation has been shown to occur in hPFN1G118V mice) and end (~202 days) stages of disease.18 This RNA-Seq data analysis is an initial step in determining the transcriptional changes caused by mutant PFN1 and may be further confirmed by comparing/contrasting RNA-Seq data from PFN1C71G mouse model.19
Overall, transcriptomes of the spinal cord tissues were highly similar across the different groups investigated—the lowest Spearman’s correlation was 0.975. However, normalized gene expression clearly distinguished the end-stage hPFN1G118V mice from the other three groups, as shown by hierarchical clustering and PCA analyses.
It has been proposed that astrocytes and microglia play important roles in the pathogenesis and progression of ALS24,25, and, consistent with this, our results indicated increased prevalence of astrocytes and microglia in end-stage hPFN1G118V mice. It is likely that changes in the heterogeneous cell populations of the spinal cord at end-stage, especially increases in glial population and activity, can be attributed to this observation. This is in agreement with findings in our recent report on the hPFN1 mouse model, in which increased astrocytic and microglial activation were revealed by immunohistochemical analysis of the ventral horn of end-stage hPFN1G118V mice18. It would be of great interest to address the role of astrocytes and microglia in the PFN1C71G mouse model in the near future.
The high degree of upregulation of the neuroinflammation signaling pathway in spinal cord tissues of end-stage PFN1G118V mice could be related to the observed increase in astrocytes and microglia in this group. Many inflammation-related pathways were up-regulated, including those of NF-kB signaling, production of nitric oxide and reactive oxygen species, interleukin signaling, and the inflammasome. Previous transcriptomic studies of murine models and of post-mortem human ALS tissues also identified upregulation of inflammatory processes9,10,13,26,27. Inflammasomes are multiprotein complexes that are activated by a variety of factors and induce activation of caspase-1, which mediates production of pro-inflammatory cytokines interleukin-1B and interleukin-18, inducers of pyropoptosis, a highly inflammatory form of programmed cell death28. Genes encoding caspase-1 and apoptosis-associated speck-like protein containing a CARD (ASC, also known as PYCARD), which are components of all types of inflammasome complexes, were significantly up-regulated in end-stage PFN1G118V spinal cords29. AIM2 and NAIP2 transcripts, which are present in AIM2 inflammasomes and NLRC4 inflammasomes, respectively, also were up-regulated in tissues of PFN1G118V mice at end-stage29. The NLRP3 inflammasome complex has been characterized most extensively and implicated in ALS and other neurodegenerative diseases that include Alzheimer’s disease, Parkinson’s disease, frontotemporal dementia, and Huntington’s disease28,30–32. Investigations of human ALS tissue samples and SOD1 mouse models have shown up-regulation of NLRP3 inflammasome activity31,32. Although transcripts encoding the NLRP3 protein were not significantly up-regulated in end-stage hPFN1G118V mice, it is possible that AIM2, NLRC4, and, potentially, NLRP3 inflammasomes play a role in PFN1-mediated death of motor neurons. The ways in which PFN1 mutation causes increased inflammation and possible up-regulation of inflammasomes in spinal cords of our mouse model may be worth examining in the future as a potential therapeutic target.
We selected 50 days as the time point to evaluate the pre-symptomatic stage in our mouse model because this is the earliest age at which PFN1 has been found in the insoluble fractions of spinal cords of these mice18. Only one gene, Prnp, was significantly differentially expressed at the pre-symptomatic stage in hPFN1G118V mice, as compared to age-matched hPFN1WT mice. Significant upregulation of Prnp was identified by RNA-Seq comparisons of hPFN1G118V and hPFN1WT mice at both time points, revealing that this gene could be expressed at significantly greater levels in mutant mice at early ages, before disease onset. However, qRT-PCR analyses did not reveal Prnp up-regulation in these two comparisons, and the correlation between RNA-Seq and qRT-PCR data improved when data from Prnp in these comparisons was removed from validation experiments. Both hPFN1G118V and hPFN1WT mice were generated with a plasmid vector (i.e., MoPrP.Xho1) that contains the mouse Prnp promotor18,33. In addition to the mouse Prnp promotor, the vector contains 5′ intronic and 3′ untranslated sequences, with an Xho1 cleavage site between exons 2 and 3 of Prnp33. It is possible that the alignment software could have aligned the endogenous Prnp gene to the intronic and untranslated sequences of the Prnp gene from mRNA generated by the transgenic expression vector. If the hPFN1G118V mice transcribe significantly more transgene than the hPFN1WT mice, misalignment of transgenic mRNA sequences as endogenous Prnp mRNA could be the cause of the up-regulation of Prnp in hPFN1G118V mice that was observed with RNA-Seq but not with qRT-PCR. Lack of any other significantly differentially expressed genes in the WY vs. MY comparison suggests that, at a transcriptomic level, 50-day-old hPFN1G118V mice are hardly distinguishable from 50-day-old hPFN1WT mice.
Given these findings, a future study is needed to examine gene expression changes at multiple time points after 50 days and before end-stage. Evaluation of spinal cord gene expression at more points in the disease, such as at early onset and full onset should be considered. Limiting gene expression studies to end-stage evaluation may indicate mainly molecular events that are secondary to causative mechanisms. Evaluating time points that occur soon after preliminary disease onset in this mouse model would also help identify molecular pathology of ALS disease progression and pathways that could be targeted therapeutically. Additionally, more time points would enhance detection of molecular changes that occur before and during the process of motor neuron death, as opposed to those that occur after motor neuron death (i.e., at end-stage).
It is possible that changes in the heterogeneous cell population of the spinal cord, especially increases in numbers and activation of glial cells that are observed at the end-stage of the disease, can blur molecular changes that occur in other cell types that are relevant to ALS, such as motor neurons. For instance, doubling the population of astrocytes may double expression levels of a given gene expressed by astrocytes, so expression changes evaluated by whole spinal cord transcriptome sequencing may not be due to increases of expression within individual cells. In future studies of hPFN1 mice, it would be worth exploring changes in gene expression with cell-type-specific RNA isolation methods that have been used with other ALS disease models. This would further elucidate whether the results we have presented are related to increased glial cell composition of the spinal cord, or due to other secondary effects. Methods developed to isolate microglia from spinal cord tissue have been used in RNA-Seq analysis of the SOD1 G93A ALS mouse model34. Additionally, embryonic stem-cell differentiation methods have been used to analyze expression profiles of astrocytes and their interplay with motor neurons in the SOD1 G93A mouse model35. In the RNA-Seq study by Phatnani et al., RNA sequencing data from both whole spinal cord tissues and from neurons and astrocytes derived from embryonic stem cells were analyzed in tandem to provide dynamic insight into the contributions of glial cells in ALS35. Previous transcriptome studies in both mouse models and humans have been conducted on laser microdissected motor neurons, enhancing detection of molecular changes in the neurons without bias from other cell types5,6,11. Use of any of these methods with the hPFN1 mouse model could add more dimension to the transcriptional effects of mutant PFN1 throughout disease progression.
This is the first study to profile the gene expression of hPFN1G118V mice, and it provides a foundation for future gene expression profiling studies of this ALS mouse model. The data presented here from whole spinal cord tissue could be used together with future studies that acutely isolate cells of the central nervous system or that examine specific cells differentiated from embryonic stems. Our findings of transcripts encoding components of AIM2 and NLRC4 inflammasomes causes us to infer that inflammasomes are promising targets to be studied in the mouse models for PFN1-mediated death of motor neurons. These molecules in the inflammasome complexes may form the basis of future studies to lead the identification of molecular features that could potentially be therapeutic targets for ALS.
Materials and Methods
Mice and tissue collection
The experiments used male hPFN1G118V mice that were pre-symptomatic (50 days old; mutant young [MY]) or at the end-stage of disease (175–245 days old; mutant old [MO]), as well as age-matched hPFN1WT mice (wild-type young [WY], and wild-type old [WO], respectively). These transgenic mice were developed with a vector construct in which hPFN1G118V or hPFN1WT cDNA was inserted at the Xho1 site of the MoPrP.Xho expression plasmid18,33. Detailed information regarding the identity of spinal cord samples (including age, genotype, and ID number) used for RNA-Seq and real-time quantitative reverse transcription (qRT-PCR) can be found in Table 1. Mice were housed under 12-hour light/dark conditions and were fed 4–5 g chow (Harlan/Teklad #7001) per day per mouse, with free access to water. Mouse identification was performed by genotyping18. Animals were sacrificed and spinal cords collected at either 50 days or at end-stage of disease for subsequent molecular analyses. hPFN1G118V mice were considered at end-stage of disease when they could not right themselves within 20 seconds18. In this study we used animals and all the experimental procedures that involved mice for this study were approved by UAMS Institutional Animal Care and Use Committee (IACUC), AUP protocol # 3730 and conducted in full accordance of the guidelines of the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. The Animal Care and Use Program at the University of Arkansas for Medical Sciences has an Assurance with the NIH (#A3063-01/D16-00035), is USDA registered (71-R-011), and AAALAC International accredited (000322). The animal facilities are located within the Division of Laboratory Animal Medicine (DLAM) in three distinct areas on campus. The animal care and use program is centrally managed by DLAM administration and is physically housed in approximately 30,000 square feet of space. DLAM has surgical, procedural, and housing facilities which focus on several research interests on campus. There are three full time veterinarians, as well as administrative staff and AALAS certified husbandry technicians to support all animal based research on this campus.
RNA library construction and sequencing
Total RNA was isolated with the TRIzol Reagent protocol (ThermoFisher, Waltham, MA) from spinal cords of hPFN1WT and hPFN1G118V mice. RNA quality and concentration were estimated with Bioanalyzer 2100 and RNA 6000 Nano Kit (Agilent Technologies, Waldbronn, Germany). RNA-Seq libraries were prepared with Illumina TruSeq Stranded mRNA LT Sample Preparation Kit (Illumina, San Diego, CA) using 1 μg of total RNA according to the manufacturer’s protocol. Sequencing was performed on the NextSeq. 500 platform (Illumina). RNA-Seq data used for downstream analysis in this study came from two separate batches of RNA libraries (denoted as batch 1 or batch 2 in Table 1).
Processing RNA-Seq data
FastQC software (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was used to generate quality-control reports of individual FASTQ files, before and after filtering. Trimmomatic36 was used to filter out low-quality reads and possible contamination from Illumina Adaptors or PCR primers. The remaining reads were aligned with TopHat37 to the UCSC mm10 mouse reference genome. Cufflinks37 was used to quantify gene expression in fragments per kb of transcript per million mapped read (FPKM). This normalization accounts for differences in library size and gene length.
Prior to batch-effect removal, the FPKM matrix of all 16 samples was log2-transformed, after adding 1 to avoid undefined values. Unexpressed genes were filtered out by removing genes with 0 expression in at least 8 samples; 18,167 genes in 16 samples survived the filtering criterion. Because samples were prepared in two batches, function ComBat38 from Bioconductor package sva39 was then used to remove possible batch effects between batches 1 and 2 by applying parametric empirical Bayesian adjustments to mean and scale. All downstream data analyses were performed on log2-transformed FPKM values after correction for batch effects.
Differential gene expression and pathway analysis
Gene expression was analyzed with the limma package, an R-based open-source software designed to analyze transcriptomic data for differential expression23. Differentially expressed genes were identified by applying a false discovery rate (FDR) cut-off of 0.05 and an absolute fold-change (FC) of 1.5. To identify significant canonical pathways in which differentially expressed genes were enriched, pathway enrichment analysis was conducted with Ingenuity Pathway Analysis software (IPA; Ingenuity Systems, Redwood City, CA).
Cell composition analysis
Changes in cell-type compositions of spinal cord samples were estimated according to expression of cell-type-specific genes for microglia, astrocytes, oligodendrocytes, neurons, and motor neurons10,22. The genes were identified from a published list of human genes10; therefore, we selected those with corresponding mouse orthologues (Supplementary Table S1). We calculated geometric means by using log2(1 + FPKM) values instead of FPKM values.
qRT-PCR validation
RNA samples from batch 1 were used for qRT-PCR validation. cDNA was synthesized from DNase-treated total RNA (1 μg) with High Capacity cDNA Reverse Transcription kit according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). qRT-PCR was performed with primers from IDT and SYBR Green PCR Master Mix (Applied Biosystems), according to the manufacturer’s instructions, and detected with the ABI QuantStudio 12 K Flex platform (Invitrogen, Carlsbad, CA). Reactions were carried out in duplicate with 52 ng of cDNA and 250 nM of each primer in a final volume reaction of 20 μl (50 °C for 2 minutes, 95 °C for 2 minutes; 45 amplification cycles at 95 °C for 15 s, 60 °C for 1 min). Relative expression levels were determined by the comparative threshold cycle (ΔΔCt) method, using GAPDH as a reference gene.
Electronic supplementary material
Acknowledgements
This work was supported by grants to MK from the University of Arkansas for Medical Sciences Startup Fund, the University of Arkansas for Medical Sciences Center for Translational Neurosciences, the National Institute of General Medical Sciences IDeA Program Award (P30 GM110702, P20 GM109005), and the National Institute of Neurological Disorders and Stroke (NS088653, NS101334). Support was provided in part by the NIH IDeA Networks of Biomedical Research Excellence (INBRE) grant (P20 GM103429) and by Center for Translational Pediatric Research (CTPR) NIH Center of Biomedical Research Excellence award (P20 GM121293). None of the funding bodies had a role in the design of the study; collection, analysis, and interpretation of data; or writing the manuscript. The manuscript was edited by the Science Communication Group at the University of Arkansas for Medical Sciences.
Author Contributions
M.K. conceived of and designed the study. Data analysis was performed by C.B., Y.R., D.F., S.B., G.G. and M.K. The manuscript was written by C.B. and M.K.
Data Availability
The data set generated and analyzed during this study is available in the GEO repository with accession number GSE113924.
Competing Interests
Dr. Kiaei and UAMS have a financial interest in the technology discussed in this publication. These financial interests have been reviewed and approved in accordance with the UAMS conflict of interest policies. Other authors have no conflict.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31132-y.
References
- 1.Kiernan MC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Sreedharan J, Brown RH., Jr. Amyotrophic lateral sclerosis: Problems and prospects. Ann Neurol. 2013;74:309–316. doi: 10.1002/ana.24012. [DOI] [PubMed] [Google Scholar]
- 3.Olsen MK, et al. Disease mechanisms revealed by transcription profiling in SOD1‐G93A transgenic mouse spinal cord. Annals of Neurology. 2001;50:730–740. doi: 10.1002/ana.1252. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira GP, Alves CJ, Chadi G. Early gene expression changes in spinal cord from SOD1(G93A) Amyotrophic Lateral Sclerosis animal model. Frontiers in Cellular Neuroscience. 2013;7:216. doi: 10.3389/fncel.2013.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perrin FE, et al. No widespread induction of cell death genes occurs in pure motoneurons in an amyotrophic lateral sclerosis mouse model. Human molecular genetics. 2005;14:3309–3320. doi: 10.1093/hmg/ddi357. [DOI] [PubMed] [Google Scholar]
- 6.Ferraiuolo L, et al. Microarray Analysis of the Cellular Pathways Involved in the Adaptation to and Progression of Motor Neuron Injury in the SOD1 G93A Mouse Model of Familial ALS. The Journal of Neuroscience. 2007;27:9201–9219. doi: 10.1523/JNEUROSCI.1470-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Arrigo A, et al. Transcriptional Profiling in the Lumbar Spinal Cord of a Mouse Model of Amyotrophic Lateral Sclerosis: A Role for Wild-Type Superoxide Dismutase 1 in Sporadic Disease? Journal of Molecular Neuroscience. 2010;41:404–415. doi: 10.1007/s12031-010-9332-2. [DOI] [PubMed] [Google Scholar]
- 8.Bandyopadhyay U, et al. RNA-Seq Profiling of Spinal Cord Motor Neurons from a Presymptomatic SOD1 ALS Mouse. PLoS One. 2013;8:e53575. doi: 10.1371/journal.pone.0053575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brohawn DG, O’Brien LC, Bennett JP., Jr. RNAseq Analyses Identify Tumor Necrosis Factor-Mediated Inflammation as a Major Abnormality in ALS Spinal Cord. PLoS One. 2016;11:e0160520. doi: 10.1371/journal.pone.0160520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Erchia AM, et al. Massive transcriptome sequencing of human spinal cord tissues provides new insights into motor neuron degeneration in ALS. Scientific Reports. 2017;7:10046. doi: 10.1038/s41598-017-10488-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang YM, et al. Gene expression profile of spinal motor neurons in sporadic amyotrophic lateral sclerosis. Annals of Neurology. 2005;57:236–251. doi: 10.1002/ana.20379. [DOI] [PubMed] [Google Scholar]
- 12.Dangond F, et al. Molecular signature of late-stage human ALS revealed by expression profiling of postmortem spinal cord gray matter. Physiological Genomics. 2004;16:229–239. doi: 10.1152/physiolgenomics.00087.2001. [DOI] [PubMed] [Google Scholar]
- 13.Offen D, et al. Spinal Cord mRNA Profile in Patients with ALS: Comparison with Transgenic Mice Expressing the Human SOD-1 Mutant. Journal of Molecular Neuroscience. 2009;38:85–93. doi: 10.1007/s12031-007-9004-z. [DOI] [PubMed] [Google Scholar]
- 14.Prudencio M, et al. Distinct brain transcriptome profiles in C9orf72-associated and sporadic ALS. Nature neuroscience. 2015;18:1175–1182. doi: 10.1038/nn.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath PR, Kirby J, Shaw PJ. Investigating cell death mechanisms in amyotrophic lateral sclerosis using transcriptomics. Front Cell Neurosci. 2013;7:259. doi: 10.3389/fncel.2013.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nature reviews. Genetics. 2009;10:57–63. doi: 10.1038/nrg2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Fung-Leung W-P, Bittner A, Ngo K, Liu X. Comparison of RNA-Seq and Microarray in Transcriptome Profiling of Activated T Cells. PLoS One. 2014;9:e78644. doi: 10.1371/journal.pone.0078644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fil D, et al. Mutant Profilin1 transgenic mice recapitulate cardinal features of motor neuron disease. Hum Mol Genet. 2017;26:686–701. doi: 10.1093/hmg/ddw429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, et al. Mutant PFN1 causes ALS phenotypes and progressive motor neuron degeneration in mice by a gain of toxicity. Proc Natl Acad Sci USA. 2016;113:E6209–E6218. doi: 10.1073/pnas.1605964113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CH, et al. Mutations in the profilin 1 gene cause familial amyotrophic lateral sclerosis. Nature. 2012;488:499–503. doi: 10.1038/nature11280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith BN, et al. Novel mutations support a role for Profilin 1 in the pathogenesis of ALS. Neurobiol Aging. 2015;36(1602):e1617–1627. doi: 10.1016/j.neurobiolaging.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L, et al. Transcriptome sequencing reveals aberrant alternative splicing in Huntington’s disease. Human molecular genetics. 2016;25:3454–3466. doi: 10.1093/hmg/ddw187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res43, 10.1093/nar/gkv007 (2015). [DOI] [PMC free article] [PubMed]
- 24.Brites D, Vaz AR. Microglia centered pathogenesis in ALS: insights in cell interconnectivity. Front Cell Neurosci. 2014;8:117. doi: 10.3389/fncel.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Hyeon SJ, Im H, Ryu H, Kim Y. Astrocytes and Microglia as Non-cell Autonomous Players in the Pathogenesis of ALS. Exp Neurobiol. 2016;25:233–240. doi: 10.5607/en.2016.25.5.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morello, G., Spampinato, A. G. & Cavallaro, S. Neuroinflammation and ALS: Transcriptomic Insights into Molecular Disease Mechanisms and Therapeutic Targets. Mediat Inflamm, 10.1155/2017/7070469 (2017). [DOI] [PMC free article] [PubMed]
- 27.Sun S, et al. Translational profiling identifies a cascade of damage initiated in motor neurons and spreading to glia in mutant SOD1-mediated ALS. Proc Natl Acad Sci USA. 2015;112:E6993–7002. doi: 10.1073/pnas.1520639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song C, et al. Aminated nanomicelles as a designer vaccine adjuvant to trigger inflammasomes and multiple arms of the innate immune response in lymph nodes. Int J Nanomedicine. 2017;12:7501–7517. doi: 10.2147/IJN.S144623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma D, Kanneganti TD. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou K, Shi L, Wang Y, Chen S, Zhang J. Recent Advances of the NLRP3 Inflammasome in Central Nervous System Disorders. J Immunol Res. 2016;2016:9238290. doi: 10.1155/2016/9238290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johann S, et al. NLRP3 Inflammasome Is Expressed by Astrocytes in the SOD1 Mouse Model of ALS and in Human Sporadic ALS Patients. Glia. 2015;63:2260–2273. doi: 10.1002/glia.22891. [DOI] [PubMed] [Google Scholar]
- 32.Debye B, et al. Neurodegeneration and NLRP3 inflammasome expression in the anterior thalamus of SOD1(G93A) ALS mice. Brain Pathol. 2018;28:14–27. doi: 10.1111/bpa.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borchelt DR, et al. A vector for expressing foreign genes in the brains and hearts of transgenic mice. Genet Anal. 1996;13:159–163. doi: 10.1016/S1050-3862(96)00167-2. [DOI] [PubMed] [Google Scholar]
- 34.Chiu IM, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013;4:385–401. doi: 10.1016/j.celrep.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phatnani HP, et al. Intricate interplay between astrocytes and motor neurons in ALS. Proc Natl Acad Sci USA. 2013;110:E756–765. doi: 10.1073/pnas.1222361110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- 39.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data set generated and analyzed during this study is available in the GEO repository with accession number GSE113924.