Abstract
Salmonella enterica serovar Enteritidis (Salmonella Enteritidis) is a globally important foodborne pathogen, and the contaminated chicken eggs are the major source of salmonellosis in humans. Salmonella Enteritidis strains are differentially susceptible to the hostile environment of egg whites. Strains with superior survival ability in egg whites are more likely to contaminate eggs and consequently infect humans. However, the genetic basis for this phenotype is unclear. We characterized two Salmonella Enteritidis strains isolated from chicken meat that had similar genetic backgrounds but large differences in survival ability in egg whites. Although genome comparisons indicated that the gene content and genomic synteny were highly conserved, variations including six insertions or deletions (INDELs) and 70 single nucleotide polymorphisms (SNPs) were observed between the two genomes. Of these, 38 variations including four INDELs and 34 non-synonymous SNPs (nsSNP) were annotated to result in amino acid substitutions or INDELs in coding proteins. These variations were located in 38 genes involved in lysozyme inhibition, vitamin biosynthesis, cell division and DNA damage response, osmotic and oxidative protection, iron-related functions, cell envelope maintenance, amino acid and carbohydrate metabolism, antimicrobial resistance, and type III secretion system. We carried out allelic replacements for two nsSNPs in bioC (biotin synthesis) and pliC (lysozyme inhibition), and two INDELs in ftsK and yqiJ (DNA damage response) by homologous recombination, and these replacements did not alter the bacterial survival ability in egg whites. However, the bacterial survival ability in egg whites was reduced when deletion mutation of the genes bioC and pliC occurred. This study provides initial correlations between observed genotypes and phenotypes and serves as an important caveat for further functional studies.
Keywords: Salmonella Enteritidis, egg white, genome sequencing, genome comparison, survival
Introduction
Salmonella is a globally important foodborne bacterial pathogen. Poultry-derived products, particularly eggs and egg products, are the major vehicles for foodborne outbreaks of salmonellosis in humans (CDC, 2010, 2015, 2016, 2018a,b,c). Of more than 2600 serovars of Salmonella, Salmonella enterica serovar Enteritidis (Salmonella Enteritidis) is one of the leading serovars related with epidemics of salmonellosis (CDC, 2010, 2015; Martelli and Davies, 2012). Salmonella Enteritidis can contaminate eggs by two possible routes and may move into the egg white from the infected reproductive organs directly before eggshell formation and after laying via hen feces or storage environments (Guard-Petter, 2001; Messens et al., 2005; Martelli and Davies, 2012).
Egg white is a hostile environment for the survival of microorganisms including Salmonella Enteritidis (Guard-Petter, 2001; Gantois et al., 2009; Baron et al., 2016). Antibacterial properties of egg white include bacteriostatic and bactericidal components, an alkaline environment and nutritional restrictions including iron and biotin chelation (Guard-Petter, 2001; Gantois et al., 2009; Baron et al., 2016). In contrast, egg yolk is a suitable medium for growth of Salmonella Enteritidis (Bradshaw et al., 1990). Thus, an important precondition for prolonged contamination of an egg by Salmonella Enteritidis is the migration of bacterial cells from the egg white to the egg yolk. The rate of migration into the yolk is positively correlated with the concentration of Salmonella Enteritidis cells in the egg white (Braun and Fehlhaber, 1995). A greater cell number translates into a greater opportunity for contamination and for transfer to humans.
Salmonella Enteritidis exhibits higher survival ability in egg whites compared to other similar species or other Salmonella serovars (Clavijo et al., 2006; Gantois et al., 2008b; De Vylder et al., 2013). Remarkably, Salmonella Enteritidis strains differ in their survival abilities in egg whites, implying that some variants are better adapted to egg whites. The genomic variations responsible for these differences are an important issue to address but are unknown at present (Clavijo et al., 2006; Shah et al., 2012). DNA–DNA hybridization studies among Salmonella Enteritidis strains with significantly different phenotypes such as survival ability in egg whites or egg-contaminating ability could not distinguish these phenotypes genetically by this method (Morales et al., 2005; Yim et al., 2010; Shah et al., 2012). Whole-genome sequencing technology is a useful analytical tool to address these phenotypic differences and has a higher resolution.
In this study, we obtained two Salmonella Enteritidis isolates with similar genetic backgrounds but notably different survival rates in egg whites. Whole genome sequencing with high coverages was conducted to explore the genomic variations between the two strains. We analyzed potential genetic markers for differential survival abilities in egg whites. This study represents a new starting point for the exploration of the genomic evolution of Salmonella Enteritidis for egg contamination.
Materials and Methods
Bacterial Strains and Eggs
The Salmonella Enteritidis strains SJTUF10978 and SJTUF10984 were obtained from Shanghai Center for Disease Control and Prevention. They were isolated from chicken meat products from two different markets in Shanghai in 2010. The strains were preserved in -80°C and grown in Luria–Bertani (LB) broth (Oxoid, United Kingdom) at 37°C with shaking before use in assays. Unfertilized specific pathogen free (SPF) eggs were purchased from Beijing Merial Vital Laboratory Animal Technology Co., Ltd. (Beijing, China) and delivered to the lab within 3 days after laying. Thereafter, eggs were incubated at 37°C for an additional 3–5 days before use.
Survival of Salmonella Enteritidis in Egg Whites
Survival rate of Salmonella Enteritidis in egg whites was measured according to a previously published method with minor modifications (Lu et al., 2003). We randomly selected eight SPF eggs and sterilized the shells with 75% ethanol before cracking. The egg white was separated from yolk and then mixed and homogenized for 10 min in a blender (easyMIX Lab Blender, AES Chemunex, Bruz, France). The mixed egg whites and normal saline (NS) were separately added into a 96-well plate at 200 μL each per well. One milliliter of overnight bacterial cultures was centrifuged at 5,000 ×g for 5 min and the pellets resuspended in 1 mL NS. The suspension was diluted with NS, and 30 μL of diluted cultures were then added to the egg white as well as to the NS in the 96-well plate in three replicates for each solution with final cell concentrations of approximately 2 × 103 CFU/mL and mixed thoroughly. Portions (50 μL) of the bacteria–NS mixture from each well were plated on LB agar plates immediately (0 h). After 24 h incubation at 37°C, the whole volume of bacteria–egg whites mixture (230 μL) from each well was plated on LB agar plates (24 h). Colonies were enumerated after incubation at 37°C for 18 h. Survival rate of bacteria in egg whites was represented as ratio of the bacteria concentration in the 24-h sample compared to that of the 0-h sample.
Growth of Salmonella Enteritidis in LB used bacteria which was cultured and diluted as above. Thirty microliters of samples were added to 200 μL LB in 96-well plates in three replicates for each solution to a final concentration of approximately 2 × 103 CFU/mL. Final concentrations were determined by plate counting as described above.
Molecular Subtyping
Before genomic sequencing, strains SJTUF10978 and SJTUF10984 were analyzed by traditional typing methods. Pulsed-field gel electrophoresis (PFGE) was performed according to the procedures described in the standard operating procedure for PulseNet PFGE of Escherichia coli O157:H7, E. coli non-O157 (STEC), Salmonella serotypes, Shigella sonnei, and Shigella flexneri1. Multiple-locus variable number tandem repeat analysis (MLVA) was performed according to the procedures described in the PulseNet standard operating procedure for analysis of MLVA data of Salmonella Enteritidis in BioNumerics – Applied BioSystems Genetic Analyzer 3130/3500 data2. The MLVA profile was represented as a string of seven integers (VNTR1–VNTR2–VNTR8–VNTR6–VNTR5–VNTR3–VNTR9). The Salmonella sequence types (STs) were identified by uploading the chromosome sequences of the two strains to MLST version 1.8 (Larsen et al., 2012) with S. enterica as the MLST scheme.
Genome Sequencing, Assembly, Finishing, and Annotation
Genomic DNA was prepared from overnight cultures using the DNeasy Blood and Tissue Kit as described by the manufacturer (Qiagen, Hilden, Germany). Libraries were constructed according to the TruSeq DNA sample preparation guide and sequenced on the Illumina HiSeq 2000 platform (Illumina, San Diego, CA, United States) to generate 2 × 100 bp paired-end sequencing reads. The reads were statistically analyzed and quality evaluated by FastQC3. Adapter sequences and low-quality reads with average quality scores <20 were removed from the data by Trimmomatic (Bolger et al., 2014). Reads that were less than 50 bp after trimming were also excluded from further genome assembly. Clean reads were assembled using the CLC Workbench 5.5 (Germantown, MD, United States). Contigs were then reordered based on comparison to the chromosome (GenBank accession no. NC_011294.1) and the plasmid pSEN (GenBank accession no. HG970000.1) of the Salmonella Enteritidis strain P125109 using progressiveMauve, version 2.3.1 (Darling et al., 2010). Gaps in the genomes were closed by sequencing PCR amplicons containing overlapping contigs on each side. The complete chromosome and plasmid sequences of SJTUF10978 and SJTUF10984 were submitted to the GenBank database and annotated with the Prokaryotic Genome Annotation Pipeline (PGAP; Version 3.24) from the National Center for Biotechnology Information (NCBI).
Genomic Characterization and Comparison
Variations between SJTUF10978 and SJTUF10984 were identified by the combination of two methods: one was mapping reads to the reference genome (NC_011294.1 and HG970000.1) using the Burrows-Wheeler Aligner (BWA, version 0.7.5a; Li and Durbin, 2009) and calling single nucleotide polymorphisms (SNPs) and small insertions or deletions (INDELs) with SAMtools (version 1.1; Mpileup/Bcftools; Li et al., 2009); another was comparing the complete genome sequences of SJTUF10978 and SJTUF10984 by progressiveMauve (Darling et al., 2010) and BLAST5. Variations were validated by mapping the sequencing reads back to the complete genomes of the two strains using Bowtie2 (Langmead and Salzberg, 2012). Genes carrying variations were named after their homologues reported in Salmonella or other bacterial species with close genetic relationships. For genes not mentioned in previous studies, the old locus tags (started by “SEN”) in the genome of Salmonella Enteritidis P125109 were used. The list of the variant genes with amino acid changes in the coding proteins were input into the PANTHER (Protein ANalysis THrough Evolutionary Relationships) database (Mi et al., 2017) for the overrepresentation test using Salmonella Typhimurium as the reference list. The significance of the result was assessed using Fisher’s Exact with FDR multiple test correction provided by this database. The non-synonymous SNPs (nsSNPs) were also analyzed by PROVEAN (Protein Variation Effect Analyzer; Choi et al., 2012; Choi and Chan, 2015), SIFT (Sim et al., 2012), PolyPhen-2 (Adzhubei et al., 2010), and SNAP2 (Hecht et al., 2015), to predict whether an amino acid substitution has an impact on the function of the coding protein. ResFinder 3.0 (Zankari et al., 2012) was used to screen antibiotic resistance genes of the two strains with a filter standard of 90% identity and 60% minimum length.
Construction of Mutants and Growth Conditions
An in-frame deletion of the bioC gene in strains SJTUF10978 and SJTUF10984 were generated by overlap extension PCR (Ho et al., 1989). A 543 bp of fragment containing the upstream region of bioC and a 566-bp fragment containing the downstream region were generated from SJTUF10978 genomic DNA with primers bioC-UpF plus bioC-UpR and bioC-DnF plus bioC-DnR. A 10-bp overlap in the primers bioC-UpR and bioC-DnF allowed amplification of an 1109-bp product by a second PCR with primers bioC-UpF and bioC-DnR. The resulting PCR product, representing a deletion of the 76–708 bp region of the bioC gene, was then treated with T4 polynucleotide kinase and ligated into the suicide plasmid pRE112 which had been digested with SmaI and dephosphorylated. The pRE112 contained the counter-selectable marker sacB1 as well as a chloramphenicol cassette. The recombinant plasmid was transformed into E. coli SM10 λpir and transformants (SM10-pREΔbioC) were recovered on LB agar supplemented with chloramphenicol. Plasmid pREΔbioC was transferred into the wide-type strains SJTUF10978 and SJTUF10984 by conjugation. The first crossover mutants with the pREΔbioC integrated into the chromosome were enriched in Selenite Cystine Broth (Beijing Land Bridge Technology, Beijing, China) containing chloramphenicol and screened on Xylose Lysine Deoxycholate Agar (Beijing Land Bridge Technology, Beijing, China) containing chloramphenicol. The first crossover mutants were then cultivated in LB with 10% (w/v) sucrose for 48 h to allow the second recombination and the loss of the suicide plasmid. The cultures were then plated on LB agar without NaCl but containing 10% (w/v) sucrose. Finally, the mutants (HΔbioC and LΔbioC, derivatives of SJTUF10978 and SJTUF10984, respectively) were negatively selected by chloramphenicol. This was confirmed by Sanger sequencing of PCR-amplified fragments. The in-frame deletion of the pliC gene in SJTUF10978 and SJTUF10984 (HΔpliC and LΔpliC, respectively) were generated using the same method as above. The double deletion of the bioC and pliC genes in SJTUF10978 was generated by introducing a pliC deletion in HΔbioC. For the substitution of the single nucleotide in the position 433 from G to T in the bioC gene in SJTUF10978 (HbioCA145S), the method was almost the same as that in the deletion mutations described above except for the first step. That is, the recombinant was constructed by direct amplification of 433T along with the flanking regions of the pliC gene using primers bioC-UpF and bioC-DnR and genomic DNA of SJTUF10984 as template. The product was ligated into plasmid pRE112. Similarly, the first step for the substitution of the single nucleotide in the position 433 from T to G in the bioC gene in SJTUF10984 (LbioCS145A) was direct amplification of 433G along with the flanking regions of bioC with primers bioC-UpF and bioC-DnR and using the genomic DNA of SJTUF10978 as the template. The product was ligated to the plasmid pRE112. The methods for the exchange of the variant sequences contained within the three genes (pliC, ftsK, and yqiJ) between SJTUF10978 and SJTUF10984 were all similar to that for the bioC gene. The primers used in the construction of mutants are shown in Supplementary Tables S3 and S4. Mutant information is presented in Table 3. In this study, the concentration of chloramphenicol was 35 μg/mL and the growth temperature for strains was 37°C.
Table 3.
Bacterial strains and plasmids used for mutant construction.
| Bacterial strains/plasmid | Characteristics | Source |
|---|---|---|
| Wild-type and mutants of SJTUF10978 | ||
| Hwt | Salmonella Enteritidis SJTUF10978, wide-type, high survival in egg whites | Chicken meat |
| HΔbioC | In-frame deletion of bioC in SJTUF10978 | This study |
| HΔpliC | In-frame deletion of pliC in SJTUF10978 | This study |
| HΔbioCΔpliC | Double in-frame deletion of bioC pliC in SJTUF10978 | This study |
| HbioCA145S | G433T substitution in bioC in SJTUF10978 | This study |
| HpliCV47A | T140C substitution in pliC in SJTUF10978 | This study |
| H-mut-ftsK | Partial deletion (C2269_G2352) in ftsK in SJTUF10978 | This study |
| H-mut-yqiJ | Gain-of-function mutation of yqiJ at position 23 (insertion of A23_T35 from SJTUF10984 yqiJ) in SJTUF10978 | This study |
| Wild-type and mutants of SJTUF10984 | ||
| Lwt | Salmonella Enteritidis SJTUF10984, wide-type, low survival in egg whites | Chicken meat |
| LΔbioC | In-frame deletion of bioC in SJTUF10984 | This study |
| LΔpliC | In-frame deletion of pliC in SJTUF10984 | This study |
| LbioCS145A | T433G substitution in bioC in SJTUF10984 | This study |
| LpliCA47V | C140T substitution in pliC in SJTUF10984 | This study |
| L-mut-ftsK | Insertion (C2269_G2352 from SJTUF10978 ftsK) at position 2269 in ftsK in SJTUF10984 | This study |
| L-mut-yqiJ | Pseudogenization mutation of yqiJ (A23_T35 deletion) in SJTUF10984 | This study |
| SM10 λpir | Escherichia coli, thi thr-1 leu6 proA2 his-4 arg E2 lacY1 galK2, ara14 xyl5 supE44, λpir | (Rubires et al., 1997) |
| pRE112 | pGP704 suicide plasmid, pir dependent, oriT, oriV, sacB, Cmr | (Edwards et al., 1998) |
Results and Discussion
Determination of Bacterial Survival Rate in Egg Whites
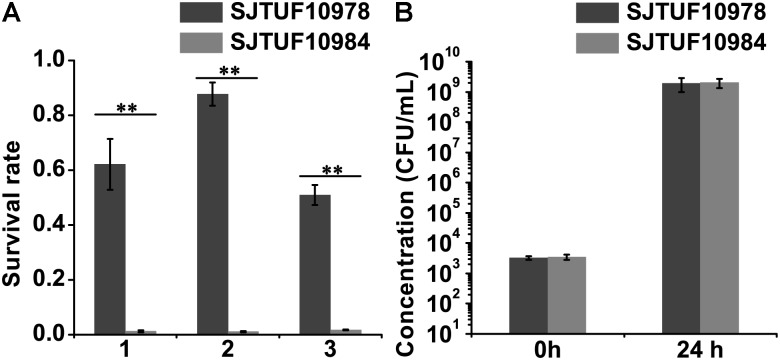
The survival rate of SJTUF10978 was significantly higher (52-fold on average) than SJTUF10984 after incubating in egg whites at 37°C for 24 h (Figure 1A). However, we found no growth differences in LB broth between the two isolates (Figure 1B). These data indicated that the higher survival ability of SJTUF10978 in egg whites was not due to general growth differences between the strains.
FIGURE 1.
Survival of Salmonella Enteritidis strains SJTUF10978 and SJTUF10984 in egg whites. (A) The survival rate represents the ratio of surviving number of bacteria after 24-h incubation to the 0-h incubation in egg whites at 37°C. Results from three experiments performed in triplicate for each were shown. After incubation, SJTUF10978 displayed significantly higher survival rates than SJTUF10984 with 62.1:1.3, 87.7:1.1, and 50.9:1.8%, respectively, in three experiments using the egg whites from different patches of SPF eggs. (B) Growth in LB as control. The means ± standard deviations of results from three experiments performed in triplicate for each were shown. ∗∗p < 0.01, two-tailed t-test.
General Genome Features
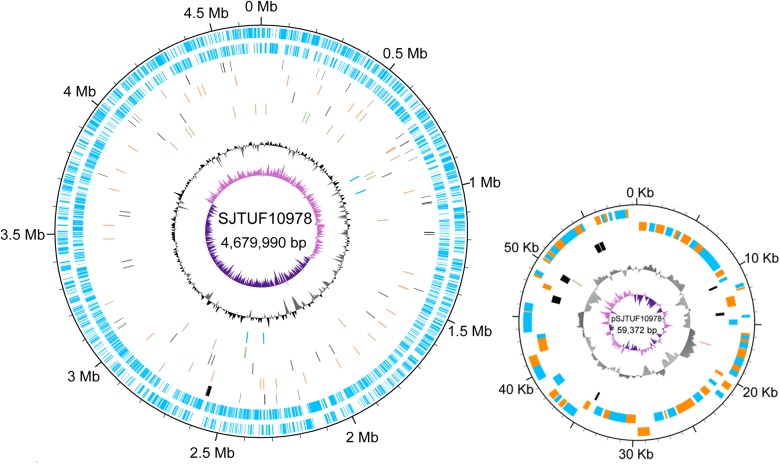
High-throughput sequencing generated ∼1 GB of data with a 327-depth coverage for SJTUF10978 and a 234-depth coverage for SJTUF10984. The de novo assembly yielded 42 contigs with an N50 of 371,755 bp for SJTUF10978 and 44 contigs with an N50 of 277,421 bp for SJTUF10984. All contigs in these two genomes were successfully mapped to the reference genome with small variations in the alignments except for a deletion of a 5,998-bp fragment in both genomes compared to the reference genome of Salmonella Enteritidis strain P125109. Gap closure was accomplished and the complete genome sequence of SJTUF10978 was comprised of a 4,679,990-bp chromosome and a 59,372-bp plasmid. Similarly, the complete genome sequence of SJTUF10984 was comprised of a 4,679,791-bp chromosome and a 59,371-bp plasmid (Figure 2 and Table 1). Both genomes have a GC content of 52.2%, comparable to the average GC content (52.1%) of S. enterica genomes reported in NCBI6. Both strains belong to the Salmonella ST 11 (ST11, alleles: aroc-5/dnan-2/hemd-3/hisd-7/pure-6/suca-6/thra-11). In addition, the same PFGE (XbaI) pattern and similar MLVA profiles: 5-4-1-10-11-3-3 in SJTUF10978 and 4-4-1-10-11-3-3 in SJTUF10984 were revealed before genome sequencing. None of the antimicrobial resistance genes in the Resfinder database (Zankari et al., 2012) was found in the two genomes.
FIGURE 2.
Circular map and genetic features of Salmonella Enteritidis strain SJTUF10978 relative to strain SJTUF10984. For the figure of the chromosome, light blue bars (first and second rings) indicate the coding sequences on the positive and negative strands, respectively. Orange, gray, green, and blue bars (fourth, fifth, sixth, and seventh rings) denote the non-synonymous SNPs, synonymous SNPs, intergenic SNPs, and INDELs between SJTUF10978 and SJTUF10984, respectively. For the figure of the plasmid of SJTUF10978, arcs in the first and second rings represent coding sequences on the positive and negative strands, respectively, and blue and orange were used to distinguish neighboring genes. The orange and green bars in the fourth ring indicate a non-synonymous SNP and an INDEL between plasmids of SJTUF10978 and SJTUF10984. For both figures, black bars (third ring) indicate pseudogenes and the black/gray and dark purple/light purple plots display GC content and GC skew ([G+C]/[G–C]), respectively (window size 10,000 bp and step size 200 bp for the chromosome, window size 500 bp, and step size 20 bp for the plasmid). The atlas was created using the Plotrix package (version 3.6-6; Lemon, 2006) within R (version 3.2.5; R Development Core Team, 2015).
Table 1.
Genomic characteristics of two Salmonella Enteritidis strains with different survival abilities in egg whites.
| SJTUF10978 | SJTUF10984 | |
|---|---|---|
| Chromosome | 4,679,990 bp | 4,679,791 bp |
| Plasmid | 59,372 bp | 59,371 bp |
| (G+C) % | 52.20% | 52.20% |
| Genes (total) | 4,854 | 4,855 |
| rRNAs | 8, 7, 7 (5S, 16S, 23S) | 8, 7, 7 (5S, 16S, 23S) |
| Pseudogenes | 183 | 181 |
| CRISPR Arrays | 2 | 2 |
| MLST | ST11 | ST11 |
| MLVA | 5-4-1-10-11-3-3 | 4-4-1-10-11-3-3 |
The 5,998-bp deletion in the two genomes compared with the P125109 reference genome was further confirmed by checking the coverage of reads mapped onto the reference genome and comparisons using progressiveMauve (Darling et al., 2010). Interestingly, this deletion was identified in only 15 Salmonella genomes by the Microbial Genomes BLAST tools in NCBI (April 18, 2018). These strains were all identified as the Enteritidis serovar by submitting their genome sequences to the SeqSero web server (Zhang et al., 2015) although the NZ_CP019383.1 was labeled as Salmonella Typhimurium by the data submitter. Of these, 12 strains were isolated from Asia (five from China, five from South Korea, and two from Thailand) and implied that the evolutionary similarity between these 12 isolates and the two isolates in our study might be due to geographical proximity.
The 5,998-bp region in the reference genome NC_011294.1 encoded a DNA methyltransferase (part of SEN4286) and restriction endonucleases (SEN4287, SEN_RS22295, SEN4291, SEN_RS22305, and part of SEN4292). Notably, the SEN4287 gene in strain SE2472 isolated from California in 1997 (Lu et al., 1999) was previously reported to have a positive effect on the survival rate of Salmonella Enteritidis in egg whites (Clavijo et al., 2006). As we were unable to compare the survival rate of SE2472 with the high survival strain SJTUF10978 in this study, we suspect that these strains may have comparable survival phenotypes in egg whites (Clavijo et al., 2006). If so, Salmonella Enteritidis strains from different areas in the world may have gone through a particularly adaptive evolution for survival in egg whites. However, if this was not the case, there must be genes other than SEN4287 that caused differential survival ability in egg whites for the two closely genetic strains we used in the present study.
Whole genome alignment of the two genomes revealed that both the chromosomes and plasmids were collinear with strong consistency in gene arrangements and > 99% shared similarity in nucleotide sequences. Genome annotation identified 4,854 genes in the genome of SJTUF10978 and 4,855 genes in the genome of SJTUF10984 (Table 1 and Figure 2). Moreover, there were 183 pseudogenes in the genome of SJTUF10978 and 181 in SJTUF10984 (Table 1 and Figure 2). Among these, 180 were present in both genomes with identical sequences while four pseudogenes only existed in one of the two genomes (sseL, yqiJ, and ratA specific to SJTUF10978; menH specific to SJTUF10984; Table 2 and Figure 2).
Table 2.
INDELs and nsSNPs in coding sequences between SJTUF10978 and SJTUF10984.
| Mutation type | Sequence change |
Gene symbol | Predicted function | |
|---|---|---|---|---|
| Nucleotide | Amino acid | |||
| Lysozyme inhibition | ||||
| nsSNP | C140T | A47V | pliC | Lysozyme inhibitor |
| Vitamin biosynthesis | ||||
| nsSNP | G1427A | G476D | ushA | Bifunctional UDP-sugar hydrolase |
| nsSNP | T433G | S145A | bioC | Biotin biosynthesis protein |
| nsSNP | T484C | X162Q | menH | 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase |
| Cell division and DNA damage response | ||||
| INDEL | C2269_G2352ins | P757_Q784ins | ftsK | DNA translocase |
| INDEL | A23_T35del | frameshifted | yqiJ | Predicted inner membrane protein |
| nsSNP | C1249A | Q417K | envC | Murein hydrolase activator |
| Osmotic and oxidative protection | ||||
| INDEL | A157_G276ins | V53_T92ins | ybiO | Mechanosensitive channel protein |
| nsSNP | C847G | Q283E | envZ | Osmolarity sensor protein |
| nsSNP | A19T | I7F | tpx | Thioredoxin-dependent thiol peroxidase |
| nsSNP | G486T | L162F | yccR | Crp/Fnr family transcriptional regulator |
| Iron-related functions | ||||
| nsSNP | T562C | Y188H | ydiU | SELO (selenoprotein O) family protein |
| nsSNP | C907T | L303F | sdaB | L-serine deaminase II |
| nsSNP | A629G | Y210C | dmsA | Dimethyl sulfoxide reductase subunit A |
| nsSNP | G916A | V306I | cfa | Cyclopropane fatty acyl phospholipid synthase |
| nsSNP | G931A | V311I | miaA | tRNA dimethylallyltransferase |
| Maintenance of cell envelope structure | ||||
| nsSNP | T338C | L113P | wecD | TDP-fucosamine acetyltransferase |
| nsSNP | G113T | G38V | fimI | Major pilin protein |
| nsSNP | T535C | S179P | stbE | Fimbrial chaperone protein |
| nsSNP | A603G | I201M | yejM | Sulfatase |
| nsSNP | G775A | A259T | ybiS | L,D-transpeptidase |
| nsSNP | G355T | A119S | ycdX | Phosphatase / putative hydrolase |
| nsSNP | T79A | C27S | yfaX | Transcriptional regulator |
| nsSNP | T1342C | C448R | cpsG | Phosphomannomutase |
| Amino acid and carbohydrate metabolism | ||||
| nsSNP | T166A | C56S | speD | S-adenosylmethionine decarboxylase |
| nsSNP | A1290T | E430D | hutH | Histidine ammonia-lyase |
| nsSNP | G455A | S152N | SEN2589 | Gluconolactonase |
| nsSNP | C614T | P205L | dsdX | D-serine permease |
| nsSNP | T382C | C128R | ugpE | Glycerol-3-phosphate transporter |
| nsSNP | T391C | W131R | SEN4302 | Phosphotransferase enzyme II C component |
| nsSNP | A1583G | E528G | actP | Acetate permease |
| Antimicrobial resistance | ||||
| nsSNP | A229C | M77L | sugE | Multidrug efflux system protein |
| nsSNP | C287G | A96G | ramR | Transcriptional regulator |
| Solitary genes | ||||
| INDEL | C579ins | frameshifted | sseL | Deubiquitinase |
| nsSNP | T932A | L311H | SEN1428 | Metal hydrolase |
| nsSNP | C1184T | A395V | SEN1429 | Carboxylesterase |
| nsSNP | C1477T | Q493X | ratA | Outer membrane protein |
| nsSNP | T541G | S181A | rsdB | Resolvase |
Sequence changes from SJTUF10984 to SJTUF10978; “X” in column 3, premature stop codon.
Genetic Variations Between SJTUF10978 and SJTUF10984
A complete genome comparison revealed 76 variations including six INDELs and 70 SNPs between SJTUF10978 and SJTUF10984 (Supplementary Table S1 and Figure 2). Four INDELs were located in coding regions in the chromosome and two were located in intergenic regions in the chromosome and the plasmid respectively. Of 70 SNPs observed in the two genomes, 34 were non-synonymous, 18 synonymous, and 18 intergenic. The coverage (average is shown for the INDELs) of the sequencing reads mapped to each variable site ranged from 88 to 465 (Supplementary Table S1). None of the synonymous and intergenic SNPs nor the two intergenic INDELs were considered. However, we cannot rule out the possibility that alterations of codon usage, regulatory sequences, or small RNAs have phenotypic effects.
The 38 variants, including 34 nsSNPs and four INDELs, were identified in the coding regions of 38 genes. Using these 38 genes as input, no gene was identified to be significantly overrepresented by the overrepresentation test in the PANTHER database (Mi et al., 2017). Manual functional analysis showed that these variant genes were involved in lysozyme inhibition, vitamin biosynthesis, cell division and DNA damage response, osmotic and oxidative protection, iron-related functions, maintenance of cell envelope structure, amino acid and carbohydrate metabolism, antimicrobial resistance, and type III secretion system (T3SS).
The SNP in pliC Is Not Responsible for the Differential Survival Ability in Egg Whites
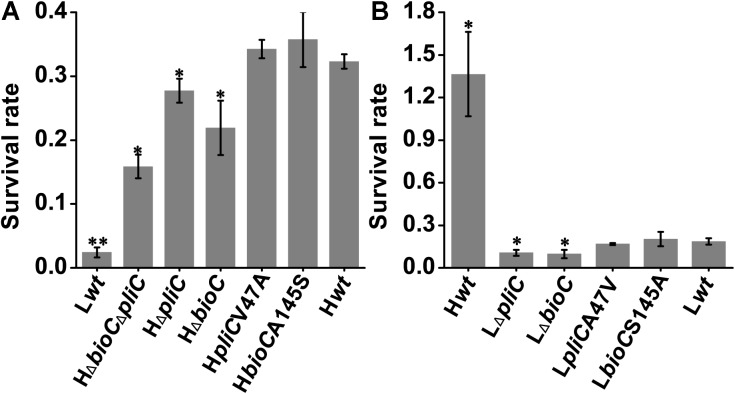
A missense variation (C140T, A47V) was found in the pliC gene in the genome comparisons (Table 2). This gene encodes a periplasmic lysozyme inhibitor of c-type lysozyme that is abundant in egg whites. C-type lysozyme is bacteriolytic and protects eggs against bacterial penetration. The probable antibacterial mechanism of this protein is peptidoglycan hydrolysis rendering the bacterial cell susceptible to osmotic lysis, or disturbing bacterial membrane structure due to the cationic and hydrophobic properties of this protein (Pellegrini et al., 2000; Ibrahim et al., 2001; Callewaert et al., 2008). In response, Salmonella Enteritidis is equipped with two kinds of lysozyme inhibitors, a periplasmic lysozyme inhibitor of c-type lysozyme (PliC) and a membrane-bound lysozyme inhibitor of c-type lysozyme (MliC; Callewaert et al., 2008). The nsSNP in pliC was predicted in silico to be deleterious for the coding protein (Supplementary Table S2). When we exchanged the SNP alleles in the pliC gene between the two strains, we found no significant changes of survival ability in egg whites (Figure 3). However, pliC gene deletion did result in decreased survival ability in egg whites for both isolates (Figure 3). This result was consistent with a Salmonella Enteritidis pliC knockout mutant that lost its in vitro inhibitory activity against lysozyme and showed increased lysozyme sensitivity (Callewaert et al., 2008).
FIGURE 3.
Survival of bioC and pliC mutants in egg whites. The survival rates of bacteria represented as the ratio of the concentration of bacteria at 24-h/0-h samples incubated at 37°C with starting inocula of approximately (A) 2 × 103 and (B) 2 × 104 CFU/mL (the initial concentration increased by an order of magnitude here because the survival rates of SJTUF10984 and its mutants in egg whites were too low to distinguish between each other when the inoculum concentration was 2 × 103 CFU/mL). Survival rate is shown as the mean ± standard deviation. Experiments were repeated three times and results from one representative experiment were presented. ∗p < 0.05 and ∗∗p < 0.01, two-tailed t-test.
Variant Genes Involved in Vitamin Biosynthesis
In this study, nsSNPs were found in three genes encoding enzymes for vitamins biosynthesis. They were in the menH (premature stop codon), bioC (missense variation), and ushA (missense variation) genes involved in the biosynthesis of menaquinone, biotin, and nicotinamide, respectively. Vitamins are important enzymatic cofactors used in basic metabolism (Begley et al., 2001; Beckett, 2007; Jiang et al., 2008). The menH gene encodes a provisional 2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase involved in menaquinone biosynthesis (Jiang et al., 2008). This gene had evolved into a pseudogene in the low survival strain SJTUF10984. Interestingly, this genotype could not be found in the NCBI database suggesting that the degradation of the menH gene may have a non-negligible effect on the phenotype of this isolate. Moreover, the menaquinone content in egg white is low (0.9 μg/100 g; Schurgers and Vermeer, 2000). Based on these observations, it was suggested that menH might be involved in survival in egg whites if menaquinone was growth limiting.
The malonyl-CoA O-methyltransferase encoding gene bioC is involved in biotin synthesis. This biological process refers to a series of enzymes encoded by the bioBFCD operon and the bioA and bioH genes (Beckett, 2007). Avidin is an extremely high affinity biotin binding protein and makes egg whites biotin limiting (Baron et al., 2016). The biotin synthase gene bioB is required for the survival of Salmonella Enteritidis in egg whites (Raspoet et al., 2014). Moreover, bioA, B, C, D, and F are transcriptionally upregulated in Salmonella Enteritidis during growth in whole egg or egg white model medium (Jakociune et al., 2016; Baron et al., 2017). In our study, the single nucleotide variation (T433G, S145A) in the bioC gene was predicted to be deleterious in silico and suggested that this nsSNP had drastic functional consequences (Supplementary Table S2). Moreover, we found this same genotype in seven sequences in the NCBI database as in SJTUF10978, but 820 were identical to SJTUF10984. This indicates the particularity of BioC 145A in SJTUF10978 evolution.
To determine whether the nsSNP in bioC resulted in differential survival abilities in egg whites, the SNP alleles in bioC were exchanged between SJTUF10978 and SJTUF10984. However, we found no significant changes of survival rate (p > 0.05) in egg whites (Figure 3). However, bioC null mutants of SJTUF10978 and SJTUF10984 exhibited decreased survival rates in comparison with their respective parent strains (Figure 3). This demonstrates that bioC plays an important role in the survival of Salmonella Enteritidis in egg whites.
We also found growth defects when these two mutants were cultured in M9 minimal medium (data not shown). This is consistent with a growth deficiency for the E. coli bioC null mutant in the same medium (Del Campillo-Campbell et al., 1967). In addition, bioC pliC double deletions made SJTUF10978 more sensitive to egg whites than either single deletions (Figure 3A). This supports the hypothesis that Salmonella Enteritidis has developed a synergetic strategy to cope with the multicomponent bactericidal effects in egg whites (Baron et al., 2016). However, the comprehensive mechanism is yet unclear. Based on the above, the bio operon plays an essential role in biotin synthesis to satisfy the growth metabolism demand of Salmonella Enteritidis when encountering the biotin-limited environment in egg whites.
It was unexpected that the SNPs in bioC and pliC did not alter the survival ability although deletions in the two genes reduced the survival ability of Salmonella Enteritidis in egg whites. In fact, the transcription of the two genes were upregulated in egg whites compared with normal medium (data to be published), which further confirmed the role of the two genes in the bacterial survival in egg whites. We thought that there were two possible reasons why the SNPs in the two genes had no effect on the survival ability of the two strains in egg whites. First, the amino acids encoded by codons containing the SNPs are probably not located in the region(s) playing a key role in protein function (conserved regions of PliC: 71–78 and 87–93 and putatively conserved methyltransferase domain of BioC: 47–138, pfam08241; Callewaert et al., 2012; Marchler-Bauer et al., 2017). Second, it might be the synergistic effect of multi-site variation that led to the survival difference of Salmonella Enteritidis in egg whites. The effect of one single site may be very weak and may even have been masked by experimental error.
Variant Genes Involved in Cell Division and DNA Damage Response
In SJTUF10984, we identified an 84-bp deletion in ftsK that was not present in SJTUF10978, resulting in a 28-amino acid deletion (Table 2). FtsK is a DNA translocase playing a key role in chromosome segregation and consists of an N-terminal transmembrane domain, a proline/glutamine rich linker, and a C-terminal P-loop ATPase motif (Iyer et al., 2004). The 28-amino acid deletion removed 2/9 tandem repeats in the linker domain, predicted by the Tandem Repeats Finder (Benson, 1999). Comparison of different FtsK proteins shows variability in the linker domain (Massey et al., 2006). The linker is necessary for full activity of chromosome dimer resolution in E. coli (Bigot et al., 2004). FtsK levels increase during cell division and in response to DNA damage (Diez et al., 1997, 2000; Wang and Lutkenhaus, 1998). In addition, ftsK expression is regulated by ppGpp of the stringent response during nutritional deprivation (Diez et al., 2000).
In the yqiJ gene of SJTUF10978, a 13-bp insertion introduced a premature stop codon (Table 2). Notably, we did not find any identical sequences for this gene from SJTUF10978 in the NCBI database. This gene encodes a putative transmembrane protein with unknown function but is predicted to be involved in the cellular response to DNA damage in E. coli (Khil and Camerini-Otero, 2002).
Salmonella Enteritidis suffers nutrient limitation and DNA damage during egg white exposure (Lu et al., 2003; Baron et al., 2016). In this context, it was of interest to see if the ftsK and yqiJ INDELs would lead to the differences of survival ability in egg whites. Therefore, the ftsK and yqiJ alleles were exchanged between the two strains but we found no significant change (p > 0.05) of survival ability in egg whites between the mutants and the wild type strains (data not shown). This essentially rules out a functional significance for the ftsK and yqiJ INDELs for the bacterial survival in egg whites. It is speculated that there may be no difference in DNA damage repair ability between the two strains.
The envC gene is also involved in cell division (Hara et al., 2002; Heidrich et al., 2002) and we identified an nsSNP in this gene between the two isolates. The envC gene encodes a murein hydrolase activator and its deletion results in the formation of long filaments and higher lysozyme sensitivity (Hara et al., 2002; Heidrich et al., 2002). The nsSNP in envC was predicted in silico to have no effect on the protein, implying a lack of relevance with the bacterial survival ability in egg whites (Supplementary Table S2).
Variant Genes in Response to Osmotic and Oxidative Stress
In SJTUF10984, an nsSNP and a 120-bp deletion were identified in the envZ and ybiO genes, respectively (Table 2). Proteins encoded by the two genes are necessary for the bacterial response to osmotic challenges (Aiba et al., 1989; Edwards et al., 2012). Moreover, the nsSNP in envZ was predicted to affect protein function using in silico analysis (Supplementary Table S2). To accommodate a wide range of hosts and natural environments, bacteria have developed mechanisms to cope with osmotic stress to maintain cellular homeostasis (Wood, 2015). The shift from other environments to egg whites would probably bring changes of osmotic pressure for Salmonella Enteritidis. EnvZ is a sensory histidine kinase in the two-component regulatory system EnvZ-OmpR involved in osmoregulation through transcriptional control of the porin genes ompF and ompC (Aiba et al., 1989; Forst et al., 1989; Lan and Igo, 1998). EnvZ-OmpR system is also an important regulator of global gene expression of Salmonella Enteritidis in the egg white model medium (Baron et al., 2017). The ybiO gene encodes a putative mechanosensitive channel involved in osmotic adaption that is activated by hypo-osmotic shock (Edwards et al., 2012). Similarly, in a previous study, another mechanosensitive channel gene (SEN3892) is shown to be involved in the survival of Salmonella Enteritidis in egg whites suggesting it may function in the hypo-osmotic environment of egg whites (Clavijo et al., 2006). Conversely, in another study, the osmolarity of egg whites is shown to be slightly lower than that of Tryptone Soy Broth and is thought to be isosmotic with the cytoplasm of Salmonella Enteritidis (Baron et al., 2016). Based on these observations, further analysis is needed to confirm whether the variations in ybiO and envZ are responsible for the differential survival of Salmonella Enteritidis strains in egg whites. Two missense variations (nsSNPs) were found in the thiol peroxidase gene tpx and the Crp/Fnr family transcriptional regulator gene yccR (Table 2). The two genes are involved in oxidative stress and nutrient deprivation responses, respectively.
Iron-Related Variant Genes
Five genes possessing single nsSNPs for each between the two isolates (Table 2) were predicted to either encode iron-containing proteins or be regulons of iron-related transcriptional regulators. These genes include the SELO (selenoprotein O) family protein gene ydiU, the L-serine deaminase II gene sdaB, the dimethyl sulfoxide reductase gene dmsA, the cyclopropane fatty acyl phospholipid synthase gene cfa, and the tRNA delta (2)-isopentenylpyrophosphate transferase gene miaA. Of these, ydiU is upregulated by the iron–sulfur cluster regulator IscR in E. coli (Giel et al., 2006; Dudkiewicz et al., 2012) and possibly involved in the iron limitation response (Outten et al., 2004; Giel et al., 2006; Wada et al., 2011). Both dmsA and cfa are regulated by Fur (ferric uptake regulator), a transcriptional regulator that controls the iron homeostasis in bacteria (Troxell et al., 2011). In addition, iron is a necessary component for DmsA and SdaB function (Su and Newman, 1991; Troxell et al., 2011). Similarly, transcriptional changes of dmsA and the L-serine deaminase I gene sdaA (similar function with sdaB) has been observed in Salmonella Enteritidis exposed to the egg white model medium (Baron et al., 2017). MiaA-catalyzed tRNA modifications are most likely involved in siderophore (enterobactin) biosynthesis (Buck and Griffiths, 1982).
Iron restriction is a key factor in the antimicrobial activities of egg whites (Guard-Petter, 2001; Gantois et al., 2009; Baron et al., 2016). In this study, although no sequence differences were found in genes directly encoding siderophores or transporters for iron acquisition, five variant genes were involved in the response to iron limitation. Moreover, deleterious effects were inferred for the nsSNPs in ydiU, sdaB, and dmsA by in silico analysis (Supplementary Table S2). This implies that our two strains possess differential adaptive capacity to iron restriction in egg whites.
Variant Genes Involved in the Maintenance of Cell Envelope Structure
We identified eight nsSNPs distributed in the genes wecD, fimI, stbE, yejM, ybiS, ycdX, yfaX, and cpsG, all of which were involved in the maintenance of cell envelope structure (Table 2). The wecD gene encodes a TDP-fucosamine acetyltransferase required in the synthesis of the enterobacterial common antigen, a glycolipid in the external leaflet of the outer membrane (Ramos-Morales et al., 2003). The fimI gene is located in the type I fimbrial operon and encodes the type I fimbrial protein subunit (Rossolini et al., 1993). The stbE gene is predicted to encode a fimbrial assembly chaperone (Lindberg et al., 1989). The yejM gene specifying an inner membrane protein with sulfatase/phosphatase activity is proven essential in E. coli. The truncated mutation of the YejM protein causes increased permeability of the outer membrane and reduced lipid A synthesis (De Lay and Cronan, 2008). The ybiS gene encodes a provisional L, D-transpeptidase that catalyzes the covalent anchoring of the Braun lipoprotein to the peptidoglycan in E. coli (Magnet et al., 2007). The Braun lipoprotein contributes to the integrity of the outer envelope structure by connecting the outer membrane to peptidoglycan (Braun, 1975). The ycdX gene is predicted to encode a phosphatase and its mutation in E. coli conferred a swarming defect (Inoue et al., 2007). The yfaX gene (alias rhmR) encodes a putative DNA-binding transcriptional regulator for the rhm operon involved in L-rhamnose metabolism (Rodionova et al., 2013). Rhamnose is one of four stoichiometric sugars in the O repeat unit of LPS and rhamnose content is positively correlated with the molecular mass of LPS. The latter is further associated with Salmonella Enteritidis host infection and egg contamination phenotypes (Guard-Petter et al., 1999; Guard-Bouldin et al., 2004). The cpsG gene specifying the phosphomannomutase is involved in capsular polysaccharide biosynthesis (Stevenson et al., 1991).
Functional prediction by in silico analysis of the effects of these eight nsSNPs indicated that six were deleterious (Supplementary Table S2). Egg white lysozyme targets peptidoglycan and its action would enhance outer membrane permeability (Callewaert et al., 2008). Moreover, LPS and flagellin are needed for Salmonella Enteritidis to respond to the bactericidal effects of egg whites (Cogan et al., 2004; Clavijo et al., 2006; Gantois et al., 2008a). Thus, we speculate that the genes in this category play roles in cell wall repair in face of lysozyme destruction (yejM and ybiS), and for the maintenance of cell wall integrity and full functions of cellular appendages (wecD, fimI, stbE, and yfaX) for bacterial survival in egg whites.
Variant Genes Responsible for Amino Acid and Carbohydrate Metabolism
Seven nsSNPs were found in genes involved in amino acid and carbohydrate metabolism. Three of these genes are the S-adenosylmethionine decarboxylase gene speD (Xie et al., 1989), the histidine ammonia-lyase gene hutH (Meiss et al., 1969), and the gluconolactonase gene SEN2589. We also identified four transporter genes (dsdX, ugpE, SEN4302, and actP) encoding a D-serine transporter (Anfora and Welch, 2006), a glycerol-3-phosphate transporter (Yang et al., 2009), a putative sugar PTS (phosphotransferase system) permease, and an acetate transporter (Gimenez et al., 2003), respectively (Table 2). Previous studies have reported that amino acid and carbohydrate metabolism is linked to the survival phenotype of Salmonella Enteritidis in egg whites determined by transposon mutant library screening (Clavijo et al., 2006) and microarray analysis (Baron et al., 2017). These studies suggested that the two isolates might have differential adaptabilities to the nutrient restriction in egg whites.
Variant Genes Involved in Antimicrobial Resistance
Two genes possessing nsSNPs were involved in antimicrobial resistance (Table 2). The sugE gene encodes for a small multidrug resistance transporter (Chung and Saier, 2002; He et al., 2011). The ramR gene encodes a transcriptional regulator that negatively regulates the expression of the AcrAB efflux system via ramA. This system is involved in resistance to antibiotics from multiple classes (Abouzeed et al., 2008; O’Regan et al., 2009). The analysis of antibiotic resistance to 10 common antibiotics was carried out for the two strains but we did not observe any resistance phenotypes (data not shown); we therefore do not know the roles of these two multidrug transporters in the two strains.
Solitary Genes
The 579C insertion in the sseL gene resulted in a premature stop in SJTUF10978 compared with SJTUF10984 (Table 2). This gene encodes a deubiquitinating enzyme and is a translocated effector of the type T3SS encoded within Salmonella pathogenicity island-2 (SPI2; Coombes et al., 2007). Studies on SseL (Salmonella secreted factor L) function focused on its deubiquitination activity when interfering with ubiquitination pathways in hosts. This enzyme has been identified as a host colonization and cytotoxicity factor and was also involved in the regulation of lipid metabolism in infected host cells (Rytkonen et al., 2007; Mesquita et al., 2012). Similarly, a T3SS encoded within SPI1 is previously identified as a necessary factor for the survival of Salmonella Enteritidis in egg whites (Clavijo et al., 2006), and is downregulated in the egg white model medium (Baron et al., 2017) and upregulated in whole eggs (Jakociune et al., 2016). These studies further implied that both T3SS systems not only have an important role in host invasion but may also have an association with the survival of Salmonella Enteritidis in egg whites. In addition, SJTUF10978 genotype for sseL was not found in the NCBI database.
Apart from sseL, pseudogenization of secreted effectors seems common in S. enterica serovars (McClelland et al., 2004; Nuccio and Baumler, 2014; Klemm and Dougan, 2016; Klemm et al., 2016; Carden et al., 2017). One of these studies found that loss of the T3SS effector gene sseI is linked to an increased systemic disease in human hosts (Carden et al., 2017). Thus, future investigations are needed to determine whether sseL degradation confers SJTUF10978 better survival in egg whites or in hens. Additionally, the sseL gene is a regulon of the two-component regulatory system SsrA-SsrB and ssrA transcription is regulated by EnvZ-OmpR (Coombes et al., 2007). The co-occurrence of the variations in envZ and sseL further indicates a possibility for the two genes to be involved in the survival of Salmonella Enteritidis in egg whites.
The gene ratA encodes a putative outer membrane protein with unknown function (Kingsley et al., 2003) and was present as a pseudogene in SJTUF10978 via a single nucleotide substitution introducing an internal stop codon (Table 2). The remaining three variant genes due to single nsSNPs were SEN1428, SEN1429, and the plasmid gene rsdB. These genes encoded a putative metal hydrolase, a carboxylesterase and a resolvase (Krause and Guiney, 1991), respectively (Table 2).
Conclusion
In the present study, we conducted the genome sequencing and functional analysis for two Salmonella Enteritidis isolates with similar genetic backgrounds but very different survival abilities in egg whites. Whole-genome sequencing and comparative analysis revealed that variant genes were involved in lysozyme inhibition, vitamin biosynthesis, cell division and DNA damage response, osmotic and oxidative protection, iron-related functions, maintenance of cell envelope structure, amino acid and carbohydrate metabolism, antimicrobial resistance, and T3SS. These may deter the effects of lysozyme, osmotic imbalances, vitamin starvation, and iron limitation. The variations we found in 21 of these genes were inferred in silico to affect functions of the encoding proteins. However, we could not directly determine whether these changes were connected with functional changes. For example, when we modified the nsSNPs in bioC and pliC, and the INDELs in ftsK and yqiJ, we found no phenotypic conversion of the mutants. Nevertheless, deletion of bioC and pliC gene did have phenotypic consequences.
Although the current results have not connected any variation to the survival phenotype of the two strains in egg whites, we suspect this difference is a comprehensive effect of many variations. In view of this, we now have collected more Salmonella Enteritidis strains in our lab. The phenotypic and genotypic analyses on these strains will be carried out in future work, which will provide more insights to the survival mechanism of Salmonella Enteritidis in egg whites. In this study, the two Salmonella Enteritidis isolates could hardly be separated from each other by traditional molecular typing methods such as PFGE and MLVA although they had 76 variations including INDELs and SNPs between the two genomes. This reminds us to be cautious about newly discovered strains in disease traceability and hazard assessment.
Data Availability
The complete genome and plasmid sequences of SJTUF10978 and SJTUF10984 for this study can be found in the GenBank database, https://www.ncbi.nlm.nih.gov/genbank/ (Accession No. CP015524 ∼ CP015527).
Author Contributions
YW, LZ, CW, HO, CS, and XS designed the study. YW performed the experiments and wrote the manuscript. YW, BJ, and LZ analyzed the data. XX, YC, CS, and XS contributed reagents, materials, and strains. All authors reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Dr. Sangwei Lu at the University of California, Berkeley, United States, for technical guidance on the experiments. We also thank Prof. Harold Corke at Shanghai Jiao Tong University for his useful suggestions.
Funding. This study was supported by the National Key R&D program of China (Grant No. 2016YFE0106100) and the National Natural Science Foundation of China (Grant No. 31230058).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02111/full#supplementary-material
References
- Abouzeed Y. M., Baucheron S., Cloeckaert A. (2008). ramR mutations involved in efflux-mediated multidrug resistance in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 52 2428–2434. 10.1128/AAC.00084-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adzhubei I. A., Schmidt S., Peshkin L., Ramensky V. E., Gerasimova A., Bork P., et al. (2010). A method and server for predicting damaging missense mutations. Nat. Methods 7 248–249. 10.1038/nmeth0410-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Mizuno T., Mizushima S. (1989). Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J. Biol. Chem. 264 8563–8567. [PubMed] [Google Scholar]
- Anfora A. T., Welch R. A. (2006). DsdX is the second D-serine transporter in uropathogenic Escherichia coli clinical isolate CFT073. J. Bacteriol. 188 6622–6628. 10.1128/jb.00634-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron F., Bonnassie S., Alabdeh M., Cochet M. F., Nau F., Guerin-Dubiard C., et al. (2017). Global gene-expression analysis of the response of Salmonella Enteritidis to egg white exposure reveals multiple egg white-imposed stress responses. Front. Microbiol. 8:829 10.3389/fmicb.2017.00829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron F., Nau F., Guerin-Dubiard C., Bonnassie S., Gautier M., Andrews S. C., et al. (2016). Egg white versus Salmonella Enteritidis! A harsh medium meets a resilient pathogen. Food Microbiol. 53(Pt B), 82–93. 10.1016/j.fm.2015.09.009 [DOI] [PubMed] [Google Scholar]
- Beckett D. (2007). Biotin sensing: universal influence of biotin status on transcription. Annu. Rev. Genet. 41 443–464. 10.1146/annurev.genet.41.042007.170450 [DOI] [PubMed] [Google Scholar]
- Begley T. P., Kinsland C., Mehl R. A., Osterman A., Dorrestein P. (2001). The Biosynthesis of Nicotinamide Adenine Dinucleotides in Bacteria, in Vitamins & Hormones. Cambridge, MA: Academic Press, 103–119. [DOI] [PubMed] [Google Scholar]
- Benson G. (1999). Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 27 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigot S., Corre J., Louarn J. M., Cornet F., Barre F. X. (2004). FtsK activities in Xer recombination, DNA mobilization and cell division involve overlapping and separate domains of the protein. Mol. Microbiol. 54 876–886. 10.1111/j.1365-2958.2004.04335.x [DOI] [PubMed] [Google Scholar]
- Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw J. G., Shah D. B., Forney E., Madden J. M. (1990). Growth of Salmonella enteritidis in yolk of shell eggs from normal and seropositive hens. J. Food Prot. 53 1033–1036. [DOI] [PubMed] [Google Scholar]
- Braun P., Fehlhaber K. (1995). Migration of Salmonella enteritidis from the albumen into the egg yolk. Int. J. Food Microbiol. 25 95–99. 10.1016/0168-1605(94)00081-g [DOI] [PubMed] [Google Scholar]
- Braun V. (1975). Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim. Biophys. Acta 415 335–377. [DOI] [PubMed] [Google Scholar]
- Buck M., Griffiths E. (1982). Iron mediated methylthiolation of tRNA as a regulator of operon expression in Escherichia coli. Nucleic Acids Res. 10 2609–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert L., Aertsen A., Deckers D., Vanoirbeek K. G., Vanderkelen L., Van Herreweghe J. M., et al. (2008). A new family of lysozyme inhibitors contributing to lysozyme tolerance in gram-negative bacteria. PLoS Pathog. 4:e1000019 10.1371/journal.ppat.1000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert L., Van Herreweghe J. M., Vanderkelen L., Leysen S., Voet A., Michiels C. W. (2012). Guards of the great wall: bacterial lysozyme inhibitors. Trends Microbiol. 20 501–510. 10.1016/j.tim.2012.06.005 [DOI] [PubMed] [Google Scholar]
- Carden S. E., Walker G. T., Honeycutt J., Lugo K., Pham T., Jacobson A., et al. (2017). Pseudogenization of the secreted effector gene sseI confers rapid systemic dissemination of S. Typhimurium ST313 within migratory dendritic cells. Cell Host Microbe 21 182–194. 10.1016/j.chom.2017.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2010). Multistate Outbreak of Human Salmonella Enteritidis Infections Associated with Shell Eggs Available at: http://www.cdc.gov/salmonella/2010/shell-eggs-12-2-10.html [Google Scholar]
- CDC (2015). Multistate Outbreak of Drug-resistant Salmonella Enteritidis Infections Linked to Raw, Frozen, Stuffed Chicken Entrees Produced by Barber Foods Available at: https://www.cdc.gov/salmonella/frozen-chicken-entrees-07-15/index.html [Google Scholar]
- CDC (2016). Multistate Outbreak of Salmonella Oranienburg Infections Linked to Good Earth Egg Company shell eggs. Available at: https://www.cdc.gov/salmonella/oranienburg-10-16/index.html [Google Scholar]
- CDC (2018a). Multistate Outbreak of Salmonella Braenderup Infections Linked to Rose Acre Farms Shell Eggs. Available at: https://www.cdc.gov/salmonella/braenderup-04-18/index.html [Google Scholar]
- CDC (2018b). Multistate Outbreak of Salmonella Typhimurium Linked to Chicken Salad Available at: https://www.cdc.gov/salmonella/typhimurium-02-18/index.html [Google Scholar]
- CDC (2018c). Outbreak of Multidrug-Resistant Salmonella Infections Linked to Raw Turkey Products Available at: https://www.cdc.gov/salmonella/reading-07-18/index.html [Google Scholar]
- Choi Y., Chan A. P. (2015). Provean web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31 2745–2747. 10.1093/bioinformatics/btv195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Sims G. E., Murphy S., Miller J. R., Chan A. P. (2012). Predicting the functional effect of amino acid substitutions and indels. PLoS One 7:e46688 10.1371/journal.pone.0046688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y. J., Saier M. H., Jr. (2002). Overexpression of the Escherichia coli sug E gene confers resistance to a narrow range of quaternary ammonium compounds. J. Bacteriol. 184 2543–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo R. I., Loui C., Andersen G. L., Riley L. W., Lu S. (2006). Identification of genes associated with survival of Salmonella enterica serovar Enteritidis in chicken egg albumen. Appl. Environ. Microbiol. 72 1055–1064. 10.1128/AEM.72.2.1055-1064.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan T. A., Jorgensen F., Lappin-Scott H. M., Benson C. E., Woodward M. J., Humphrey T. J. (2004). Flagella and curli fimbriae are important for the growth of Salmonella enterica serovars in hen eggs. Microbiology 150(Pt 4), 1063–1071. 10.1099/mic.0.26791-0 [DOI] [PubMed] [Google Scholar]
- Coombes B. K., Lowden M. J., Bishop J. L., Wickham M. E., Brown N. F., Duong N., et al. (2007). SseL is a Salmonella-specific translocated effector integrated into the SsrB-controlled Salmonella pathogenicity island 2 type III secretion system. Infect. Immun. 75 574–580. 10.1128/IAI.00985-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling A. E., Mau B., Perna N. T. (2010). progressiveMauve: multiple genome alignment with gene gain, loss and rearrangement. PLoS One 5:e11147 10.1371/journal.pone.0011147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lay N. R., Cronan J. E. (2008). Genetic interaction between the Escherichia coli AcpT phosphopantetheinyl transferase and the YejM inner membrane protein. Genetics 178 1327–1337. 10.1534/genetics.107.081836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vylder J., Raspoet R., Dewulf J., Haesebrouck F., Ducatelle R., Van Immerseel F. (2013). Salmonella Enteritidis is superior in egg white survival compared with other Salmonella serotypes. Poult. Sci. 92 842–845. 10.3382/ps.2012-02668 [DOI] [PubMed] [Google Scholar]
- Del Campillo-Campbell A., Kayajanian G., Campbell A., Adhya S. (1967). Biotin-requiring mutants of Escherichia coli K-12. J. Bacteriol. 94 2065–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez A., Gustavsson N., Nystrom T. (2000). The universal stress protein A of Escherichia coli is required for resistance to DNA damaging agents and is regulated by a RecA/FtsK-dependent regulatory pathway. Mol. Microbiol. 36 1494–1503. 10.1046/j.1365-2958.2000.01979.x [DOI] [PubMed] [Google Scholar]
- Diez A. A., Farewell A., Nannmark U., Nystrom T. (1997). A mutation in the ftsK gene of Escherichia coli affects cell-cell separation, stationary-phase survival, stress adaptation, and expression of the gene encoding the stress protein UspA. J. Bacteriol. 179 5878–5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudkiewicz M., Szczepinska T., Grynberg M., Pawlowski K. (2012). A novel protein kinase-like domain in a selenoprotein, widespread in the tree of life. PLoS One 7:e32138 10.1371/journal.pone.0032138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. D., Black S., Rasmussen T., Rasmussen A., Stokes N. R., Stephen T. L., et al. (2012). Characterization of three novel mechanosensitive channel activities in Escherichia coli. Channels 6 272–281. 10.4161/chan.20998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. A., Keller L. H., Schifferli D. M. (1998). Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 207 149–157. 10.1016/S0378-1119(97)00619-7 [DOI] [PubMed] [Google Scholar]
- Forst S., Delgado J., Inouye M. (1989). Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 86 6052–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Gast R., Humphrey T. J., et al. (2009). Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiol. Rev. 33 718–738. 10.1111/j.1574-6976.2008.00161.x [DOI] [PubMed] [Google Scholar]
- Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Van Immerseel F. (2008a). Salmonella enterica serovar Enteritidis genes induced during oviduct colonization and egg contamination in laying hens. Appl. Environ. Microbiol. 74 6616–6622. 10.1128/AEM.01087-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I., Eeckhaut V., Pasmans F., Haesebrouck F., Ducatelle R., Van Immerseel F. (2008b). A comparative study on the pathogenesis of egg contamination by different serotypes of Salmonella. Avian Pathol. 37 399–406. 10.1080/03079450802216611 [DOI] [PubMed] [Google Scholar]
- Giel J. L., Rodionov D., Liu M., Blattner F. R., Kiley P. J. (2006). IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol. Microbiol. 60 1058–1075. 10.1111/j.1365-2958.2006.05160.x [DOI] [PubMed] [Google Scholar]
- Gimenez R., Nunez M. F., Badia J., Aguilar J., Baldoma L. (2003). The gene yjcG, cotranscribed with the gene acs, encodes an acetate permease in Escherichia coli. J. Bacteriol. 185 6448–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard-Bouldin J., Gast R. K., Humphrey T. J., Henzler D. J., Morales C., Coles K. (2004). Subpopulation characteristics of egg-contaminating Salmonella enterica serovar Enteritidis as defined by the lipopolysaccharide O chain. Appl. Environ. Microbiol. 70 2756–2763. 10.1128/Aem.70.5.2756-2763.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guard-Petter J. (2001). The chicken, the egg and Salmonella enteritidis. Environ. Microbiol. 3 421–430. [DOI] [PubMed] [Google Scholar]
- Guard-Petter J., Parker C. T., Asokan K., Carlson R. W. (1999). Clinical and veterinary isolates of Salmonella enterica serovar Enteritidis defective in lipopolysaccharide O-chain polymerization. Appl. Environ. Microbiol. 65 2195–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H., Narita S., Karibian D., Park J. T., Yamamoto Y., Nishimura Y. (2002). Identification and characterization of the Escherichia coli envC gene encoding a periplasmic coiled-coil protein with putative peptidase activity. FEMS Microbiol. Lett. 212 229–236. [DOI] [PubMed] [Google Scholar]
- He G. X., Zhang C., Crow R. R., Thorpe C., Chen H., Kumar S., et al. (2011). SugE, a new member of the SMR family of transporters, contributes to antimicrobial resistance in Enterobacter cloacae. Antimicrob. Agents Chemother. 55 3954–3957. 10.1128/AAC.00094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht M., Bromberg Y., Rost B. (2015). Better prediction of functional effects for sequence variants. BMC Genomics 16(Suppl. 8):S1. 10.1186/1471-2164-16-s8-s1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidrich C., Ursinus A., Berger J., Schwarz H., Holtje J. V. (2002). Effects of multiple deletions of murein hydrolases on viability, septum cleavage, and sensitivity to large toxic molecules in Escherichia coli. J. Bacteriol. 184 6093–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989). Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77 51–59. [DOI] [PubMed] [Google Scholar]
- Ibrahim H. R., Thomas U., Pellegrini A. (2001). A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J. Biol. Chem. 276 43767–43774. 10.1074/jbc.M106317200 [DOI] [PubMed] [Google Scholar]
- Inoue T., Shingaki R., Hirose S., Waki K., Mori H., Fukui K. (2007). Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J. Bacteriol. 189 950–957. 10.1128/jb.01294-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L. M., Makarova K. S., Koonin E. V., Aravind L. (2004). Comparative genomics of the FtsK-HerA superfamily of pumping ATPases: implications for the origins of chromosome segregation, cell division and viral capsid packaging. Nucleic Acids Res. 32 5260–5279. 10.1093/nar/gkh828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakociune D., Herrero-Fresno A., Jelsbak L., Olsen J. E. (2016). Highly expressed amino acid biosynthesis genes revealed by global gene expression analysis of Salmonella enterica serovar Enteritidis during growth in whole egg are not essential for this growth. Int. J. Food Microbiol. 224 40–46. 10.1016/j.ijfoodmicro.2016.02.015 [DOI] [PubMed] [Google Scholar]
- Jiang M., Chen X., Guo Z. F., Cao Y., Chen M., Guo Z. (2008). Identification and characterization of (1R,6R)-2-succinyl-6-hydroxy-2,4-cyclohexadiene-1-carboxylate synthase in the menaquinone biosynthesis of Escherichia coli. Biochemistry 47 3426–3434. 10.1021/bi7023755 [DOI] [PubMed] [Google Scholar]
- Khil P. P., Camerini-Otero R. D. (2002). Over 1000 genes are involved in the DNA damage response of Escherichia coli. Mol. Microbiol. 44 89–105. 10.1046/j.1365-2958.2002.02878.x [DOI] [PubMed] [Google Scholar]
- Kingsley R. A., Humphries A. D., Weening E. H., De Zoete M. R., Winter S., Papaconstantinopoulou A., et al. (2003). Molecular and phenotypic analysis of the CS54 island of Salmonella enterica serotype Typhimurium: identification of intestinal colonization and persistence determinants. Infect. Immun. 71 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm E., Dougan G. (2016). Advances in understanding bacterial pathogenesis gained from whole-genome sequencing and phylogenetics. Cell Host Microbe 19 599–610. 10.1016/j.chom.2016.04.015 [DOI] [PubMed] [Google Scholar]
- Klemm E. J., Gkrania-Klotsas E., Hadfield J., Forbester J. L., Harris S. R., Hale C., et al. (2016). Emergence of host-adapted Salmonella Enteritidis through rapid evolution in an immunocompromised host. Nat. Microbiol. 1:15023 10.1038/Nmicrobiol.2015.23 [DOI] [PubMed] [Google Scholar]
- Krause M., Guiney D. G. (1991). Identification of a multimer resolution system involved in stabilization of the Salmonella dublin virulence plasmid pSDL2. J. Bacteriol. 173 5754–5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan C. Y., Igo M. M. (1998). Differential expression of the OmpF and OmpC porin proteins in Escherichia coli K-12 depends upon the level of active OmpR. J. Bacteriol. 180 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B., Salzberg S. L. (2012). Fast gapped-read alignment with Bowtie 2. Nat. Methods 9 357–U354. 10.1038/Nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen M. V., Cosentino S., Rasmussen S., Friis C., Hasman H., Marvig R. L., et al. (2012). Multilocus sequence typing of total-genome-sequenced bacteria. J. Clin. Microbiol. 50 1355–1361. 10.1128/JCM.06094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon J. (2006). Plotrix: a package in the red light district of R. R-News 6 8–12. [Google Scholar]
- Li H., Durbin R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 1754–1760. 10.1093/bioinformatics/btp324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., et al. (2009). The sequence alignment/map format and samtools. Bioinformatics 25 2078–2079. 10.1093/bioinformatics/btp352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg F., Tennent J. M., Hultgren S. J., Lund B., Normark S. (1989). PapD, a periplasmic transport protein in P-pilus biogenesis. J. Bacteriol. 171 6052–6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Killoran P. B., Riley L. W. (2003). Association of Salmonella enterica serovar Enteritidis yafD with resistance to chicken egg albumen. Infect. Immun. 71 6734–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S., Manges A. R., Xu Y., Fang F. C., Riley L. W. (1999). Analysis of virulence of clinical isolates of Salmonella Enteritidis in vivo and in vitro. Infect. Immun. 67 5651–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet S., Bellais S., Dubost L., Fourgeaud M., Mainardi J. L., Petit-Frere S., et al. (2007). Identification of the L,D-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J. Bacteriol. 189 3927–3931. 10.1128/JB.00084-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Bo Y., Han L., He J., Lanczycki C. J., Lu S., et al. (2017). CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 45 D200–D203. 10.1093/nar/gkw1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli F., Davies R. H. (2012). Salmonella serovars isolated from table eggs: an overview. Food Res. Int. 45 745–754. 10.1016/j.foodres.2011.03.054 [DOI] [Google Scholar]
- Massey T. H., Mercogliano C. P., Yates J., Sherratt D. J., Lowe J. (2006). Double-stranded DNA translocation: structure and mechanism of hexameric FtsK. Mol. Cell. 23 457–469. 10.1016/j.molcel.2006.06.019 [DOI] [PubMed] [Google Scholar]
- McClelland M., Sanderson K. E., Clifton S. W., Latreille P., Porwollik S., Sabo A., et al. (2004). Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat. Genet. 36 1268–1274. 10.1038/ng1470 [DOI] [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. (1969). Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J. Biol. Chem. 244 5382–5391. [PubMed] [Google Scholar]
- Mesquita F. S., Thomas M., Sachse M., Santos A. J., Figueira R., Holden D. W. (2012). The Salmonella deubiquitinase SseL inhibits selective autophagy of cytosolic aggregates. PLoS Pathog. 8:e1002743 10.1371/journal.ppat.1002743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messens W., Grijspeerdt K., Herman L. (2005). Eggshell penetration by Salmonella: a review. Worlds Poult. Sci. J. 61 71–85. 10.1079/Wps200443 [DOI] [Google Scholar]
- Mi H. Y., Huang X. S., Muruganujan A., Tang H. M., Mills C., Kang D., et al. (2017). PANTHER version 11: expanded annotation data from gene ontology and reactome pathways, and data analysis tool enhancements. Nucleic Acids Res. 45 D183–D189. 10.1093/nar/gkw1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales C. A., Porwollik S., Frye J. G., Kinde H., McClelland M., Guard-Bouldin J. (2005). Correlation of phenotype with the genotype of egg-contaminating Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 71 4388–4399. 10.1128/AEM.71.8.4388-4399.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio S. P., Baumler A. J. (2014). Comparative analysis of Salmonella genomes identifies a metabolic network for escalating growth in the inflamed gut. mBio 5:e00929-14. 10.1128/mBio.00929-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan E., Quinn T., Pages J. M., McCusker M., Piddock L., Fanning S. (2009). Multiple regulatory pathways associated with high-level ciprofloxacin and multidrug resistance in Salmonella enterica serovar enteritidis: involvement of RamA and other global regulators. Antimicrob. Agents Chemother. 53 1080–1087. 10.1128/AAC.01005-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outten F. W., Djaman O., Storz G. (2004). A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol. Microbiol. 52 861–872. 10.1111/j.1365-2958.2004.04025.x [DOI] [PubMed] [Google Scholar]
- Pellegrini A., Thomas U., Wild P., Schraner E., von Fellenberg R. (2000). Effect of lysozyme or modified lysozyme fragments on DNA and RNA synthesis and membrane permeability of Escherichia coli. Microbiol. Res. 155 69–77. 10.1016/S0944-5013(00)80040-3 [DOI] [PubMed] [Google Scholar]
- R Development Core Team. (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Ramos-Morales F., Prieto A. I., Beuzon C. R., Holden D. W., Casadesus J. (2003). Role for Salmonella enterica enterobacterial common antigen in bile resistance and virulence. J. Bacteriol. 185 5328–5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspoet R., Shearer N., Appia-Ayme C., Haesebrouck F., Ducatelle R., Thompson A., et al. (2014). A genome-wide screen identifies Salmonella Enteritidis lipopolysaccharide biosynthesis and the HtrA heat shock protein as crucial factors involved in egg white persistence at chicken body temperature. Poult. Sci. 93 1263–1269. 10.3382/ps.2013-03711 [DOI] [PubMed] [Google Scholar]
- Rodionova I. A., Li X., Thiel V., Stolyar S., Stanton K., Fredrickson J. K., et al. (2013). Comparative genomics and functional analysis of rhamnose catabolic pathways and regulons in bacteria. Front. Microbiol. 4:407 10.3389/fmicb.2013.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossolini G. M., Muscas P., Chiesurin A., Satta G. (1993). Analysis of the Salmonella fim gene cluster: identification of a new gene (fimI) encoding a fimbrin-like protein and located downstream from the fimA gene. FEMS Microbiol. Lett. 114 259–265. [DOI] [PubMed] [Google Scholar]
- Rubires X., Saigi F., Pique N., Climent N., Merino S., Alberti S., et al. (1997). A gene (wbbL) from Serratia marcescens N28b (O4) complements the rfb-50 mutation of Escherichia coli K-12 derivatives. J. Bacteriol. 1797581–7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytkonen A., Poh J., Garmendia J., Boyle C., Thompson A., Liu M., et al. (2007). SseL, A Salmonella deubiquitinase required for macrophage killing and virulence. Proc. Natl. Acad. Sci. U.S.A. 104 3502–3507. 10.1073/pnas.0610095104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurgers L. J., Vermeer C. (2000). Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis 30 298–307. [DOI] [PubMed] [Google Scholar]
- Shah D. H., Casavant C., Hawley Q., Addwebi T., Call D. R., Guard J. (2012). Salmonella Enteritidis strains from poultry exhibit differential responses to acid stress, oxidative stress, and survival in the egg albumen. Foodborne Pathog. Dis. 9 258–264. 10.1089/fpd.2011.1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim N. L., Kumar P., Hu J., Henikoff S., Schneider G., Ng P. C. (2012). SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 40 W452–W457. 10.1093/nar/gks539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G., Lee S. J., Romana L. K., Reeves P. R. (1991). The gene cluster of Salmonella strain LT2 includes a second mannose pathway: sequence of two genes and relationship to genes in the rfb gene cluster. Mol. Gen. Genet. 227 173–180. [DOI] [PubMed] [Google Scholar]
- Su H., Newman E. B. (1991). A novel L-serine deaminase activity in Escherichia coli K-12. J. Bacteriol. 173 2473–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxell B., Fink R. C., Porwollik S., McClelland M., Hassan H. M. (2011). The Fur regulon in anaerobically grown Salmonella enterica sv. Typhimurium: identification of new Fur targets. BMC Microbiol. 11:236 10.1186/1471-2180-11-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T., Morizane T., Abo T., Tominaga A., Inoue-Tanaka K., Kutsukake K. (2011). EAL domain protein YdiV acts as an anti-FlhD4C2 factor responsible for nutritional control of the flagellar regulon in Salmonella enterica Serovar Typhimurium. J. Bacteriol. 193 1600–1611. 10.1128/JB.01494-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. L., Lutkenhaus J. (1998). FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Mol. Microbiol. 29 731–740. 10.1046/j.1365-2958.1998.00958.x [DOI] [PubMed] [Google Scholar]
- Wood J. M. (2015). Bacterial responses to osmotic challenges. J. Gen. Physiol. 145 381–388. 10.1085/jgp.201411296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Tabor C. W., Tabor H. (1989). Spermidine biosynthesis in Escherichia coli: promoter and termination regions of the speED operon. J. Bacteriol. 171 4457–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K., Wang M., Metcalf W. W. (2009). Uptake of glycerol-2-phosphate via the ugp-encoded transporter in Escherichia coli K-12. J. Bacteriol. 191 4667–4670. 10.1128/JB.00235-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim L., Betancor L., Martinez A., Giossa G., Bryant C., Maskell D., et al. (2010). Differential phenotypic diversity among epidemic-spanning Salmonella enterica serovar Enteritidis isolates from humans or animals. Appl. Environ. Microbiol. 76 6812–6820. 10.1128/AEM.00497-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., et al. (2012). Identification of acquired antimicrobial resistance genes. J. Antimicrobial Chemotherapy 67 2640–2644. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S. K., Yin Y. L., Jones M. B., Zhang Z. Z., Kaiser B. L. D., Dinsmore B. A., et al. (2015). Salmonella serotype determination utilizing high-throughput genome sequencing data. J. Clin. Microbiol. 53 1685–1692. 10.1128/Jcm.00323-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete genome and plasmid sequences of SJTUF10978 and SJTUF10984 for this study can be found in the GenBank database, https://www.ncbi.nlm.nih.gov/genbank/ (Accession No. CP015524 ∼ CP015527).